Abstract
The present study investigated the mechanism underlying the protective effect of dexmedetomidine (Dex) on hippocampal neuronal HT22 cell apoptosis induced by the anesthetics isoflurane and bupivacaine. The cellular morphology was observed using a phase contrast microscope. The effects of anesthetics on cell proliferation were assayed using a Cell Counting Kit-8 (CCK-8). The levels of apoptosis were examined by flow cytometry utilizing Annexin V-fluorescein isothiocyanate/propidium iodide double staining, and the protein expression levels of cleaved caspase-3, phosphorylated phosphoinositide 3′-kinase (p-PI3K), p-protein kinase B (p-AKT), hypoxia inducible factor (HIF-α), pyruvate kinase M2 (PKM2), B-cell lymphoma (Bcl-2)-associated X protein (Bax), Bcl-2 and cytochrome c were detected by western blot analysis. In vitro treatment with anesthetics was identified to decrease cell proliferation (P<0.01), the effect of which was then markedly inhibited by treatment with Dex (P<0.01) or a PI3K/AKT agonist. Exposure to anesthetics induced apoptosis in HT22 cells (75.4%), which was significantly attenuated by co-treatment with Dex (26.2%) or the PI3K/AKT agonist (28.1%). Analysis of the protein expression levels revealed that exposure to anesthetics resulted in the activation of cleaved caspase-3, Bax, cytochrome c, HIF-α and PKM2 and decreased the expression levels of Bcl-2, p-PI3K and p-AKT. However, these changes were inhibited by treatment with Dex or the PI3K/AKT agonist. Dex protected hippocampal neuronal HT22 cells from anesthetic-induced apoptosis through the promotion of the PI3K/AKT pathway and inhibition of the HIF-α/PKM2 axis.
Keywords: dexmedetomidine, isoflurane, bupivacaine, apoptosis, HT22 cells
Introduction
Anesthetic agents are used to overcome intraoperative and postoperative pain for adult and pediatric surgeries (1). Among them, isoflurane is the typical inhalation anesthetic primarily used in general anesthesia and bupivacaine is an amide, and used as a local anesthetic (2,3). However, a number of previous studies have suggested that anesthetics, including isoflurane and bupivacaine, may unavoidably induce neuron injury, particularly in older populations, even at normal clinical doses (3,4). Isoflurane was demonstrated to induce neuroapoptosis and inhibit protein kinase B (AKT) activity in the hippocampus of neonatal rats (4). Bupivacaine treatment resulted in significant levels of apoptosis in human proximal tubular HK-2 cells (5,6).
Apoptosis is the process of programmed cell death, accompanied by nuclear fragmentation, cell shrinkage, chromatin condensation, membrane blebbing and the formation of apoptotic bodies (7). Neuron apoptosis is the primary cause of neurotoxicity (8), and the mechanisms of anesthesia-induced apoptosis have not been well studied. Increased expression levels of the proapoptotic protein B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax) results in mitochondrial membrane breakage and activation of caspases, which results in cell apoptosis (8). Previously, a number of studies suggested that neuronal apoptosis made mice and human susceptible to a series of acute and chronic diseases. including Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS) and Parkinson's disease (PD) (9). Raynaud and Marcilhac (10) suggested that progressively decreasing numbers of neurons accounted for the irreversible pathogenesis of AD in adult brain tissue. Increased numbers of apoptotic neuronal cells and expression of caspase 3 and Bax were identified in the PD nigra compared with age-matched controls (11). PD was originally attributed to neuronal loss within the substantia nigra pars compacta and a concomitant loss of dopamine. Therefore, there may be a close association between neuronal apoptosis and human disease.
An important factor known to regulate cell apoptosis is hypoxia-inducible factor 1 (HIF-1). The HIF-1α subunit of HIF-1 is the master regulator of the cellular response to hypoxia (12). HIF-1 contains an oxygen-regulated α subunit and a constitutive HIF-1β subunit, which regulates the transcription of a number of genes involved in cell proliferation, angiogenesis and glycolysis (13). Pyruvate kinase M2 (PKM2), as a HIF-1 target gene, transcribes an important metabolic enzyme that is also a regulatory protein of HIF-1 activity (13).
Previous studies have identified that the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway serves a major role in regulating the homeostasis between cell survival and apoptosis (14–16). AKT-associated serine-threonine kinases regulate cell survival and serve an important role in the pathogenesis of degenerative diseases and in cancer (15). Increasing evidence has suggested that a number of chemical drugs activate apoptosis in cells by inhibiting the PI3K/AKT pathway (16). However, it remains unknown whether dexmedetomidine (Dex) affects the PI3K/AKT pathway during apoptosis in neuronal cells, particularly in hippocampal neuronal HT22 cells.
Dex, as a typical α2 adrenergic receptor agonist, attenuates isoflurane- and bupivacaine-induced neuronal apoptosis (6,17). However, the exact mechanisms underlying its anti-apoptotic activity are largely unknown. In the present study, it was identified that the Dex protected hippocampal neuronal HT22 cells from isoflurane or bupivacaine-induced apoptosis primarily through suppressing HIF-α/PKM2 and activating the PI3K-AKT pathway.
Materials and methods
Cells and cell culture
The mouse HT22 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml streptomycin and 100 U/ml penicillin (Beijing Xias Biotechnology Co., Ltd., Beijing, China), and maintained at 37°C with 5% CO2 in a humidified atmosphere. All experiments were performed on logarithmically growing cells.
In order to conduct the following assays, cells were seeded into plates and divided into seven groups: the HT22 cell control group; the Isoflurane (1.12 mM) group; the Isoflurane (1.12 mM) + Dex (200 µM) group; the Isoflurane (1.12 mM) + IGF-1 (100 nM) group; the Bupivacaine (900 µM) group; the Bupivacaine (900 µM) + Dex (200 µM) group; and the Bupivacaine (900 µM) + IGF-1 (100 nM) group.
Reagents
Primary antibodies against PKM2 (cat. no. sc-365684), HIF-α (cat. no. sc-13515), cleaved caspase-3 (cat. no. sc-271759), AKT (cat. no. sc-8312), phosphorylated (p)-AKT (cat. no. sc-271964), Bax (cat. no. sc-20067), Bcl-2 (cat. no. sc-23960) and cytochrome c (cat. no. sc-13156) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Trypsin was purchased from Beijing Solarbio Science & Technology Co., Ltd., (Beijing, China). A Cell Counting Kit-8 (CCK-8) was obtained from Beyotime Institute of Biotechnology (Haimen, China). An annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) double staining apoptosis kit was purchased from Nanjing KeyGen Biotech Co., Ltd., (Nanjing, China). Dex was purchased from sigma (Sigma Aldrich; Merck KGaA, Darmstadt, Germany; cat. no. SML0956). IGF 1 was purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
Senescence-associated β-galactosidase (SA β-gal) staining
Hippocampal neuronal HT22 cells exposed to sub-cytotoxic oxidative stress undergo stress-induced premature senescence, which may be identified using SA β-gal staining (18). HT22 cells were exposed to 100 mM tert Buty1 hydroperoxide (t-BHP) for 2 h at 37°C.
The SA β-gal staining kit (Beyotime Institute of Biotechnology) was used to detect senescent cells according to the manufacturer's protocol. Briefly, cells were seeded into 96-well culture plates (Corning Incorporated, Corning, NY, USA) at a density of 5×103 cells/well and cultured for 24 h. Then, the cells were washed twice with PBS and fixed in 1 ml 3% formaldehyde at room temperature for 15 min. Following fixation, cells were washed with PBS three times and stained with 1 ml freshly prepared cell staining working solution (containing 10 µl SA β-gal staining solution A, 10 µl SA β-gal staining solution B, 930 µl SA β-gal staining solution C and 50 µl X-Gal solution; obtained from Beyotime Institute of Biotechnology) at 37°C for 12 h in the dark. Finally, a light microscope (magnification, ×200; Olympus Corporation, Tokyo, Japan) was used to identify the blue-stained cells.
Cell proliferation assay
Cells were seeded into 96-well culture plates (Corning Incorporated) at a density of 5×103 cells/well and cultured for 24 h. Then, the cells were divided into seven groups and cultured for 24 h at 37°C. Then, 10 µl/well CCK-8 solution was added into the cell culture supernatant at 37°C for 2 h. Thereafter, the optical density was measured at 450 nm wavelength using a microplate reader (Sigma-Aldrich; Merck KGaA).
Observation of morphological changes
HT22 cells were seeded into 24-well culture plates (Corning Incorporated) at a density of 2×104 cells/well and divided into seven groups. Then, cellular morphology was observed using a phase contrast microscope (magnification, ×200; Leica Microsystems GmbH, Wetzlar, Germany).
Flow cytometry analysis of annexin V-FITC/PI double staining
The annexin V-FITC/PI double staining assay was performed using quantitative flow cytometry. Cells were harvested at 37°C for 24 h. Subsequently, harvested cells were using 0.25% trypsin, centrifuged at 352 × g for 5 min at 4°C and then washed twice with PBS. The supernatant was discarded, and the pellet was resuspended with 300 µl binding buffer (Hepes 10 mM, NaCl 140 mM, CaCl2 2.5 mM, pH 7.4 in tri-distilled water). Cells were incubated at 37°C with 5 µl FITC-conjugated Annexin V for 15 min and then incubated with 10 µl PI for 15 min at room temperature in the dark. The samples were analyzed by a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The fluorescent scatter plots indicate three cell populations: Live (Annexin V-FITC−/PI−), necrotic (Annexin V-FITC+/PI+), and apoptotic (Annexin V-FITC+/PI−). Quadrant analysis (BD FACSuite™ v 1.0; BD Biosciences) was performed on the gated fluorescent scatter plot to examine the percentage of live, necrotic and apoptotic cell populations.
Western blot analysis
HT22 cells were divided into seven groups and cultured for 24 h at 37°C. Then, cells were harvested; washed twice with PBS and then lysed in lysis buffer (50 mM HEPES pH 7.4, 1% Triton X-100, 2 mM sodium orthovanadate, 100 mM sodium fluoride, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 µg/ml aprotinin and 10 µg/ml leupeptin) at 4°C for 60 min. Lysates were centrifuged at 15,000 × g for 10 min at 4°C, and the supernatants were used for western blot analysis. Protein content of the supernatants was determined using a Bio-Rad protein assay reagent (Bio Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts (50 µg) of the total protein were separated by 10–11% gel electrophoresis and electrophoretically transferred to nitrocellulose membranes (NC membranes, 0.45 µm; Thermo Fisher Scientific, Inc.) at 4°C for 2 h. The NC membranes were then blocked with 5% skimmed milk at room temperature for 1 h. Proteins were detected with the primary antibodies (all 1:1,000) against PKM2, HIF-α, cleaved caspase-3, AKT, p-AKT, Bax, Bcl-2 and cytochrome c overnight at 4°C, followed by a horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. sc-2789; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Protein bands were visualized by using enhanced chemiluminescence (BeyoECL Plus; Beyotime Institute of Biotechnology) as the HRP substrate. Band density of the specific protein was analyzed with Quantity one image software (Image Lab™ software version 5.1; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data obtained by at least three independent experiments were expressed as mean ± standard deviation. Comparison of multiple groups was performed by one-way analysis of variance followed by Dunnett's test using GraphPad Prism v7 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Dex inhibits anesthetic-induced apoptotic body formation and attenuates the proliferation inhibition of HT22 cells
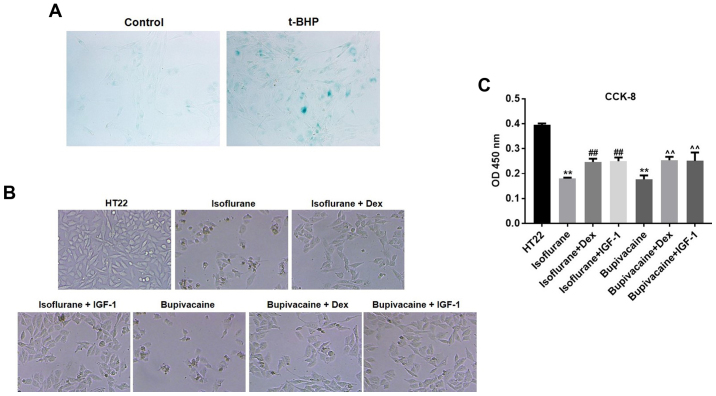
HT22 cell senescence was first induced by t-BHP, which was then confirmed by the β-gal staining (Fig. 1A). Next, to determine the features of HT22 cell growth, the morphological changes were observed by phase contrast microscopy. Marked membrane blebbing, cell shrinkage and formation of apoptotic bodies were observed following treatment with isoflurane and bupivacaine. However, the changes were less significant in the presence of Dex or insulin-like growth factor 1 (IGF-1), the PI3K/AKT agonist (Fig. 1B). To additionally evaluate the potential effect of Dex on cell proliferation, a CCK-8 assay was conducted. As demonstrated in Fig. 1B, the growth of HT22 cells was markedly inhibited by isoflurane or bupivacaine compared with the control group (P<0.01). However, Dex significantly decreased the isoflurane or bupivacaine-mediated proliferation inhibition of HT22 cells (Fig. 1C).
Figure 1.
Dex protects against anesthetic-induced HT22 cell apoptosis. (A) HT22 cells were pretreated with 100 mM t-BHP for 2 h and subjected to senescence-associated β-galactosidase staining. (B) HT22 cells were treated with Dex and/or anesthetics for 24 h. The morphological changes were observed by phase contrast microscopy. Magnification ×200 (C) Cell proliferation were determined using a CCK-8 Kit. n=3; data are presented as mean ± standard deviation. **P<0.01 vs. HT22; ##P<0.01 vs. Isoflurane; and ^^P<0.01 vs. Bupivacaine. t-BHP, tert-Butyl hydroperoxide; Dex, dexmedetomidine; CCK-8, Cell Counting Kit-8; OD, optical density; IGF-1, insulin-like growth factor 1.
Treatment with Dex rescues HT22 cells from anesthetic-induced apoptosis
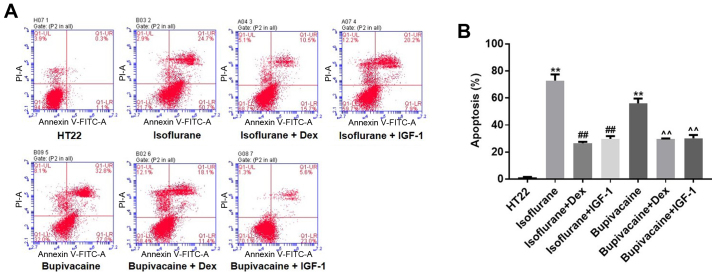
Annexin V and PI double staining was used to examine HT22 cell apoptosis. As indicated in Fig. 2A, isoflurane (1.12 mM) treatment for 24 h resulted in 75.4% apoptosis compared with the vehicle (1.4%). Similarly, exposing HT22 cells to 900 µM bupivacaine for 24 h resulted in 59.8% apoptosis. However, Dex treatment decreased isoflurane- or bupivacaine-induced cell apoptosis to 26.2 and 29.5%, respectively. Similarly, the percentage of isoflurane or bupivacaine-induced apoptotic cells decreased to 28.1 and 28.6%, respectively, following incubation with IGF-1 (Fig. 2B). In conclusion, it was confirmed that treatment with Dex protected HT22 cells from anesthetic-induced apoptosis.
Figure 2.
Apoptosis of HT22 cells is assessed by Annexin V-FITC/PI staining. (A) Flow cytometry analysis of HT22 cell viability and apoptosis at 24 h after indicated treatments. The Annexin V-FITC+/PI− cells (viable apoptotic cells) and Annexin V-FITC+/PI+ cells (non-viable apoptotic cells) were considered apoptotic cells and counted. (B) The data are presented as the mean ± standard deviation of the results from three independent experiments. **P<0.01 vs. HT22; ##P<0.01 vs. Isoflurane; and ^^P<0.01 vs. Bupivacaine. FITC, fluorescein isothiocyanate; PI, propidium iodine; IGF-1, insulin-like growth factor 1; Dex, dexmedetomidine.
Anesthetics induce apoptosis via activating the HIF-α/PKM2 pathway and inhibiting the PI3K-AKT pathway in HT22 cells, which is reversed by Dex
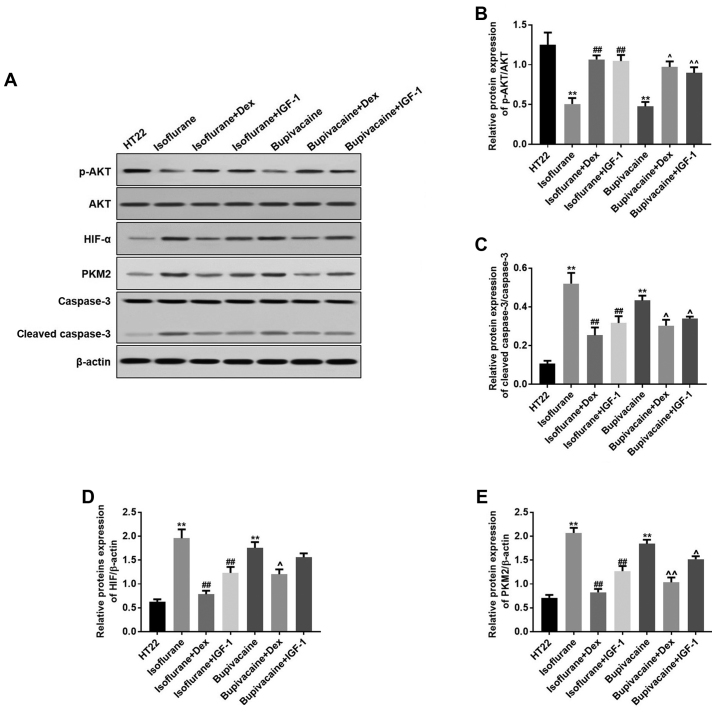
Isoflurane or bupivacaine significantly decreased the protein expression of p-PI3K and p-AKT; while treatment with Dex or IGF-1 increased their expression levels. Concomitantly, Dex or IGF-1 treatment resulted in equivalent inhibitory effects on the isoflurane- or bupivacaine-induced expression of these proteins (Fig. 3A). Data from the western blot analysis indicated the same effects (Fig. 3B-D).
Figure 3.
Dex regulates the proteins expression levels of HIF-α, PKM2, p-AKT and cleaved caspase-3 in vitro. (A) HIF-α, PKM2, p-AKT and cleaved caspase-3 levels were detected by western blot analysis. β-actin was used as a loading control. (B) The quantitative analysis of the ratio of p-Akt to Akt. (C) Cleaved caspase-3, (D) HIF-α and (E) PKM2 levels were analyzed by one-way analysis of variance and Dunnett's post-hoc test. n=3; data are presented as mean ± standard deviation. **P<0.01 vs. HT22; ##P<0.01 vs. Isoflurane; ^P<0.05 and ^^P<0.01 vs. Bupivacaine. HIF-α, hypoxia-inducible factor α; PKM2, pyruvate kinase M2; p, phosphorylated; AKT, protein kinase B; IGF-1, insulin-like growth factor 1; Dex, dexmedetomidine.
Anesthetics-induced apoptosis may occur through a mitochondria-dependent intrinsic apoptosis pathway
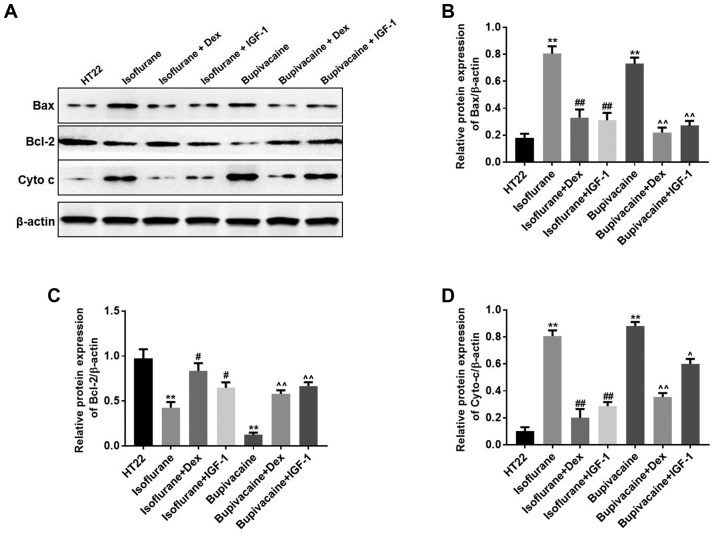
Western blot analysis was performed to additionally examine the mechanism of anesthetics-induced cell death. It has been established that the release of cytochrome c into the cytoplasm and an increase of the Bax:Bcl-2 ratio leads to apoptosis (4). Bcl-2 is a potent inhibitor of apoptotic cell death, while Bax accelerates apoptosis by contributing to the permeabilization of the outer mitochondrial membrane (4). In the present study, when considering the intrinsic apoptosis pathway, the translocation of the anti-apoptotic protein Bcl-2 to the mitochondria was markedly decreased, while the pro-apoptotic translocation of Bax to the mitochondria and the release of cytochrome c into cytoplasm were increased in the anesthetics-treated groups. However, treatment with Dex or IGF-1 inhibited these effects in HT22 cells (Fig. 4A). The results indicated that the intrinsic apoptosis pathway was upregulated by anesthetics in the hippocampal neuronal HT22 cells. Data from the western blot analysis indicated similar results (Fig. 4B-D).
Figure 4.
Anesthetics induce cell death primarily through the intrinsic apoptosis pathway. (A) The expression of Bax, Bcl-2 and cyto c were detected by western blot analysis. β-actin was used as an equal loading control. The quantitative analysis of the levels of (B) Bax, (C) Bcl-2 and (D) cyto c by one-way analysis of variance and Dunnett's post-hoc test. n=3; data are presented as mean ± standard deviation; **P<0.01 vs. HT22; ##P<0.01 Isoflurane + Dex vs. Isoflurane; #P<0.05 Isoflurane + IGF 1 vs. Isoflurane; ^^P<0.01 Bupivacaine + Dex vs. Bupivacaine and ^P<0.05 Bupivacaine + IGF 1 vs. Bupivacaine. cyto c, cytochrome c; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; IGF-1, insulin-like growth factor 1; Dex, dexmedetomidine.
Discussion
To assess the protective mechanism of Dex on the anesthetic-induced damage in the elderly population, an aging model with t-BHP-stimulated HT22 cells was designed in the present study. Exposure of HT22 cells to 100 mM tert-Butyl hydroperoxide (t-BHP) for 2 h decreased their proliferative life span and increased the proportion of cells positive for the SA β-gal activity (18). The results demonstrated that the apoptosis of HT22 cells induced by anesthetic treatment was caused by HIF-α/PKM2 activation and PI3K-AKT pathway downregulation. Dex simultaneously inhibited the HIF-α/PKM2 activation and PI3K-AKT downregulation in anesthetic-treated HT22 cells, thereby attenuating the intrinsic apoptosis pathway. These data provided novel insights into the process of anesthetic injury in HT22 cells and the protective potential of Dex on anesthetic-treated HT22 cells. Consistent with previous data, isoflurane or bupivacaine treatment increased the expression of cleaved caspase-3 and promoted the expression of HIF-α and PKM2.
There are a number of factors affecting neuronal cells apoptosis. Oxidative stress in neuronal cells leads to apoptosis through the release of cytochrome c from the mitochondria and caspase-3 activation (19). In addition, tumor necrosis factor-α released by neuronal cells is associated with glutamate-induced neuronal cell death (20). Lauritzen et al (21) identified that rat mature cerebellar granule cells die through apoptosis when cultured in a medium containing physiological concentrations of K+. A number of studies have suggested that anesthetics induce neurodegeneration in multiple brain regions (22,23). However, the present study specially focused on hippocampal neuronal HT22 cells, as previous studies had demonstrated that isoflurane induced a severe hippocampal lesion in neonatal rats, accompanied by an abnormal response to contextual fear conditioning (22).
Anesthetics including isoflurane and bupivacaine are the most common clinical drugs used during surgical procedures and are generally safe (23,24). Isoflurane has been demonstrated to be neuroprotective and neurotoxic (25–28). Short-time exposure of isoflurane provides neuroprotection via the moderate activation of inositol triphosphate (IP3) receptors and activation of AKT-mediated neuroprotection. However, long-time exposure of isoflurane induces neurotoxicity via overactivation of IP3 receptors and activates excessive Ca2+ release from the endoplasmic reticulum (25–28). Previously, increasing data have indicated that anesthetics are neurotoxic even at normal clinical doses (29,30). The primary neurotoxic effect is mediated through the activation of apoptotic death (22). Previous studies have suggested that isoflurane induced neurocognitive impairment and neuroapoptosis in neonatal rats (22). It has also been demonstrated that isoflurane induced neuroapoptosis throughout the cortex, thalamus and hippocampus regions, accompanying increased caspase-3 levels (22). Bupivacaine, as a local anesthetic, has been demonstrated to induce neural dysfunction and cell apoptosis in vitro (31). Bupivacaine led to the inhibition of mitochondrial respiratory complexes, decreased mitochondrial membrane potential and overproduction of reactive oxygen species (ROS), with cytochrome c liberation and activation of the caspase-3-dependent apoptosis pathway (32,33).
Dex is usually used as an antianxiety treatment, sedative and analgesic. Dex may relieve stress and maintain the stable function of the cardiovascular system (34). During the anesthesia recovery phase, Dex maintained patients in a continuous calm state with good respiratory function (35). Dex is an α2-adrenergic agonist, and exhibited neuroprotective effects against ischemic cerebral injury through activating the α2-adrenergic receptors and binding at imidazoline 1 and 2 receptors (4). Dex attenuated isoflurane-induced injury in the developing brain, providing neurocognitive protection (4). Dex attenuated bupivacaine-induced cytotoxicity in the mouse neuroblastoma N2 cell line, primarily by decreasing the release of ROS and the expression of caspase-3, and ultimately inhibiting apoptosis in N2 cells (17). Consistent with the aforementioned results, the present study identified that Dex protected the hippocampal neuronal HT22 cells against isoflurane-and bupivacaine-induced apoptosis. However, a previous study suggested that Dex itself induced neuroapoptosis in vivo and in vitro, respectively, and although Dex exhibited neuroprotective effect at clinical doses, the high cumulative doses and concentrations induced neuroapoptosis (36).
Apoptosis is activated by a series of caspases, namely the cysteine aspartic-specific proteases. The apoptotic pathway is activated by intracellular and extracellular signals. There are two different pathways leading to apoptosis: The intrinsic (mitochondrial) and extrinsic (death receptor) pathways (37). The intrinsic pathway is associated with changes in the permeability transition of the outer mitochondrial membrane, and this permeability is primarily controlled by the Bcl-2 family of proteins, including Bax, through regulating the formation of apoptotic protein-conducting pores in the mitochondrial membrane (37). Then, permeabilization allows the release of intermembrane proteins cytochrome c, which functions to activate caspase-9, thereby promoting the expression of caspase-3, activating apoptosis (37). Cleaved caspase-3 expression is a marker of apoptotic cell death (37). In the present study, western blot analysis data demonstrated that isoflurane and bupivacaine increased the expression of cleaved caspase-3, Bax, Bcl-2 and cytochrome c in the hippocampal neuronal HT22 cells, while Dex inhibited this increase. This suggests that the intrinsic apoptosis pathway was upregulated by treatment with anesthetics in the hippocampal neuronal HT22 cells.
In the present study, isoflurane or bupivacaine significantly decreased the protein expression levels of p-PI3K and p-AKT, while treatment with Dex or IGF-1 increased the expression of p-PI3K and p-AKT. This indicates that the neuroprotective effects of Dex may be mediated by activating the PI3K/AKT pathway. The results of the present study are consistent with the study of Li et al (4), which suggested that Dex treatment induced neuroprotective effects against isoflurane-induced neuroapoptosis in the hippocampus of neonatal rats by preserving PI3K/AKT pathway activity. HIF-1α and PKM2 are associated with glucose metabolism and mitochondrial respiratory chain (38). In the present study, Dex protected hippocampal neuronal HT22 cells from isoflurane- or bupivacaine-induced apoptosis primarily through suppressing the HIF-α/PKM2 axis. Thereby, the anti-apoptosis effect of Dex may be associated with the regulation of the HIF-α/PKM2 pathway. However, the detailed interactions how Dex preserves PI3K/AKT activity and suppresses the HIF-α/PKM2 pathway remain unclear and require additional investigation.
In conclusion, the present study suggested that Dex treatment protected against anesthetic-induced intrinsic apoptosis in vitro, indicating that it exhibits anti-apoptotic qualities. It was demonstrated that the neuroprotective effect of Dex against anesthetic-induced cell apoptosis occurred primarily through preserving PI3K/AKT activity and suppressing the HIF-α/PKM2 pathway. These data provide not only novel insight into the complex associations between anesthetics and cell death, but also the possibility for the treatment of anesthetic-induced injury using Dex.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical and Health Science and Technology Plan of Zhejiang Province (2014kyb082, 2018KY390).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
FB, XK and JW contributed to the experimental design, tissue collection, the execution of experiments and were the major contributors in developing the first draft of the manuscript. QX performed staining assay, and analyzed and interpreted the data. FB reviewed and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–1928. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 2.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik O, Kaye AD, Kaye A, Belani K, Urman RD. Emerging roles of liposomal bupivacaine in anesthesia practice. J Anaesthesiol Clin Pharmacol. 2017;33:151–156. doi: 10.4103/joacp.JOACP_375_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Zeng M, Chen W, Liu C, Wang F, Han X, Zuo Z, Peng S. Dexmedetomidine reduces isoflurane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PLoS One. 2014;9:e93639. doi: 10.1371/journal.pone.0093639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Zhang QG, Lai LY, Wen XJ, Zheng T, Cheung CW, Zhou SQ, Xu SY. Neuroprotective effect of ginkgolide B on bupivacaine-induced apoptosis in SH-SY5Y cells. Oxid Med Cell Longev. 2013;2013:159864. doi: 10.1155/2013/159864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng J, Drobish JK, Liang G, Wu Z, Liu C, Joseph DJ, Abdou H, Eckenhoff MF, Wei H. Anesthetic preconditioning inhibits isoflurane-mediated apoptosis in the developing rat brain. Anesth Analg. 2014;119:939–946. doi: 10.1213/ANE.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malikova J, Zdarilova A, Hlobilkova A. Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:5–12. doi: 10.5507/bp.2006.001. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Chen XL, Zheng JY, Zhou JW, Ma ZL. Astragaloside IV protects new born rats from anesthesia-induced apoptosis in the developing brain. Exp Ther Med. 2016;12:1829–1835. doi: 10.3892/etm.2016.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol. 2004;72:1–25. doi: 10.1016/j.pneurobio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer's disease. FEBS J. 2006;273:3437–3443. doi: 10.1111/j.1742-4658.2006.05352.x. [DOI] [PubMed] [Google Scholar]
- 11.Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 12.Koh MY, Darnay BG, Powis G. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1alpha, leading to its oxygen-independent degradation. Mol Cell Biol. 2008;28:7081–7095. doi: 10.1128/MCB.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wit RH, Mujić-Delić A, van Senten JR, Fraile-Ramos A, Siderius M, Smit MJ. Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1α/PKM2 axis in glioblastoma cells. Oncotarget. 2016;7:67966–67985. doi: 10.18632/oncotarget.11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng Y, Zhang J, Liu Y. PI3K/Akt is involved in bufalin-induced apoptosis in gastric cancer cells. Anticancer Drugs. 2009;20:59–64. doi: 10.1097/CAD.0b013e3283160fd6. [DOI] [PubMed] [Google Scholar]
- 15.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: Size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 16.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 17.Tüfek A, Kaya S, Tokgöz O, Firat U, Evliyaoğlu O, Çelik F, Karaman H. The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clin Invest Med. 2013;36:E95–E102. doi: 10.25011/cim.v36i2.19572. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Chen X, Zhu Y, Zeng Y, Jin J. A senescence model induced by tertbutyl-hydroperoxide in rats. J Fujian Medical Univ. 2003;37:19–22. [Google Scholar]
- 19.Annunziato L, Amoroso S, Pannaccione A, Cataldi M, Pignataro G, D'Alessio A, Sirabella R, Secondo A, Sibaud L, Di Renzo GF. Apoptosis induced in neuronal cells by oxidative stress: Role played by caspases and intracellular calcium ions. Toxicol Lett. 2003;139:125–133. doi: 10.1016/S0378-4274(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 20.Kogo J, Takeba Y, Kumai T, Kitaoka Y, Matsumoto N, Ueno S, Kobayashi S. Involvement of TNF-alpha in glutamate-induced apoptosis in a differentiated neuronal cell line. Brain Res. 2006;1122:201–208. doi: 10.1016/j.brainres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Lauritzen I, Zanzouri M, Honoré E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis. Role of task leak K+ channels. J Biol Chem. 2003;278:32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- 22.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 23.Auroy Y, Benhamou D, Bargues L, Ecoffey C, Falissard B, Mercier FJ, Bouaziz H, Samii K. Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service. Anesthesiology. 2002;97:1274–1280. doi: 10.1097/00000542-200211000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Park CJ, Park SA, Yoon TG, Lee SJ, Yum KW, Kim HJ. Bupivacaine induces apoptosis via ROS in the Schwann cell line. J Dent Res. 2005;84:852–857. doi: 10.1177/154405910508400914. [DOI] [PubMed] [Google Scholar]
- 25.Liang G, Wang Q, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei H. A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg. 2008;106:492–500. doi: 10.1213/ane.0b013e3181605b71. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Yang Z, Liang G, Wu Z, Peng Y, Joseph DJ, Inan S, Wei H. Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology. 2013;118:537–549. doi: 10.1097/ALN.0b013e3182833fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–250. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Liang G, Chen Q, Joseph DJ, Meng Q, Eckenhoff RG, Eckenhoff MF, Wei H. Anesthetic-induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J Pharmacol Exp Ther. 2010;333:14–22. doi: 10.1124/jpet.109.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths JD, Le NV, Grant S, Bjorksten A, Hebbard P, Royse C. Symptomatic local anaesthetic toxicity and plasma ropivacaine concentrations after transversus abdominis plane block for Caesarean section. Br J Anaesth. 2013;110:996–1000. doi: 10.1093/bja/aet015. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs NM, Rodoreda P. Primary anaesthetic deaths in Western Australia from 1985–2008: Causation and preventability. Anaesth Intensive Care. 2013;41:302–310. doi: 10.1177/0310057X1304100305. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Castro R, Patel S, Garavito-Aguilar ZV, Rosenberg A, Recio-Pinto E, Zhang J, Blanck TJ, Xu F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997–1007. doi: 10.1213/ane.0b013e31819385e1. [DOI] [PubMed] [Google Scholar]
- 32.Arai Y, Kondo T, Tanabe K, Zhao QL, Li FJ, Ogawa R, Li M, Kasuya M. Enhancement of hyperthermia-induced apoptosis by local anesthetics on human histiocytic lymphoma U937 cells. J Biol Chem. 2002;277:18986–18993. doi: 10.1074/jbc.M108084200. [DOI] [PubMed] [Google Scholar]
- 33.Cela O, Piccoli C, Scrima R, Quarato G, Marolla A, Cinnella G, Dambrosio M, Capitanio N. Bupivacaine uncouples the mitochondrial oxidative phosphorylation, inhibits respiratory chain complexes I and III and enhances ROS production: Results of a study on cell cultures. Mitochondrion. 2010;10:487–496. doi: 10.1016/j.mito.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: A systematic review and meta-analysis. Clinics (Sao Paulo) 2014;69:777–786. doi: 10.6061/clinics/2014(11)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia ZQ, Chen SQ, Yao X, Xie CB, Wen SH, Liu KX. Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: A meta-analysis of randomized clinical trials. J Surg Res. 2013;185:833–843. doi: 10.1016/j.jss.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Liu JR, Yuki K, Baek C, Han XH, Soriano SG. Dexmedetomidine-induced neuroapoptosis is dependent on its cumulative dose. Anesth Analg. 2016;123:1008–1017. doi: 10.1213/ANE.0000000000001527. [DOI] [PubMed] [Google Scholar]
- 37.Kim KH, Seo HS, Choi HS, Choi I, Shin YC, Ko SG. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch Pharm Res. 2011;34:1363–1372. doi: 10.1007/s12272-011-0817-5. [DOI] [PubMed] [Google Scholar]
- 38.Chen G, Feng W, Zhang S, Bian K, Yang Y, Fang C, Chen M, Yang J, Zou X. Metformin inhibits gastric cancer via the inhibition of HIF1α/PKM2 signaling. Am J Cancer Res. 2015;5:1423–1434. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.