Abstract
Spinal cord injury (SCI) results in inflammation, and TLR4, which is an inflammatory factor, has an important role in the pathological injury that occurs following SCI. Recently, bone marrow stromal cells (BMSCs) have been demonstrated to be a novel treatment in SCI. However, the underlying mechanism of neuroprotection in SCI by BMSCs remains unclear. The present study was designed to investigate the therapeutic mechanism of BMSCs in SCI by analysis of Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) expression. The present results demonstrated that BMSC transplantation promoted functional recovery and tissue repair in SCI rats. Interestingly, it also reduced the expression of TLR4 and NF-κB after SCI. Furthermore, it was demonstrated that BMSCs downregulated the expression of apoptosis factor caspase-12 in the SCI rat model. The present results demonstrated that BMSCs may have incorporated into the spinal cord to improve locomotor function after SCI, partly via the TLR4/NF-κB signaling pathway. To the best of our knowledge, this is the first study to determine that BMSCs prevented secondary injury and enhanced functional recovery in SCI via inhibition of TLR4/NF-κB-mediated inflammation.
Keywords: spinal cord injury, bone marrow stromal cells, inflammation, nuclear factor-κB, Toll-like receptor-4
Introduction
Spinal cord injury (SCI) causes serious disability and is a medical problem worldwide (1). SCI has two defined phases, consisting of primary and secondary injury mechanisms that lead to an excessive inflammatory response (2,3). Disruption of the spinal tract results in neuronal apoptosis, and impedes neuronal repair and regeneration. A key challenge is how to reduce inflammation, improve axonal regeneration and functional recovery after SCI (4). Recently, transplantation of bone marrow stromal cells (BMSCs) has emerged as a novel treatment for SCI (5–7). BMSCs are a population of heterogeneous mesenchymal cells located in the bone marrow that possess unlimited proliferative capacity and multidifferentiation properties (8,9). Furthermore, it has been reported that BMSCs have an important role in the immunomodulation of innate and adaptive immune processes in vitro and in vivo (10,11). BMSCs transplantation may benefit injured neurons, through the release of various kinds of factors, which indirectly influence the process of inflammation by regulating the expression of inflammatory cytokines from a variety of immune cell types after SCI (12–15). However, the mechanisms underlying the regulation of inflammation by BMSCs in the injured spinal cord remain unclear.
Secondary SCI is accompanied by a series of intracellular metabolisms, such as inflammatory cell infiltration. After SCI, the blood-brain barrier (BBB) is disrupted and inflammatory cells produce potentially toxic molecules, including free oxygen radicals, cytokines and chemokines which may inhibit axon regeneration of the spinal lesion (2,4). Toll-like receptors (TLRs) are a transmembrane receptor family. Activation of the TLRs has a critical role in the innate immune response (16). Toll-like receptor 4 (TLR4) is an important member that is associated with SCI-induced inflammation. Accumulating evidence indicates the involvement of TLR4 in inducing spinal inflammation, including that in lateral sclerosis, ischemia reperfusion injury and trauma (17,18). As one of the most important downstream molecules in the TLR signaling pathways, nuclear factor (NF)-κB is a transcriptional factor required for transcriptional activation of its target genes, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 (19,20).
Therefore, in the present study, a modified Allen's weight-drop SCI rat model was established and BMSCs were transplanted into the injured spinal cord. Locomotion recovery and pathological changes in the spinal cord of the SCI rat model were analyzed after BMSC transplantation. Furthermore, the effect of BMSCs on modulating the expressions of TLR4 and NF-κB in the injured spinal cord was investigated. The present study may challenge the classical view of stem cell transplant therapy for SCI, not only through neuronal differentiation, but also in reducing inflammation.
Materials and methods
Ethics statement
The experimental procedures were approved by the Animal Ethics Committee of Zhejiang University (Hangzhou, China) and were performed according to institutional guidelines. All efforts were made to minimize the number of rats used and their suffering.
Primary BMSC culture and characterization
Primary rat BMSCs were isolated as previously described (7). BMSCs were harvested from the femur of 3-week-old Sprague-Dawley (SD) female rats. Bone marrow was removed and diluted with an equal volume of Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), which was subsequently centrifuged at 1,200 × g for 7 min. The supernatant was removed, and the pellet was inoculated into plastic flasks containing DMEM supplemented with 10% fetal bovine serum (FBS; 10% w/v; Gibco; Thermo Fisher Scientific, Inc.), 1% L-glutamine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 1% penicillin and streptomycin. The flasks were incubated at 37°C in a humidified tissue culture incubator containing 5% CO2 and 95% air. The medium was replaced every 3 days, and cells were passaged at 1:4 when 90% confluence was reached, using 0.25% trypsin. All stem cells in this experiment were performed with cells in passage 3.
SCI model
Thirty 6-week-old SD female rats were purchased from Zhejiang Experimental Animal Center (Hangzhou, China) and divided into three groups at random: sham operation (control) group, SCI group and BMSC-treated SCI group. Rats were anesthetized with an intraperitoneal injection of 40 mg/kg sodium pentobarbital. The vertebral column of the rats was then exposed, and a laminectomy carried out at T10 vertebrae. A weight of 10 g was dropped from a height of 5 cm onto the exposed spinal cord to cause moderate contusion at the T10 vertebrae in the SCI group and BMSC treatment group rats (6). The sham operation rats received the same surgical procedure, with no injury. After injury, 10 µl DMEM containing 1×106 BMSCs was injected into the center of the injured spinal cords of the BMSC treatment group rats, using electrode microneedles. The same volume of cell culture media was injected into the SCI and sham operation animals. All rats were subcutaneously injected with ampicillin (100 mg/kg) daily for the first 7 days to prevent infection. Twice per day, the bladder was emptied manually. A certain number of rats were sacrificed for immunohistochemical staining of TLR4, NF-κB, and caspase-12 48 h after SCI, while others were sacrificed 7 days after SCI, for growth-associated protein 43 (GAP-43) immunofluorescence staining, hematoxylin and eosin (H&E) Staining and toluidine blue (Nissl).
Assessment of motor function
The Basso, Beattie, and Bresnahan (BBB) locomotor scale was used to evaluate the rats after transplantation (21). The ranging scale from 0 (complete paralysis) to 21 (normal locomotion) was applied to evaluate the motor function in an open field for 2–3 min in all groups at days 1, 7, 14 and 21 after surgery in all three groups.
H&E and Nissl staining
For pathological analysis, the sections were respectively subjected to H&E and Nissl staining. Five rats from each group were anesthetized with an intraperitoneal injection of 60 mg/kg sodium pentobarbital, and perfused with 4% paraformaldehyde in PBS 7 days after SCI. The lesion epicenter (4 mm) of the injured spinal cord was removed, fixed for 24 h and prepared for cryostat sectioning. The transverse sections (10 µm thick) were mounted in silane-coated slides. Rat sections were subsequently stained with cresyl violet (0.3%; VWR International, Buffalo Grove, IL, USA) for H&E and Nissl Staining. All images were collected in the same area of the section using an Olympus BX61 microscope (Olympus Corporation, Tokyo, Japan). Area measurements were performed using Image-Pro Plus 5.0 image analysis software (Media Cybernetics Inc., Atlanta, GA, USA).
Immunohistochemical and immunofluorescence staining
Tissue sections from all groups were washed in 0.01 M PBS containing 0.3% Triton X-100 (pH 7.4, PBS-T) for immunohistochemical analysis, prior to immersion in 2% normal horse serum in PBS for 120 min at 37°C and incubation overnight at 4°C with polyclonal rabbit anti-NF-κB, TLR4 and caspase-12 (1:200; Boster Biotechnology Co., Ltd., Wuhan, China) antibodies. Sections were washing with PBS and subsequently incubated with HRP goat anti-rabbit immunoglobulin G (IgG; 1:200; Boster Biotechnology Co., Ltd.) secondary antibody for 1 h. Hematoxylin was used to counterstain nuclei. The samples were mounted in Fluoromount/Plus (Diagnostic BioSystems, Pleasanton, CA, USA). Images were obtained on an Axio Imager M1 microscope with AxioVision software (Carl Zeiss, Tokyo, Japan).
Immunofluorescence analysis was used to detect the expression of GAP-43 in all groups. The sections were incubated overnight with GAP-43 antibody (1:100; Boster Biotechnology Co., Ltd.) overnight at 4°C, washed three times with PBS and subsequently incubated with fluorescent-conjugated secondary antibody. Slides were counterstained with DAPI for 5 min and coverslipped. All images were captured in the same area of the section using an Olympus FluoView FV1000 Confocal laser scanning microscope.
Statistical analysis
Data are presented as mean ± standard deviation. One-way analysis of variance (ANOVA) with a post-hoc Tukey's test was used for comparisons between more than two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
BBB scores
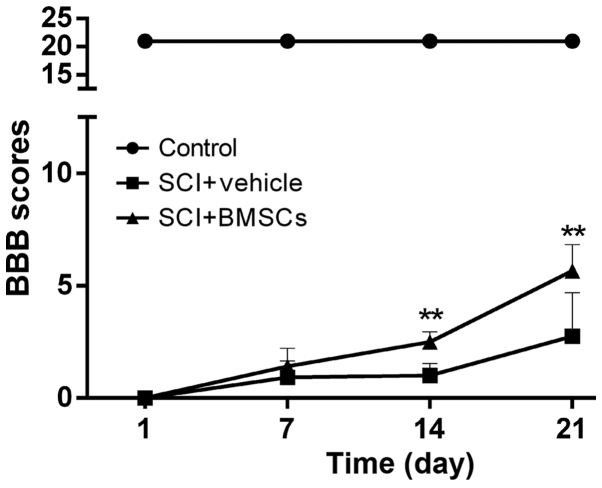
1 day after SCI establishment, rats in the SCI and BMSC-treated groups displayed typical paraplegia syndrome; the tail was dropped and both hind limbs were paralyzed with muscle strength scores of 0. Rats were followed up at 14 and 21 days after BMSC transplantation. The BBB score in the BMSCs treatment group was significantly higher than that of the SCI group (P<0.05), while the animals in the sham operation group walked normally. Comparison of BMSC treatment rats at different time points revealed that the BBB score exhibited a gradual upward trend at 7, 14 and 21 days after SCI. The improvement in the BMSC treatment rats was significantly different from the SCI rats at 14 and 21 days after BMSCs transplantation (P<0.05; Fig. 1).
Figure 1.
BBB Scores at 1, 7, 14 and 21 days after bone marrow stromal cell transplantation in each group. Values are presented as the mean ± standard deviation. **P<0.01 vs. SCI rats. BBB, blood-brain barrier.
H&E and Nissl staining assay
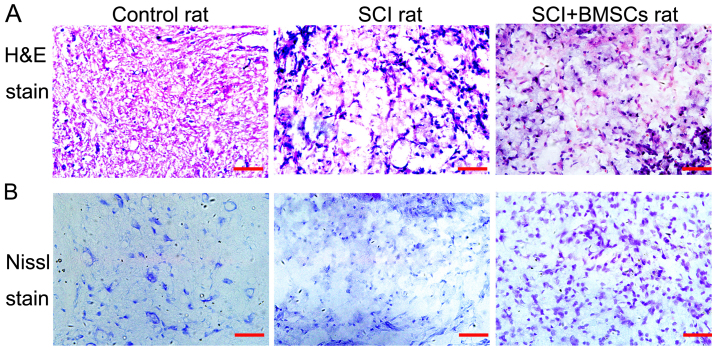
7 days after BMSC transplantation, H&E staining was used to analyze pathological changes after SCI. The results demonstrated that neurons in the sham operation group appeared normal, with intact, round, full nuclei and clear nucleoli. However, neuronal swelling and shrunken neurons with darkly stained, condensed nuclei, as well as significant loss and damage to neuronal and glial cells, were observed in the SCI and SCI+BMSCs groups (Fig. 2). Furthermore, the tissues appeared disorderly and irregularly arranged. However, the pathological changes observed improved with BMSC treatment, as the cavity of the SCI+BMSC group was reduced, compared with the SCI group. In addition, Nissl staining was used to investigate the extent of neuronal demyelination at the injury site. It was observed that the sections from the sham group contained myelin sheaths of different diameters in an orderly arrangement with even distribution whereas demyelination was present after injury, with disorderly arrangement and irregular distribution, accompanied by numerous swellings and near-disruption in the SCI group. However, these changes were improved in the BMSCs treatment group (Fig. 2B). Both H&E staining and Nissl staining results indicated that BMSC treatment improved the pathological changes induced after SCI.
Figure 2.
(A) H&E staining and (B) Nissl staining in the spinal cord of each group, 7 days after the BMSC group received treatment. Scale bar=50 µm (magnification, ×400). H&E, hematoxylin and eosin; BMSC, bone marrow stromal cell; SCI, spinal cord injury.
TLR4, NF-κB, and caspase-12 expression in the injured spinal cord
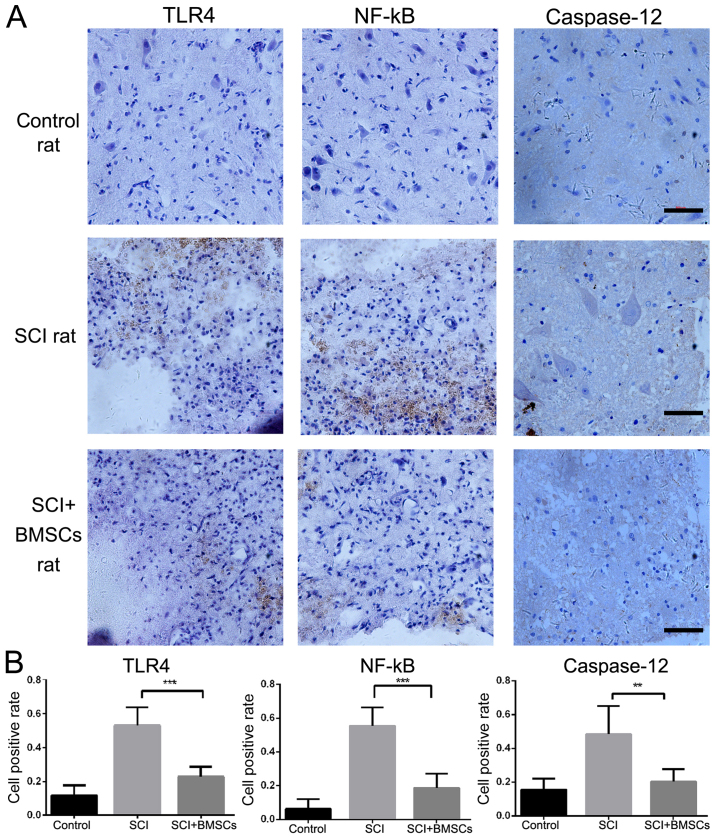
As cavity formation was reduced under BMSCs treatment, whether BMSCs transplantation reduced the inflammatory response and neuronal apoptosis was investigated. The expression of the inflammatory factors TLR4 and NF-κB, as well as apoptosis factor caspase-12, was detected by immunohistochemical staining. The results revealed that both TLR4 and NF-κB expression increased after SCI, However, BMSC treatment resulted in a significant decrease in expression, compared with the SCI group (P<0.01). The expression of caspase-12 was markedly upregulated after SCI. BMSC treatment resulted in significantly lower caspase-12 levels compared with the SCI group (P<0.05; Fig. 3), indicating that neuronal apoptosis caused by SCI was significantly improved by BMSC treatment. Taken together, the results demonstrated that BMSC transplant reduced the inflammatory response and apoptotic cell death following SCI.
Figure 3.
(A) Immunohistochemical staining of TLR4, NF-κB and caspase-12 expression, (B) the proportion of TLR4, NF-κB and caspase-12 positive cells in each group, 24 h post-surgery. Scale bar=500 µm (magnification, ×400). ***P<0.01, vs. SCI rats, **P<0.05, vs. SCI rats. TLR4, Toll-like receptor 4; NF-κB, nuclear factor-κB; BMSC, bone marrow stromal cell; SCI, spinal cord injury.
GAP43 expression is promoted by BMSC transplantation
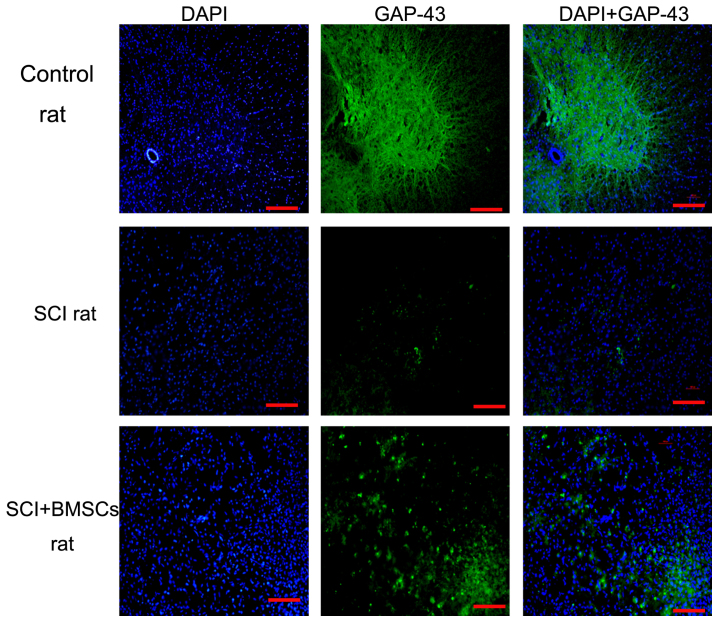
It was hypothesized that decreased inflammation and apoptosis may promote axon regeneration. The present study focused on spinal GAP43 expression 7 days after BMSC transplantation. GAP43 is highly expressed in the axons, and is the most widely used marker for nerve regeneration. By immunofluorescence staining, it was demonstrated that GAP43-positive fibers were significantly increased in response to BMSC treatment (Fig. 4), indicating that BMSC treatment increased GAP43 expression and may have supported axon regeneration.
Figure 4.
Immunofluorescence detection of GAP-43 expression in each group, 7 days after BMSC transplantation. Green staining indicates GAP-43 expression. Blue staining (DAPI) indicates the nuclei. GAP-43+DAPI=merges. Scale bar=500 µm (magnification, ×40). GAP-43, growth-associated protein 43; BMSC, bone marrow stromal cell; SCI, spinal cord injury.
Discussion
SCI is a serious disease that eventually results in loss of movement and sensation below the lesion (15,22). After SCI occurs, the inflammatory response has an important role in the pathogenesis of the injured spinal cord (23,24). The present study demonstrated that BMSC transplantation suppressed the expression of TLR4 and NF-κB in the injured spinal cord after trauma. Furthermore, locomotor deficits, pathological changes in the spinal cord and caspase-12 expression were markedly ameliorated following BMSCs transplantation to SCI rats. To the best of our knowledge, the present study is the first to demonstrate that BMSCs transplantation improved functional recovery following SCI by inhibiting the inflammatory response via TLR4/NF-κB signaling.
After SCI, inflammatory cell infiltration and hypoxia occur around the original injury site. These inflammatory responses begin immediately after injury, and continue in the days and weeks following SCI. This process results in further tissue damage, leading to the loss of motor and sensory function below the lesion (3). The inflammatory response in the injured spinal cord is a key mechanism mediating the secondary injury stage after SCI (2,25). The cells at the site of injury trigger the inflammatory response through the activation of multiple receptors, including TLR4, in immune-competent cells, neurons and astrocytes (26). These receptors promote the activation of NF-κB, a multifunctional transcription factor that controls several pro-inflammatory and stress responses, which may ultimately induce apoptosis, neuronal degeneration and disease progression (27). A previous study revealed that the ideal treatment for SCI would focus on preventing inflammation in the injured spinal cord, improving the microenvironment around the injured lesion and promoting axonal regeneration (27). Our results demonstrated that SCI activated TLR4/NF-κB signaling and increased caspase-12 expression in the injured spinal cord. Furthermore, BMSC transplantation significantly improved the pathological changes observed and rat locomotor function; this may have been through the suppression of TLR4 and NF-κB expression in the injured spinal cord. Furthermore, the number of caspase-12 positive cells was notably decreased in BMSC-treated SCI rats.
In conclusion, the present study demonstrated that BMSC transplantation to the injured spinal cord may contribute to functional restoration in SCI rats. BMSC treatment gradually improved the injured spinal cord tissue and dramatically decreased the number of caspase-12 positive cells, potentially through downregulation of TLR4/NF-κB expression. The results provided a novel insight into the therapeutic potential of BMSCs, which may lead to the development of a novel therapeutic approach to prevent inflammation following SCI.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang Provincial Natural Science Foundation of China (grant no. LY15H090002) and the Public Applied Technology Research Project of Zhejiang Province (grant no. 2015C37081).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
SB performed the experiments and wrote the manuscript. HZ performed the experiments and analyzed the data. LW contributed in designing the study and revising the manuscript.
Ethics approval and consent to participate
The experimental procedures were approved by the Animal Ethics Committee of Zhejiang University (Hangzhou, China) and were performed according to institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rubiano AM, Carney N, Chesnut R, Puyana JC. Global neurotrauma research challenges and opportunities. Nature. 2015;527:S193–S197. doi: 10.1038/nature16035. [DOI] [PubMed] [Google Scholar]
- 2.Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53:14–18. doi: 10.1038/sc.2014.184. [DOI] [PubMed] [Google Scholar]
- 3.Orr MB, Simkin J, Bailey WM, Kadambi NS, McVicar AL, Veldhorst AK, Gensel JC. Compression Decreases Anatomical and Functional Recovery and Alters Inflammation after Contusive Spinal Cord Injury. J Neurotrauma. 2017;34:2342–2352. doi: 10.1089/neu.2016.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada S. The pathophysiological role of acute inflammation after spinal cord injury. Inflamm Regen. 2016;36:20. doi: 10.1186/s41232-016-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L, Lin H, Bai S, Zheng L, Zhang X. Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration. Neurochem Int. 2018;115:80–84. doi: 10.1016/j.neuint.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Pu Y, Meng K, Gu C, Wang L, Zhang X. Thrombospondin-1 modified bone marrow mesenchymal stem cells (BMSCs) promote neurite outgrowth and functional recovery in rats with spinal cord injury. Oncotarget. 2017;8:96276–96289. doi: 10.18632/oncotarget.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu C, Li H, Wang C, Song X, Ding Y, Zheng M, Liu W, Chen Y, Zhang X, Wang L. Bone marrow mesenchymal stem cells decrease CHOP expression and neuronal apoptosis after spinal cord injury. Neurosci Lett. 2017;636:282–289. doi: 10.1016/j.neulet.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Tang X, Chen F, Lin Q, You Y, Ke J, Zhao S. Bone marrow mesenchymal stem cells repair the hippocampal neurons and increase the expression of IGF-1 after cardiac arrest in rats. Exp Ther Med. 2017;14:4312–4320. doi: 10.3892/etm.2017.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Chen J, Chai W, Ni M, Sun X, Tian D. Glycitin regulates osteoblasts through TGF-β or AKT signaling pathways in bone marrow stem cells. Exp Ther Med. 2016;12:3063–3067. doi: 10.3892/etm.2016.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng YH, Deng YY, Lai W, Zheng SY, Bian HN, Liu ZA, Huang ZF, Sun CW, Li HH, Luo HM, et al. Effect of bone marrow mesenchymal stem cells on the polarization of macrophages. Mol Med Rep. 2018;17:4449–4459. doi: 10.3892/mmr.2018.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ock SA, Baregundi Subbarao R, Lee YM, Lee JH, Jeon RH, Lee SL, Park JK, Hwang SC, Rho GJ. Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue. Stem Cells Int. 2016;2016:9581350. doi: 10.1155/2016/9581350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ide C, Nakai Y, Nakano N, Seo TB, Yamada Y, Endo K, Noda T, Saito F, Suzuki Y, Fukushima M, et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Hawryluk GW, Mothe A, Wang J, Wang S, Tator C, Fehlings MG. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21:2222–2238. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W, Yang Y, Yang JY, Liang M, Song J. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway. Int J Mol Med. 2016;37:1075–1082. doi: 10.3892/ijmm.2016.2498. [DOI] [PubMed] [Google Scholar]
- 15.Cho SR, Kim YR, Kang HS, Yim SH, Park CI, Min YH, Lee BH, Shin JC, Lim JB. Functional Recovery after the Transplantation of Neurally Differentiated Mesenchymal Stem Cells Derived from Bone Marrow in a Rat Model of Spinal Cord Injury. Cell Transplant. 2016;25:1423. doi: 10.3727/096368916X692078. [DOI] [PubMed] [Google Scholar]
- 16.Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol. 2014;426:1246–1264. doi: 10.1016/j.jmb.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kigerl KA, Popovich PG. Toll-like receptors in spinal cord injury. Curr Top Microbiol Immunol. 2009;336:121–136. doi: 10.1007/978-3-642-00549-7_7. [DOI] [PubMed] [Google Scholar]
- 18.Heiman A, Pallottie A, Heary RF, Elkabes S. Toll-like receptors in central nervous system injury and disease: A focus on the spinal cord. Brain Behav Immun. 2014;42:232–245. doi: 10.1016/j.bbi.2014.06.203. [DOI] [PubMed] [Google Scholar]
- 19.Ntoufa S, Vilia MG, Stamatopoulos K, Ghia P, Muzio M. Toll-like receptors signaling: A complex network for NF-κB activation in B-cell lymphoid malignancies. Semin Cancer Biol. 2016;39:15–25. doi: 10.1016/j.semcancer.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Wang Y, Ouyang X. Beyond toll-like receptors: Porphyromonas gingivalis induces IL-6, IL-8, and VCAM-1 expression through NOD-mediated NF-κB and ERK signaling pathways in periodontal fibroblasts. Inflammation. 2014;37:522–533. doi: 10.1007/s10753-013-9766-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhou R, Alvarado L, Ogilvie R, Chong SL, Shaw O, Mushahwar VK. Non-gait-specific intervention for the rehabilitation of walking after SCI: Role of the arms. J Neurophysiol. 2018;119:2194–2211. doi: 10.1152/jn.00569.2017. [DOI] [PubMed] [Google Scholar]
- 22.Kong X, Gao J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941–954. doi: 10.1111/jcmm.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Liu C, Chen S, Zhao H, Zhou K, Wang W, Yuan Y, Li Z, Guo Y, Shen Z, et al. Activation of the Nrf2/ARE signaling pathway by probucol contributes to inhibiting inflammation and neuronal apoptosis after spinal cord injury. Oncotarget. 2017;8:52078–52093. doi: 10.18632/oncotarget.19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Q, Li C, Yu L. Gambogic acid inhibits spinal cord injury and inflammation through suppressing the p38 and Akt signaling pathways. Mol Med Rep. 2018;17:2026–2032. doi: 10.3892/mmr.2017.8026. [DOI] [PubMed] [Google Scholar]
- 25.Yuan B, Liu D, Liu X. Spinal cord stimulation exerts analgesia effects in chronic constriction injury rats via suppression of the TLR4/NF-κB pathway. Neurosci Lett. 2014;581:63–68. doi: 10.1016/j.neulet.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 26.He Z, Zhou Y, Lin L, Wang Q, Khor S, Mao Y, Li J, Zhen Z, Chen J, Gao Z, et al. Dl-3-n-butylphthalide attenuates acute inflammatory activation in rats with spinal cord injury by inhibiting microglial TLR4/NF-κB signalling. J Cell Mol Med. 2017;21:3010–3022. doi: 10.1111/jcmm.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Chen X, Huang X, Qin C, Fang Y, Liu Y, Zhang G, Pan D, Wang W, Xie M. Soluble epoxide hydrolase inhibition provides multi-target therapeutic effects in rats after spinal cord injury. Mol Neurobiol. 2016;53:1565–1578. doi: 10.1007/s12035-015-9118-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.