Abstract
In view of the high incidence of metabolic syndrome and the role of exercise in promoting metabolic disorders, the present study aimed to investigate the therapeutic effects of swimming intervention on metabolic syndrome. In the present study, a total of 100 patients with metabolic syndrome and 100 healthy individuals were included. Fasting blood was extracted from each participant, and the serum levels of interleukin (IL)-1, high sensitivity C-reactive protein (hs-CRP), tumor necrosis factor α (TNF-α) and IL-8 were measured by sandwich enzyme-linked immunosorbent assay. Patients were randomly divided into five groups (groups A-E). Patients in group A was treated with conventional drug treatment. Besides conventional treatment, patients in groups B-E were also subjected to swimming intervention for 15, 30, 45 and 60 min each time, respectively, four times a week for 3 months. Changes in the homeostatic model assessment of β-cell function and insulin resistance (HOMA-IR) score, and in the serum levels of IL-1, hs-CRP, TNF-α and IL-8 were recorded. Furthermore, muscle tissues were collected from patients, and the expression levels of insulin receptor substrate-1 (IRS-1), glucose transporter type 4 (GLUT4) and protein kinase B (Akt) in the tissues were detected by western blot assay. The results revealed that HOMA-IR and the serum levels of IL-1, hs-CRP, TNF-α and IL-8 were significantly higher in metabolic syndrome patients as compared with those in the normal controls, while swimming intervention reduced HOMA-IR and these serum levels to different extents. Swimming intervention also promoted IRS-1 and Akt phosphorylation, and increased GLUT4 expression level. Thus, it is concluded that swimming intervention may improve metabolic syndrome through multiple pathways.
Keywords: metabolic syndrome, swimming intervention, inflammatory factors, Insulin receptor substrate-1, glucose transporter type 4, phosphoinositide 3-kinase/protein kinase B
Introduction
Metabolic syndrome is a clustering of medical conditions that increases the risk of type 2 diabetes mellitus and cardiovascular disease (1). In the United States, the prevalence of the metabolic syndrome is >20% and increases with aging (2). With changes in people's diet structure and increased incidence of obesity, the incidence of metabolic syndrome is predicted to be significantly increased in the near future (3). Patients with metabolic syndrome usually present atherogenic dyslipidemia, visceral adiposity, insulin resistance, elevated blood pressure, endothelial dysfunction, genetic susceptibility and chronic stress. Among these, insulin resistance is the major cause of the development of type 2 diabetes mellitus in these patients (4), while chronic inflammation, which is characterized by abnormal production of certain cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor α (TNF-α), is closely correlated with the development of insulin resistance (5). Therefore, improving chronic inflammation will benefit the recovery of metabolic syndrome.
Exercise therapy is a treatment strategy to improve certain medical conditions through physical activity. Exercise therapy has displayed promising therapeutic effects in the treatment of various human diseases, including stroke (6), chronic fatigue syndrome (7) and chronic low back pain (8). In addition, exercise has also been used as an anti-inflammatory therapy in the treatment of certain diseases, such as rheumatic diseases (9), indicating its potential in improving insulin resistance in patients with metabolic syndrome. A recent study has revealed that diet structure management and exercise therapy can improve certain aspects of metabolic syndrome, including the endothelial function (10). However, the effects of exercise therapy on chronic inflammation and insulin resistance in patients with metabolic syndrome have not been well studied to date.
In the present study, patients with metabolic syndrome were treated with swimming intervention, and the effects of this therapy on insulin resistance and key inflammatory factors were investigated. Furthermore, the effects of swimming intervention on key insulin signal transduction pathways were explored.
Patients and methods
Patients
A total of 100 patients with metabolic syndrome admitted between January 2015 and January 2017 at the University Hospital of Chuzhou University (Chuzhou, China) were selected. All patients were diagnosed according to the criteria established by a previous study (11). Patients who met three of the following four criteria were diagnosed with metabolic syndrome: i) Overweight and/or obese, with a body mass index of ≥25; ii) had a fasting plasma glucose of ≥6.1 mmol/l (110 mg/dl) and/or 2-h plasma glucose of ≥7.8 mmol/l (140 mg/dl), and/or had been diagnosed with diabetes and treated; iii) had systolic/diastolic blood pressure of ≥140/90 mmHg, and/or had been diagnosed with hypertension and treated; and iv) had a fasting triglyceride level of ≥1.7 mmol/l (150 mg/dl), and/or a high-density lipoprotein cholesterol level of <0.9 mmol/l (35 mg/dl) for males and <1.0 mmol/l (39 mg/dl) for females. The enrolled patients included 44 males and 56 females, and their age ranged between 22 and 76 years, with a mean age of 46±19.2 years. In addition, 100 normal healthy individuals were also selected to serve as the control group, including 49 males and 51 females with an age ranging between 25 and 73 years, and a mean age of 43±17.8 years. No significant differences in terms of the age and sex were detected between the patient and control groups. The homeostatic model assessment of β-cell function and insulin resistance (HOMA-IR) in serum was calculated using fasting insulin (FINS) and fasting blood glucose (FBG) with the following formula: HOMA-IR=FINS/(22.5×10−FBG) (12). HOMA-IR in normal adult is generally <2.7. A higher HOMA-IR indicates that a patient possesses a metabolic disorder.
Patients were randomly divided into five groups, including groups A-E (n=20 per group). Patients in group A were treated with conventional drug treatment using metformin (250 mg per time, twice per day) and insulin sensitizers (thiazolidinediones; 20 mg, once per day). Besides conventional treatment, patients in group B-E were also subjected to swimming intervention for 15, 30, 45 and 60 min each time, respectively, four times a week for 3 months. This study was approved by the Ethics Committee of Chuzhou University, and all patients signed informed consent forms prior to participation.
Serum and tissue collection and processing
Fasting blood (10 ml) was extracted from each participant in the morning of the day before and at 3 months after swimming intervention. Blood samples were kept at room temperature for 1 h, followed by centrifugation at 1,200 × g for 10 min at room temperature to collect the serum. Serum samples were stored at −80°C prior to use. Patients were asked to rest for 3 h, and muscle biopsies (150–200 mg) were obtained from the vastus lateralis muscle under local anesthesia using a modified Bergstrom needle following treatment.
Measurement of serum levels of high sensitivity C-reactive protein (hs-CRP), TNF-a, IL-1 and IL-8
The serum levels of IL-1 (cat. no. DLB50), hs-CRP (cat. no. DCRP00; both R&D Systems, Inc., Minneapolis, MN, USA), TNF-α (cat. no. RPN5967; GE Healthcare, Chicago, IL, USA) and IL-8 (cat. no. D8000C; R&D Systems, Inc.) were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using corresponding ELISA kit. Serum samples were diluted to 1:10 in dilution buffer and then the 100-µl mixture was transferred to the ELISA plate (GE Healthcare). Next, the assay was performed according to the manufacturer's protocol.
Western blot analysis
A radioimmunoprecipitation assay solution (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to extract total protein from the muscle tissues obtained from three patients each from the healthy control, group A and group E (13). A plasma membrane protein extraction kit (cat. no. ab65400; Abcam, Cambridge, MA, USA) was used to extract the plasma membrane protein, and protein samples were quantified by a bicinchoninic acid assay. Next, 20 µg protein from each sample was subjected to 10% SDS-PAGE to separate proteins with different molecular weights, followed by transfer to a polyvinylidene difluoride membrane. Membranes were then blocked with 5% skimmed milk at room temperature for 2 h. Subsequent to washing using TBST membrane (0.3% Tween), The membranes was with TBS with 0.3% Tween and then incubated overnight at 4°C with the corresponding primary antibodies, including rabbit anti-insulin receptor substrate-1 (IRS1; 1:2,000; cat. no. ab52167), anti-p-IRS1 (phospho-Y896; 1:2,000; cat. no. ab4873), anti-glucose transporter type 4 (GLUT4; 1:2,000; cat. no. ab33780), anti-p-protein kinase B (Akt; 1:2,000; cat. no. ab18206), anti-Akt (1:2,000; cat. no. ab126811) and rabbit anti-β-actin (1:1,000; cat. no. ab8226; all purchased from Abcam) antibodies. Subsequent to washing, membranes were further incubated with anti-rabbit horseradish peroxidase-conjugated IgG secondary antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room temperature for 4 h. Following further washing, the enhanced chemiluminescence method (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was applied to detect the signals. Image J software was used to normalize the relative expression level of each protein to that of the endogenous control β-actin.
Statistical analysis
IBM SPSS software (version 19.0; IBM Corp., Armonk, NY, USA) was used for statistical analysis of the data. Normal distribution data were expressed as the mean ± standard deviation. A Student's t-test was used for comparisons between two groups of normally distributed data, while analysis of variance and least significant difference tests were performed for comparisons among multiple groups. P<0.05 was considered to denote a statistically significant difference.
Results
Comparison of insulin resistance between patients with metabolic syndrome and the control group
As shown in Fig. 1, HOMA-IR score was significantly higher in patients with metabolic syndrome in comparison with that in the control group (P<0.01), indicating the existence of insulin resistance in metabolic syndrome patients.
Figure 1.
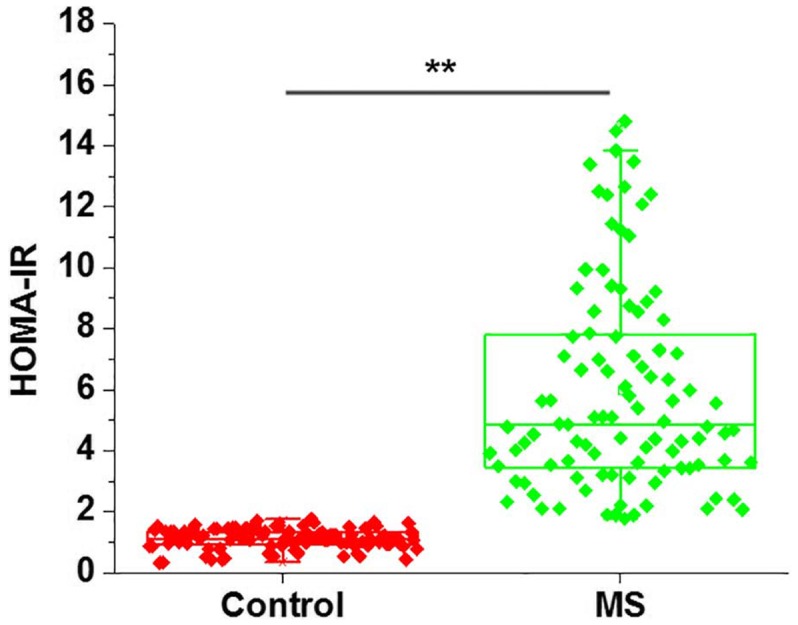
Comparison of HOMA-IR between MS patients and normal control group. HOMA-IR was calculated using the following formula: HOMA-IR=Fins/(22.5e-InFBG) (12) HOMA-IR score was significantly higher in patients with MS in comparison with the control group. **P<0.01 vs. control group. MS, metabolic syndrome; HOMA-IR, homeostatic model assessment of β-cell function and insulin resistance.
Comparison of serum levels of IL-1, hs-CRP, TNF-α and IL-8 between patients with metabolic syndrome and the control group
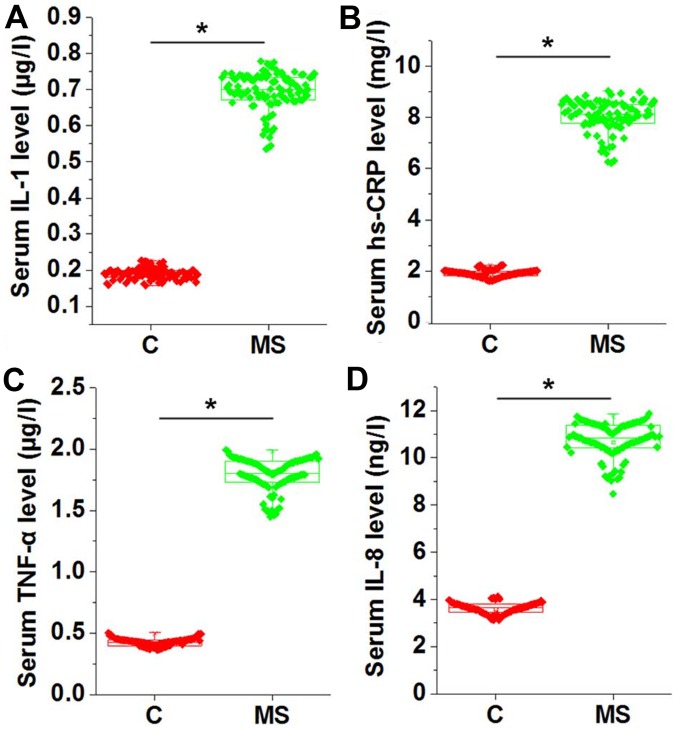
Chronic inflammation is common in patients with metabolic syndrome. Therefore, the levels of several serum inflammatory factors, including IL-1, hs-CRP, TNF-α and IL-8, were measured and compared between the two groups. As shown in Fig. 2, the serum levels of IL-1 (Fig. 2A), hs-CRP (Fig. 2B), TNF-α (Fig. 2C) and IL-8 (Fig. 2D) were significantly higher in patients with metabolic syndrome as compared with those in the normal controls (P<0.05), indicating the presence of inflammatory response in those patients.
Figure 2.
Comparison of serum levels of (A) IL-1, (B) hs-CRP, (C) TNF-α and (D) IL-8 between patients with MS and the control group. The serum levels were measured by ELISA and found to be significantly higher in patients with MS compared with the normal controls. *P<0.05. C, healthy controls; MS, metabolic syndrome; IL, interleukin; hs-CRP, high sensitivity C-reactive protein; TNF-α, tumor necrosis factor α.
Effects of swimming intervention on HOMA-IR and serum IL-1, hs-CRP, TNF-α and IL-8 levels in patients with metabolic syndrome
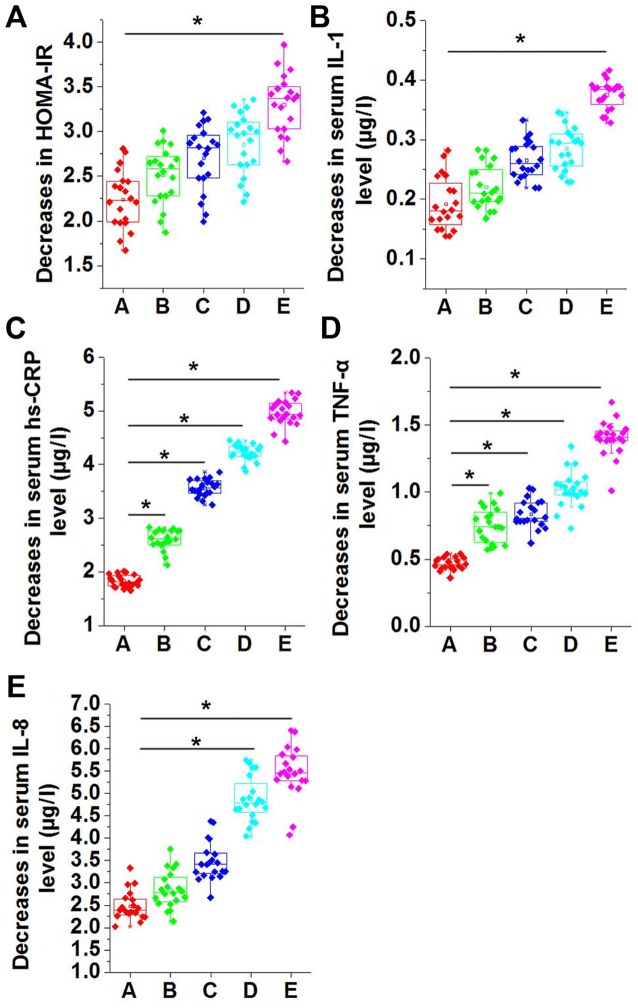
The 100 patients were randomly divided into groups A-E, and no statistically significant differences were identified in HOMA-IR score and the serum levels of IL-1, hs-CRP, TNF-α and IL-8 among the five groups prior to swimming intervention. Following swimming intervention for 3 months, the decrease in HOMA-IR increased with the increase in the swimming time per session; however, no significant differences were detected among groups A-D (Fig. 3A). As compared with group A, which did not receive swimming intervention, the decrease in HOMA-IR was significantly higher in group E, which was subjected to 60 min of swimming intervention at each session (P<0.05; Fig. 3A). Similarly, compared with group A, the decrease in the serum levels of IL-1 (Fig. 3B), hs-CRP (Fig. 3C), TNF-α (Fig. 3D) and IL-8 (Fig. 3E) were also higher in groups B-E. Decreases in serum levels of IL-1 (Fig. 3B), hs-CRP (Fig. 3C), TNF-α (Fig. 3D) and IL-8 (Fig. 3E) increased continually in groups B-E.
Figure 3.
Comparison of decreases in (A) HOMA-IR, and serum levels of (B) IL-1, (C) hs-CRP, (D) TNF-α and (E) IL-8 in different groups of patients with MS. The decrease in HOMA-IR score increased along with the increase in swimming time per session. No significant differences in HOMA-IR were detected among groups A-D, while the score significantly increased in group E compared with group A. Decreases in serum levels of IL-1, hs-CRP, TNF-α and IL-8 were significantly higher in groups B-E compared with group A. *P<0.05. HOMA-IR, homeostatic model assessment of β-cell function and insulin resistance; IL, interleukin; hs-CRP, high sensitivity C-reactive protein; TNF-α, tumor necrosis factor α; group A, metabolic syndrome with no swimming intervention; groups B-E, swimming intervention for 15, 30, 45 and 60 min per session, respectively.
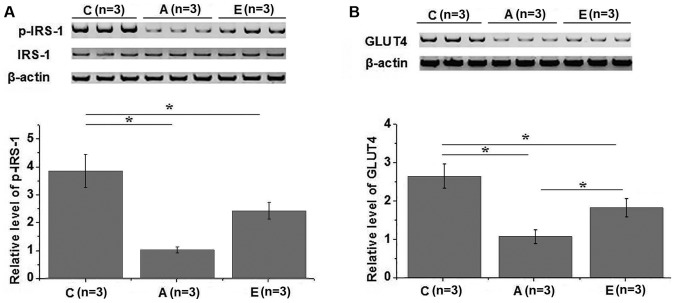
Effects of swimming intervention on IRS-1 phosphorylation and GLUT4 expression
Western blot analysis was performed to investigate the effects of swimming intervention on IRS-1 and GLUT4. In total, 3 healthy controls, 3 group A patients and 3 group E patients were included in this experiment. Compared with the healthy controls, no significant changes in the expression level of IRS-1 were observed in groups A and E (Fig. 4A). However, the phosphorylation level of IRS-1 was significantly lower in groups A and E in comparison with that in the healthy control group (P<0.05). In addition, compared with group A, the phosphorylation level of IRS-1 was significantly higher in group E (P<0.05; Fig. 4A). Similarly, the expression level of GLUT4 in the plasma membrane was significantly lower in groups A and E as compared with that in the control group (P<0.05), while it was significantly higher in group E compared with group A (Fig. 4B). IRS-1 and GLUT4 serve pivotal roles in insulin signal transduction (14,15); therefore, the aforementioned data suggest that swimming intervention may promote IRS-1 phosphorylation and GLUT4 expression to improve insulin resistance.
Figure 4.
Effects of swimming intervention on (A) IRS-1 phosphorylation and (B) GLUT4 expression, detected by western blot analysis. Swimming intervention promoted IRS-1 phosphorylation and GLUT4 expression in patients with metabolic syndrome. *P<0.05. IRS-1, insulin receptor substrate-1; GLUT-4, glucose transporter type 4; C, healthy controls; group A, metabolic syndrome with no swimming intervention; group E, swimming intervention for 60 min per session.
Effects of swimming intervention on phosphoinositide 3-kinase (PI3K)/Akt pathway
The PI3K/Akt signaling pathway is also involved in insulin signal transduction (16). Therefore, the effects of swimming intervention on PI3K/Akt pathway were also investigated in the present study. No significant differences in the expression level of Akt were observed among different groups. However, the phosphorylation level of Akt was significantly lower in groups A and E as compared with that in the control group (P<0.05). The phosphorylation level of Akt was also significantly higher in group E in comparison with that in group A (P<0.05; Fig. 5). These data suggest that swimming intervention is able to improve insulin resistance by activating the PI3K/Akt signaling pathway.
Figure 5.
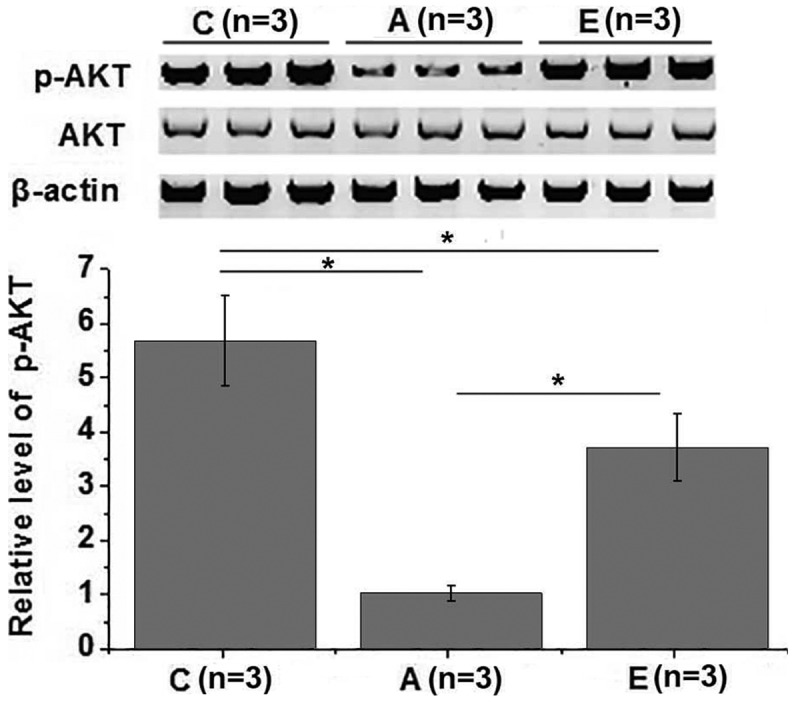
Effects of swimming intervention on PI3K/Akt pathway. Swimming intervention promoted the phosphorylation of Akt in patients with metabolic syndrome. *P<0.05. PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; C, healthy controls; group A, metabolic syndrome with no swimming intervention; group E, swimming intervention for 60 min per session.
Discussion
In recent years, the prevalence of metabolic syndrome has markedly increased in both developed and developing countries (17). Insulin resistance, as a common medical condition in metabolic syndrome, is closely correlated with the development of type 2 diabetes mellitus (1). Although the cutoff score of HOMA-IR separating metabolic syndrome patients varies in different regions, increased HOMA-IR scores compared with healthy people usually indicate aggregated pathological conditions of metabolic syndrome (18). Consistent with previous studies, in the present study, HOMA-IR scores were significantly higher in metabolic syndrome patients in comparison with those in normal control individuals, indicating the existence of insulin resistance in these patients. Chronic inflammation has been proven to be a major cause of the development of insulin resistance (5). A recent study has demonstrated that, when insulin resistance occurs, the levels of inflammation-associated factors, including osteopontin, monocyte chemoattractant protein 1, fractalkine, TNF-α and IL-6, will be significantly increased in the human body, leading to inflammatory reactions (19). Similar results were reported in the current study, which demonstrated that the serum levels of the pro-inflammatory factors IL-1, hs-CRP, TNF-α and IL-8 were significantly increased in patients with metabolic syndrome compared with the normal control individuals.
Exercise therapy aims to improve certain medical conditions through the application of physical activity. Numerous studies have reported that different types of exercise therapies, such as cycling, walking and swimming, can relieve swelling, pain and inflammation caused by injuries and different types of chronic diseases (20). In a study of patients with major depression, Knapen et al (21) have demonstrated that exercise therapy, as a valuable complementary treatment to the traditional therapies, was able to significantly reduce the risk of depression-induce medical conditions, including metabolic syndrome, type 2 diabetes and cardiovascular diseases. Furthermore, the authors reported that exercise therapy also improved the body image, which in turn improved the quality of life of patients. In another study, Almeida et al (22) reported that swimming intervention for 5 weeks was sufficient to reduce increased expression levels of brain-derived neurotrophic factor and nerve growth factor induced by nerve injury without significantly affecting glial-derived neurotrophic factor. Swimming intervention also inhibited phosphorylation of phospholipase Cγ1, and reversed microglia hyperactivity and astrocytes in the dorsal horn following nerve injury, thus improving neuropathic pain (22). Recent studies have also indicated that the exercise habits of individuals are closely associated with insulin resistance in the body, and a well-designed exercise therapy plan can effectively improve insulin resistance and inhibit the development of its complications (23). Furthermore, different types of exercise therapies can also regulate the expression of inflammation-associated factors though different pathways, including epigenetic modifications, which in turn inhibits inflammatory responses (24). In the present study, patients with metabolic syndrome were treated with swimming intervention for 3 months at a frequency of four times per week. Compared with patients who did not receive swimming intervention, the HOMA-IR score and serum levels of key pro-inflammatory factors IL-1, hs-CRP, TNF-α and IL-8 were significantly reduced in patients treated with swimming intervention. The therapeutic effects of swimming intervention were increased with the increase in the intensity of exercise. These data suggest that swimming intervention is able to improve insulin resistance and inhibit inflammatory reactions in patients with metabolic syndrome.
IRS-1 serves a pivotal role in insulin signal transduction, and the polymorphisms of IRS-1 expression are closely correlated with insulin resistance (25). GLUT-4 is an insulin-regulated glucose transporter that promotes the transportation of circulating glucose into muscle and fat cells to be processed, which in turn reduces the level of glucose in the blood (26). Translocation of GLUT-4 to the plasma membrane is critical for the transduction of insulin signaling. In the present study, the phosphorylation level of IRS-1 and expression level of GLUT-4 were significantly reduced in muscle tissues of metabolic syndrome patients, while swimming intervention promoted IRS-1 phosphorylation and GLUT-4 translocation to plasma membrane. Furthermore, the PI3K/Akt pathway has important functions in insulin signal transduction (27). In the current study, the phosphorylation level of Akt was significantly lower in metabolic syndrome patients as compared with that in the normal controls, while swimming intervention increased the phosphorylation level of IRS-1. These data suggest that swimming intervention activated IRS-1 and PI3K/Akt pathway, and promoted GLUT-4 translocation to plasma membrane, thus improving the metabolic syndrome.
Only HOMA-IR scoring was used to reflect the degree of insulin resistance due to the limited resources, which is a limitation of the present study. Our future study will detect more indexes, including the blood glucose level and glycosylated hemoglobin, to further verify the conclusions of the current study. Besides PI3K/Akt pathway, the insulin signaling transduction is also affected by other pathways, such as the Ras/ERK signaling pathway (28). Our further studies will also focus on the effects of swimming intervention on those pathways.
In conclusion, swimming intervention reduced the HOMA-IR score and serum levels of IL-1, hs-CRP, TNF-α and IL-8. In addition, it promoted IRS-1 and Akt phosphorylation, and GLUT4 translocation, therefore improving the metabolic syndrome. However, the present study is limited by the small sample size, and future studies with bigger sample size are required to confirm the conclusions of the current study.
Acknowledgements
Not applicable.
Funding
The study was financially supported by grants from the Key Project of Natural Science of Anhui Provincial Education Department (grant no. KJ2017A427), the School Grade Project of Chuzhou University (grant no. 2014sk09), and the 2018 Anhui College Excellent Youth Backbone Personnel Domestic Interview Research and Study Project (grant no. gxgnfx2018048).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
JT and LG designed the experiments, and read and approved the manuscript. JT performed experiments and collected the data. LG analyzed and interpreted data, and wrote the manuscript.
Ethics approval and consent to participate
The current study was approved by the University Hospital of Chuzhou University. All participants signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.O'neill S, O'driscoll L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22:516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Xu H, Barnes GT, Yang Q, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwakkel G, van Peppen R, Wagenaar RC, Wood Dauphinee S, Richards C, Ashburn A, Miller K, Lincoln N, Partridge C, Wellwood I, Langhorne P. Effects of augmented exercise therapy time after stroke. Stroke. 2004;35:2529–2539. doi: 10.1161/01.STR.0000143153.76460.7d. [DOI] [PubMed] [Google Scholar]
- 7.Larun L, Brurberg KG, Odgaard-Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2016;10:CD003200. doi: 10.1002/14651858.CD003200.pub5. [DOI] [PubMed] [Google Scholar]
- 8.Hayden JA, Cartwright JL, Riley RD, vanTulder MW the Chronic Low Back Pain IPD Meta-Analysis Group, corp-author. Exercise therapy for chronic low back pain: Protocol for an individual participant data meta-analysis. Syst Rev. 2012;1:64. doi: 10.1186/2046-4053-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzawa Y, Sugiyama S, Sugamura K, Sumida H, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H, Nakayama N, et al. Successful diet and exercise therapy as evaluated on self-assessment score significantly improves endothelial function in metabolic syndrome patients. Circ J. 2013;77:2807–2815. doi: 10.1253/circj.CJ-13-0549. [DOI] [PubMed] [Google Scholar]
- 11.Ma CM, Yin FZ, Liu XL, Wang R, Lou DH, Lu Q. How to simplify the diagnostic criteria of metabolic syndrome in adolescents. Pediatr Neonatol. 2017;58:178–184. doi: 10.1016/j.pedneo.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: The software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol. 2014;306:C879–C886. doi: 10.1152/ajpcell.00069.2014. [DOI] [PubMed] [Google Scholar]
- 15.Sun XJ, Miralpeix M, Myers MG, Jr, Glasheen EM, Backer JM, Kahn CR, White MF. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992;267:22662–22672. [PubMed] [Google Scholar]
- 16.Yao H, Han X, Han X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am J Cardiovasc Drugs. 2014;14:433–442. doi: 10.1007/s40256-014-0089-9. [DOI] [PubMed] [Google Scholar]
- 17.Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–20. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, Quintela AG. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13:47. doi: 10.1186/1472-6823-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, Andreozzi F, Jenkinson C, Cersosimo E, Federici M, et al. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol. 2014;51:123–131. doi: 10.1007/s00592-013-0543-1. [DOI] [PubMed] [Google Scholar]
- 20.Moore G, Durstine JL, Painter P. American College of Sports Medicine: ACSM's Exercise Management for Persons with Chronic Diseases and Disabilities, 4E(M) Human Kinetics. 2016 [Google Scholar]
- 21.Knapen J, Vancampfort D, Moriën Y, Marchal Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil Rehabil. 2015;37:1490–1495. doi: 10.3109/09638288.2014.972579. [DOI] [PubMed] [Google Scholar]
- 22.Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR, Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156:504–513. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 23.Fedewa MV, Gist NH, Evans EMa, Dishman RK. Exercise and insulin resistance in youth: A meta-analysis. Pediatrics. 2014;133:e163–e174. doi: 10.1542/peds.2013-2718. [DOI] [PubMed] [Google Scholar]
- 24.Horsburgh S, Robson-Ansley P, Adams R, Smith C. Exercise and inflammation-related epigenetic modifications: Focus on DNA methylation. Exerc Immunol Rev. 2015;21:26–41. [PubMed] [Google Scholar]
- 25.Giandalia A, Pappalardo MA, Russo GT, Romeo EL, Alibrandi A, Di Bari F, Vita R, Cucinotta D, Benvenga S. Influence of peroxisome proliferator-activated receptor (PPAR)-(gamma) exon 2 (Pro12Ala) and exon 6 (His447His) and Gly972Arg insulin receptor substrate (IRS)-1 polymorphisms on insulin resistance (IR) and beta cell function in southern mediterranean women with polycystic ovary syndrome (PCOS) J Clin Transl Endocrinol. 2017;13:1–8. doi: 10.1016/j.jcte.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sano H, Peck GR, Blachon S, Lienhard GE. A potential link between insulin signaling and GLUT4 translocation: Association of Rab10-GTP with the exocyst subunit Exoc6/6b. Biochem Biophys Res Commun. 2015;465:601–605. doi: 10.1016/j.bbrc.2015.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y, Liang D, Zhang R, Zhang S, Wang H, Cao F. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 2011;353:305–313. doi: 10.1007/s11010-011-0799-0. [DOI] [PubMed] [Google Scholar]
- 28.Gomes AP, Blenis JA. Nexus for cellular homeostasis: The interplay between metabolic and signal transduction pathways. Curr Opin Biotechnol. 2015;34:110–117. doi: 10.1016/j.copbio.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.