Abstract
Correlation of the severity of chronic kidney disease (CKD) with serum inflammation, osteoporosis and vitamin D deficiency was investigated. A total of 78 patients suffering from CKD who presented to the Union Hospital from December 2015 to December 2017 were selected randomly and divided into three groups based on the severity of the disease. Comparisons of interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), indicators of osteoporosis [serum phosphate, serum calcium and bone mineral density (BMD)], content of 25(OH)D, serum sodium, serum potassium and BUN were conducted among groups. The correlation of in vivo creatinine (Cr) with C-reactive protein (CRP), TNF-α, BMD and vitamin D deficiency were analyzed. With the aggravation of illness, IL-6, CRP, TNF-α, serum phosphate, serum sodium, serum potassium and blood urea nitrogen (BUN) were increased gradually, while serum calcium, BMD and vitamin D were decreased significantly (P<0.05). The content of Cr in patients suffering from osteoporosis was significantly higher than that in normal group (P<0.05). The Cr of patients in the group with abnormal CRP was significantly higher than that with normal CRP (P<0.05). Analysis showed that there is positive correlation between Cr and CRP (r=0.6961, P<0.001), as well as between Cr and TNF-α (r=0.8969, P<0.001); and negative correlation between Cr and BMD (r=0.5472, P<0.001), and between Cr and 25(OH)D (r=0.4733, P<0.001). The severity of CKD is correlated with serum inflammation, osteoporosis and vitamin D deficiency. The higher the severity of the illness, the worse the condition of osteoporosis will be.
Keywords: chronic kidney disease, inflammation, osteoporosis, vitamin D deficiency
Introduction
Clinical statistics have shown that chronic kidney disease (CKD) ranks third in the world, in terms of incidence rate and number of infected individuals, next to cancer and heart diseases (1,2). It is caused by interaction of several kinds of nephropathy, so patients with renal diseases may develop CKD if they don't receive timely diagnosis and treatment (3,4). Clinical treatment can lead CKD in remission, but this disease may cause complications such as the frequent occurrence of inflammation, decrease in serum calcium, osteoporosis and vitamin D deficiency. Therefore, attention should be paid to the prevention of the above complications and finding the successful treatment in clinic (5–8). In this study, the correlation of CKD with inflammatory factors, osteoporosis and vitamin D deficiency were analyzed, providing the basis for the follow-up clinical treatment.
Patients and methods
General data
A total of 78 patients with CKD presented to the Union Hospital (Wuhan, China) from December 2015 to December 2017 were selected and divided into three groups according to the severity of the disease: CKD I–II stage group, CKD III–IV stage group and CKD V stage group. Among patients in CKD I–II stage group, there were 12 males and 13 females with body mass index (BMI) of 25.5±2.76 kg/m2, height of 169.54±17.54 cm and average age of 58.43±5.65 years. In CKD III–IV stage group, there were 14 males and 12 females with BMI of 24.9±3.09 kg/m2, height of 170.01±17.09 cm and average age of 59.65±6.09 years. In CKD V stage group, there were 12 males and 15 females with BMI of 25.2±2.76 kg/m2, height of 167.76±16.43 cm and average age of 58.21±5.32 years.
Inclusion criteria: patients who had good adherence and no mental diseases and whose main organs such as heart, liver and kidney were not injured.
Exclusion criteria: patients who recently had infection symptoms or were suffering from cancer or osteoporosis.
The study was approved by the Ethics Committee of the Union Hospital and informed consents were signed by the patients or the guardians.
Methods
Venous blood samples (8 ml) were collected from all patients on an empty stomach. After leaving the samples to stand for 30 min, serum was separated through centrifugation at 8,000 × g for 15 min at 4°C. Inflammatory factors interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were detected by enzyme-linked immunoassay. C-reactive protein (CRP) was measured by an auto-chemistry analyzer (SmartChem; Row2 Technologies, Inc., Parsippany, NJ, USA).
Indicators of osteoporosis: serum phosphate and serum calcium were both measured by an automatic biochemical analyzer (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China). When serum albumin level of the patient was <40 g/l, the corrected blood calcium level was calculated. Bone mineral density (BMD) was measured by a bone density meter (MetriScan; Alara, Inc., San Jose, CA, USA) (9).
Determination of 25(OH)D: vitamin D deficiency was defined as 25(OH)D was <15 ng/ml, and the detection of this factor was carried out by magnetic particle-based chemiluminescence (Roche Pharma AG, Grenzach-Wyhlen, Germany) (10). Determination of serum sodium and serum potassium: ion selective electrode method and the auto-chemistry analyzer were used. Serum creatinine (Cr): It was analyzed using the auto-chemistry analyzer after enzymatic assay. Blood urea nitrogen (BUN): it was determined by ultraviolet-glutamic acid dehydrogenase assay and analyzed by the auto-chemistry analyzer.
Statistical analysis
The statistical software, Statistical Product and Service Solutions (SPSS; SPSS, Inc., Chicago, IL, USA) 17.0, was used for the statistical analysis of the data. t-test was used for enumeration data which were expressed as mean ± standard deviation (SD). ANOVA was used for comparison between multiple groups and the post hoc test was the Least Significant Difference test. Pearson's analysis was employed for correlation analysis between variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of general data of patients among different groups
It was found that there were no differences in age, sex, BMI and height of patients among the groups (Table I).
Table I.
Comparison of the general data of patients among different groups.
| Index | CKD I–II stage | CKD III–IV stage | CKD V stage |
|---|---|---|---|
| Age (years) | 58.43±5.65 | 59.65±6.09 | 58.21±5.32 |
| Sex (male/female) | 12/13 | 14/12 | 12/15 |
| BMI (kg/m2) | 25.5±2.76 | 24.9±3.09 | 25.2±2.76 |
| Height (cm) | 169.54±17.54 | 170.01±17.09 | 167.76±16.43 |
CKD, chronic kidney disease; BMD, body mass index.
Comparisons of inflammatory factors, osteoporosis indicators and vitamin D deficiency of patients among different groups. Compared with those in the CKD I–II stage group, IL-6, CRP, TNF-α, serum phosphate, serum sodium, serum potassium and BUN of the patients in the other two groups were significantly increased, but serum calcium, BMD and 25(OH)D were significantly decreased. IL-6, CRP, TNF-α, serum phosphate, serum sodium, serum potassium and BUN in the CKD V stage group were significantly higher than those in the CKD III–IV stage group, but serum calcium, BMD and 25(OH)D were lower than those in the CKD III–IV stage group, which showed statistically significant difference (P<0.05) (Table II).
Table II.
Comparison of inflammatory factors, osteoporosis indicators and vitamin D of patients among different groups.
| Index | CKD I–II stage | CKD III–IV stage | CKD V stage |
|---|---|---|---|
| IL-6 (pg/ml) | 99.2±9.3 | 125.3±10.3a | 189.2±10.7a,b |
| CRP (µg/ml) | 2.9±0.2 | 6.4±0.6a | 20.3±2.0a,b |
| TNF-α (ng/ml) | 1.22±0.12 | 1.79±0.18a | 2.23±0.23a,b |
| Serum calcium (mmol/l) | 2.2±0.2 | 2.0±0.2a | 1.5±0.2a,b |
| Serum phosphate (mmol/l) | 1.3±0.1 | 1.6±0.1a | 2.0±0.2a,b |
| BMD | 0.923±0.086 | 0.823±0.098a | 0.723±0.067a,b |
| 25(OH)D (ng/ml) | 20.5±2.3 | 14.9±1.7a | 6.3±0.8a,b |
| Serum sodium (mmol/l) | 134.43±11.56 | 146.67±14.09a | 152.09±15.21a,b |
| Serum potassium (mmol/l) | 3.86±0.45 | 4.32±0.39a | 4.78±0.49a,b |
| BUN (mml/l) | 13.67±1.65 | 14.87±1.98a | 16.02±1.99a,b |
P<0.05, compared with CKD I–II stage group
P<0.05, compared with CKD III–IV stage group. CKD, chronic kidney disease; IL-6, interleukin-6; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; BMD, bone mineral density; BUN, blood urea nitrogen.
Comparison of Cr between normal and osteoporosis group
Patients were divided into two groups: normal and osteoporosis group on the basis of BMD. It was found that Cr content of patients with osteoporosis was noticeably higher than that in normal group, which showed a statistically significant difference (P<0.05) (Fig. 1).
Figure 1.
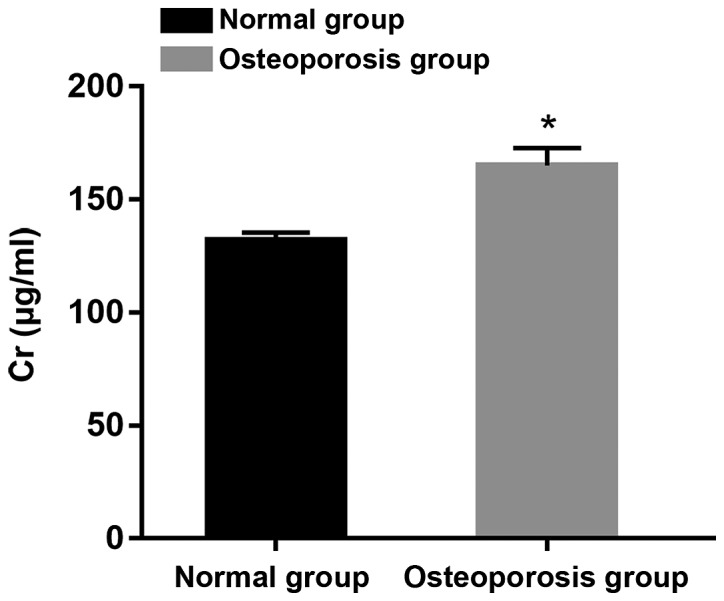
Comparison of Cr between normal and osteoporosis group. *P<0.05. Cr, creatinine.
Comparison of Cr content between groups with normal and abnormal CRP
According to the comparison between the two groups of patients, it was suggested that the Cr content in the group with abnormal CRP was significantly higher than that in the normal group (P<0.05) (Fig. 2).
Figure 2.
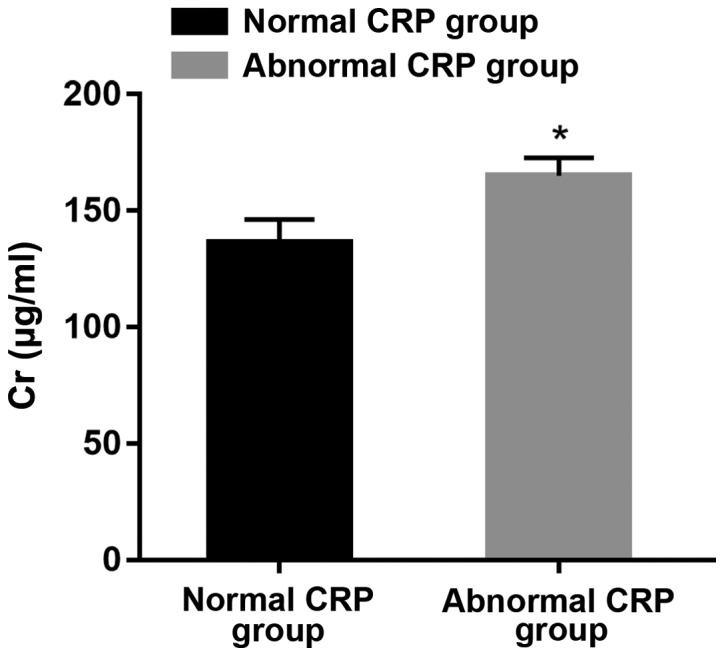
Comparison of Cr content between groups with normal and abnormal CRP. *P<0.05. Cr, creatinine; CRP, C-reactive protein.
Correlation analysis of Cr and CRP
Through the correlation analysis, it was found that there was a positive correlation between Cr and CRP (r=0.6961, P<0.001). Thus, with the increase of Cr level, the content of CRP was higher (Fig. 3).
Figure 3.
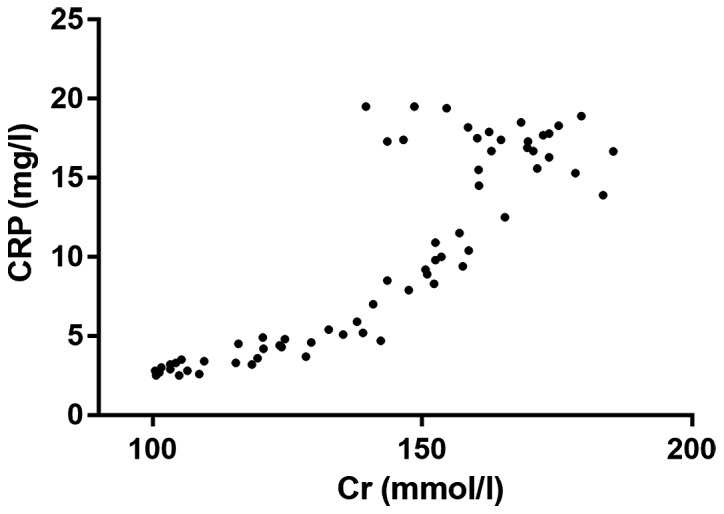
Correlation analysis of Cr and CRP. Cr, creatinine; CRP, C-reactive protein.
Analysis of the relationship between Cr and TNF-α
The correlation analysis showed that there was a positive correlation between Cr and TNF-α (r=0.8969, P<0.001), which meant that with the increase of Cr level in the body, the content of TNF-α in vivo was higher (Fig. 4).
Figure 4.
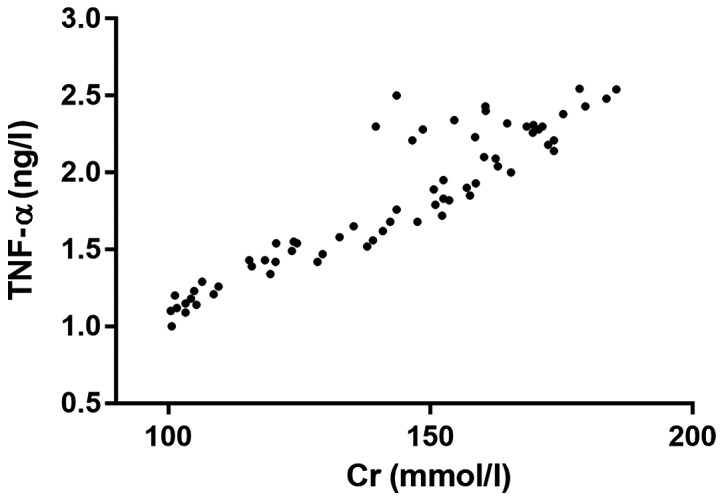
Analysis of the relationship between Cr and TNF-α. Cr, creatinine; TNF-α, tumor necrosis factor-α.
Correlation analysis of Cr and BMD
The correlation analysis showed that there was a negative correlation between Cr and BMD (r=0.5472, P<0.001). This meant that with the increase of Cr level, BMD in vivo was lower (Fig. 5).
Figure 5.
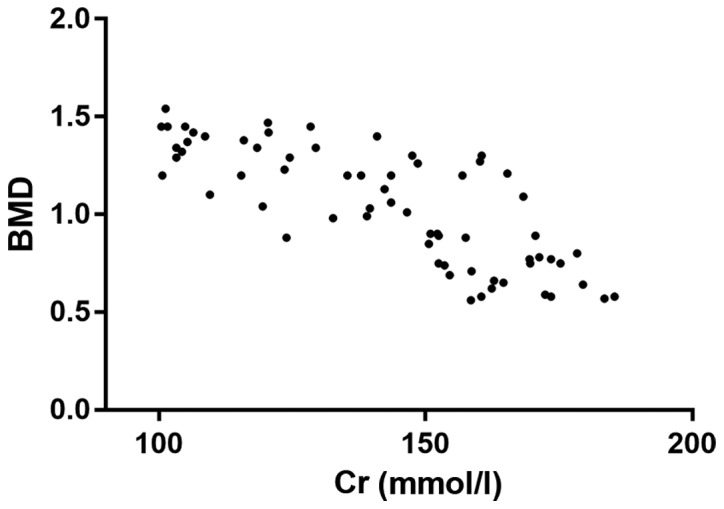
Correlation analysis of Cr and BMD. Cr, creatinine; BMD, bone mineral density.
Analysis of the correlation between Cr and 25(OH)D
Through the correlation analysis, it was found that there was a negative correlation between Cr and 25(OH)D (r=0.4733, P<0.001). In other words, with the increase of Cr level, 25(OH)D was lower (Fig. 6).
Figure 6.
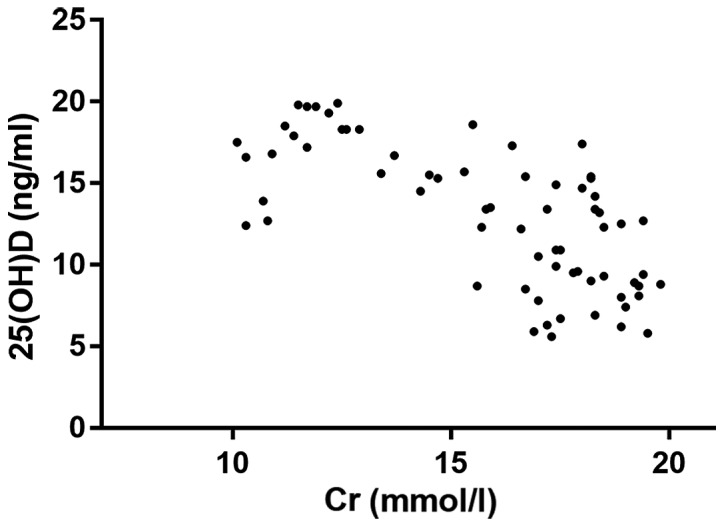
Correlation analysis of Cr and 25(OH)D. Cr, creatinine.
Discussion
CKD has a high morbidity rate in clinical practice and causes great inconvenience to patient life and work (11). It leads to many complications including inflammatory infection, osteoporosis and vitamin D deficiency (12,13). To cure and prevent CKD effectively, in-depth studies of the relation of CKD with inflammatory factors, osteoporosis and vitamin D deficiency should be conducted, providing more therapy targets for CKD.
Studies have shown that patients with CKD will suffer from various inflammations, of which the pathogenic factors are mainly renal disease and infection immunodeficiency. Inflammatory infection is not caused by bacteria or viruses, but by higher concentration of IL-6 released by T lymphocytes and increased expression levels of CRP and TNF-α after the antigen stimulation in CKD patients (14,15). Continuous accumulation of these inflammatory factors produces physiological damage to kidneys and in turn leads to microinflammation in the kidneys, which causes the increased content of inflammation such as IL-6 (16–18). This study showed that there are correlations between the severity of renal inflammation and inflammatory factors including IL-6, TNF-α and CRP. The more severe the disease is, the higher the content of the above inflammatory factors is, which indicates that the severity of CKD is positively correlated with inflammation.
Abnormalities of serum phosphate and serum calcium are detected in patients with CKD, in which the content of serum phosphate is continuously elevated, and that of serum calcium is continuously decreased with the aggravation of the disease. This is considered to be related to metabolic acidosis clinically. Acidosis will destroy homeostasis of serum phosphate, hinder the active transport of small intestinal mucosa and cause passive diffusion of phosphate and abnormal transfer of internal loading of phosphorus. The significant decrease in content of serum calcium in patients with CKD is believed clinically to be associated with elevation of serum phosphate, abnormal arginine intake of calcium and vitamin D deficiency. As an important indicator of bone metabolism in patients, the marked reduction of BMD indicates the reduction of bone mass and high possibility of bone fracture and osteoporosis (19,20). The present study showed that with the deterioration of CKD, the value of BMD is decreased significantly, indicating that patients' osteoporosis is more severe with the worsening of the disease condition. Related research has shown that too high content of serum phosphate will lead to over release of inflammatory factors in patients. In addition, serum phosphate stimulates monocyte and macrophage to release massive inflammatory factors and activates the nuclear factor κB (NF-κB) signal pathway, leading to increased content of TNF-α. It is speculated that there may be a relation of BMD and IL-6 with the above mechanism.
Vitamin D deficiency is mainly evaluated through the detection of 25(OH)D that in vivo is combined with the corresponding receptor so as to form interaction between serum phosphate and serum calcium. Vitamin D deficiency is very common in patients with CKD, and clinical studies have found that vitamin D deficiency has become an important risk factor for CKD. In this study, the inflammation degree in CKD was found to be negatively correlated with 25(OH)D, and as the condition became more severe, the content of this factor was lower, which is similar to findings of previous clinical studies (21). Hence, diagnosis, treatment and prevention of CKD can be conducted by measuring the content of this factor.
In summary, the severity of CKD in patients is correlated with inflammatory factors, osteoporosis and vitamin D deficiency. In addition, more serious disease condition causes the release of more inflammatory factors, higher content of serum phosphate, lower content of serum calcium and more severe vitamin D deficiency as well as osteoporosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CL was responsible for the data analyses and wrote the manuscript. CL and HL collected the clinical and laboratory information. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Union Hospital (Wuhan, China) and informed consents were signed by the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen J, Gu D, Chen CS, Wu X, Hamm LL, Muntner P, Batuman V, Lee CH, Whelton PK, He J. Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrol Dial Transplant. 2007;22:1100–1106. doi: 10.1093/ndt/gfl759. [DOI] [PubMed] [Google Scholar]
- 2.Cruz MC, Andrade C, Urrutia M, Draibe S, Nogueira-Martins LA, Sesso Rde C. Quality of life in patients with chronic kidney disease. Clinics (Sao Paulo) 2011;66:991–995. doi: 10.1590/S1807-59322011000600012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jepson RE. Current understanding of the pathogenesis of progressive chronic kidney disease in cats. Vet Clin North Am Small Anim Pract. 2016;46:1015–1048. doi: 10.1016/j.cvsm.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93:803–813. doi: 10.1016/j.kint.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Arnold R, Issar T, Krishnan AV, Pussell BA. Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis. 2016;5:2048004016677687. doi: 10.1177/2048004016677687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huan L, Yuezhong L, Chao W, HaiTao T. The urine albumin-to-creatinine ratio is a reliable indicator for evaluating complications of chronic kidney disease and progression in IgA nephropathy in China. Clinics (Sao Paulo) 2016;71:243–250. doi: 10.6061/clinics/2016(05)01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lips P, Goldsmith D, de Jongh R. Vitamin D and osteoporosis in chronic kidney disease. J Nephrol. 2017;30:671–675. doi: 10.1007/s40620-017-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shigematsu T, Muraoka R, Sugimoto T, Nishizawa Y. Risedronate therapy in patients with mild-to-moderate chronic kidney disease with osteoporosis: Post-hoc analysis of data from the risedronate phase III clinical trials. BMC Nephrol. 2017;18:66. doi: 10.1186/s12882-017-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marciniak C, Gabet J, Lee J, Ma M, Brander K, Wysocki N. Osteoporosis in adults with cerebral palsy: Feasibility of DXA screening and risk factors for low bone density. Osteoporos Int. 2016;27:1477–1484. doi: 10.1007/s00198-015-3393-6. [DOI] [PubMed] [Google Scholar]
- 10.Ward C, Contino K, Patel A, Mbei EE, Roy S, Hunter K, Gandhi S. The association of serum 25-Hydroxyvitamin D status in patients with osteoarthritis in the primary care office. N Am J Med Sci. 2016;8:47–55. doi: 10.4103/1947-2714.175216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pongpirul W, Pongpirul K, Ananworanich J, Klinbuayaem V, Avihingsanon A, Prasithsirikul W. Chronic kidney disease incidence and survival of Thai HIV-infected patients. AIDS. 2018;32:393–398. doi: 10.1097/QAD.0000000000001698. [DOI] [PubMed] [Google Scholar]
- 12.Michishita R, Matsuda T, Kawakami S, Tanaka S, Kiyonaga A, Tanaka H, Morito N, Higaki Y. The association between changes in lifestyle behaviors and the incidence of chronic kidney disease (CKD) in middle-aged and older men. J Epidemiol. 2017;27:389–397. doi: 10.1016/j.je.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochi M, Kohagura K, Shiohira Y, Iseki K, Ohya Y. Chronic kidney disease, inflammation, and cardiovascular disease risk in rheumatoid arthritis. J Cardiol. 2018;71:277–283. doi: 10.1016/j.jjcc.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Li ZY, Zheng Y, Chen Y, Pan M, Zheng SB, Huang W, Zhou ZH, Ye HY. Brazilin ameliorates diabetic nephropathy and inflammation in db/db mice. Inflammation. 2017;40:1365–1374. doi: 10.1007/s10753-017-0579-4. [DOI] [PubMed] [Google Scholar]
- 15.Batchu SN, Hughson A, Gerloff J, Fowell DJ, Korshunov VA. Role of Axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension. 2013;62:302–309. doi: 10.1161/HYPERTENSIONAHA.113.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Bello JA, Gómez-Díaz RA, Contreras-Rodríguez A, Talavera JO, Mondragón-González R, Sanchez-Barbosa L, Diaz-Flores M, Valladares-Salgado A, Gallardo JM, Aguilar-Kitsu A, et al. Carotid intima media thickness, oxidative stress, and inflammation in children with chronic kidney disease. Pediatr Nephrol. 2014;29:273–281. doi: 10.1007/s00467-013-2626-1. [DOI] [PubMed] [Google Scholar]
- 17.Lim GB. Arrhythmias: IL-6 and risk of atrial fibrillation in chronic kidney disease. Nat Rev Cardiol. 2016;13:183. doi: 10.1038/nrcardio.2016.23. [DOI] [PubMed] [Google Scholar]
- 18.Xun C, Zhao Y. Potential role of soluble TNF-α receptors in diagnosis of patients with chronic kidney disease. Ann Clin Lab Sci. 2017;47:310–314. [PubMed] [Google Scholar]
- 19.Peltonen S, Biancari F, Lindgren L, Mäkisalo H, Honkanen E, Lepäntalo M. Outcome of infrainguinal bypass surgery for critical leg ischaemia in patients with chronic renal failure. Eur J Vasc Endovasc Surg. 1998;15:122–127. doi: 10.1016/S1078-5884(98)80132-9. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Mavuduru RS, Sharma V. Pan-ureteric transitional cell carcinoma in a patient of autosomal dominant polycystic kidney disease with chronic renal failure. NDT Plus. 2009;2:76–77. doi: 10.1093/ndtplus/sfn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin A, Le Barbier M, Er L, Andress D, Sigrist MK, Djurdjev O. Incident isolated 1,25(OH)(2)D(3) deficiency is more common than 25(OH)D deficiency in CKD. J Nephrol. 2012;25:204–210. doi: 10.5301/JN.2011.8429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


