Abstract
In peripheral arterial disease (PAD), angiogenesis is the major process involved in repairing the microvasculature in the ischemic lower limb. Curcumin, a monomer isolated from turmeric roots, has been demonstrated to have pro- and anti-angiogenic effects under different circumstances. Previous studies have indicated that curcumin treatment improves tissue repair and perfusion recovery in a mouse model of diabetic PAD. However, the effects of curcumin on PAD under non-diabetic conditions has remained elusive, In the present study, mice with PAD and a normal glycaemic profile were treated with curcumin, which improved perfusion recovery, increased capillary density and elevated microRNA (miR)-93 expression in ischemic muscle tissue. In cultured endothelial cells under simulated ischemia, curcumin improved endothelial cell viability and enhanced tube formation. However, following miR-93 knockdown using a microRNA inhibitor, endothelial cell tube formation was inhibited. Furthermore, in the presence of the miR-93 inhibitor, curcumin did not alter endothelial cell viability or tube formation. These results demonstrate that curcumin had beneficial effects in non-diabetic PAD by improving angiogenesis, which may have been achieved partially via the promotion of miR-93 expression.
Keywords: curcumin, angiogenesis, peripheral arterial disease, microRNA-93, endothelial cells
Introduction
Peripheral arterial disease (PAD), caused by occlusion of the arteries extending to the lower extremities, is a growing medical problem that affects >200 million individuals worldwide (1–3). Delivery of oxygen, nutrients and other mediators to ischemic sites in patients with PAD via the blood circulation is dependent on neovascularization, including angiogenesis and arteriogenesis (4–7). However, at present, no medications are available to induce functional neovascularization and thereby treat patients with PAD (8–10).
Curcumin is a bright-yellow compound isolated from the root of Curcuma longa, which is a member of the ginger family, and has traditionally been used to treat a variety of clinical conditions, including cancer, Alzheimer's disease and insulin resistance (11–13). Previous studies suggested that curcumin induces therapeutic angiogenesis and improves hind limb perfusion recovery after surgical femoral artery ligation in diabetic mice (14). However, whether curcumin provides a therapeutic benefit in PAD without diabetes has remained elusive. Considering that a large proportion of patients with PAD do not have any accompanying diabetes mellitus (2,15), the present study was performed in order to investigate the potential effects of curcumin on perfusion recovery in a non-diabetic mouse model of PAD, and to elucidate the mechanism of action of angiogenic microRNA.
Materials and methods
Murine hindlimb ischemia (HLI)
Unilateral HLI was generated via surgical ligation and excision of the femoral artery to create an experimental PAD model, as described previously (16). In the present study, 32 male BALB/c mice (age, 14 weeks; weight, 20–25 g) were anesthetized with 3% isoflurane. Immediately after HLI, the mice were randomized into two groups (n=16 in each): In the control group, the mice received 300 µl olive oil only, and in the curcumin group, the mice received 1,000 mg/kg curcumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in 300 µl olive oil. All mice received treatment by gavage, once per day for two weeks.
All procedures of the present study followed the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication no. 85–23, revised 1996). The experimental protocol was approved by the Committee on Animal Experiments of Wuhan University School of Medicine (Wuhan, China). The BALB/c mice were obtained from the Experimental Animal Center of Wuhan University (Wuhan, China). Mice were housed in a specific pathogen-free laboratory environment at a temperature of 25°C and a constant humidity of 50±10% with free access to food and water under a 12-h light/dark cycle.
Perfusion recovery
Mice were anesthetized and subjected to a non-invasive assessment of ischemic and non-ischemic limb perfusion using a laser Doppler perfusion imaging system (LDPI; Perimed Instruments AB, Stockholm, Sweden) at 0, 7, 14, 21 and 28 days after HLI, as described previously (17). Perfusion of the ischemic limb was quantified and normalized to the non-surgical limb, and the results are presented as a percentage of the values in the non-ischemic side.
Immunofluorescence
Mice were sacrificed in a CO2 chamber at 28 days after HLI, and the gastrocnemius anterior muscles from the ischemic side were cryo-sectioned in 6-µm sections. Anti-CD31 antibody (rat anti-mouse CD31; cat. no. 550274; 1:100 dilution; BD Pharmingen, San Jose, CA, USA) was applied to acetone-fixed sections (fixed for −20°C for 10 min) of ischemic gastrocnemius muscle tissue, followed by incubation overnight at 4°C with an Alexa Fluor 555 anti-rabbit secondary antibody (1:400 dilution; cat. no. BM2004; Boster Biological Technology, Wuhan, China). Images were acquired using an Olympus IX71 high-magnification microscope (Olympus, Tokyo, Japan). Capillary densities were analyzed by counting in four randomly selected high-power fields (magnification, ×100) and expressed as the number of CD31+ cells per field.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from tissue or cells using a PureLink® RNA Mini kit (cat. no. 12183018A; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. Real-time qPCR for microRNA (miR) quantification and a miR assay (assay no. 001090; cat. no: 4427975; Thermo Fisher Scientific, Inc.) were used for RT-qPCR according to the manufacturer's protocols. Small nucleolar RNA MBII-202 (assay no. 001095; Thermo Fisher Scientific, Inc.) served as an internal control for miR quantification. The quantification cycle (Cq) value obtained for each gene was normalized to that of the respective internal control (ΔCq). Each gene was then further normalized to the average ΔCq value of its control group (ΔΔCq). The final fold expression changes were calculated using the 2−ΔΔCq equation (18).
Cell culture and in vitro transfection
Human umbilical vein endothelial cells (HUVECs) were isolated from a donor umbilical cord, as described previously (19), and then cultured in endothelial cell growth medium (Cell Applications, Inc., San Diego, CA, USA) supplemented with 10% fetal bovine serum (Wuhan Boster Biological Technology). To mimic endothelial cells under ischemic conditions as a model for HLI, HUVECs were subjected to hypoxia (2% oxygen; BioSpherix, Lacona, NY, USA) and serum starvation (HSS). The use of HUVECs was approved by the Institutional Review Boards of Wuhan University (Wuhan, China).
In vitro transfection of miRNA inhibitors was used to knock down miR-93 expression in HUVECs, as described previously (20). In brief, a reverse transfection protocol using neofx transfection agent (Ambion, Austin, TX, USA) was used to transfect miR-93 inhibitor, or miRNA inhibitor negative control (cat. no. 4464084; Thermo Fisher Scientific, Inc) into HUVECs for 48 h.
Cellular viability and angiogenesis assay
For assessment of cellular viability, HUVECs transfected with an miR-93 inhibitor or control miR were seeded into a 96-well plate at a density of 1×104 cells/well (n=8/group), and then cultured under HSS conditions with/without curcumin (10 µM) for 48 h. Subsequently, the cell viability was assessed using tetrazolium dye incorporation (BioVision, Milpitas, CA, USA).
In vitro angiogenesis assays were performed as previously described (20), under HSS conditions. In brief, HUVECs transfected with miR-93 inhibitor or control were seeded in 96-well dishes coated with growth factor-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at a density of 1×104 cells/well, and then exposed to HSS conditions in the presence of curcumin (10 µM) or vehicle alone for 12 h to assess tube formation. Each set of conditions was replicated in 6 wells from the 96-well dish. The degree of tube formation was determined by measuring the length of the tubes and the number of loops in each well under a magnification, ×40 using ImageJ software 1.15K (National Institutes of Health, Bethesda, MD, USA). Each experiment was repeated using at least two different batches of HUVECs in total.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 7.0 software (GraphPad Inc., La Jolla, CA, USA). An unpaired t-test was used for comparisons between two groups; comparisons between ≥3 groups were performed with one-way analysis of variance and Tukey's post-hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Curcumin improves perfusion recovery, angiogenesis and causes upregulation of miR-93 expression in experimental PAD
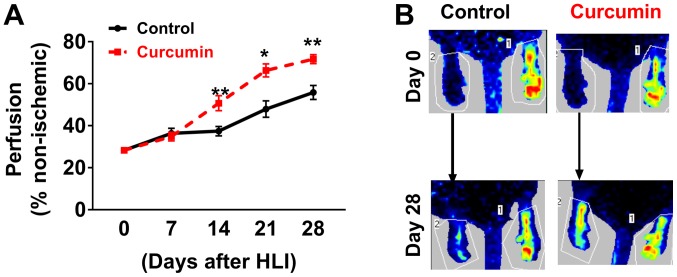
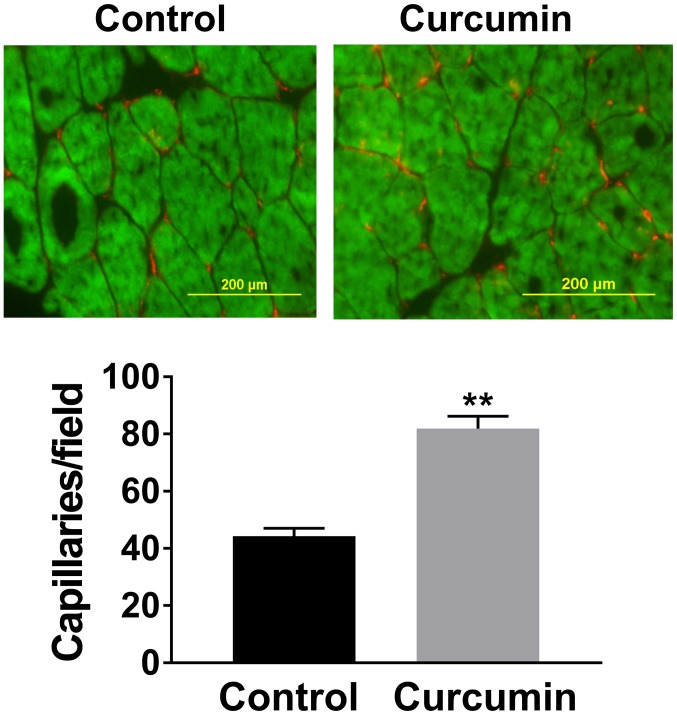
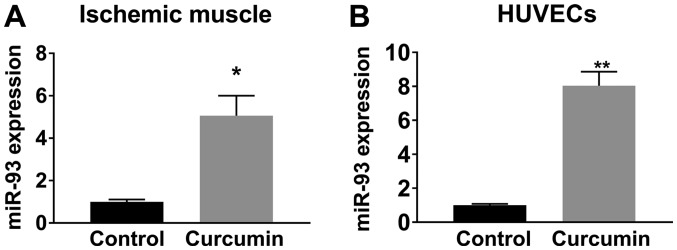
LDPI imaging revealed that BALB/c mice receiving curcumin experienced better perfusion recovery at 14, 21 and 28 days after HLI compared with those receiving olive oil (Fig. 1). Immunostaining with CD31 to visualize capillaries indicated that after 28 days of post-HLI treatment, the gastrocnemius anterior muscles from the ischemic side of the mice receiving curcumin exhibited a higher capillary density compared with those receiving olive oil (81.9±4.3 vs. 44.3±2.7 capillaries/field, n=10/group; P<0.01; Fig. 2). A previous study indicated that miR-93 is a potent regulator of angiogenesis in the ischemic limbs of patients with PAD and in animal models (20). Given that curcumin induced angiogenesis as a therapeutic benefit after HLI, the level of miR-93 was assessed, revealing that curcumin treatment increased miR-93 expression by ~5 fold (n=5/group) in the ischemic muscle at 7 days after HLI (Fig. 3A).
Figure 1.
(A) LDPI revealed significantly increased perfusion recovery in BALB/c mice treated with curcumin at 14, 21 and 28 days after HLI. (B) Representative LDPI profiles in mice on days 0 and 28. Values are expressed as the mean ± standard error of the mean (n=10/group). *P<0.05; **P<0.01 vs. control. LDPI, laser Doppler perfusion imaging; HLI, hind limb ischemia.
Figure 2.
Curcumin treatment increased the capillary density in ischemic gastrocnemius muscle tissue at 28 days after hind limb ischemia, as compared with that in mice receiving vehicle (scale bar, 200 µM). Values are expressed as the mean ± standard error of the mean (n=10/group). Green staining represents skeletal muscle, red staining indicates capillaries in ischemic muscle tissue. **P<0.01 vs. control.
Figure 3.
Curcumin treatment caused an upregulation in miR-93 expression (A) in ischemic muscle tissues (5.05±0.94 fold) at 7 days after hind limb ischemia and (B) in HUVECs (8.03±0.82 fold) after 12 h under hypoxia and serum starvation conditions. Values are expressed as the mean ± standard error of the mean (n=10/group) *P<0.05; **P<0.01 vs. control. miR, microRNA; HUVECs, human umbilical cord vascular endothelial cells.
Curcumin therapy increases angiogenesis under hypoxia
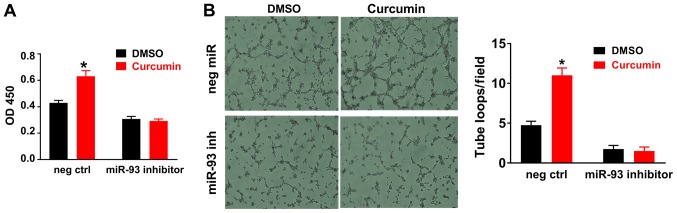
In HUVECs cultured under HSS conditions (mimicking in vivo ischemia), curcumin treatment for 12 h significantly increased miR-93 expression (Fig. 3B), consistent with the in vivo results obtained with ischemic muscle tissue. In addition, curcumin increased endothelial cell viability (Fig. 4A) and tube formation (Fig. 4B) in vitro under HSS conditions. It was also revealed that miR-93 knockdown using a miR-93 inhibitor reduced angiogenesis and curcumin-induced angiogenesis and endothelial cell survival were attenuated when miR-93 was knocked down by an miR-93 inhibitor in vitro (Fig. 4A and B).
Figure 4.
Curcumin treatment increases the cell viability and tube formation of cultured HUVECs under HSS conditions. (A) HUVECs were incubated with curcumin or DMSO for 48 h, and curcumin significantly increased the cell viability. (B) HUVECs were seeded on 96-well plates coated with Matrigel; curcumin treatment for 12 h significantly increased tube formation, as indicated by increased loops (magnification, ×40). DMSO (0.02%, volume concentration in the medium) served as a negative control for curcumin, as curcumin used in the in vitro study was dissolved in DMSO. miR-93 knockdown reduces angiogenesis in vitro, furthermore following miR-93 knockdown, curcumin did not alter endothelial cell viability or tube formation. Values are expressed as the mean ± standard error of the mean. *P<0.05 vs. control. HUVECs, human umbilical cord vascular endothelial cells; HSS, hypoxia and serum starvation; OD 450, optical density at 450 nm; neg ctrl, negative control; miR, microRNA; inh, inhibitor; DMSO, dimethylsulfoxide.
Discussion
To the best of our knowledge, the present study is the first to demonstrate that curcumin improves angiogenesis and perfusion recovery in non-diabetic experimental PAD. Furthermore, it was indicated that curcumin treatment increased miR-93 expression in ischemic muscle tissue and cultured endothelial cells, and that miR-93 elevation may be involved in curcumin-induced therapeutic angiogenesis under ischemic conditions.
Previous studies have demonstrated that curcumin has a protective effect on ischemic limbs in diabetic mouse models (14,15). However, the effects of curcumin on limb ischemia in non-diabetic subjects have remained to be assessed. Angiogenesis is an important process of new blood vessel formation, which includes the stimulation, promotion and stabilization of endothelial cells; it is a key factor in the perfusion recovery of tissue following ischemia. Curcumin has a pro-angiogenic effect on wound healing and HLI in type 1 diabetes (21). However, it has been indicated to have anti-angiogenic effects in pituitary adenomas and hepatic cancer (11,22). Taken together, curcumin exhibits bi-directional effects under different disease conditions. Therefore, under non-diabetic conditions, the effects of curcumin on angiogenesis in PAD require further study.
A noteworthy result of the present study is that curcumin improves perfusion recovery after HLI through the induction of miR-93 upregulation in ischemic endothelial cells. miRs are a group of small non-coding RNAs containing ~22 nucleotides that function through RNA silencing and the post-transcriptional regulation of gene expression (23–27). miR-93 has been reported to act as a potent mediator to induce neovascularization in PAD. In a mouse model of PAD, miR-93 knockdown was reported to reduce angiogenesis and perfusion recovery; conversely, miR-93 overexpression improved perfusion recovery and angiogenesis by targeting cell cycle regulatory pathways (21). A more recent study indicated that miR-93 induces macrophage M2 polarization, which eventually leads to enhanced angiogenesis and arteriogenesis in a mouse model of PAD (28). In the present study, treatment with curcumin was identified to cause an upregulation of miR-93 in ischemic muscle tissue and endothelial cells. In addition, miR-93 inhibition blocked curcumin-induced therapeutic angiogenesis in vitro. This may suggest that miR-93 is involved in the therapeutic effects of curcumin on PAD.
At present, limited therapies are available for PAD, and no known treatment is capable of increasing neovascularization in the ischemic limbs of patients with PAD (10,17). Combined with the previous result that curcumin improves outcomes in diabetic PAD, the present study suggests that curcumin may serve as an effective alternative treatment approach for PAD in non-diabetic subjects.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JZ and RH conceived and designed the current study. JZ, QW and RH wrote and edited the manuscript. JZ, QW, GR, JQ and RH performed the experiments, and read and approved the final manuscript.
Ethics approval and consent to participate
The experimental animal protocol was approved by the Committee on Animal Experiments of Wuhan University School of Medicine. The use of HUVECs isolated from donor umbilical cords was approved by the Institutional Review Boards of Wuhan University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Guerchet M, Aboyans V, Mbelesso P, Mouanga AM, Salazar J, Bandzouzi B, Tabo A, Clément JP, Preux PM, Lacroix P. Epidemiology of peripheral artery disease in elder general population of two cities of Central Africa: Bangui and Brazzaville. Eur J Vasc Endovasc Surg. 2012;44:164–169. doi: 10.1016/j.ejvs.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: Epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 4.Espinola-Klein C, Savvidis S. Peripheral arterial disease. Epidemiology, symptoms and diagnosis. Internist (Berl) 2009;50:919–926. doi: 10.1007/s00108-009-2364-4. (In German) [DOI] [PubMed] [Google Scholar]
- 5.Grundmann S, Piek JJ, Pasterkamp G, Hoefer IE. Arteriogenesis: Basic mechanisms and therapeutic stimulation. Eur J Clin Invest. 2007;37:755–766. doi: 10.1111/j.1365-2362.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT. Treatment of peripheral arterial disease-extending ‘intervention’ to ‘therapeutic choice’. N Engl J Med. 2006;354:1944–1947. doi: 10.1056/NEJMe068037. [DOI] [PubMed] [Google Scholar]
- 7.Joh JH, Joo SH, Park HC. Simultaneous hybrid revascularization for symptomatic lower extremity arterial occlusive disease. Exp Ther Med. 2014;7:804–810. doi: 10.3892/etm.2014.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aviles RJ, Annex BH, Lederman RJ. Testing clinical therapeutic angiogenesis using basic fibroblast growth factor (FGF-2) Brit J Pharmacol. 2003;140:637–646. doi: 10.1038/sj.bjp.0705493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens CD, Conte MS. Medical management of peripheral arterial disease: Bridging the ‘Gap’? Circulation. 2012;126:1319–1321. doi: 10.1161/CIRCULATIONAHA.112.129692. [DOI] [PubMed] [Google Scholar]
- 10.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387–396. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 11.Allegra A, Innao V, Russo S, Gerace D, Alonci A, Musolino C. Anticancer activity of curcumin and its analogues: Preclinical and clinical studies. Cancer Invest. 2017;35:1–22. doi: 10.1080/07357907.2016.1247166. [DOI] [PubMed] [Google Scholar]
- 12.Goozee KG, Shah TM, Sohrabi HR, Rainey-Smith SR, Brown B, Verdile G, Martins RN. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer's disease. Br J Nutr. 2016;115:449–465. doi: 10.1017/S0007114515004687. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Osorio AS, Monroy A, Alavez S. Curcumin and insulin resistance-Molecular targets and clinical evidences. Biofactors. 2016;42:561–580. doi: 10.1002/biof.1302. [DOI] [PubMed] [Google Scholar]
- 14.You J, Sun J, Ma T, Yang Z, Wang X, Zhang Z, Li J, Wang L, Ii M, Yang J, Shen Z. Curcumin induces therapeutic angiogenesis in a diabetic mouse hindlimb ischemia model via modulating the function of endothelial progenitor cells. Stem Cell Res Ther. 2017;8:182. doi: 10.1186/s13287-017-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett PM, Wall CAM, Stack AG. Peripheral artery disease prevalence and mortality trends of United States dialysis population: 1995–2005. Irish J Med Sci. 2010;179:S409–S410. [Google Scholar]
- 16.Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: Implications for investigation of therapeutic agents. J Appl Physiol (1985) 2004;97:773–780. doi: 10.1152/japplphysiol.00107.2004. [DOI] [PubMed] [Google Scholar]
- 17.Albadawi H, Oklu R, Cormier NR, O'Keefe RM, Heaton JT, Kobler JB, Austen WG, Watkins MT. Hind limb ischemia-reperfusion injury in diet-induced obese mice. J Surg Res. 2014;190:683–691. doi: 10.1016/j.jss.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007;2:481–485. doi: 10.1038/nprot.2007.54. [DOI] [PubMed] [Google Scholar]
- 20.Hazarika S, Farber CR, Dokun AO, Pitsillides AN, Wang T, Lye RJ, Annex BH. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation. 2013;127:1818–1828. doi: 10.1161/CIRCULATIONAHA.112.000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elavarasu S, Suthanthiran T, Thangavelu A, Alex S, Palanisamy VK, Kumar TS. Evaluation of superoxide dismutase levels in local drug delivery system containing 0.2% curcumin strip as an adjunct to scaling and root planing in chronic periodontitis: A clinical and biochemical study. J Pharm Bioallied Sci. 2016;8(Suppl 1):S48–S52. doi: 10.4103/0975-7406.191967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang F, Yao Y, Wu J, Liu Q, Zhang J, Pu X, Zhang Q, Xia L. Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-kB/VEGF signaling. Am J Transl Res. 2017;9:5538–5547. [PMC free article] [PubMed] [Google Scholar]
- 23.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 24.González-Duarte RJ, Cázares-Ordoñez V, Ávila-Chávez E. The microRNA biogenesis machinery: Regulation by steroid hormones and alterations in cancer. Rev Invest Clin. 2014;66:460–464. [PubMed] [Google Scholar]
- 25.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 26.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Yuan P, He Y. Role of microRNAs in peripheral artery disease (review) Mol Med Rep. 2012;6:695–700. doi: 10.3892/mmr.2012.978. [DOI] [PubMed] [Google Scholar]
- 28.Ganta VC, Choi MH, Kutateladze A, Fox TE, Farber CR, Annex BH. A MicroRNA93-interferon regulatory factor-9-immunoresponsive gene-1-itaconic acid pathway modulatesM2-like macrophage polarization to revascularize ischemic muscle. Circulation. 2017;135:2403–2425. doi: 10.1161/CIRCULATIONAHA.116.025490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.