Abstract
Non-small cell lung cancer (NSCLC) and mesothelioma are renowned for being diagnosed at a late stage and poor prognosis. Although surgery, chemotherapy, and radiotherapy have yielded successful outcomes, the improvement on the survival rate of NSCLC and mesothelioma have been less marked. Recently, adoptive immunotherapy, particularly chimeric antigen receptor T (CAR-T) cell therapy demonstrated promise for improving the survival of acute lymphoblastic leukemia with minimum toxicity. However, its application in solid tumors still warrants in-depth investigations and multiple consistent trial results, particularly in eliminating ‘off-tumor’ toxicity. To explore CAR-T therapy in NSCLC and mesothelioma, second-generation CAR-T cells were constructed targeting mesothelin (MSLN), which is abundant in NSCLC and mesothelioma but is under expressed in normal tissues. The second-generation design incorporated co-stimulatory CD28 and 4-1BB signaling domains to enhance the proliferation. Following the successful analysis of CAR-T cells by flow cytometry, cytotoxicity experiments were performed using the LDH kit to verify the killing effect of CAR-T cells on target cells. Otherwise, the in vivo killing tumor activity of MSLN CAR-T cells was verified by constructing a mouse model using tumor-derived cells from patients to inoculate the mice. When the effector-to-target ratio is >0.5:1, CAR-T MSLN cells exhibited significantly higher ability to kill tumor cells than T cells. In in vivo experiments, mice whose tail vein was injected with CAR-T MSLN cells demonstrated significantly slower tumor growth. Without continuous administration, both groups became gradually synchronized in growth of tumor size, which suggests that the persistence of CAR-T cells is an important issue in preclinical studies.
Keywords: chimeric antigen receptor T, tumor, mesothelin, cell therapy
Introduction
Lung cancer is a common malignancy which causes one million worldwide mortalities each year (1). Non-small cell lung cancer (NSCLC) accounts for 70–85% lung cancer cases, among which 40% patients reach late stage prior to diagnosis (2). The multidisciplinary paradigm of therapies focused on surgery, chemotherapy and radiotherapy has advanced greatly in recent decades; however, the 5-year survival rate has remained almost constant (3–5). Mesothelioma developed from lung and breast cancers is also a malignant disease associated with aggressive disease progress and extensive economic burden (6,7). As the median overall survival in late stage was only 1 year, both NSCLC and mesothelioma are refractory to standard chemotherapy and only a marginal proportion of patients survive (8–10). Therefore, novel therapies are required.
Previously, adoptive immunotherapy demonstrated promise for prolonging the survival with minimum toxicity (11). The antitumor activity of adoptive therapies was exerted by lymphokine-activated killer cells (LAKs), tumor-infiltrating lymphocytes (TILs), cytokine-induced killer cells (CIKs), dendritic and cytokine-induced killer cells (DC-CIKs), natural killer (NK) cells, engineered T cells and chimeric antigen receptor T cells (CAR-T cells), among which CAR-T cell therapy has achieved remarkable efficacy in acute lymphoblastic leukemia (ALL) (12). CAR-T cells recognize surface antigens independently from major histocompatibility complex restriction, mostly via single chain variable fragments (scFvs), which are derived from tumor antigen-reactive antibodies (13). When targeting tumor surface antigens, the cluster of differentiation (CD)3ζ chain domain and CD28 and/or 4-1BB costimulatory domains will be activated to enhance T cell proliferation, cytokine secretion, resistance to apoptosis and in vivo persistence (13–15). Nevertheless, ‘on target, off tumor’ toxicity is a major challenge in CAR-T therapy, in which the antigen is also expressed in normal tissues (16). Therefore, constructing CAR-T cells that target tumor tissues with negligible off-tumor toxicity is of critical importance.
Mesothelin (MSLN) is an immunogenic glycoprotein that is abundant in ovarian cancers, NSCLC and mesotheliomas (17). Due to its low expression in normal mesothelial cells, MSLN is an ideal candidate for targeted immunotherapy in mesotheliomas (18). In the present study, second-generation CAR-T cells targeting MSLN, the scFvs, which have affinities to intracellular domain of co-stimulatory factor CD28, 4-1BB and CD3ζ, were constructed. In both ex vivo and in vivo experiments, this approach was demonstrated to exert potent effects on tumor clearance. At the cellular level, the CAR-T cells constructed from healthy individuals seemed to have more potent effect than those derived from patients, indicating the potential advantage of allogenic CAR-T therapy. The significantly elevated targeting of CAR-T cells can be achieved with a 0.5:1 effector to target (E:T) ratio, and the antitumor effect of CAR-T cells increase rapidly with increases of the E:T ratio. When it reached 40:1, 78% cells were damaged. In an in vivo mouse model, the difference in growth rate of tumor size was significant at day 5, after which both groups became synchronized in growth of tumor size. These findings suggest that CAR-T cells targeting MSLN could inhibit tumor growth both in vivo and ex vivo, although a sophisticated methodology that enhances the effect of CAR-T cells is required to continuously suppress the tumor.
Materials and methods
Construct of pCAR-MSLN recombinant lentiviral expression vector and viral production
Genetic synthesis of CAR targeting MSLN was outsourced to iCARTab Biomed (Suzhou, China). The whole-gene sequence was sub-cloned to lentiviral vector pCAR-puro following cleavage via EcoRI-XbaI restriction enzyme (Takara Bio, Inc., Otsu, Japan). pCAR-puro, which contains CD28 and 4-1BB signaling modules, was developed specifically for CAR-T therapy studies. The cloned sequence of MSLN CAR in the resulting recombinant vector pCAR-MSLN was confirmed by Sanger sequencing (19), followed by extraction using a Qiagen Plasmid Maxi kit (Qiagen GmbH, Hilden, Germany). The pCAR-MSLN vectors were then transfected into 293T cells (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) as previously described (20). Titration of lentivirus was performed by quantitative polymerase chain reaction using woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and albumin (ALB) genes as reference. Premix Taq™ kit (cat. no. R004Q; Takara Bio, Inc.) was used for the qPCR assay, according to the manufacturer's protocol. The sequences of the primers and probes for WPRE and ALB are shown in the Table I. PCR was performed under the following conditions: 50 cycle denaturation at 95°C for 15 sec, annealing at 60°C for 60 sec and elongation at 72°C for 30 sec. The Cq value of lentivirus carrying pCAR-MSLN (Table II) was calculated using the 2−ΔΔCq method (21). The vector copy number and titer were calculated as follows:
Table I.
Primer and probe sequences used for the quantitative polymerase chain reaction analysis.
| Gene | Sequence (5′-3′) |
|---|---|
| WPRE | |
| Forward primer | GGCACTGACAATTCCGTGGT |
| Reverse primer | AGGGACGTAGCAGAAGGACG |
| Probe | FAM-ACGTCCTTTCCATGGCTGCTCGC-BHQ |
| ALB | |
| Forward primer | GCTGTCATCTCTTGTGGGCTGT |
| Reverse primer | ACTCATGGGAGCTGCTGGTTC |
| Probe | FAM-CCTGTCATGCCCACACAAATCTCTCC-BHQ |
WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; ALB, albumin; FAM, fluorescein; BHQ, Black Hole Quencher® Dye.
Table II.
Cq value of lentivirus carrying pCAR-MSLN.
| Sample | WPRE | ALB | ||
|---|---|---|---|---|
| Lentivirus | 23.30 | 23.71 | 27.29 | 27.62 |
The two experimental replicates are presented. pCAR-MSLN, chimeric antigen receptor-mesothelin plasmid; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; ALB, albumin.
T cell sampling and preparation of CAR-T cells
A total of 5 patients (4 male and 1 female; age, 64.80±2.77) and one 45-year-old female healthy control were recruited to provide T cells. Blood was obtained at Shanghai Chest Hospital (Shanghai, China) in December 2015. T cells derived from the healthy control and patients were used in separate experiments. Blood samples (50 ml) were obtained from each donor. T cells were isolated by Lymphoprep (StemCell Technologies, Vancouver, Canada). The blood sample was added to the upper layer of the Lymphoprep and then centrifuged at 800 × g for 20 min at room temperature to obtain a mononuclear cell layer. Then separated mononuclear cells were added to a new centrifuge tube with 50 µl/ml sorted magnetic beads mix (EasySep™ Human T Cell Enrichment kit; StemCell Technologies) and incubated for 10 min at room temperature. The tube was then inserted into a magnetic pole (EasySep™ Magnet; cat. no. 18000; Stem Cell Technologies) and allowed to stand at room temperature for 5 min. Following the incubation, the cells were removed and then re-suspended in RPMI-1640 supplemented with 10% FBS (both Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA) and 100 U/ml interleukin (IL)-2 (R&D Systems, Inc., Minneapolis, MN, USA). When the cell density reached 1×106 cells/ml, a mixture of 100 U/ml IL-2, 100 ng/ml anti-CD3 antibodies (OKT3; cat. no. 14-0037-82) and 250 ng/ml anti-CD28 antibodies (cat. no. 14-0289-82; both eBioscience; Thermo Fisher Scientific Inc.) was added to the medium. Following 48 h of culture at 37°C, 1.83×108 TU/ml lentivirus carrying pCAR-MSLN was added to the cell culture, together with 8 µg/ml polybrene (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and then incubated for 24 h at 37°C and 50% CO2. The mixture of cell culture and virus was centrifuged at 250 × g for 10 min at room temperature and the supernatant containing additional viruses was removed. The T cells were then re-suspended in fresh medium, and incubated at the same condition for 3–6 days to produce CAR-T cells.
The present study was approved by the Institutional Review Board of the Shanghai Chest Hospital (Shanghai, China) and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent prior to any study-related procedure.
Detection of MSLN-CAR expression in recombinant CAR-T cells
As protein L was able bind to the light chain of mouse antibody (22), the affinity system comprised of biotin-tagged protein L and phycoerythrin (PE)-conjugated streptavidin could be used to detect the expression of MSLN CAR in CAR-T cells. CAR-T cells were continuously cultured in RPMI-1640 medium for 2–5 days at 37°C. Collected cells were adjusted to a density of 1×106/ml and centrifuged at 500 × g for 5 min at 37°C. The cell pellets was washed 3 times with PBS and centrifuged at 500 × g for 5 min at 37°C. A total of 100 µl Protein L (500 ng; ACROBiosystems, Inc., Newark, DE, USA) in PBS was added to the cell pellet and incubated for 30 min at room temperature. Cells were washed 3 times with PBS and centrifuged at 500 × g for 5 min at 37°C then incubated with PE-streptavidin (eBioscience, Thermo Fisher Scientific Inc.) for 30 min in the dark at room temperature, washed 3 times with PBS and centrifuged at 500 × g for 5 min at 37°C. The CAR-T cells were then resuspended in 500 µl PBS and analyzed using a BD Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The data was analyzed by Flowjo software (version 10; FlowJo, LLC, Ashland, OR, USA).
Ex vivo cytotoxicity assay
Cytotoxicity assay was performed by measuring the percentage of cell lysis using the LDH Assay kit (Promega Corporation, Madison, WI, USA). CAR-T cells were washed with sterile PBS (Gibco; Thermo Fisher Scientific, Inc.), resuspended with serum-free RPMI-1640 and mixed into target cells, namely HeLa cells or CHO-K1 cells (both Cell Bank of the Chinese Academy of Sciences) overexpressing MSLN (CHO-K1-MSLN cells) at a gradient ratio of effector to target (E:T ratios). T cells were used as a control. A total of 9 wells were used for triplicate experiment to measure the spontaneous lysis of target and effector cells and the maximum number of lysis using lysis agent CytoTox 96 Non-Radioactive Cytotoxicity assay (cat. no. G1780; Promega Corporation). The ‘Target Spontaneous’ and ‘Target Maximum’ wells were seeded with 5×104 target tumor cells, and ‘Effector Spontaneous’ wells were seeded with CAR-T MSLN cells according to different E:T ratios. Following culturing for 6 h at 37°C, 10X lysis agent was added to the ‘Target Maximum’ well and incubated at 37°C and 50% CO2 for 45 min. Following complete lysis of target cells in ‘Target Maximum’ wells, the plate was centrifuged at 1,200 × g at room temperature for 5 min, and the 50-µl supernatant of each well was transferred to another plate. Assay buffer was mixed with substrate mix and aliquoted to each well. Following termination with stop solution, the absorbance of the mixture at an optical density of 490 nm was measured via a microplate reader. The percentage of lysis in experimental and control well was calculated as follows:
Construct of CHO-K1-MSLN
The MSLN transcript NM_005823.5 was synthesized by GenScript Biotech Corp (Nanjing, China) and subcloned into Lenti-CMV-Puro vectors (iCARTab Biomedical. Co. Ltd.) as previously described (23). Following Sanger confirmation as previously described (19), vectors were extracted and then transduced into packaging cells using polyetherimide (Polyscience, Inc., Warrington, PA, USA), and Lenti-MSLN viruses were isolated by adding PBS supplemented with 20% sucrose to the culture medium of the packed cells. Then, the mixture was centrifuged at 20,000 × g for 2 h at 4°C; the viruses, which were in the precipitate, were then removed. CHO-K1 cells were then transfected with Lenti-MSLN viruses. Following the centrifugation of CHO-K1 culture mixed with Lenti-MSLN viruses at 800 × g at room temperature for 30 min and removal of the supernatant, CHO-K1-MSLN cells were re-suspended in fresh medium and cultured for 5 days.
Flow cytometry detection of MSLN
HeLa or CHO-K1-MSLN cells were divided into two groups, which were blocked by a FcR blocking reagent (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) for 30 min at room temperature were then incubated with either allophycocyanin (APC)-MSLN antibodies (cat. no. FAB32652A; R&D Systems, Inc.) or rat immunoglobulin G2A APC isotype control (cat. no. IC006A; R&D Systems, Inc.) for 30 min at room temperature. The cells were then suspended in 500 µl PBS and analyzed using a BD Accuri C6 flow cytometer. The data was analyzed by Flowjo software.
In vivo validation of antitumor effect
A total of 15 male NPG mice (weight, 18–22 g; Beijing Biocytogen Co., Ltd., Beijing, China) aged 3–4 weeks were housed in used in ventilated cages (5 mice/cage) at 20–26°C with 30–70% humidity and alternate lighting according to 12 h intervals. The cages were ~300×180×150 mm. Dried granule food was sterilized by radiation irradiation. The mice had free access to the food and sterile water. A small section of patients' tumor tissue was isolated. Collagenase type II (cat. no. 17101015; Thermo Fisher Scientific Inc.) digested the tumors into a single cell suspension and blocked by a FcR blocking reagent (Miltenyi Biotec GmbH) for 30 min at room temperature. The cells were then incubated with APC-conjugated MSLN antibodies (cat. no. FAB32652A; 1:100; R&D Systems, Inc.) for 30 min at room temperature and then washed 3 times with PBS. The expression of MSLN was analysed on the membrane surface of tumor-derived cells from patients using flow cytometry and the data was analysed by Flowjo software (version 10; FlowJo, LLC). Following the irradiation of the mice with 0.8 Gy cobalt-60 for 24 h, NSCLC tissues (diameter, 2–3 mm) from 5 patients with high MSLN expression were subcutaneously inoculated into the right hackle of NPG mice. The tumor grew rapidly following transplant, and following 26 days growth, the size of tumor reached a mean of 20–30 mm3. The mice were then randomly allocated to control and experimental groups based on the tumor volume (n=7/group). One mouse was not used in the current study. A total of 8×106 CAR-T cells or T cells were administered via tail vein infusion to the experimental and control groups, respectively. The size of tumors was measured by vernier calipers every 5 days, for a consecutive 15 days. The volume of the tumor was calculated as: Volume (mm3)=(AxB2)/2, where A represents the long diameter of tumor tissue and B represents the short diameter.
Statistical analysis
Data were analyzed by SPSS 18.0 (SPSS, Inc., Chicago, IL, USA). All data are presented as mean ± standard deviation. Statistical analysis of ex vivo tumor cell lysis was performed with Wilcoxon matched pairs signed rank test, and the in vivo experiment was analyzed with independent sample t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Successful construction of pCAR-MSLN recombinant lentiviral expression vector
Second generation CAR molecules were designed for the present study. The lentiviral vector pCAR-MSLN integrated with anti-MSLN CAR also contains co-stimulator, CD28 and 4-1BB. The vectors were excised by EcoRI-XbaI, and electrophoresis demonstrated that they were ~2,200 bp in length, which was close to 2,171 bp, as calculated by adding together the number of base pairs of DNA expressing the anti-MSLN scFv peptide, CD28 and 4-1BB retrieved from the NCBI databse (www.ncbi.nlm.nih.gov). The pCAR-MSLN vectors were amplified and confirmed by Sanger sequencing. No mutations were detected in the recombinant lentiviral vector pCAR-MSLN (data not shown).
Titration of recombinant lentivirus containing pCAR-MSLN
The pCAR-MSLN vectors were transfected to packaging 293T cells. qPCR was performed to titrate the virus. WPRE oligos amplified the WPRE sequence present in almost all later generation lentiviral vectors. ALB oligos were used to normalize the genomic DNA. Based on the Cq value of WPRE and ALB in pCAR-MSLN-containing 293T cells, two experimental replicates yielded the following number of lentivirus copies: WPRE, 23.30 and 23.71 and ALB, 27.29 and 27.62 (Table II). The titer of pCAR-MSLN in 293T was quantified as 1.83×108 TU/ml (data not shown).
MSLN CAR expression in recombinant CAR-T cells
MSLN CAR expression was detected by flow cytometry. The output graph of flow cytometry indicated a markedly difference in MSLN CAR between CAR-T cells and control T cells (Fig. 1), suggesting the successful construction of MSLN CAR-T cells by transfecting recombinant lentiviruses to primary T cells.
Figure 1.
Expression level of mesothelin-CAR on CAR-T membrane detected by flow cytometry. Left panel presents control cells and right panel presents CAR-T cells. CAR, chimeric antigen receptor.
CAR-T cells are detrimental to tumor cells
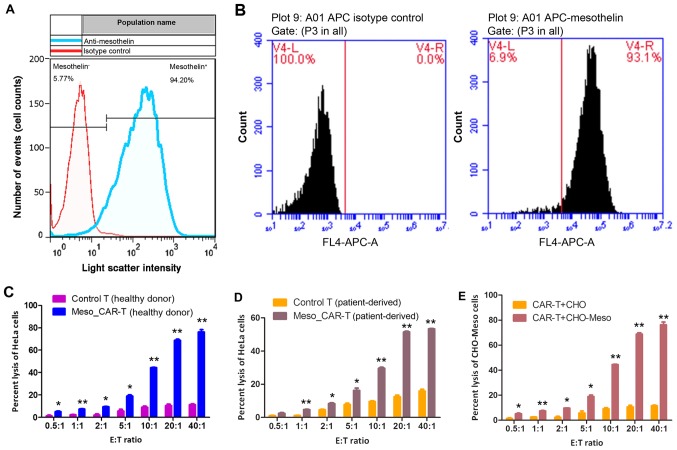
HeLa cells were chosen as target cells to validate the effect of MSLN CAR-T cells. To confirm the targetability of HeLa cells, the expression of MSLN was measured. Flow cytometry demonstrated that 94.20% cells express MSLN, and 5.77% HeLa cells were MSLN negative (Fig. 2A). As the whole HeLa cell culture exhibited high expression of MSLN, such discrepancy within HeLa cells may be due to heterogeneity of cancer cells. Similarly, recombinant CHO-K1-MSLN exhibited abundant MSLN expression, where 93.1% CHO-K1-MSLN cells overexpressed MSLN, and 6.9% of them carried low content of MSLN (Fig. 2B).
Figure 2.
(A) Detection of MSLN on HeLa cells by flow cytometry. (B) MSLN expression on CHO-K1-MSLN cell membrane detected by flow cytometry. Left panel is histogram of CHO-K1 cells, and right panel is CHO-K1-MSLN cells. LDH cytotoxicity assay of HeLa cells targeted by (C) allogenic and (D) autologous CAR-T MSLN cells. (E) LDH cytotoxicity assay of CHO-K1-MSLN cells targeted by allogenic CAR-T MSLN cells. *P<0.05, **P<0.01 vs. Control T. MSLN, mesothelin; LDH, lactate dehydrogenase; E:T, effector-to-target; CAR, chimeric antigen receptor; APC, allophycocyanin.
Following the confirmation of targetability of HeLa cell and CHO-K1-MSLN cells, the antitumor effect of CAR-T cells was verified by in vitro experiments. When the E:T ratio reached 0.5:1, the antitumor effect of CAR-T cells was significantly higher than control T cells (P<0.05; Fig. 2C and D), as indicated by LDH assay of tumor cells. The CAR-T cells constructed from the healthy donor and patients exhibited significantly more potent antitumor effects compared with their respective T cells (all P<0.05; Fig. 2C and D).
To confirm that CAR-T cells could exert the same effect on other types of cells, recombinant CHO-K1-MSLN overexpressing MSLN was used as a target of CAR-T cells constructed from healthy individual. In accordance with HeLa cells, the significantly elevated targeting of CAR-T cells was achieved with 0.5:1 E:T ratio, and the antitumor effect of CAR-T cells increased rapidly with increases of the E:T ratio (P=0.04). When this reached 40:1, 78% cells were lysed (Fig. 2E).
The in vivo antitumor effect of CAR-T cells
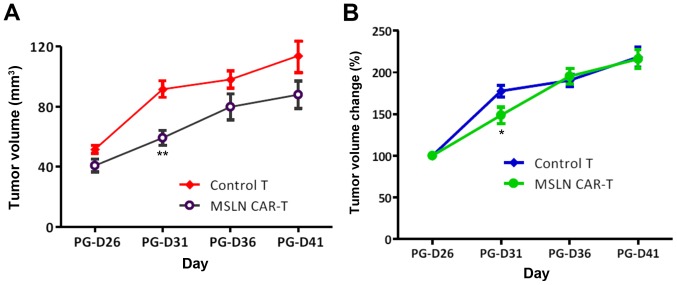
With the effective E:T ratio obtained from in vitro experiments, NPG mice were used to validate in vivo antitumor activity. All tumors grew following tail vein injection, whereas those infused with CAR-T cells grew slower. The difference in growth rate of tumor size was significant at PG-D31 (P=0.03), whereas subsequently, both groups gradually synchronized in tumor growth rate without continuous injection (Fig. 3). This result suggests that a sophisticated methodology that enhances the effect of CAR-T cells is required to continuously suppress the tumor.
Figure 3.
(A) Tumor volume of tumor-bearing NPG mice infused with CAR-T MSLN cells. (B) Tumor volume change of tumor-bearing NPG mice infused with CAR-T MSLN cells. *P<0.05, **P<0.01 vs. Control T. CAR, chimeric antigen receptor; MSLN, mesothelin.
Discussion
The first immunotherapy used clinically was the injection of Streptococcus erysipelas and the Bacillus prodigiosus to treat inoperable sarcoma, in which the anticancer effect was observed and drew extensive research interests (1). There are numerous therapeutic methodologies designed to activate the immune system to kill tumor cells, which are divided into the following four categories based on the underlying mechanisms (24): Adoptive cell therapy, tumor vaccine, monoclonal antibody and other non-specific cytokines. Adoptive cell therapy has long been established in cancer treatment, which involves transferring in vitro cultured lymphocytes back to cancer patients. Adoptive cell therapy could remedy the immune inactivity following radio-chemotherapy (25). Currently, the most used lymphocyte subgroups in clinical settings include: LAK, TIL, CIK, DC-CIK, NK cells, γδT cells, CAR-T cells and TCR-T cells (26). Both LAK and TIL were activated by IL-2, yet the antitumor potency of TIL was 50–100-fold higher than LAK as TIL were isolated from tumor infiltrating lymphocytes (27). They were successfully applied in treating sarcoma (28), but time spent in culturing and the difficulties in separating TIL constitute the present challenge in improving the efficacy. DC cells could recognize tumor antigens and CIK cells secrete cytotoxic factors, and combination of DC and CIK was demonstrated to improve efficacy of chemotherapy, mitigate side effects and prolong life expectancy of patients (29). However, immune tolerance and immune escape are major barriers for DC-CIK therapy (30). NK cell-based therapy has achieved marked efficacy in NSCLC, myeloma, breast cancer, renal cell carcinoma and colorectal carcinoma (31), and γδT cell-based therapy has been effective in treating renal cell carcinoma and prostatic cancer (32). However, the targetability and tumor-killing capacity of both methods fell short of expectations (33).
Engineered T cells that target a tumor antigen via T-cell receptors (TCRs) or a CAR exhibited promise in rapid stimulation of tumor immunity and reducing tumor burdens (34,35). TCRs are restricted to leukocyte antigen, limiting their application as a mainstream therapeutic strategy, whereas CARs may be engineered to directly target proteins, carbohydrates or glycolipids on the cell surface, providing design flexibility and diversity (36). Compared with first-generation CARs, which contains CD3ζ cytoplasmic domain, second-generation CARs integrate intracellular signaling domains from various costimulatory factors, such as CD28, 4-1BB or inducible T cell costimulator, to augment the activation signal by CD3ζ and promote amplification of T cells (37). Such dual signaling may eliminate deficiency of T cells and enhance the persistence and function of T cells (36). For example, CD28 can bind to phosphoinositide 3-kinase (PI3K) via YMNM cytoplasmic domain, thereby initiating the PI3K-protein kinase B pathway to promote proliferation of T cells (38); 4-1BB can be transiently induced by TCR and CD28 signaling through extracellular signal-regulated kinase and c-Jun N-terminal kinase signaling pathways, resulting in fast proliferation and durable functioning of CD4+ and CD8+ T cells (36).
In the present study, second-generation CAR-T cells targeting MSLN were constructed, of which the scFvs have affinities to the intracellular domains of co-stimulatory factor CD28, 4-1BB and CD3ζ (39). In ex vivo and in vivo experiments, this approach was demonstrated to exert a potent effect on tumor clearance. The lentiviral vector was used to deliver engineered DNAs, which demonstrated high transduction efficiency. Another advantage of employing lentiviral vectors is that it avoids the integration of foreign genomics into non-dividing human primary cells, which has been a concern for retroviral vectors, therefore eliminating the undesired risk of insertional oncogenesis (40–42).
Although CAR-T therapy has great potential for killing tumors, the ‘on target, off tumor’ toxicity poses a major concern. An approach to minimize such toxicity is to engineer additional antibodies targeting specific antigens that are differentially, if not exclusively, expressed in tumor than normal tissues (43). CD19 is ubiquitously expressed in malignant and normal B cells, but the normal B cells expressing CD19 are hematopoietic or approaching cell death (44). Therefore, CD19 is a nearly ideal target for B cell malignancies. Recently, treatment of B cell malignancies achieved a breaking advancement with CAR-T cell therapy: Multiple clinical trials have revealed that CAR-T cells targeting CD19 could treat refractory lymphoma with response rates over 50% (45,46), and in myeloma, CAR-T cells engineered to target CD19+ demonstrated efficacy in eradicating the disease (47). Other target antigens include tumor-associated glycoprotein 72 for metastatic colorectal cancer (48), folate receptor-α for ovarian cancer (49), L1-cell adhesion molecule for metastatic neuroblastoma (50), and CD22 for ALL (51), in which 5 targets have entered phase 2 trials: GD2 (NCT02765243), CD22 (NCT03196830), CD20 (NCT03196830), CD30 (NCT03196830) and carcinoembryonic antigen (NCT01723306) (52). Although these studies envisage great potentials of second generation CAR-T cell therapy, antigens that are rarely expressed in normal cells but abundant in malignancies are still rare (53). Two outstanding tumor targets for solid tumor are ERBB2 and MSLN, which were applied in 8 and 6 cancer types, respectively (54).
MSLN is a glycoprotein anchored to the plasma membrane, which has minimal expression in normal tissue but abundant expression in solid tumors, including mesothelioma, ovarian cancer, pancreatic cancer and lung cancer (18,54–56) Multiple studies (57–59) have suggested that MSLN expression is correlated with poor prognosis. It can activate nuclear factor-κB, mitogen-activated protein kinase and PI3K intracellular pathways that contribute to cell proliferation and resistance to apoptosis (60–62). In addition, overexpression of MSLN could lead to excessive expression of matrix metalloproteinase-9, promoting migration and infiltration (63). The initial clinical MSLN-specific CAR-T therapy was conducted in 2 patients affected with malignant pleural mesotheliomas and pancreatic cancer, respectively (64). Antitumor potency was achieved by infusion of mRNA-engineered CAR-T MSLN cells with acceptable safety despite the transient nature of CAR-T cells and the absence of pretreated lymphodepletion.
In the present study, it was demonstrated that CAR-T cells derived from healthy individuals exhibited better effects than those derived from patients, indicating that allogenic T cells may be more effective in suppressing malignancies than autologous T cells at the cell level. The T cells derived from patients may have undergone exhaustion, which is a state of dysfunction that commonly arise from chronic infections and cancers (65). The mechanisms underlying T cell exhaustion comprise elevated multiple inhibitory signaling, including programmed cell death protein 1, lymphocyte activation gene 3, CD160, T cell immunoglobulin and mucin-domain containing-3, T cell immunoreceptor with Ig and ITIM domains and cytotoxic T lymphocyte-associated protein 4 (66–70), resulting in loss of T cell effector functions, altered metabolism and a parallel but ineffective transcriptional program (71). Allogenic T cells circumvent the exhaustion conditioning of T cells, and thus are more effective towards tumor cells (72,73).
Compared with autologous CAR-T cells, allogenic approach allows for expanded manufacturing of ‘off-the-shelf’ CAR-T cells for numerous recipients. Although the cellular level of antitumor efficacy of allogenic CAR-T cells are more potent than autologous CAR-T cells, graft-versus-host disease (GVHD) remains a major impediment to the successful adoption of allogenic CAR-T cells. Recently, Ghosh et al (74) demonstrated that alloreactive T cells expressing CD28-costimulated CD19 CARs produced enhanced stimulation, leading to overt mitigation of effector function and clonal deletion, and significantly decreased occurrence of GVHD. A recent case of reducing GVHD in CAR-T cell therapy was conducted on an infant with CD19+ ALL for whom autologous T cells could not be obtained, yet the endogenous TCR was deleted to prevent GVHD (75).
There are also a number of limitations in the present study, which will be improved upon in further investigations. For example, the cell line used was HeLa, a cervical cancer cell line, which is a considerable confounding factor. The single cell line applied in the present study may not be strong enough evidence to support the targetability of MSLN CAR-T cells, therefore cells with higher MSLN expression may be more suitable. In vivo cytotoxicity assay is required to complement the in vitro assay of the present study. Apart from the suppression on the growth of tumor, the tumor elimination effect of MSLN CAR-T cells will be helpful in addressing the stability of MSLN CAR-T cell therapy. The use of the 293T cell line constitutes another limitation: Stepanenko and Dmitrenko recently raised a concern for the use of this cell line (76), as it demonstrated no evident tissue-specific gene expression signature, which may compromise the resembling certain tissue-origin tumors. Furthermore, the compound phenotype and unstable, heterogeneous karyotype made it difficult to allow consistent and rigorous comparison between different experimental groups.
The lack of blank control made it difficult to examine the specificity of MSLN CAR-T cells. Case-control comparison was performed for elucidating cytotoxicity and tumor suppressing effect of MSLN targeted CAR-T cells via using un-engineered T cells as control, and MSLN-abundant HeLa cells as target. Results suggested that MSLN CAR-T cells have more potent cytotoxicity than T cells, yet this advantageous effect was due to the cumulative influence of MSLN targeting T cells and the targetability of MSLN antigen. This finding is primarily sufficient to support the conclusion that MSLN CAR-T cells are superior than T cells in killing MSLN expressing tumors. However, the specificity was not addressed clearly for a lack of blank control such as non-MSLN expressing cells or non-tumor epithelial cells. This is a major limitation of the present study. CAR-T cells were initially designed to improve the specificity of T cells, which has been demonstrated to be successful (77,78), although specificity remains problematic in a CAR-T therapy study (79). Improving specificity is a major issue in CAR-T cell engineering, which can be addressed by improving the targetability of chimeric antigen receptors, or adding other tumor-specific antigen receptors. Unlike clinical trials, the present study was limited to and focused on exploring the therapeutic potential of MSLN CAR-T cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by Western Medicine Guidance Project of Shanghai Science and Technology Commission (grant no. 11411961300).
Availability of data and materials
All data generated or analyzed during the current study are included in this published article.
Authors' contributions
XF performed the major experiments. LY analyzed the data and was a major contributor in writing the manuscript. YL performed the statistical analysis and provided important suggestions in manuscript writing. LL designed the experiment and provided advice during manuscript revision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of the Shanghai Chest Hospital (Shanghai, China) and all patients provided written informed consent.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions, et al The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Ricciardi GR, Russo A, Franchina T, Ferraro G, Zanghì M, Picone A, Scimone A, Adamo V. NSCLC and HER2: Between lights and shadows. J Thorac Oncol. 2014;9:1750–1762. doi: 10.1097/JTO.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DB, Peng C, Sosman JA. Nivolumab in melanoma: Latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7:97–106. doi: 10.1177/1758834014567469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–378. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- 6.Weill H, Hughes JM, Churg AM. Changing trends in US mesothelioma incidence. Occup Environ Med. 2004;61:438–441. doi: 10.1136/oem.2003.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 8.Schiller JH, Harrington D, Belani C, Langer CP, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 9.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS. At the bedside: Adoptive cell therapy for melanoma-clinical development. J Leukoc Biol. 2014;95:875–882. doi: 10.1189/jlb.0513293. [DOI] [PubMed] [Google Scholar]
- 12.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreitman RJ, Hassan R, FitzGerald DJ, Pastan I. Phase I Trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74:2907–2912. doi: 10.1158/0008-5472.CAN-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan R, Bera T, Pastan I. Mesothelin: A new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 19.Deng YM, Spirason N, Iannello P, Jelley L, Lau H, Barr IG. A simplified Sanger sequencing method for full genome sequencing of multiple subtypes of human influenza A viruses. J Clin Virol. 2015;68:43–48. doi: 10.1016/j.jcv.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, Yeh R, Capacio V, Olszewska M, Hosey J, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Akerstrom B, Bjorck L. Protein L: An immunoglobulin light chain-binding bacterial protein. Characterization of binding and physicochemical properties. J Biol Chem. 1989;264:19740–19746. [PubMed] [Google Scholar]
- 23.Taghian DG, Nickoloff JA. Subcloning strategies and protocols. Methods Mol Biol. 1996;58:221–235. doi: 10.1385/0-89603-402-X:221. [DOI] [PubMed] [Google Scholar]
- 24.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currie GA. Eighty years of immunotherapy: A review of immunological methods used for the treatment of human cancer. Br J Cancer. 1972;26:141–153. doi: 10.1038/bjc.1972.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg SA. IL-2: The first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Ren B, Li H, Yu J, Cao S, Hao X, Ren X. Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol Immunother. 2013;62:65–73. doi: 10.1007/s00262-012-1311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Y, Tao Q, Wang H, Xiong S, Zhang R, Chen T, Tao L, Zhai Z. Dendritic cells decreased the concomitant expanded tregs and tregs related IL-35 in cytokine-induced killer cells and increased their cytotoxicity against leukemia cells. PLoS One. 2014;9:e93591. doi: 10.1371/journal.pone.0093591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 32.Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: A systematic review of clinical trials. OncoImmunology. 2014;3:e27572. doi: 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 34.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: Engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/S1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 35.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 36.van der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garçon F, Patton DT, Emery JL, Hirsch E, Rottapel R, Sasaki T, Okkenhaug K. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- 39.Fesnak AD, June CH, Levine BL. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durand S, Cimarelli A. The inside out of lentiviral vectors. Viruses. 2011;3:132–159. doi: 10.3390/v3020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A, Di Serio C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava S, Riddell SR. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper LJ, Al-Kadhimi Z, DiGiusto D, Kalos M, Colcher D, Raubitschek A, Forman SJ, Jensen MC. Development and application of CD19-specific T cells for adoptive immunotherapy of B cell malignancies. Blood Cells Mol Dis. 2004;33:83–89. doi: 10.1016/j.bcmd.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuster SJ, Svoboda J, Nasta SD, Porter DL, Chong EA, Landsburg DJ, Mato AR, Lacey SF, Melenhorst JJ, Chew A, et al. Sustained remissions following chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. Blood. 2015;126:183. [Google Scholar]
- 47.Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, Zheng Z, Vogl DT, Cohen AD, Weiss BM, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med. 2015;373:1040–1047. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer. 2017;5:22. doi: 10.1186/s40425-017-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, Jensen MC. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 51.Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, FitzGerald DJ, Barrett DM, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1174. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arabi F, Torabi-Rahvar M, Shariati A, Ahmadbeigi N, Naderi M. Antigenic targets of CAR T Cell Therapy. A retrospective view on clinical trials. Exp Cell Res. 2018;369:1–10. doi: 10.1016/j.yexcr.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Mami-Chouaib F, Echchakir H, Dorothee G, Vergnon I, Chouaib S. Antitumor cytotoxic T-lymphocyte response in human lung carcinoma: Identification of a tumor-associated antigen. Immunol Rev. 2002;188:114–121. doi: 10.1034/j.1600-065X.2002.18810.x. [DOI] [PubMed] [Google Scholar]
- 54.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 55.Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho M, Bera TK, Willingham MC, Onda M, Hassan R, Fitzgerald DJ, Pastan I. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 57.Huang CY, Cheng WF, Lee CN, Su YN, Chien SC, Tzeng YL, Hsieh CY, Chen CA. Serum mesothelin in epithelial ovarian carcinoma: A new screening marker and prognostic factor. Anticancer Res. 2006;26:4721–4728. [PubMed] [Google Scholar]
- 58.Han SH, Joo M, Kim H, Chang S. Mesothelin expression in gastric adenocarcinoma and its relation to clinical outcomes. J Pathol Transl Med. 2017;51:122–128. doi: 10.4132/jptm.2016.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas A, Chen Y, Steinberg SM, Luo J, Pack S, Raffeld M, Abdullaev Z, Alewine C, Rajan A, Giaccone G, et al. High mesothelin expression in advanced lung adenocarcinoma is associated with KRAS mutations and a poor prognosis. Oncotarget. 2015;6:11694–11703. doi: 10.18632/oncotarget.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin confers pancreatic cancer cell resistance to TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and IL-6/Mcl-1 overexpression. Mol Cancer. 2011;10:106. doi: 10.1186/1476-4598-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang MC, Chen CA, Chen PJ, Chiang YC, Chen YL, Mao TL, Lin HW, Lin Chiang WH, Cheng WF. Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem J. 2012;442:293–302. doi: 10.1042/BJ20110282. [DOI] [PubMed] [Google Scholar]
- 62.Chang MC, Chen CA, Hsieh CY, Lee CN, Su YN, Hu YH, Cheng WF. Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K pathway. Biochem J. 2009;424:449–458. doi: 10.1042/BJ20082196. [DOI] [PubMed] [Google Scholar]
- 63.Cristaudo A, Foddis R, Vivaldi A, Guglielmi G, Dipalma N, Filiberti R, Neri M, Ceppi M, Paganuzzi M, Ivaldi GP, et al. Clinical significance of serum mesothelin in patients with mesothelioma and lung cancer. Clin Cancer Res. 2007;13:5076–5081. doi: 10.1158/1078-0432.CCR-07-0629. [DOI] [PubMed] [Google Scholar]
- 64.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 70.Callahan MK, Wolchok JD. At the Bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 72.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et al. Bioactivity and safety of IL13R 2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21:4062–4072. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomez GG. Adoptive T cell therapies for glioblastoma. J Cancer Clin Trials. 2015;1:1–5. [Google Scholar]
- 74.Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, Gunset G, Perna F, Kreines FM, Levy ER, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23:242–249. doi: 10.1038/nm.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qasim W, Amrolia P, Samarasinghe S, Ghorashian S, Zhan H, Stafford S, et al. First clinical application of talen engineered universal car19 T cells in B-ALL. Blood. 2015;126:2046. [Google Scholar]
- 76.Stepanenko AA, Dmitrenko VV. HEK293 in cell biology and cancer research: Phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene. 2015;569:182–190. doi: 10.1016/j.gene.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 77.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, Hackett PB, Kohn DB, Shpall EJ, Champlin RE, Cooper LJ. Redirecting specificity of T-cell populations for CD19 using the sleeping beauty system. Cancer Res. 2008;68:2961–2971. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci USA. 2016;113:e450–e448. doi: 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the current study are included in this published article.