Abstract
Several lines of evidence implicate serotonin (5-hydroxytryptamine, 5-HT)in regulating personality traits and mood control. Serotonergic neurons are classically thought to be tonic regular-firing, “clock-like” neurons. Neurotransmission by serotonin is tightly regulated by the serotonin transporter (SERT) and by autoreceptors (serotonin receptors expressed by serotonin neurons) through negative feedback inhibition at the cell bodies and dendrites (5-HT1A receptors) of the dorsal raphe nuclei or at the axon terminals (5-HT1B receptors). In dorsal raphe neurons, the release of serotonin from vesicles in the soma, dendrites, and/or axonal varicosities is independent of classical synapses and can be induced by neuron depolarization, by the stimulation of L-type calcium channels, by activation of glutamatergic receptors, and/or by activation of 5-HT2 receptors. The resulting serotonin release displays a slow kinetic and a large diffusion. This process called volume transmission may ultimately affect the rate of discharge of serotonergic neurons, and their tonic activity. The therapeutic effects induced by serotonin-selective reuptake inhibitor (SSRI) antidepressants are initially triggered by blocking SERT but rely on consequences of chronic exposure, i.e., a selective desensitization of somatodendritic 5-HT1A autoreceptors. Agonist stimulation of 5-HT2B receptors mimicked behavioral and neurogenic SSRI actions, and increased extracellular serotonin in dorsal raphe. By contrast, a lack of effects of SSRIs was observed in the absence of 5-HT2B receptors (knockout-KO), even restricted to serotonergic neurons (Htr2b5-HTKO mice). The absence of 5-HT2B receptors in serotonergic neurons is associated with a higher 5-HT1A-autoreceptor reactivity and thus a lower firing activity of these neurons. In agreement, mice with overexpression of 5-HT1A autoreceptor show decreased neuronal activity and increased depression-like behavior that is resistant to SSRI treatment. We propose thus that the serotonergic tone results from the opposite control exerted by somatodendritic (Gi-coupled) 5-HT1A and (Gq-coupled) 5-HT2B receptors on dorsal raphe neurons. Therefore, 5-HT2B receptors may contribute to SSRI therapeutic effects by their positive regulation of adult raphe serotonergic neurons. Deciphering the molecular mechanism controlling extrasynaptic release of serotonin, and how autoreceptors interact in regulating the tonic activity of serotonergic neurons, is critical to fully understand the therapeutic effect of SSRIs.
Keywords: serotonin receptors, somatodendritic release, volume transmission, antidepressants, autoreceptors
Introduction
In any given year, nearly 40% of the population in European countries is affected, directly or indirectly, by mental illness (Insel and Sahakian, 2012). Mental illness or psychiatric diseases are heterogeneous pathologies and much effort remains necessary to improve diagnosis and therapies. For example, 30–40% of patients with major depression do not respond to current treatments, which suggests that ontogeny of the disease may vary among individuals, and that novel pathways and therapeutic targets have to be identified. Serotonin (5-hydroxytryptamine, 5-HT) is implicated in the processing of perception, emotion, and cognitions and has been involved in various psychiatric disorders (Krishnan and Nestler, 2008). Several lines of evidence implicate serotonin in regulating personality traits and mood control. Indeed, serotonin has also been implicated in the etiology of several mood disorders, including autism spectrum disorders (ASD), major depressive disorder (MDD), schizophrenia or bipolar disorder (BD) (Vadodaria et al., 2018). Accordingly, a growing interest in understanding the molecular and cellular effect of many therapeutic compounds has emerged: serotonin transporter (SERT) is the main target of serotonin selective reuptake inhibitor (SSRI) antidepressants, and 5-HT2 receptors are targets of atypical antipsychotics.
Variations in serotonin levels may affect mood and motivation but functions of endogenous serotonin remain controversial. It has been recently suggested that serotonin enables organisms to adapt to dynamic environments by controlling neuronal plasticity and behavior (Matias et al., 2017). Therefore, the clinical benefits of improving serotonin function would stem from facilitating adaptive changes to negative affects rather than positively modulating the emotional states (Branchi, 2011). Serotonergic neurons are classically thought to display regular tonic firing, or “clock-like,” neurons (Jacobs and Azmitia, 1992), whereas phasic firing in bursts is associated with specific behaviors. Phasic and tonic firing of serotonergic neurons have also been proposed to have opposite functions. However, the respective contribution of serotonergic mode of firing to behavior remains unclear. Tonic firing of serotonin neuron population activity seems related to the extra-synaptic tonic serotonin levels and burst firing to the rapid, high-amplitude, and intra-synaptic phasic serotonin release.
However, how the positive modulation of serotonin tone translates into raised mood or decreased anxiety is not yet understood and the precise relationship between certain behaviors and brain serotonin levels remains unclear. For instance, anxiolysis as a result of reducing brain serotonin is well established, suggesting that serotonin increases anxiety. However, anxiety is often paired with depression, which is classically associated with low serotonin levels (Jennings et al., 2010). Also, SSRIs are effective in treating both disorders, but only in a fraction of patients. Therefore, the precise relationship between serotonin levels and behavior is still to be established. Studies to date have not provided a sufficiently detailed understanding of how tonic serotonin neuron activity can be related to serotonin levels. In this review, we will summarize the known molecular mechanisms controlling tonic release of serotonin, in which autoreceptors (serotonin receptors expressed by serotonin neurons) and SERT participate in regulating the excitability of serotonergic neurons. An understanding of the detailed dynamics of serotonin dendritic release might clarify how serotonin governs behavior, which is critical to fully understand the therapeutic effect of SSRIs.
The Two Modes of Monoamine and Serotonin Transmission
In the brain, neuronal communication is mediated by two major modes of chemical transmission. In the presynaptic terminal, neurotransmitters are released rapidly and locally, and signal to post-synaptic partners for synaptic transmission. In “non-synaptic” transmission, by contrast, neuromodulators diffuse over a large area to stimulate surrounding cells including glial cells (Agnati et al., 1995). In fast neurotransmission, the active zone, which is formed by defined and ordered protein network and docks synaptic vesicles, releases neurotransmitters in millisecond timing. By enhancing their release probability, this neurotransmission allows ordered vesicles to fuse in front of post-synaptic neurotransmitter receptors (Südhof, 2012). The non-synaptic mode of transmission does not take place between two pre- and post-synaptic elements as described above, and neuromodulators are released in a pseudo-open space. Thus, non-synaptic transmission is defined as “volume transmission” (Agnati et al., 1995; Zoli et al., 1999) and lasts for seconds. Precise organization of secretion is not necessary for volume transmission. This signal, which is slow and diffuses in a space larger than the synaptic cleft, involves a low concentration of neurotransmitters.
Monoamine (including serotonin) release has been subdivided into tonic and phasic modes. Tonic release controls the large variation in extracellular monoamine through basal and non-synchronous firing of neurons; by contrast, in phasic release, synchronized burst firing results in a fast, large, and transient neuromodulator increase (Grace, 2016). These neurochemical findings correspond to different neuronal activities. For example, the tonic activity of serotonin neurons can be related to extra-synaptic serotonin-containing vesicle release; the burst firing can be related to the rapid, high-amplitude, intra-synaptic phasic serotonin-containing vesicle release. Tonic firing is characterized by low frequency (0.1–3 Hz), and is classically defined as having clock-like, pace-maker regularity. Phasic firing characterized with burst of higher firing rates (up to 17 Hz) has indeed been reported in serotonin neurons (Allers and Sharp, 2003; Kocsis et al., 2006; Hajós et al., 2007). The precise control of neuronal activity that differentiates these two modes of release is not yet well understood.
The existence of serotonin volume transmission has been supported by several observations, (1) the distribution of serotonergic receptors and transporter not facing post-synaptic densities suggests that they detect serotonin released extrasynaptically (Ridet et al., 1994; Bunin and Wightman, 1999); this is notably the case for the 5-HT1A receptor, which is known to play an autoreceptor function in the dorsal raphe (Kia et al., 1996; Riad et al., 2000); (2) serotonin- and vesicular transporter (VMAT2)-positive vesicles are found not only in axonal varicosities, but also in the soma and dendrites; these VMAT2-positive vesicles are located independently of post-synaptic elements (Chazal and Ralston, 1987; Descarries and Mechawar, 2000), suggesting that non-synaptic vesicular storage and release can also occur in the somatodendritic compartment; (3) finally, it has been shown that similar amount of serotonin can be found at the somatic or dendritic level compared to axonal terminals (Bruns et al., 2000; Kaushalya et al., 2008b); in addition, extracellular concentrations of serotonin can increase in response to single stimulation pulses (Bunin and Wightman, 1999). Extrasynaptic release mechanisms likely occur by regulated exocytosis of vesicles (Trueta and De-Miguel, 2012) leading a widespread release in the extracellular space.
In axons, serotonin can be released from presynaptic terminals, but also from extra-synaptic sites (varicosities). In axonal varicosities, in dendrites and in soma, serotonin is released via volume transmission. The tonic activity of serotonin neurons being related to extra-synaptic serotonin release is likely to use volume transmission. However, the vesicular release machinery for this mode of transmission may be different from that used for synaptic transmission.
Vesicular Complexes Involved in Serotonin Release by Volume Transmission
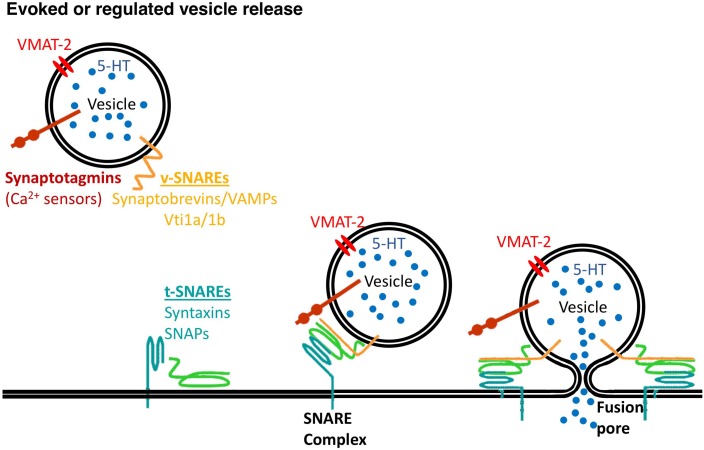
Members of the family of soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptors (SNAREs) are involved in intracellular vesicular trafficking. The association of SNARE proteins expressed by interacting membranes triggers exocytosis by forming complexes through four coiled-coil SNARE motifs (Jahn and Scheller, 2006). Evoked synaptic vesicle release needs the canonical SNARE proteins, including the vesicle-associated SNAREs (v-SNAREs) synaptobrevin 2 that interacts with target membrane SNAREs (t-SNAREs) syntaxin 1 and SNAP-25 that are required for vesicle fusion (Figure 1 and Table 1).
FIGURE 1.
Vesicle release needs the SNARE proteins. v-SNARE proteins, synaptobrevins/VAMPs, Vti1a/1b and t-SNARE proteins, syntaxins and synaptosomal-associated proteins (SNAPs) mediate synaptic vesicles fusion to the plasma membrane with a contribution of calcium sensors, synaptotagmins.
Table 1.
Vesicles-associated molecules and mRNA expression in serotonergic neurons.
| Molecule | Type | Expression in 5-HT NeuronsD |
|---|---|---|
| Vesicular SNAREs (v-SNAREs)B,R | ||
| Synaptobrevin 1/VAMP1 | NC | ++ |
| Synaptobrevin 2/VAMP2 | C | ++++ |
| Vamp3 | NC | + |
| Vamp4 | NC | ++ |
| Vamp7 | NC | + |
| Vti1a | NC | + |
| Vti1b | NC | ++ |
| Target membrane SNAREs (t-SNAREs)B,R | ||
| Syntaxin Stx1a | C | + |
| Stx1b | C | +++ |
| Stx2 | NC | + |
| Stx3 | NC | + |
| Stx4a | NC | ++ |
| Stx5a | NC | + |
| Stx6 | NC | + |
| Stx7 | NC | ++ |
| Stx8 | NC | + |
| Stx12 | NC | +++ |
| Stx16 | NC | ++ |
| Stx17 | NC | + |
| Stx18 | NC | + |
| SNAP-25 | C | +++++ |
| SNAP-29 | NC | + |
| Calcium sensorsB,R | ||
| Synaptotagmin Syt1 | ++++ | |
| Syt2 | + | |
| Syt3 | + | |
| Syt4 | +++ | |
| Syt5 | ++ | |
| Syt6 | + | |
| Syt7 | + | |
| Syt9 | ++ | |
| Syt11 | +++ | |
| Syt12 | + | |
| Syt13 | +++ | |
| Syt16 | + | |
| Syt17 | + | |
C, Canonical SNAREs; NC, non-canonical SNAREs; data are from D(Okaty et al., 2015), R(Ramirez and Kavalali, 2012), and B(Burré, 2007).
Volume transmission likely involves a particular vesicular machinery. Vesicular transporters traffic to synaptic vesicles as well as large dense core vesicles (Fei et al., 2008). It has been shown that, in transfected neurons, VMAT-2 is spontaneously targeted to the regulated secretory pathway and is sufficient to drive regulated exocytotic release of monoamine (Li et al., 2005). In midbrain, it has been recently reported that axons of dopamine neurons contain non-synaptic release sites (varicosities) that are required for action potential-triggered dopamine release in 30% of dopamine vesicle clusters, leading to the conclusion that a large proportion dopamine varicosities release dopamine independently of action potentials and thus use a different exocytotic release machinery (Liu et al., 2018).
If synaptic transmission mechanisms are well described, volume transmission mechanisms remain to be precisely investigated. Vesicles exocytosis might use similar machinery to the evoked transmitter-release exocytosis of neurons and neurosecretory cells. Regulated release likely uses the non-canonical SNARE proteins, present in serotonergic neurons (Okaty et al., 2015) and listed in Table 1 including VAMP4, VAMP7 (Raingo et al., 2012; Bal et al., 2013), Vti1a or Vti1b (Kunwar et al., 2011; Ramirez et al., 2012), see for reviews (Burré, 2007; Ramirez and Kavalali, 2012). Whether volume transmission uses a mechanism more closely related to regulated vesicular release rather than classical synaptic release has to be further investigated.
Models of Somatodendritic Serotonin Release
The mechanisms of non-synaptic serotonin release are difficult to study in physiological situations. Therefore, only few models of non-synaptic serotonin release have been described. Serotonin can be non-synaptically released at somatodendritic, pure somatic and/or pure dendritic compartments, with different control mechanisms (de Kock et al., 2006; Kaushalya et al., 2008a; Colgan et al., 2009; Leon-Pinzon et al., 2014).
In Leeches
One of the best described model is the leech Retzius giant serotonergic neurons, in which low electrical stimulation (induced by a single action potential) causes the somatodendritic release of serotonin as evaluated by amperometry (Bruns et al., 2000). This release lasts several seconds following initial stimulation (Trueta et al., 2003), allowing serotonin to spread to several micrometers. The initial stimulation triggers the opening of L-type calcium channels (Trueta et al., 2003), the release of serotonin from few serotonin-containing vesicles, which then via 5-HT2-receptor activation produces a Ca2+ release from intracellular calcium stocks amplifying the release of serotonin from serotonin-containing vesicles (Trueta et al., 2004; Trueta and De-Miguel, 2012; Leon-Pinzon et al., 2014). In summary, somatodendritic release/exocytosis of serotonin occurs following low electrical stimulation and opening the L-type calcium channels. Ca2+-induced Ca2+ release is reinforced by activation of 5-HT2 receptors, which, by their coupling to the PLC pathway, amplify the serotonin release in a feed-forward manner (Leon-Pinzon et al., 2014; Figure 2). The resulting positive feedback loop maintains exocytosis for the following several seconds until the last vesicles in the cluster have fused (Trueta and De-Miguel, 2012; Leon-Pinzon et al., 2014). Taking into account the fact that some serotonergic neurons are capable of releasing glutamate, the co-release of this neuromodulator by simultaneous stimulation of the 5-HT2 receptors and NMDA receptors would induce a stronger signal and thus a rapid and strong reinforcement of serotonin transmission.
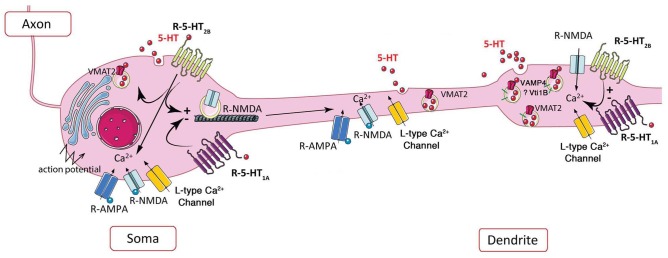
FIGURE 2.
Schematic representation of the potential role of 5-HT2B receptor in extrasynaptic release of serotonin. Left, in soma, the somatic release of serotonin depends on AMPA and NMDA receptors, L-type calcium channels, and action potentials, 5-HT1A and potentially 5-HT2B receptor via its coupling to PKC (Trueta et al., 2003, 2004; de Kock et al., 2006; Colgan et al., 2012; Leon-Pinzon et al., 2014). Activation of 5-HT1A and 5-HT2B receptors may decrease or increase the membrane expression of NMDA receptors, respectively (Yuen et al., 2005, 2008). Right, the NMDA receptor-dependent dendritic release is controlled by L-type calcium channels (Colgan et al., 2012), negatively by 5-HT1A and positively by 5-HT2B receptors at dendritic “puncta” independently of action potential (Colgan et al., 2012).
In Rats
At somatodendritic level of dorsal raphe neurons, the presence of VMAT2 allows the accumulation of serotonin in vesicles (Chazal and Ralston, 1987). As shown by amperometry and 2-Photon calcium imaging, the non-synaptic somatodendritic release of serotonin-containing vesicles can be induced by the stimulation of calcium channels, or by activation of glutamatergic NMDA receptors in the absence of action potentials (de Kock et al., 2006). Using 3-Photon microscopy in living rat brain slices along with immunofluorescence and electron microscopy, vesicular serotonin release from soma and dendrites in the dorsal raphe was visualized for the first time (Kaushalya et al., 2008a; Colgan et al., 2012). These authors clearly established that punctate fluorescence does represent serotonin based on properties of multiphoton wavelength excitation, its detection in microdialysis serotonergic neurons, and its depletion upon exposure to serotonin synthesis inhibitors. Moreover, the presence of clusters of serotonin vesicles in dendrites was confirmed by (i) the immunolocalization of VMAT2 and the dendritic marker MAP2 with serotonin, (ii) the localization of VMAT2 vesicle clusters by electron microscopy in dendrites of serotonergic neuron, (iii) the size of dendritic serotonin/VMAT2 clusters comparable to the size of dendritic puncta and larger than terminal boutons, and (iv) the serotonin release from dendritic vesicles upon electrical stimulation or exposure to glutamate agonists, which requires extracellular Ca2+ and is blocked by the VMAT2 inhibitors. In the soma of serotonergic neurons, calcium channel- and NMDA receptor-activation by action potentials increases serotonin release (Figure 2-left); in proximal dendrites, both AMPA and NMDA receptor activation by back propagating action potentials may facilitate serotonin release; in contrast to standard release from axon terminals triggered by glutamate receptors, dendritic release of serotonin is independent of action potentials and requires L-type Ca2+ channels, but not sodium channels (Colgan et al., 2012; Figure 2-right).
Thus, unlike synaptic dendritic release in other spiking neurons, the dendritic release/exocytosis of serotonin is based on dendritic glutamatergic excitation without requirement for back-propagating action potentials, and is characterized by its sensitivity to NMDA, L-type Ca2+ channel blocker nimodipine. Furthermore, it was reported that upon electrical stimulation, the serotonin releasable pool is 300 times lower in comparison with dopamine despite comparable tissue content. Serotonin may be stored in vesicles or other compartments that do not exocytose consistent with a small quantity of serotonin available for release (Hashemi et al., 2012; Jennings, 2013). Hence, dorsal raphe dendrites release serotonin, and this function is physiologically and pharmacologically unique, although the molecular effectors and regulators of these dendritic non-synaptic events remain to be described in details.
Serotonin Tone and Serotonergic Autoreceptors
5-HT1 Receptors
Neurotransmission by serotonin is tightly regulated by autoreceptors through negative feedback inhibition at somatodendritic levels (5-HT1A receptors) of the raphe nuclei or at axonal levels (5-HT1B receptors). The 5-HT1A autoreceptor is found in the soma and dendrites of serotonergic neurons of raphe (Kia et al., 1996; Riad et al., 2000). In the raphe, the 5-HT1A autoreceptor-mediated inhibition was for long time believed to be the only homeostatic feedback mechanism controlling the tonic firing rate, pacemaker-like, of serotonergic neurons, mainly based on in vitro data, for review see (Piñeyro and Blier, 1999; Vizi et al., 2010). However, accumulating results are weakening the traditional model postulating that serotonin neuron autoinhibition is mediated exclusively by the hyperpolarizing 5-HT1A autoreceptor and that is the main factor controlling the pacemaker-like firing rate of serotonergic neurons, for review see (Andrade et al., 2015).
At somatodendritic levels, a reduction of expression of 5-HT1A autoreceptors produces strong antidepressant effects, probably due to a reduction of the negative feedback on serotonergic neuron activity (Bortolozzi et al., 2012). Moreover, the genetic suppression of 5-HT1A autoreceptors causes an anxiety-like behavior in the basal state, and a higher increase in serotonin release compared to wild-type mice in response to stress (Richardson-Jones et al., 2010). Deletion of either 5-HT1A or 5-HT1B autoreceptors (somatodendritic and axonal, respectively) does not modify brain serotonergic tone as assessed by microdialysis (Guilloux et al., 2011). Moreover, while complete deletion of both receptors in Htr1a/1b-/- mice affected the acute response to SSRIs in the forced swim test, the chronic effects of SSRIs were still observed in anxiety test (Guilloux et al., 2011). In mice with overexpression of 5-HT1A autoreceptor, hypothermic response is increased, and both serotonin content and neuronal activity are decreased in the dorsal raphe. These mice display increased anxiety- and depression-like behaviors that are resistant to chronic antidepressant treatment (Vahid-Ansari et al., 2017). In addition, blockade of 5-HT1A autoreceptors in dorsal raphe brain slices was found to have surprisingly no effect on the firing of the serotonergic neurons as reviewed in Liu et al. (2005). There is thus a discrepancy in 5-HT1A receptors acting as a regulator of pace-maker homeostasis of serotonergic neurons between in vivo and in vitro studies.
Other studies showed that serotonergic cell groups can be interconnected, the dorsal raphe in particular receiving serotonergic inputs from the caudal raphe (Bang et al., 2012), which may implicate different types of serotonergic neurons. 5-HT1A receptors participate in serotonergic neurons with different electrophysiological profiles, the inhibitory effect of 5-HT1A receptors being superior in dorsal raphe than in median raphe neurons, suggesting greater negative feedback in the dorsal raphe (Beck et al., 2004). Similarly, Teissier et al. (2015) identified opposed consequences of dorsal vs. median raphe serotonergic neuron inhibition, suggesting that median raphe hyperactivity increases anxiety, whereas low dorsal over median raphe serotonergic activity ratio increases depression-like behavior. These observations suggest a heterogeneity of serotonergic neurons, which are interconnected but not necessarily located in the same serotonergic nucleus. It will thus be worth testing the effect of altering volume transmission in various raphe nuclei.
5-HT2 Receptors
On dorsal raphe slices, most serotonin neurons are hyperpolarized following the opening of GIRK channels by the application of a 5-HT1A receptor agonist. In the presence of 5-HT1A-receptor antagonists, it has been reported that serotonin induces a depolarization, which can be blocked by different antagonists specific of Gq-coupled 5-HT2 receptors (Craven et al., 2001). In another study using rat brain slices, the stimulation of 5-HT1A receptors also hyperpolarized most serotonin neurons, and about half of these neurons show also a depolarization in response to 5-HT2 receptor agonists (Marinelli et al., 2004). These data suggest that 5-HT2 receptors expressed by subsets of serotonergic neurons could participate in serotonin somatodendritic volume transmission. Local agonist stimulation of 5-HT2B receptors in dorsal raphe increased extracellular serotonin, supporting an excitatory effect of this receptor on serotonergic neuron activity (Doly et al., 2008). Furthermore, a fraction of raphe serotonergic neurons coexpress both 5-HT1A and 5-HT2B receptors (Diaz et al., 2012). These observations confirmed that serotonergic neurons are heterogeneous by expressing different serotonin receptors and that both 5-HT1A and 5-HT2 receptors could participate in serotonin tone regulation.
Putative positive regulation of dorsal raphe by 5-HT2B receptors has been proposed (McDevitt and Neumaier, 2011). Strikingly, acute and long-term effects of SSRIs both in behavior and neurogenesis were eliminated after genetic ablation of 5-HT2B receptors or upon selective antagonist treatment (Diaz et al., 2012). Conversely, pharmacological experiments indicated that acute agonist stimulation of 5-HT2B receptors mimicked acute SSRI action (Diaz and Maroteaux, 2011) and that chronic agonist stimulation of 5-HT2B receptors mimicked chronic SSRI action on behavior and neurogenesis, which were abolished in mice knocked-out (KO) for the 5-HT2B receptor gene (Htr2b-/-) (Diaz et al., 2012). Accordingly, conditional KO mice for 5-HT2B receptors only in serotonergic neurons (Htr2b-cKO5-HT mice), reproduced the lack of SSRI effects; these mice also displayed a reduced tonic firing frequency of dorsal raphe serotonin neurons, and a stronger hypothermic effect of 5-HT1A-autoreceptor stimulation (Belmer et al., 2018). The increased excitability of serotonergic neurons observed upon selective 5-HT2B-receptor overexpression in raphe serotonergic neurons confirmed the cell autonomous effect of this receptor. The excess of inhibitory control exerted by 5-HT1A receptors in Htr2b-cKO5-HT mice may thus explain the lack of response to chronic SSRI in these mice. Conversely, the raphe neurons from mice expressing reduced amount of 5-HT1A receptors (5-HT1A-Low) are more likely to fire at higher rates than control mice, consistent with decreased autoinhibition (Richardson-Jones et al., 2010). In parallel, Philippe et al. (2018) showed that an increased 5-HT1A-autoreceptor binding and function led to reduced serotonergic tone, increased anxiety-depression-like behaviors, and induced mice to be resistant to chronic fluoxetine. A higher 5-HT1A-autoreceptor reactivity and a lower firing activity of these neurons was observed in Htr2b-cKO5-HT mice (Belmer et al., 2018). Confirmation of these findings have been obtained in mice expressing the activator Gq-coupled DREADDS hM3Dq (similar to 5-HT2B receptor’s coupling) in serotonergic neurons, which demonstrates, upon stimulation, an increase in serotonergic neurons firing rates (Teissier et al., 2015) and an antidepressant-like behavioral response (Urban et al., 2016). On the contrary, mice expressing the inhibitory Gi-coupled DREADDS hM4Di (similar to 5-HT1A receptor’s coupling) in serotonergic neurons display, upon stimulation, a decrease in serotonin neuronal firing rates (Teissier et al., 2015). The serotonergic tone may thus result from the opposite control exerted by cross-regulation between Gi-coupled 5-HT1A and Gq-coupled 5-HT2B receptors on serotonergic neurons (Belmer and Maroteaux, 2018).
Interestingly, frog motor neurons showed potentiation of NMDA-induced depolarization by serotonin. The underlying mechanism involves: (1) activation of 5-HT2B receptors; (2) activation of a Gq-protein; (3) a transduction mechanism causing an influx of extracellular Ca2+ through L-type calcium channels; (4) binding of Ca2+ to calmodulin; and (5) reduction of the open-channel block of the NMDA receptor produced by physiological concentration of Mg2+ ions (Holohean and Hackman, 2004). Furthermore, Bigford et al. (2012), showed that either 5-HT2B or 5-HT2C receptor antiserum immunoprecipitated GluN1 subunit of NMDA receptors, suggesting that these receptor subtypes are able to interact in complexes with NMDA receptors and our unpublished data confirmed a 5-HT2B- and GluN1-receptor association. Independently, the 5-HT2B receptor, which is expressed in stomach and cardiomyocytes, has been reported to act via L-type calcium channels in both tissues (Cox and Cohen, 1996; Bai et al., 2010) and activation of 5-HT2B receptors triggered also intracellular calcium release from ryanodine-sensitive stores as shown in the leech somatodendritic release of serotonin (Leon-Pinzon et al., 2014). Together, these data indicate that somatodendritic release of serotonin is a model in which 5-HT2B receptors could participate and regulate the excitability of serotonergic neurons together with 5-HT1A receptors.
The mechanism by which these two receptors interact remains to be described as well as the associated partners and intracellular pathways involved in the regulation of serotonergic tone at the level of serotonin neurons themselves.
Volume Transmission, SERT, and SSRI Antidepressants
The serotonin transporter SERT by regulating extracellular levels of serotonin is a major partner in the regulation of serotonin tone (Piñeyro and Blier, 1999; Vizi et al., 2010). Under normal conditions, evoked extracellular serotonin concentration shows strong firing frequency-dependence. Mice lacking SERT (KO mice) or treated with SSRIs display extracellular serotonin concentrations evoked by stimulation that tend to similar high levels at all frequencies, while in SERT overexpressing mice, evoked extracellular serotonin concentrations tend to equal low levels (Jennings et al., 2010). These findings, therefore, indicate that SERT plays a role of a frequency pass filter in regulating extracellular serotonin concentrations evoked by stimulation. The role of SERT in setting basal extracellular serotonin concentrations and detailed contribution to serotonergic tonic and volume transmission has yet to be investigated in vivo.
The therapeutic effects of SSRIs are initially triggered by blocking SERT. Microdialysis experiments have shown that acute SSRI injections increase extracellular levels of serotonin by approximately 400% in the dorsal raphe and nearly 200% in forebrain terminal regions (Invernizzi et al., 1992, 1997). The SSRI-dependent increases in extracellular serotonin concentration require Ca2+-dependent vesicular release, which should induce somatodendritic 5-HT1A autoreceptor-mediated decreases in spontaneous release of serotonergic neurons (Gartside et al., 1995; Hajos et al., 1995). Following SSRI injection, although basal serotonergic firing rates should decrease, the tonic activity increases extracellular serotonin levels (Dankoski et al., 2016). Interestingly, local infusion in the dorsal raphe of a 5-HT2B receptor agonist through the microdialysis probe produced an increase in extracellular serotonin concentration that could be blocked by 5-HT2B receptor antagonist (Doly et al., 2008) and mimicked the SSRIs effects. These data support a contribution of this receptor subtype in carrier-dependent serotonin accumulation.
One mechanism by which SERT can contribute to the enhancement of extracellular serotonin includes reversed transport, i.e., by carrier-mediated efflux (Forrest et al., 2008; Sitte and Freissmuth, 2015). The “club drug” 3,4-methylenedioxy methamphetamine (MDMA, ecstasy) binds preferentially to and reverses the activity of SERT, by causing release of serotonin from vesicles. Acute pharmacological inhibition or genetic ablation of 5-HT2B receptors in KO mice completely abolished MDMA-induced hyperlocomotion, sensitization, and serotonin release. Furthermore, the 5-HT2B receptor dependence of MDMA-stimulated release of endogenous serotonin relies on its expression in serotonergic neurons as recently demonstrated in mice lacking 5-HT2B receptors only in serotonergic neurons (Htr2b-cKO5-HT mice) (Belmer et al., 2018). These data support also a contribution of this receptor subtype in carrier-dependent serotonin efflux. Unlike serotonin release in soma or terminals, dendritic serotonin release in response to AMPA or NMDA receptor stimulation requires L-type Ca2+ channels. AMPA-evoked serotonin release measured with varying fluoxetine concentrations showed that somatic serotonin release has fivefold greater sensitivity to fluoxetine than responses from dendritic puncta (Colgan et al., 2012). Differences in SERT regulation, localization and/or function may explain this difference, since SERT immunoreactivity has been mainly found at the plasma membrane in extrasynaptic location including axonal varicosities, whereas in soma and dendrites it was mainly observed intracellularly (Vizi et al., 2010; Belmer et al., 2017).
The therapeutic effects induced by SSRIs rely on long-term neuroadaptations. Since the activation of 5-HT1A autoreceptor decreases the activity of serotonin neurons (Commons, 2008), more than 2 weeks of SSRI treatment are necessary to observe a decreased expression of 5-HT1A receptors in serotonergic neurons (Popa et al., 2010). This decrease in expression of 5-HT1A receptors, which is followed by an increase in the firing of serotonergic neurons, has been proposed to explain the clinical delay of the antidepressant effect of SSRIs (Adell et al., 2002; Santarelli et al., 2003; Richardson-Jones et al., 2010; Rainer et al., 2012). In SERT KO mice, 5-HT1A autoreceptors are desensitized in raphe nuclei, while they remain intact in post-synaptic neurons (Fabre et al., 2000). This desensitization of 5-HT1A autoreceptors in the raphe is thought to be due to the chronic accumulation of extracellular serotonin in the absence of uptake (Soiza-Reilly et al., 2015). The lack of acute and chronic SSRI efficacy observed in Htr2b-cKO5-HT mice is associated with a reduced tonic firing frequency of dorsal raphe serotonin neurons, whereas the selective 5-HT2B-receptor overexpression in raphe serotonergic neurons increases the excitability of these neurons (Belmer et al., 2018). Together with the observation that agonist stimulation of 5-HT2B receptors is sufficient to reproduce SSRI effects including raphe serotonin accumulation, these results support that the reduction in 5-HT1A receptor activity drives the antidepressant efficacy that may involve SERT regulation.
Colgan et al. (2012) proposed that the differential regulation between somatic vs. dendritic serotonin release may explain the antidepressant effects of inhibitors of NMDA receptors like ketamine (Machado-Vieira et al., 2009; Casamassima et al., 2010). Ketamine, has recently been shown to increase serotonin in prefrontal cortex, which correlates with antidepressant-like activity in the forced swimming test; its antidepressant-like activity requires activation of raphe AMPA receptors that recruits the prefrontal cortex neural circuit (Pham et al., 2017). Furthermore, AMPA receptor-dependent serotonin release and subsequent 5-HT1A receptor stimulation may be involved in the actions of an mGlu2/3 receptor antagonist and ketamine in the NSF test (Fukumoto et al., 2014). However, it has been reported that a direct activation of AMPA receptors by ketamine metabolites and mTOR signaling is sufficient to increase synaptogenesis in prefrontal cortical pyramidal neurons and to enhance serotonergic neurotransmission via descending inputs to the raphe nuclei or even by a direct inhibition of NMDA receptors localized on GABAergic interneurons, for reviews see (Artigas et al., 2018; Zanos and Gould, 2018). It is therefore unlikely that the rapid antidepressant effects of NMDA receptor inhibitors act through a control of serotonergic tone, which would require time to be efficient, but through a direct control of upstream targets.
Genetic Variants of Molecules Putatively Associated to Volume Transmission
Interestingly, human polymorphisms associated to psychiatric diseases have been found in genes encoding molecules putatively involved in somatodendritic release, including voltage-gated L-type calcium channel subunit, 5-HT2B receptor, 5-HT1A receptor, VMAT-2, or SERT. Single-nucleotide polymorphisms (SNPs) in the α1 subunit (CACNA1C) of the L-type calcium channels Cav1.2 rank among the most consistent and replicable genetics findings in psychiatry and have been associated with schizophrenia, bipolar disorder and major depression (Casamassima et al., 2010; Dedic et al., 2018) and more recently with treatment resistant depression (Fabbri et al., 2018). In humans, a loss-of-function SNP of 5-HT2B receptors is associated with serotonin-dependent phenotypes, including impulsivity and suicidality (Bevilacqua et al., 2010). Association studies with the functional 5-HT1A receptor promoter SNP rs6295 showed that patients present early deficits in cognitive, fear and stress reactivity that may lead to depression (Albert and Fiori, 2014). A specific haplotype in SLC18A2, the gene encoding VMAT-2, was significantly associated with depression symptoms in men (Christiansen et al., 2007). Furthermore, a significant association was found between post-traumatic stress disorder (diagnosis) and SNPs in SLC18A2 (Solovieff et al., 2014). Carriers of the short allele of the promoter polymorphism of SERT gene (5-HTTPR) have increased anxiety-related traits and elevated risk of depression (Pezawas et al., 2005). Evidence points to a lower response to SSRIs among Caucasian patients with the 5-HTTPR short genotype and among (Asian) patients with the STin2 10/12 genotype (Smits et al., 2008). However, humans carrying the short variant of the 5-HTTPR outperform subjects carrying the long allele in an array of cognitive and social tasks (Homberg and Lesch, 2011). So, one has to be careful in interpreting data from human gene polymorphism, without extensive characterization of their physiological consequences. These human polymorphisms that are associated to psychiatric diseases have then to be validated in models of serotonin somatodendritic release.
Conclusion
Our understanding of serotonin transmission has been limited by technical problems. This review has summarized different mode of serotonin transmission and how they could impact behavioral and antidepressant efficacy. A better description of the molecular mechanisms involved in regulating serotonin somatodendritic release in vivo, using for example 3-Photons microscopy, is necessary to identify the impact of various modes of serotonin release and to unravel the mechanisms of tonic serotonin level regulation. These data should indicate if different modes of serotonin release mediate distinct behavioral effects. Understanding whether and how serotonin tone is controlled may also increase our understanding how its impact on behavior. By deciphering the molecular mechanisms of serotonin release that regulate firing patterns we should be able to increase our knowledge of serotonin function in physiological and pathophysiological situations. This should ultimately allow us to improve treatment of psychiatric disorders involving serotonin, such as depression.
Author Contributions
All authors collected references, wrote the manuscript, and prepared the figures.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Research in the Maroteaux laboratory has been supported in part by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Sorbonne Université Sciences – Pierre et Marie Curie, and by grants from the Fondation pour la Recherche sur le Cerveau, the Fondation de France, the Fondation pour la Recherche Médicale “Equipe FRM DEQ2014039529,” the French Ministry of Research (Agence Nationale pour la Recherche ANR-17-CE16-0008 and the Investissements d’Avenir programme ANR-11-IDEX-0004-02) and the DIM Cerveau et Pensée from Region Ile de France. LM’s team is part of the École des Neurosciences de Paris Ile-de-France network and of the Bio-Psy Labex and as such this work was supported by French state funds managed by the ANR within the Investissements d’Avenir programme under reference ANR-11-IDEX-0004-02.
References
- Adell A., Celada P., Abellan M. T., Artigas F. (2002). Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Brain Res. Rev. 39 154–180. 10.1016/S0165-0173(02)00182-0 [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Zoli M., Strömberg I., Fuxe K. (1995). Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69 711–726. 10.1016/0306-4522(95)00308-6 [DOI] [PubMed] [Google Scholar]
- Albert P. R., Fiori L. M. (2014). Transcriptional dys-regulation in anxiety and major depression: 5-HT1A gene promoter architecture as a therapeutic opportunity. Curr. Pharm. Des. 20 3738–3750. 10.2174/13816128113196660740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers K. A., Sharp T. (2003). Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 122 193–204. 10.1016/S0306-4522(03)00518-9 [DOI] [PubMed] [Google Scholar]
- Andrade R., Huereca D., Lyons J. G., Andrade E. M., McGregor K. M. (2015). 5-HT1A receptor-mediated autoinhibition and the control of serotonergic cell firing. ACS Chem. Neurosci. 6 1110–1115. 10.1021/acschemneuro.5b00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigas F., Celada P., Bortolozzi A. (2018). Can we increase the speed and efficacy of antidepressant treatments? Part II. Glutamatergic and RNA interference strategies. Eur. Neuropsychopharm. 28 457–482. 10.1016/j.euroneuro.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Bai C.-F., Liu J.-C., Zhao R., Cao W., Liu S.-B., Zhang X.-N., et al. (2010). Role of 5-HT2B receptor in cardiomyocyte apoptosis of norepinephrine-induced cardiomyopathy model in rats. Clin. Exp. Pharmacol. Physiol. 37 e145–e151. 10.1111/j.1440-1681.2010.05388.x [DOI] [PubMed] [Google Scholar]
- Bal M., Leitz J., Reese A. L., Ramirez D. M. O., Durakoglugil M., Herz J., et al. (2013). Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron 80 934–946. 10.1016/j.neuron.2013.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S. J., Jensen P., Dymecki S. M., Commons K. G. (2012). Projections and interconnections of genetically defined serotonin neurons in mice. Eur. J. Neurosci. 35 85–96. 10.1111/j.1460-9568.2011.07936.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S. G., Pan Y.-Z., Akanwa A. C., Kirby L. G. (2004). Median and dorsal raphe neurons are not electrophysiologically identical. J. Neurophysiol. 91 994–1005. 10.1152/jn.00744.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmer A., Klenowski P. M., Patkar O. L., Bartlett S. E. (2017). Mapping the connectivity of serotonin transporter immunoreactive axons to excitatory and inhibitory neurochemical synapses in the mouse limbic brain. Brain Struct. Funct. 222 1297–1314. 10.1007/s00429-016-1278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmer A., Maroteaux L. (2018). Regulation of raphe serotonin neurons by serotonin 1A and 2B receptors. Neuropsychopharmacology 44 218–219. 10.1038/s41386-018-0214-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmer A., Quentin E., Diaz S. L., Guiard B. P., Fernandez S. P., Doly S., et al. (2018). Positive regulation of raphe serotonin neurons by serotonin 2B receptors. Neuropsychopharmacology 43 1623–1632. 10.1038/s41386-018-0013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L., Doly S., Kaprio J., Yuan Q., Tikkanen R., Paunio T., et al. (2010). A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature 468 1061–1066. 10.1038/nature09629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigford G. E., Chaudhry N. S., Keane R. W., Holohean A. M. (2012). 5-Hydroxytryptamine 5HT2C receptors form a protein complex with N-methyl-D-aspartate GluN2A subunits and activate phosphorylation of Src protein to modulate motoneuronal depolarization. J. Biol. Chem. 287 11049–11059. 10.1074/jbc.M111.277806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A., Castañé A., Semakova J., Santana N., Alvarado G., Cortés R., et al. (2012). Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol. Psychiatry 17 612–623. 10.1038/mp.2011.92 [DOI] [PubMed] [Google Scholar]
- Branchi I. (2011). The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology 36 339–351. 10.1016/j.psyneuen.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Bruns D., Riedel D., Klingauf J., Jahn R. (2000). Quantal release of serotonin. Neuron 28 205–220. 10.1016/S0896-6273(00)00097-0 [DOI] [PubMed] [Google Scholar]
- Bunin M. A., Wightman R. M. (1999). Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci. 22 377–382. 10.1016/S0166-2236(99)01410-1 [DOI] [PubMed] [Google Scholar]
- Burré J., Volknandt W. (2007). The synaptic vesicle proteome. J. Neurochem. 101 1448–1462. 10.1111/j.1471-4159.2007.04453.x [DOI] [PubMed] [Google Scholar]
- Casamassima F., Hay A. C., Benedetti A., Lattanzi L., Cassano G. B., Perlis R. H. (2010). L-type calcium channels and psychiatric disorders: a brief review. Am. J. Med. Genet. B 153B 1373–1390. 10.1002/ajmg.b.31122 [DOI] [PubMed] [Google Scholar]
- Chazal G., Ralston H. J. (1987). Serotonin-containing structures in the nucleus raphe dorsalis of the cat: an ultrastructural analysis of dendrites, presynaptic dendrites, and axon terminals. J. Comp. Neurol. 259 317–329. 10.1002/cne.902590302 [DOI] [PubMed] [Google Scholar]
- Christiansen L., Tan Q., Iachina M., Bathum L., Kruse T. A., McGue M., et al. (2007). Candidate gene polymorphisms in the serotonergic pathway: influence on depression symptomatology in an elderly population. Biol. Psychiatry 61 223–230. 10.1016/j.biopsych.2006.03.046 [DOI] [PubMed] [Google Scholar]
- Colgan L. A., Cavolo S. L., Commons K. G., Levitan E. S. (2012). Action potential-independent and pharmacologically unique vesicular serotonin release from dendrites. J. Neurosci. 32 15737–15746. 10.1523/JNEUROSCI.0020-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan L. A., Putzier I., Levitan E. S. (2009). Activity-dependent vesicular monoamine transporter-mediated depletion of the nucleus supports somatic release by serotonin neurons. J. Neurosci. 29 15878–15887. 10.1523/JNEUROSCI.4210-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons K. G. (2008). Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system. Eur. J. Neurosci. 27 2611–2618. 10.1111/j.1460-9568.2008.06235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. A., Cohen M. L. (1996). 5-HT2B receptor signaling in the rat stomach fundus: dependence on calcium influx, calcium release and protein kinase C. Behav. Brain Res. 73 289–292. 10.1016/0166-4328(96)00125-8 [DOI] [PubMed] [Google Scholar]
- Craven R. M., Grahame-Smith D. G., Newberry N. R. (2001). 5-HT1A and 5-HT2 receptors differentially regulate the excitability of 5-HT-containing neurones of the guinea pig dorsal raphe nucleus in vitro. Brain Res. 899 159–168. 10.1016/S0006-8993(01)02221-1 [DOI] [PubMed] [Google Scholar]
- Dankoski E. C., Carroll S., Wightman R. M. (2016). Acute selective serotonin reuptake inhibitors regulate the dorsal raphe nucleus causing amplification of terminal serotonin release. J. Neurochem. 136 1131–1141. 10.1111/jnc.13528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock C. P. J., Cornelisse L. N., Burnashev N., Lodder J. C., Timmerman A. J., Couey J. J., et al. (2006). NMDA receptors trigger neurosecretion of 5-HT within dorsal raphe nucleus of the rat in the absence of action potential firing. J. Physiol. 577 891–905. 10.1113/jphysiol.2006.115311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedic N., Pöhlmann M. L., Richter J. S., Mehta D., Czamara D., Metzger M. W., et al. (2018). Cross-disorder risk gene CACNA1C differentially modulates susceptibility to psychiatric disorders during development and adulthood. Mol. Psychiatry 23 533–543. 10.1038/mp.2017.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L., Mechawar N. (2000). Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 125 27–47. 10.1016/S0079-6123(00)25005-X [DOI] [PubMed] [Google Scholar]
- Diaz S. L., Doly S., Narboux-Neme N., Fernandez S., Mazot P., Banas S., et al. (2012). 5-HT2B receptors are required for serotonin-selective antidepressant actions. Mol. Psychiatry 17 154–163. 10.1038/mp.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S. L., Maroteaux L. (2011). Implication of 5-HT2B receptors in the serotonin syndrome. Neuropharmacology 61 495–502. 10.1016/j.neuropharm.2011.01.025 [DOI] [PubMed] [Google Scholar]
- Doly S., Valjent E., Setola V., Callebert J., Herve D., Launay J. M., et al. (2008). Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J. Neurosci. 28 2933–2940. 10.1523/JNEUROSCI.5723-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri C., Corponi F., Albani D., Raimondi I., Forloni G., Schruers K., et al. (2018). Pleiotropic genes in psychiatry: calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog. Neuropsychopharmacol. Biol. Psychiatry 81 203–210. 10.1016/j.pnpbp.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Fabre V., Beaufour C., Evrard A., Rioux A., Hanoun N., Lesch K. P., et al. (2000). Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur. J. Neurosci. 12 2299–2310. 10.1046/j.1460-9568.2000.00126.x [DOI] [PubMed] [Google Scholar]
- Fei H., Grygoruk A., Brooks E. S., Chen A., Krantz D. E. (2008). Trafficking of vesicular neurotransmitter transporters. Traffic 9 1425–1436. 10.1111/j.1600-0854.2008.00771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest L. R., Zhang Y. W., Jacobs M. T., Gesmonde J., Xie L., Honig B. H., et al. (2008). Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. U.S.A. 105 10338–10343. 10.1073/pnas.0804659105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K., Iijima M., Chaki S. (2014). Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology 231 2291–2298. 10.1007/s00213-013-3378-0 [DOI] [PubMed] [Google Scholar]
- Gartside S. E., Umbers V., Hajós M., Sharp T. (1995). Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br. J. Pharmacol. 115 1064–1070. 10.1111/j.1476-5381.1995.tb15919.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A. (2016). Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 17 524–532. 10.1038/nrn.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux J.-P., David D. J. P., Xia L., Nguyen H. T., Rainer Q., Guiard B. P., et al. (2011). Characterization of 5-HT(1A/1B)-/- mice: an animal model sensitive to anxiolytic treatments. Neuropharmacology 61 478–488. 10.1016/j.neuropharm.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Hajós M., Allers K. A., Jennings K., Sharp T., Charette G., Sík A., et al. (2007). Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphe nucleus using juxtacellular labelling methods. Eur. J. Neurosci. 25 119–126. 10.1111/j.1460-9568.2006.05276.x [DOI] [PubMed] [Google Scholar]
- Hajos M., Gartside S. E., Villa A. E., Sharp T. (1995). Evidence for a repetitive (burst) firing pattern in a sub-population of 5-hydroxytryptamine neurons in the dorsal and median raphe nuclei of the rat. Neuroscience 69 189–197. 10.1016/0306-4522(95)00227-A [DOI] [PubMed] [Google Scholar]
- Hashemi P., Dankoski E. C., Lama R., Wood K. M., Takmakov P., Wightman R. M. (2012). Brain dopamine and serotonin differ in regulation and its consequences. Proc. Natl. Acad. Sci. U.S.A. 109 11510–11515. 10.1073/pnas.1201547109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holohean A. M., Hackman J. C. (2004). Mechanisms intrinsic to 5-HT2B receptor-induced potentiation of NMDA receptor responses in frog motoneurones. Br. J. Pharmacol. 143 351–360. 10.1038/sj.bjp.0705935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg J. R., Lesch K.-P. (2011). Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry 69 513–519. 10.1016/j.biopsych.2010.09.024 [DOI] [PubMed] [Google Scholar]
- Insel T. R., Sahakian B. J. (2012). Drug research: a plan for mental illness. Nature 483:269. 10.1038/483269a [DOI] [PubMed] [Google Scholar]
- Invernizzi R., Belli S., Samanin R. (1992). Citalopram’s ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug’s effect in the frontal cortex. Brain Res. 584 322–324. 10.1016/0006-8993(92)90914-U [DOI] [PubMed] [Google Scholar]
- Invernizzi R., Velasco C., Bramante M., Longo A., Samanin R. (1997). Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology 36 467–473. 10.1016/S0028-3908(97)00060-9 [DOI] [PubMed] [Google Scholar]
- Jacobs B. L., Azmitia E. C. (1992). Structure and function of the brain serotonin system. Physiol. Rev. 72 165–229. 10.1152/physrev.1992.72.1.165 [DOI] [PubMed] [Google Scholar]
- Jahn R., Scheller R. H. (2006). SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 7 631–643. 10.1038/nrm2002 [DOI] [PubMed] [Google Scholar]
- Jennings K. A. (2013). A comparison of the subsecond dynamics of neurotransmission of dopamine and serotonin. ACS Chem. Neurosci. 4 704–714. 10.1021/cn4000605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings K. A., Lesch K.-P., Sharp T., Cragg S. J. (2010). Non-linear relationship between 5-HT transporter gene expression and frequency sensitivity of 5-HT signals. J. Neurochem. 115 965–973. 10.1111/j.1471-4159.2010.07001.x [DOI] [PubMed] [Google Scholar]
- Kaushalya S. K., Desai R., Arumugam S., Ghosh H., Balaji J., Maiti S. (2008a). Three-photon microscopy shows that somatic release can be a quantitatively significant component of serotonergic neurotransmission in the mammalian brain. J. Neurosci. Res. 86 3469–3480. 10.1002/jnr.21794 [DOI] [PubMed] [Google Scholar]
- Kaushalya S. K., Nag S., Ghosh H., Arumugam S., Maiti S. (2008b). A high-resolution large area serotonin map of a live rat brain section. Neuroreport 19 717–721. 10.1097/WNR.0b013e3282fd6946 [DOI] [PubMed] [Google Scholar]
- Kia H. K., Miquel M. C., Brisorgueil M. J., Daval G., Riad M., El Mestikawy S., et al. (1996). Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 365 289–305. [DOI] [PubMed] [Google Scholar]
- Kocsis B., Varga V., Dahan L., Sik A. (2006). Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl. Acad. Sci. U.S.A. 103 1059–1064. 10.1073/pnas.0508360103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E. J. (2008). The molecular neurobiology of depression. Nature 455 894–902. 10.1038/nature07455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar A. J., Rickmann M., Backofen B., Browski S. M., Rosenbusch J., Schöning S., et al. (2011). Lack of the endosomal SNAREs vti1a and vti1b led to significant impairments in neuronal development. Proc. Natl. Acad. Sci. U.S.A. 108 2575–2580. 10.1073/pnas.1013891108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Pinzon C., Cercós M. G., Noguez P., Trueta C., De-Miguel F. F. (2014). Exocytosis of serotonin from the neuronal soma is sustained by a serotonin and calcium-dependent feedback loop. Front. Cell. Neurosci. 8:169. 10.3389/fncel.2014.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Waites C. L., Staal R. G., Dobryy Y., Park J., Sulzer D. L., et al. (2005). Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron 48 619–633. 10.1016/j.neuron.2005.09.033 [DOI] [PubMed] [Google Scholar]
- Liu C., Kershberg L., Wang J., Schneeberger S., Kaeser P. S. (2018). Dopamine Secretion is mediated by sparse active zone-like release sites. Cell 172 706–718.e15. 10.1016/j.cell.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.-J., Lambe E. K., Aghajanian G. K. (2005). Somatodendritic autoreceptor regulation of serotonergic neurons: dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur. J. Neurosci. 21 945–958. 10.1111/j.1460-9568.2005.03930.x [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R., Salvadore G., Diazgranados N., Zarate C. A. (2009). Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol. Ther. 123 143–150. 10.1016/j.pharmthera.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S., Schnell S. A., Hack S. P., Christie M. J., Wessendorf M. W., Vaughan C. W. (2004). Serotonergic and nonserotonergic dorsal raphe neurons are pharmacologically and electrophysiologically heterogeneous. J. Neurophysiol. 92 3532–3537. 10.1152/jn.00437.2004 [DOI] [PubMed] [Google Scholar]
- Matias S., Lottem E., Dugué G. P., Mainen Z. F. (2017). Activity patterns of serotonin neurons underlying cognitive flexibility. eLife 6:e20552. 10.7554/eLife.20552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt R. A., Neumaier J. F. (2011). Regulation of dorsal raphe nucleus function by serotonin autoreceptors: a behavioral perspective. J. Chem. Neuroanat. 41 234–246. 10.1016/j.jchemneu.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty B. W., Freret M. E., Rood B. D., Brust R. D., Hennessy M. L., Debairos D., et al. (2015). Multi-scale molecular deconstruction of the serotonin neuron system. Neuron 88 774–791. 10.1016/j.neuron.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Drabant E. M., Verchinski B. A., Munoz K. E., Kolachana B. S., et al. (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 8 828–834. 10.1038/nn1463 [DOI] [PubMed] [Google Scholar]
- Pham T. H., Mendez-David I., Defaix C., Guiard B. P., Tritschler L., David D. J., et al. (2017). Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 112 198–209. 10.1016/j.neuropharm.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Philippe T. J., Vahid-Ansari F., Donaldson Z. R., Le François B., Zahrai A., Turcotte-Cardin V., et al. (2018). Loss of MeCP2 in adult 5-HT neurons induces 5-HT1A autoreceptors, with opposite sex-dependent anxiety and depression phenotypes. Sci. Rep. 8:5788. 10.1038/s41598-018-24167-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeyro G., Blier P. (1999). Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol. Rev. 51 533–591. [PubMed] [Google Scholar]
- Popa D., Cerdan J., Reperant C., Guiard B. P., Guilloux J. P., David D. J., et al. (2010). A longitudinal study of 5-HT outflow during chronic fluoxetine treatment using a new technique of chronic microdialysis in a highly emotional mouse strain. Eur. J. Pharmacol. 628 83–90. 10.1016/j.ejphar.2009.11.037 [DOI] [PubMed] [Google Scholar]
- Rainer Q., Nguyen H. T., Quesseveur G., Gardier A. M., David D. J., Guiard B. P. (2012). Functional status of somatodendritic serotonin 1A autoreceptor after long-term treatment with fluoxetine in a mouse model of anxiety/depression based on repeated corticosterone administration. Mol. Pharmacol. 81 106–112. 10.1124/mol.111.075796 [DOI] [PubMed] [Google Scholar]
- Raingo J., Khvotchev M., Liu P., Darios F., Li Y. C., Ramirez D. M. O., et al. (2012). VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat. Neurosci. 15 738–745. 10.1038/nn.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez D. M. O., Kavalali E. T. (2012). The role of non-canonical SNAREs in synaptic vesicle recycling. Cell. Logist. 2 20–27. 10.4161/cl.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez D. M. O., Khvotchev M., Trauterman B., Kavalali E. T. (2012). Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron 73 121–134. 10.1016/j.neuron.2011.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M., Garcia S., Watkins K. C., Jodoin N., Doucet E., Langlois X., et al. (2000). Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 417 181–194. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones J. W., Craige C. P., Guiard B. P., Stephen A., Metzger K. L., Kung H. F., et al. (2010). 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65 40–52. 10.1016/j.neuron.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet J. L., Tamir H., Privat A. (1994). Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord: a light and electron microscopic study using an anti-idiotypic antiserum. J. Neurosci. Res. 38 109–121. 10.1002/jnr.490380114 [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301 805–809. 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- Sitte H. H., Freissmuth M. (2015). Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol. Sci. 36 41–50. 10.1016/j.tips.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits K. M., Smits L. J., Peeters F. P., Schouten J. S., Janssen R. G., Smeets H. J., et al. (2008). The influence of 5-HTTLPR and STin2 polymorphisms in the serotonin transporter gene on treatment effect of selective serotonin reuptake inhibitors in depressive patients. Psychiatr. Genet. 18 184–190. 10.1097/YPG.0b013e3283050aca [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M., Goodfellow N. M., Lambe E. K., Commons K. G. (2015). Enhanced 5-HT1A receptor-dependent feedback control over dorsal raphe serotonin neurons in the SERT knockout mouse. Neuropharmacology 89 185–192. 10.1016/j.neuropharm.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieff N., Roberts A. L., Ratanatharathorn A., Haloosim M., De Vivo I., King A. P., et al. (2014). Genetic association analysis of 300 genes identifies a risk haplotype in SLC18A2 for post-traumatic stress disorder in two independent samples. Neuropsychopharmacology 39 1872–1879. 10.1038/npp.2014.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C. (2012). The presynaptic active zone. Neuron 75 11–25. 10.1016/j.neuron.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier A., Chemiakine A., Inbar B., Bagchi S., Ray R. S., Palmiter R. D., et al. (2015). Activity of raphé serotonergic neurons controls emotional behaviors. Cell Rep. 13 1965–1976. 10.1016/j.celrep.2015.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueta C., De-Miguel F. F. (2012). Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front. Physiol. 3:319. 10.3389/fphys.2012.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueta C., Méndez B., De-Miguel F. F. (2003). Somatic exocytosis of serotonin mediated by L-type calcium channels in cultured leech neurones. J. Physiol. 547 405–416. 10.1113/jphysiol.2002.030684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueta C., Sánchez-Armass S., Morales M. A., De-Miguel F. F. (2004). Calcium-induced calcium release contributes to somatic secretion of serotonin in leech retzius neurons. J. Neurobiol. 61 309–316. 10.1002/neu.20055 [DOI] [PubMed] [Google Scholar]
- Urban D. J., Zhu H., Marcinkiewcz C. A., Michaelides M., Oshibuchi H., Rhea D., et al. (2016). Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41 1404–1415. 10.1038/npp.2015.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadodaria K. C., Stern S., Marchetto M. C., Gage F. H. (2018). Serotonin in psychiatry: in vitro disease modeling using patient-derived neurons. Cell Tissue Res. 371 161–170. 10.1007/s00441-017-2670-4 [DOI] [PubMed] [Google Scholar]
- Vahid-Ansari F., Daigle M., Manzini M. C., Tanaka K. F., Hen R., Geddes S. D., et al. (2017). Abrogated Freud-1/Cc2d1a repression of 5-HT1A autoreceptors induces fluoxetine-resistant anxiety/depression-like behavior. J. Neurosci. 37 11967–11978. 10.1523/JNEUROSCI.1668-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S., Fekete A., Karoly R., Mike A. (2010). Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 160 785–809. 10.1111/j.1476-5381.2009.00624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E. Y., Jiang Q., Chen P., Feng J., Yan Z. (2008). Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J. Biol. Chem. 283 17194–17204. 10.1074/jbc.M801713200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E. Y., Jiang Q., Chen P., Gu Z., Feng J., Yan Z. (2005). Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J. Neurosci. 25 5488–5501. 10.1523/JNEUROSCI.1187-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Gould T. D. (2018). Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 23 801–811. 10.1038/mp.2017.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M., Jansson A., Syková E., Agnati L. F., Fuxe K. (1999). Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol. Sci. 20 142–150. 10.1016/S0165-6147(99)01343-7 [DOI] [PubMed] [Google Scholar]