Abstract
Transforming growth factor (TGF)-β1 may stimulate the activation of hepatic stellate cells (HSCs), resulting in the development of liver fibrosis. As micro RNA (miRNA)-122 is known to be associated with liver inflammation, its effects on the epithelial-mesenchymal transition (EMT) of HSCs through the inhibition of the TGF-β1/drosophila mothers against decapentaplegic protein 4 (Smad4) signaling pathway were investigated. The MTT assay was performed to explore the optimum TGF-β1 concentration suitable for HSC stimulation. Fluorescence microscopy was used to observe the transfection efficiency and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis were used to observe gene and protein expression levels of α-smooth muscle actin (α-SMA), E-cadherin, N-cadherin and Smad4, respectively, in HSCs treated with TGF-β1 or TGF-β1 and miRNA-122. MTT assay results indicated that the concentration of 10 µg/l TGF-β1 was suitable for maximum growth and survival of HSCs. Notably, the mRNA expression levels of N-cadherin and α-SMA were significantly increased (each, P<0.05), but the expression levels of E-cadherin were decreased following 10 µg/l TGF-β1 treatment. Similar results were observed regarding the protein expression levels of N-cadherin, α-SMA and E-cadherin. Furthermore, the expression of F-actin was increased in the 10 µg/l TGF-β1 treated group compared with the 0 µg/l TGF-β1 treaded group and stretching of the muscle fiber filament was observed. miRNA-122 lentiviral vector transfection significantly decreased the mRNA expression of N-cadherin and increased the mRNA expression of E-cadherin in HSCs stimulated with TGF-β1, as evident from RT-qPCR results. Similar results were also observed regarding the protein expression levels of N-cadherin and E-cadherin. The expression levels of Smad4, the primary component of the TGF-β1 signaling pathway, were significantly lower in cells treated with TGF-β1 and miRNA-122 (P<0.01) compared those treated with TGF-β1. Thus, miRNA-122 may inhibit the activation and EMT of HSCs stimulated by TGF-β1.
Keywords: microRNA-122, epithelial mesenchymal transition, liver fibrosis, transforming growth factor-β1/smads signaling pathway
Introduction
Liver fibrosis is an important stage in the development of various liver diseases, which is associated with the activation of hepatic stellate cells (HSCs) (1), the promotion of extracellular matrix synthesis and degradation, the induction of inflammatory and fibrous correlation factor expression and the mediation of liver cell apoptosis (2). Transforming growth factor (TGF)-β1 serves a vital role in the activation and transformation of HSCs, and promotes the expression of extracellular matrix molecules in these cells (3,4). In this process, TGF-β1 combines with the intracellular receptor, drosophila mothers against decapentaplegic protein (Smad), and mediates TGF-β1 signaling pathways (5).
A previous study has suggested that the activated TGF-β1 signaling pathway may induce epithelial-mesenchymal transition (EMT) of HSCs (6). During this process, the expression of the epithelial component, E-cadherin, is downregulated, while that of the mesenchymal component, α-smooth muscle actin (α-SMA)/vitamin E, is upregulated (7,8). The inhibition of epithelial-mesenchymal changes in HSCs may prevent the development of hepatic fibrosis (9,10).
microRNAs (miRNAs) are 20–24 nucleotides long, endogenous, highly conserved, non-coding small single-stranded RNAs that inhibit the regulation of gene expression through an interpreter and participate in the metabolism of liver cells and physiological processes, including the stress response (11). miRNA-122 accounts for 70% of all identified miRNAs in the liver and serves a pivotal role in liver physiology (12). miRNA-122 is associated with various pathological changes in the liver, including chronic inflammation of the liver (13), liver fibrosis (14,15), cirrhosis (16) and liver cancer, and is considered a non-invasive diagnostic predictor of liver fibrosis (14). In addition, miRNA-122 can act as a tumor suppressor miRNA and regulate metastasis of liver cancer (17,18). Previous findings have indicated that the decrease in miRA-122 expression is negatively correlated with proliferation, migration and hepatocellular carcinoma cell invasion (19). The Wnt/β-catenin signaling pathway regulates miRNA-122-mediated regulation of the proliferation and apoptosis of liver cancer cells (20) and transformation of the epithelium of liver cancer (21). However, it is not known whether microRNA may inhibit the transformation of the epithelium of HSCs and suppress the development of hepatic fibrosis. Therefore, the effect of miRNA-122 on EMT activation in HSCs was evaluated to explore its therapeutic potential for the treatment of liver fibrosis.
Materials and methods
Cell lines and culture
A rat HSC-T6 cell line (cat. no. FS-0060; American Type Culture Collection, Manassas, VA, USA) was maintained in Dulbeccos minimum essential medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin at 37°C in a humidified atmosphere containing 5% CO2.
MTT assay
The MTT method was used to determine the optimal concentration of TGF-β1 required to induce the logarithmic growth phase in rat HSCs. Cells were treated with 0.25% trypsin and seeded at a density of 104 cells after vaccination in 96-well plates. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 for 12 h and treated with 0, 5, 10 and 20 ng/ml of TGF-β1 (PeproTech, Inc., Rocky Hill, NJ, USA). The cells were observed for a total of 4, 8, 24, 48 and 72 h, and incubated with 20 µl/ml MTT solution at 37°C in a humidified atmosphere containing 5% CO2. Cells were treated with 150 µl dimethyl sulfoxide and incubated at room temperature for 10 min under constant shaking. The absorbance was measured at a wavelength of 490 nm. The survival rate of the control group was considered as 100% and used to obtain the survival rate of other groups as follows after 12 h TGF-β1 treatment: Cell survival rate (%)=(experimental group OD-blank group OD)/(control group OD-blank group OD) ×100. This experiment was repeated four times and the results were indicated as the mean ± standard deviation.
Immunofluorescence staining
Cells from the logarithmic phase were washed with phosphate-buffered saline (PBS) and treated with 0.25% ethylenediaminetetraacetic acid-trypsin. Cells were seeded at a density of 2×104 cells, following vaccination in 6-well plates and cultured for 24 h at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, cells were fixed with 4% paraformaldehyde for 20 min, then washed three times with PBS containing 0.1% Triton X-100 for 20 min. Samples were further washed three times with PBS and incubated with 7.5% bovine serum albumin (cat. no. A3912-10; Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) at room temperature for 1 h. Following blocking, cells were treated with mouse anti- F-actin (cat. no. ab-205; 1:100; Abcam, Cambridge, UK) antibody overnight at 4°C. Cells were washed three times with PBS and subjected to treatment with FITC-conjugated IgG (1:50; cat. no. AS001; ABclonal Biotech Co., Ltd., Wuhan, China) for 1h at 37°C. Following this, 4′,6-diamidino-2-phenylindole nuclear staining was performed for 15 min and cells were observed using a fluorescence microscope (magnification, ×100).
Transfection of miRNA-122 slow virus vector
miRNA-122 slow virus vector (cat. no. 15993-1; vector: Ubi-MCS-SV40-EGFP-IRES-puromycin) was designed and synthesized by the Shanghai Jikai Medical Laboratory (Shanghai Genechem Co., Ltd.; sequence: 5′-ACCGGTCCACGGAGGAGTCTGTGACAAAGAAGGAGGGTGAAGGGGAGGTTAGCACCCTTGTGCCTACAGACTCTCCTTAGCAGAGCTCTGGAGTGTGACAATGGTGTTTGTGTCCAAAACATCAAACGCCATCATCACACTAAACAGCTACTGCTAGGCTATCCGTCTACTCCGTGCGCGACTTGACGTCTGCCCTCTCAGAGCAAGAAGTTTTGTCTTATGTACTCTCCGTCATAGGTAATTTATGGCTAGC-3). CON238 (Ubi-MCS-SV40-EGFP-IRES-puromycin) was used as a negetive virus vector. In this part, HSC cells treated with 10 µg/l TGF-β1 only and HSC cells treated with 10 µg/l TGF-β1 and CON238 were utilized as control groups. HSC cells treated with 10 µg/l TGF-β1 and micro-122 slow virus vector were utilized as experimental groups. First, HSCs were diluted to obtain 3×104−3×106 cells/ml. Following this, a total of 2 ml cell suspension was added to each well of a 6-well plate. Cells were allowed to adhere to the surface of the plate. HSC cells were then cultivated with 2 ml fresh culture medium (DMEM containing 10% fetal bovine serum) containing 5 ug/ml polybrene (cat. no. REVG0001; Shanghai Genechem Co., Ltd.) Preliminary experiments revealed an MOI of 10 (Drop in the infection by degree of × = virus volume/cell number; data not shown), the micro-122 slow virus vector and CON238 negative virus vector were diluted to 1×108 tu/ml using Enhanced Infection Solution (cat. no. ENI.S. REVG0002; Shanghai Genechem Co., Ltd.). Subsequently, a total of 20 µl virus solution was added to the three groups and incubated for 6 h at 37°C. HSC Cells were then washed with PBS and further incubated with DMEM with 10% fetal bovine serum for 48 h at 37°C. Cells were observed using a fluorescence microscope.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from 1×107 cells/ml using the TRIzol reagent (cat. no. 15596-026; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to manufacturers instructions. The concentration of total RNA was measured using an Eppendorf Bio Photometer. First-strand cDNA was synthesized from 5 µg total RNA per sample in a 20-µl system including 1 µl random 6-mers, 1 µl dNTP mixture, 0.5 µl PrimeScript RTase, 0.5 µl Rnase and 17 µl ddH20 using an RT-qPCR kit (cat. no. DRRO14A; Takara Biotechnology Co., Ltd., Dalian, China). The following gene-specific primers were used: α-SMA forward, 5′-CCACTGCTGCTTCCTCTTC-3′ and reverse, 5′-CGCCGACTCCATTCCAAT-3′; N-cadherin forward, 5′-TATGGTGGTGGTGATGACTGA-3′ and reverse, 5′-CGGTGCTAGTGGACTACAGA-3′; E-cadherin forward, 5′-GCTCGCTGAACTCCTCTGA-3′ and reverse, 5′-TCGCCGCCACCATACATA-3′; Smad4 forward, 5′-ACTTCCCCAACATTCCTGTG-3′ and reverse, 5′-ATCCATTCTGCTGCTGTCCT-3′; GAPDH forward, 5′-ACGGCAAGTTCAACGGCACAG-3′ and reverse, 5′-GAAGACGCCAGTAGACTCCACGAC-3′; miRNA-122, 5′-GCTGTGGAGTGTGACAATGGTG-3′; U6 forward, 5′-CGCTTCGGCAGCACATATACT-3′ and reverse, 5′-GAATTTGCGTGTCATCCTTGC-3′. The reaction steps were as follows: Stage 1 Prevariant: 95°C for 5 min; Stage 2 Cyclic reaction: 95°C for 10 min and 60°C for 30 sec for 40 cycles. Stage 3 dissociation curve: 95°C for 15 sec, 60°C for 60 sec and 90°C for 15 sec for one cycle. The 2−ΔΔCq method (22) was used to quantify the relative gene expression levels. U6 and GAPDH were used as reference genes.
Western blot analysis
Total protein was extracted from 1×107/ml HSC cells using radioimmunoprecipitation assay buffer (cat. no. P0013; Beyotime Institute of Biotechnology, Haimen, China). Subsequently, protein concentration was determined using a BCA protein kit (Beyotime Institute of Biotechnology). Protein (100 µg/lane) was separated on 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Following blocking with 5% skimmed milk at room temperature for 2 h, the membrane was incubated with the following primary antibodies overnight at 4°C: ianti-α-SMA (cat. no. ab5694; 1:500), anti-N-cadherin (cat. no. ab76011; 1:500), anti E-cadherin (cat. no. ab76055; 1:1,000; all Abcam), anti-Smad4 (cat. no. 51144-1-AP; 1:1,000; Proteintech Group, Inc., Chicago, IL, USA) and anti-GAPDH (cat. no. EM31010-011: 1,000; Beijing Emarbio Science & Technology Co., Ltd., Beijing, China) overnight at 4°C. The membrane was then incubated with horseradish peroxidase conjugated goat anti-mouse Immunoglobulin G (1:2,000; cat. no. AS003; ABclonal Biotech Co., Ltd.) for a further 2 h at room temperature. An ECL Plus ultra-sensitive luminescent liquid (Applygen Technologies, Inc., Beijing, China) was utilized to develop the membrane. Protein bands were analyzed using Quantity One v4.6.2 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The density of each target band was calculated relative to GAPDH to determine relative expression levels.
Statistical analysis
Results were reported as the mean ± standard deviation and analyzed using SPSS software (version 21.0 for Windows; IBM Corp., Armonk, NY, USA) was used for all statistical analyses. One-way analysis of variance followed by the least significant difference test. Two-group comparisons were performed using the Student t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
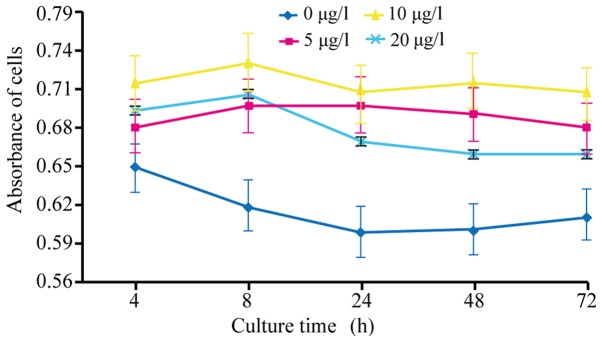
TGF-â1 at 10 ìg/l promotes the viability and survival of HSCs
For the effective activation of HSCs, the most suitable concentration of TGF-â1 was determined. Cells were treated with different concentrations (0, 5, 10 and 20 ìg/l) of TGF-â1. MTT assay results revealed that treatment with 10 ìg/l TGF-â1 for 72 h resulted in maximum cell viability (Fig. 1). Using 0 ìg/l TGF-â1 treatment as the control (100% survival), it was revealed that 10 ìg/l TGF-â1 demonstrated the highest survival rate after 48 h (Table I).
Figure 1.
Detection of HSC-T6 proliferation by MTT with different concentrations of TGF-β1. TGF-β1, transforming growth factor-β1.
Table I.
Effect of different concentration of TGF-β1 on the cell survival rate on 48 h.
| Groups | Concentration (µg/l) | Absorption value | Survival rate (%) |
|---|---|---|---|
| Control | 0 | 0.615±0.020 | 100.00 |
| TGF-β1 | 5 | 0.692±0.021a | 112.52 |
| 10 | 0.714±0.022b | 116.15 | |
| 20 | 0.668±0.002a | 108.56 |
P<0.05
P<0.01 compared with control group. Data were presented as mean ± standard deviation (n=4). TGF, transforming growth factor.
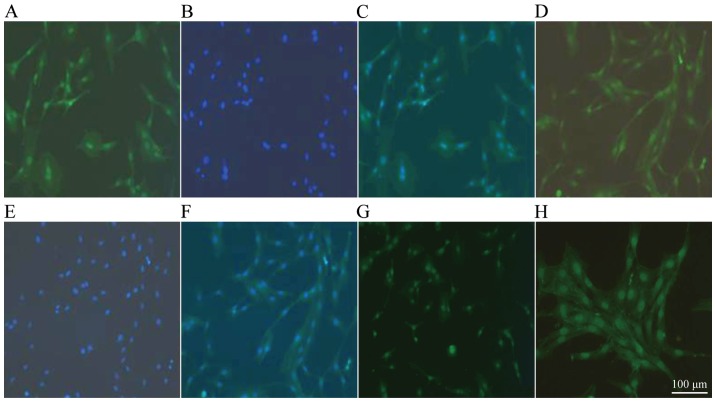
Upregulation and morphological changes in F-actin indicate the activation of HSCs
Cell migration is associated with cytoskeleton remodeling of actin, including F-actin upregulation and morphological changes, indicating the activation of HSCs (23). Results indicated an increase in the expression of F-actin in HSCs following treatment with 10 µg/l TGF-β1. Notably, increased expression of the stress fiber in the cell is indicative of the activation and proliferation of HSCs (24). In the control group, the cytoskeleton exhibited diffused distribution, irregular and uneven length of actin filaments and bright nuclei fluorescence (Fig. 2A-C; magnification, ×100). In the TGF-β1 group, F-actin green fluorescence was concentrated and clustered, and the fluorescence intensity was homogenous within the cell membrane and cytoplasm (Fig. 2D-F; magnification, ×100); F-actin was relatively thick and long along the longitudinal axis of the cells and arranged parallel to the thin strips of stress fibers (Fig. 2G and H; magnification, ×100).
Figure 2.
Cytoskeletal structure changes of F-actin induced by TGF-β1 observed by immunofluorescence. (A-C) Control group and (D-F) 10 µg/l TGF-β1 group. (A and D) F-actin skeletal structure; (B and E) 4,6-diamidino-2-phenylindole stained nuclei; (C) combination of (A) and (B); and (F) combination of (D) and (E) (all magnification, ×100). (G) Control group (magnification, ×100); and (H) 10 µg/l TGF-β1 group (magnification, ×200). TGF-β1, transforming growth factor-β1.
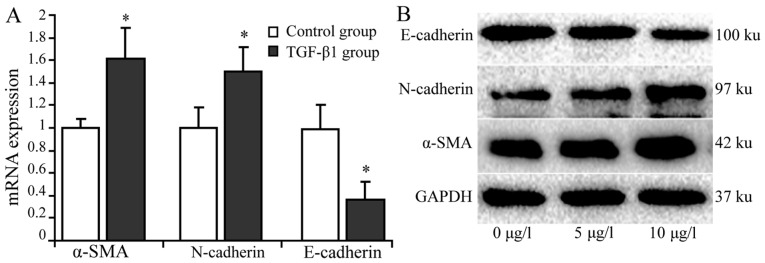
TGF-β1 upregulates á-SMA but downregulates E-cadherin expression in activated HSCs
RT-qPCR and western blot analysis were used to detect the expression of α-SMA, N-cadherin and E-cadherin in HSCs treated with 10 µg/l TGF-β1. The results indicated that the relative expression levels of α-SMA mRNA and protein was significantly higher in the treatment group compared with the control group. The relative expression of N-cadherin mRNA was marginally higher in the treatment group compared with the control group. N-cadherin protein expression was markedly higher in the treatment group compared with the control group. The mRNA expression levels of E-cadherin were significantly lower in the treatment group compared with the control group (P<0.05), and similar results were indicated with regard to E-cadherin protein expression (Fig. 3).
Figure 3.
Expression of α-SMA, N-cadherin and E-cadherin in hepatic stellate cells induced with 10 µg/l TGF-β1. mRNA and protein expression levels of α-SMA, N-cadherin and E-cadherin were determined in hepatic stellate cells using reverse transcription-quantitative polymerase chain reaction and western blot analysis. (A) mRNA Expression of α-SMA, N-cadherin and E-cadherin in hepatic stellate cells treated with 10 µg TGF-β1. (B) Protein expression of α-SMA, N-cadherin and E-cadherin in hepatic stellate cells with different of TGF-β1 concentrations. *P<0.05 vs. the control group.
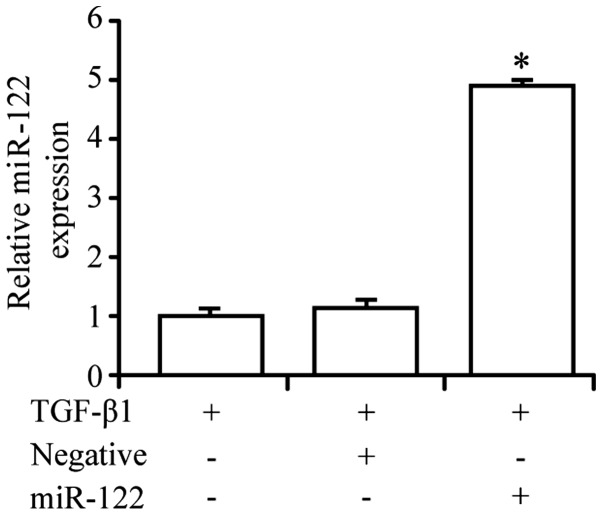
Expression of miRNA-122 is upregulated in HSCs transfected with miRNA-122 lentiviral vector
Fluorescence microscopy revealed green fluorescence in HSCs transfected with miRNA-122 lentiviral vectors into the cell cytoplasm. Nuclei exhibited bright fluorescence, and the fluorescence intensity was stronger than that of the cytoplasm. The expression level of miRNA-122 in the transfected cells (4.92±0.09) was significantly higher (P<0.05) than that observed in cells transfected with the negative control vector (1.21±0.08) and the blank control group (1.00±0.11; Fig. 4).
Figure 4.
miRNA-122 expression in hepatic stellate cells transfected with miRNA-122 lentiviral vector. Reverse transcription-quantitative polymerase chain reaction was used to compare the expression of miRNA-122 in the miRNA-122 lentiviral vector group compared with control groups *P<0.05 vs. TGF-β1 groups or TGF-β1 and negative lentiviral vector groups.
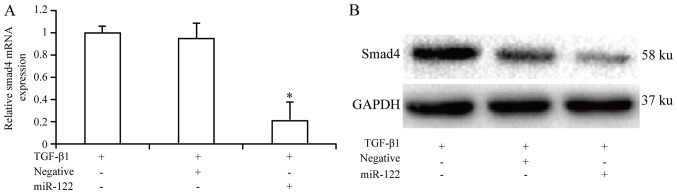
Smad4 serves as the key protein in the TGF-â1 signaling pathway
The expression of Smad4 may result in the inhibition of the activation and biological activity of HSCs induced by the TGF-â1 signaling pathway. Therefore, the expression of Smad4 was studied following miRNA-122 transfection in HSCs. According to the results in Fig. 5, Smad4 expression was significantly (P<0.05) higher in cells treated with TGF-β1 compared with cells treated with TGF-β1 and miRNA-122. Thus, miRNA-122 inhibited the activation of HSCs induced by TGF-β1.
Figure 5.
Expression of Smad4 in hepatic stellate cells treated with TGF-β1 or TGF-β1 and miRNA-122. *P<0.05 vs. TGF-β1 groups or TGF-β1 and negative lentiviral vector groups. (A) Relative mRNA expression of Smad4 in hepatic stellate cells treated with 10 µg TGF-β1. (B) Relative protein expression of Smad4 in hepatic stellate cells.
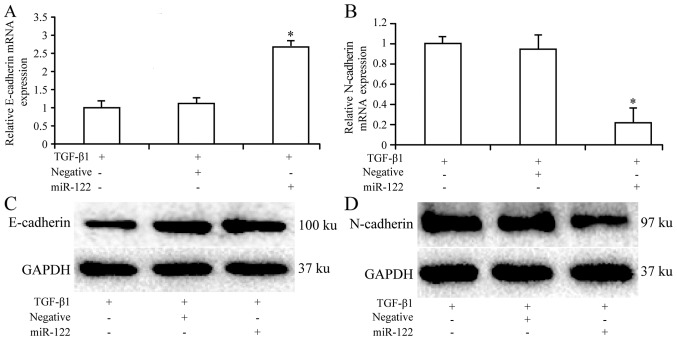
Downregulation of N-cadherin expression and overexpression of E-cadherin in HSCs treated with miRNA-122 and TGF-â1 indicates that miRNA-122 may inhibit EMT of HSCs induced by TGF-â1
TGF-β1 and empty plasmid groups were used as control groups. The results suggested that the mRNA expression of E-cadherin was significantly higher (P<0.05) in the treatment group (2.73±0.16) compared with the negative vector transfection group (1.17±0.12) and blank control group (1.01±0.20). In addition, E-cadherin protein expression was higher in the treatment group (0.87±0.07) compared with the control groups (0.36±0.11 and 0.41±0.130). The mRNA and protein expression of N-cadherin was lower in the treatment group (0.21±0.13 and 0.32±0.07, respectively) compared with the two control groups (0.92±0.12 and 0.88±0.14, respectively, for negative vector transfection group) and (1.02±0.20 and 0.21±0.07, respectively, for blank control group; Fig. 6). Notably the difference regarding mRNA N-cadherin expression was statistically significant (P<0.05).
Figure 6.
Expression of E-cadherin and N-cadherin in hepatic stellate cells treated with TGF-β1 or TGF-β1 and miRNA-122. (A and B) mRNA and (C and D) protein expression levels of E-cadherin and N-cadherin were determined using reverse transcription-quantitative polymerase chain reaction and western blot analysis. *P<0.05 vs. TGF-β1 groups or TGF-β1 and negative lentiviral vector groups.
Discussion
The activation of HSCs is a major cause of liver fibrosis and cirrhosis, and effective inhibition of the proliferation and activation of HSCs is a key step in the prevention of liver fibrosis development (25). EMT in differentiated cells is caused by external factors that impart mature epithelial characteristics to cells, characterized with the loss of cell polarity and change in the cytoskeleton structure and migration ability (26). EMT has long been determined a characteristic of tumor cells. Previous findings on renal fibrosis, pulmonary fibrosis and peritoneal fibrosis have focused on EMT (27). A study in the mouse model of carbon tetrachloride-induced liver fibrosis indicated that the lower expression of E-cadherin and higher expression of α-SMA directly affected EMT in liver fibrosis (6). However, to the best of our knowledge, no report has described the series of processes that lead to the activation of HSCs.
The present study investigated whether the activation of HSCs induced by TGF-β1 causes the transformation of the epithelium. It was first explored whether TGF-β1 may activate HSCs, and results indicated that the activity and proliferation of HSCs increased with an increase in the concentration of TGF-β1; however, concentrations higher than 10 µg/l failed to induce a significant increase in HSC activity and proliferation. Therefore, the concentration of 10 µg/l TGF-β1 was used for further experiments. The HSC activation by TGF-β1 was studied, and it was observed that myofibroid cells with stellate caused reconstruction of the cytoskeleton. F-actin is an important skeletal protein involved in the formation of the cytoskeleton (28). In addition to maintaining the normal morphology of the cells, F-actin also participates in biological processes through stress regulation (29). An increase in the expression of F-actin and stretching of the muscle fiber filament was observed in a previous study (30). In addition, the effects of TGF-β1 on the expression of E-cadherin and N-cadherin were evaluated in HSCs. N-cadherin gene and protein expression levels increased, but the gene and protein expression levels of the epithelial component E-cadherin were downregulated. Thus, TGF-β1 induced HSC activation and caused EMT.
The inhibition of the TGF-β1 signaling pathway may suppress the activation of HSCs and reduce the development of hepatic fibrosis. To identify the target gene or protein that effectively inhibited this regulation, miRNA-122, which is expressed specifically in the liver and accounts for 70% of all hepatic microRNAs, was used. miRNA-122 serves an important role in liver development, differentiation and maintenance under normal physiological processes and may cause a series of liver diseases, including inflammation, cirrhosis/liver fibrosis, fat metabolism and cancer (13,16). The present study indicated miRNA-122 may inhibit the regulation of the TGF-β1 signaling pathway in HSCs.
A previous study suggested that the expression of miRNA-122 was associated with the inflammatory response of the liver (31). In addition, miRNA-122 expression is significantly lower in chronic inflammation than in normal conditions and early inflammation (32). Previous findings have indicated that miRNA-122 is considered a marker of liver fibrosis (14). An miRNA-122 lentiviral vector was constructed and successfully transfected into HSCs in the present study. Successful transformants were selected using the green fluorescent protein expression method.
The expression of E-cadherin/N-cadherin was studied in HSCs treated with miRNA-122 lentiviral vector alone or in combination with TGF-β1. It was revealed that the increase in the expression of miRNA-122 resulted in a significant increase in E-cadherin expression in cells transfected with miRNA-122 lentiviral vector when compared with those treated with empty plasmid group or TGF-β1 and miRNA-122. Conversely, the expression of N-cadherin significantly decreased with an increase in miR-122 expression, resulting in the inhibition of EMT of HSCs. Thus, the upregulation in miRNA-122 expression may result in the inhibition of EMT of HSCs. This result was in line with reports from Omran et al and Nakamura et al (14,15), wherein the morphology and functional study demonstrated that the overexpression of miRNA-122 may effectively promote the differentiation and maturation of liver cells. The expression of miRNA-122 at appropriate concentrations may adjust the balance between liver cell proliferation and differentiation, as well as that between EMT and mesenchymal epithelial transition. To clarify the role of the TGF-β1 signaling pathway in this process, changes in the expression of the key protein Smad4 in the TGF-β1 signaling pathway were studied. Results indicated that HSCs stimulated by TGF-β1 exhibited upregulated expression of Smad4; however, miRNA-122 treatment significantly decreased the expression of Smad4, which suggested the inhibitory effect of miRNA-122 on the activation of HSCs induced by TGF-β1 and EMT.
Taken together, the present findings demonstrated the effects of miRNA-122 on HSC epithelium. However, the regulation of HSC activation is typically a complex signaling network. It was suggested that miRNA-122 may regulate the activation of HSCs mediated by TGF-β1 signaling pathways; whether the exact mechanism involves direct interactions between the two is unknown. Therefore, further studies must be performed to study the regulatory effects of miRNA-122 in liver fibrosis in order to extend its application as a potential agent against liver fibrosis.
Acknowledgements
Hepatic stellate cells HSC-T6 cells were kindly provided by Professor Hong Shi of the Traditional Chinese and Western Medicine Institute, Fujian University of Chinese Medicine (Fuzhou, China).
Funding
The present study was funded by the Fuzhou Science and Technology Project (grant nos. 2014-S-137-1 and 2014-S-137-5) and the Fujian Science and Technology Guiding Project (grant no. 2015D002).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors contributions
LW and BC performed the experiments, participated in data collection and drafted the manuscript. QZ and WL performed statistical analyses and designed the present study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tomita K, Teratani T, Suzuki T, Shimizu M, Sato H, Narimatsu K, Usui S, Furuhashi H, Kimura A, Nishiyama K, et al. Acyl-CoA: Cholesterol acyltransferase 1 mediates liver fibrosis by regulating free cholesterol accumulation in hepatic stellate cells. J Hepatol. 2014;61:98–106. doi: 10.1016/j.jhep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Duval F, Moreno-Cuevas JE, González-Garza MT, Rodríguez-Montalvo C, Cruz-Vega DE. Liver fibrosis and protection mechanisms action of medicinal plants targeting apoptosis of hepatocytes and hepatic stellate cells. Adv Pharmacol Sci. 2014;2014:373295. doi: 10.1155/2014/373295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang AT, Hu DD, Wang P, Cong M, Liu TH, Zhang D, Sun YM, Zhao WS, Jia JD, You H. TGF-β1 induces the dual regulation of hepatic progenitor cells with both anti- and proliver fibrosis. Stem Cells Int. 2016;2016:1492694. doi: 10.1155/2016/1492694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63:2010–2019. doi: 10.1046/j.1523-1755.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 5.Dropmann A, Dediulia T, Breitkopf-Heinlein K, Korhonen H, Janicot M, Weber SN, Thomas M, Piiper A, Bertran E, Fabregat I, et al. TGF-β1 and TGF-β2 abundance in liver diseases of mice and men. Oncotarget. 2016;7:19499–19518. doi: 10.18632/oncotarget.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan L, Ye L, Zhuang L, Zou X, Liu S, Zhang Y, Zhang L, Jin C, Huang Y. VEGFC/VEGFR3 axis mediates TGFβ1-induced epithelial-to-mesenchymal transition in non-small cell lung cancer cells. PLoS One. 2018;13:e0200452. doi: 10.1371/journal.pone.0200452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang QL, Tao YY, Yuan JL, Shen L, Liu CH. Salvianolic acid B preventsepithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biol. 2010;11:31. doi: 10.1186/1471-2121-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi WR, Yang CQ, Shi Q. Transforming growth factor-β1 induced epithelial-mesenchymal transition in hepatic fibrosis. Hepatogastroenterology. 2012;59:1960–1963. doi: 10.5754/hge11750. [DOI] [PubMed] [Google Scholar]
- 9.Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A. miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol. 2008;48:648–656. doi: 10.1016/j.jhep.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Jia Y, Zheng R, Guo Y, Wang Y, Guo H, Fei M, Sun S. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clin Chem. 2010;56:1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 11.Lewis Starkey PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, French NS, Dhaun N, Webb DJ, Costello EM, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 12.Su TH, Chen M, Liu CJ, Chen CL, Ting TT, Tseng TC, Chen PJ, Kao JH, Chen DS. Serum microRNA-122 level correlates with Virologic responses to pegylated interferon therapy in chronic hepatitis C. Proc Natl Acad Sci USA. 2013;110:7844–7849. doi: 10.1073/pnas.1306138110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding X, Ding J, Ning J, Yi F, Chen J, Zhao D, Zheng J, Liang Z, Hu Z, Du Q. Circulating microRNA-122 as a potential biomarker for liver injury. Mol Med Rep. 2012;5:1428–1432. doi: 10.3892/mmr.2012.838. [DOI] [PubMed] [Google Scholar]
- 14.Omran AA, Osman KS, Kamel HM, Abdel-Naem EA, Hasan DE. MicroRNA-122 as a novel non-invasive marker of liver fibrosis in hepatitis C virus patients. Clin Lab. 2016;62:1329–1337. doi: 10.7754/Clin.Lab.2015.151141. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M, Kanda T, Jiang X, Haga Y, Takahashi K, Wu S, Yasui S, Nakamoto S, Yokosuka O. Serum microRNA-122 and Wisteria floribunda agglutinin-positive Mac-2 binding protein are useful tools for liquid biopsy of the patients with hepatitis B virus and advanced liver fibrosis. PLoS One. 2017;12:e0177302. doi: 10.1371/journal.pone.0177302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waidmann O, Köberle V, Brunner F, Zeuzem S, Piiper A, Kronenberger B. Serum microRNA-122 predicts survival in patients with liver cirrhosis. PLoS One. 2012;7:e45652. doi: 10.1371/journal.pone.0045652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 18.Qiao DD, Yang J, Lei XF, Mi GL, Li SL, Li K, Xu CQ, Yang HL. Expression of microRNA-122 and microRNA-22 in HBV-related liver cancer and the correlation with clinical features. Eur Rev Med Pharmacol Sci. 2017;21:742–747. [PubMed] [Google Scholar]
- 19.Wang N, Wang Q, Shen D, Sun X, Cao X, Wu D. Downregulation of microRNA-122 promotes proliferation, migration, and invasion of human hepatocellular carcinoma cells by activating epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:2035–2047. doi: 10.2147/OTT.S92378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Zhu X, Wu L, Yang R, Yang Z, Wang Q, Wu F. MicroRNA-122 suppresses cell proliferation and induces cell apoptosis in hepatocellular carcinoma by directly targeting Wnt/β-catenin pathway. Liver Int. 2012;32:752–760. doi: 10.1111/j.1478-3231.2011.02750.x. [DOI] [PubMed] [Google Scholar]
- 21.Jin Y, Wang J, Han J, Luo D, Sun Z. MiR-122 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Snail1 and Snail2 and suppressing WNT/β-cadherin signaling pathway. Exp Cell Res. 2017;360:210–217. doi: 10.1016/j.yexcr.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Ma L, Yang X, Wei R, Ye T, Zhou JK, Wen M, Wen RT, Li P, Dong B, Liu L, et al. MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression. Cell Death Dis. 2018;9:718. doi: 10.1038/s41419-018-0752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui X, Zhang X, Yin Q, Meng A, Su S, Jing X, Li H, Guan X, Li X, Liu S, Cheng M. F-actin cytoskeleton reorganization is associated with hepatic stellate cell activation. Mol Med Rep. 2014;9:1641–1647. doi: 10.3892/mmr.2014.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu F, Ji S, Su L, Wan L, Zhang S, Dai C, Wang Y, Fu J, Zhang Q. Adipose-derived mesenchymal stem cells inhibit activation of hepatic stellate cells in vitro and ameliorate rat liver fibrosis in vivo. J Formos Med Assoc. 2015;114:130–138. doi: 10.1016/j.jfma.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Shen Y, Hong J, Xia Q, Zhou F, Liu X. The contribution of TGF-β in Epithelial-Mesenchymal Transition (EMT): Down-regulation of E-cadherin via snail. Neoplasma. 2015;62:1–15. doi: 10.4149/neo_2015_002. [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Nie HY, Gao X, Yang J, Pu J, Chen Z, Cui X, Wang Y, Wang H, Jia G. Epithelial-mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-beta/Smad signaling pathway. Toxicol Lett. 2014;226:150–162. doi: 10.1016/j.toxlet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Molè-Bajer J, Bajer AS, Inoué S. Three-dimensional localization and redistribution of F-actin in higher plant mitosis and cell plate formation. Cell Motil Cytoskeleton. 1988;10:217–228. doi: 10.1002/cm.970100126. [DOI] [PubMed] [Google Scholar]
- 29.Gong X, Fan Y, Zhang Y, Luo C, Duan X, Yang L, Pan J. Inserted rest period resensitizes MC3T3-E1 cells to fluid shear stress in a time-dependent manner via F-actin-regulated mechanosensitive channel(s) Biosci Biotechnol Biochem. 2014;78:565–573. doi: 10.1080/09168451.2014.895657. [DOI] [PubMed] [Google Scholar]
- 30.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boesch-Saadatmandi C, Wagner AE, Wolffram S, Rimbach G. Effect of quercetin on inflammatory gene expression in mice liver in vivo-role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol Res. 2012;65:523–530. doi: 10.1016/j.phrs.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Bala S, Petrasek J, Ward J, Alao H, Levin I, Szabo G. Serum microrna-122 and mir-155 as biomarkers of liver injury and inflammation in models of acute and chronic liver disease. Gastroenterology. 2012;140:906. doi: 10.1016/S0016-5085(11)63758-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.