Abstract
BACKGROUND: To evaluate the efficacy and toxicities of regorafenib plus irinotecan, dose-escalated on the basis of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping, in previously heavily treated metastatic colorectal cancer (mCRC) and the prognostic values of EGFR expression, KRAS mutations, and tumor sidedness. METHODS: Forty-one patients with mCRC with disease progression after treatment with fluoropyrimidines, oxaliplatin, irinotecan, anti-VEGF, and anti-EGFR MoAbs were subjected to UGT1A1 genotyping and received regorafenib combined with FOLFIRI with dose-escalated irinotecan. RESULTS: The median follow-up period was 10.0 months (1.3-23.5 months). The overall disease control rate was 58.5%, whereas the median progression-free survival (PFS) and overall survival (OS) were 6.0 months and 12.0 months, respectively. KRAS mutations were significantly associated with positive EGFR expression (P = .026). KRAS mutations significantly correlated with a shorter OS than KRAS wild-type (6.0 vs. 14.4 months, P = .014) but had no significant association with PFS. Positive EGFR expression had an inverse correlation with PFS (2.5 vs. 14.0 months, P = .039) and OS (9.6 vs. 19.7 months, P = .044). Moreover, left-sided tumors associated with superior PFS (2.0 vs. 7.0 months, P < .0001) and OS (4.0 vs. 13.0 months, P < .0001), and tumor sidedness was an independent prognostic factor by the multivariate analysis. CONCLUSION: Regorafenib and FOLFIRI concomitant therapy with dose-escalated irinotecan seemed to be potentially practicable with satisfactory oncological results. KRAS mutations and EGFR expression might be predictors of poor oncological outcomes; however, left-sided mCRCs would be more beneficial for concomitant regorafenib and FOLFIRI therapy.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related deaths worldwide; an estimated 1.4 million newly diagnosed cases and nearly 700,000 deaths were reported in 2012 [1]. Metastases coincide with approximately one-fourth of the CRC cases at the time of diagnosis (synchronous), and the initially local diseases progress to metastases in approximately 40% of the rest of the cases (metachronous), accounting for up to 60% of CRC cases that require treatment for metastatic diseases [2].
Metastatic CRCs (mCRCs) are principally managed with chemotherapy composed of fluoropyrimidines and either oxaliplatin or irinotecan combined with monoclonal antibodies (MoAbs) targeting vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR) if RAS is wild type. Regorafenib is an oral multikinase inhibitor that targets and inhibits receptor tyrosine kinases (RTKs) in the Ras/Raf/MEK/ERK pathway, which participates in the signaling of oncogenesis, angiogenesis, and cancer proliferation and metastasis [3]. Regorafenib monotherapy serves as a salvage treatment for progressive mCRCs that are unresponsive to fluoropyrimidines, irinotecan, oxaliplatin, and anti-VEGF and anti-EGFR MoAbs. In the global CORRECT study, which compared regorafenib monotherapy and placebo in previously treated mCRC, regorafenib monotherapy yielded a significantly superior disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) compared with placebo in previously treated mCRC (41% vs.15%, P < .0001; 1.9 vs.1.7 months, P < .0001; 6.4 vs.5.0 months, P = .0052, respectively) [4]. Another CONCUR trial in the Asian population revealed similar significant differences in DRC, PFS, and OS between regorafenib monotherapy and placebo (51.5% vs. 7.4%, P < .0001; 3.2 vs.1.7 months, P < .0001; 8.8 vs.6.3 months; P = .00016, respectively) [5]. For grade >3 adverse events (AEs) of regorafenib, such as hand-foot skin reaction (HFSR), anemia, thrombocytopenia, and small intestine hemorrhage, daily doses of regorafenib from 160 mg to 120 mg are usually reduced, and the oncological outcomes may be comparable [6]. Furthermore, uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping is approved by the Food and Drug Administration of the United States for predicting irinotecan-related severe diarrhea and neutropenia [7] because UGT1A1 protein is the main enzyme responsible for glucuronidation of the active metabolite of irinotecan 7-ethyl-10-hydroxycamptothecin (SN-38) and therefore metabolizes and detoxifies irinotecan [8]. However, UGT1A1 genotyping also predicts the maximum dose of irinotecan that can be tolerated and enables dose adjustment of irinotecan [9], [10], [11]. We previously introduced dose-reduced regorafenib combined with dose-adjusted irinotecan based on individual UGT1A1 genotyping along with 5-fluorouracil (5-FU) plus leucovorin (FOLFIRI regimen) with satisfactory oncological results [12].
The right-sided colon differs from the left-sided colon and rectum both in embryological origin and in blood supply, where the right-sided colon arises from midgut, blood supply comes from superior mesentery vessels, and the left-sided colon and rectum are hindgut structures supplied from inferior mesentery vessels. The prognostic impact of tumor sidedness also differs in that right-sided mCRCs have poor prognosis and have limited benefit from systemic chemo- and targeted therapy. The CALGB/SWOG 80405 study demonstrated that KRAS wild-type metastatic right-sided colon cancers had a shorter median OS than metastatic left-sided colon cancers (19.4 months vs. 33.3 months, hazard ratio [HR] = 1.55, 95% confidence interval [CI] = 1.32-1.82, P < .0001). In those who received bevacizumab, OS was 24.2 months for right-sided mCRCs and 31.4 months for left-sided mCRCs (HR = 1.32, 95% CI = 1.05-1.65, P = .01), whereas OS was 16.7 months for right-sided tumors and 36 months for left-sided tumors treated with cetuximab (HR = 1.87, 95% CI = 1.48-2.32, P < .0001). In addition, bevacizumab showed better outcomes than cetuximab in right-sided tumors regardless of KRAS status. On the contrary, KRAS wild-type left-sided mCRCs had more benefit from cetuximab than bevacizumab [13].
KRAS mutations have been clearly identified as a predictor marker for response to anti-EGFR MoAbs in patients with mCRC, and anti-EGFR MoAbs are therefore exclusively administered for KRAS wild-type tumors [14], [15], [16], [17], [18], [19], [20]. Although EGFR expression may be a negative prognostic factor in CRC after curative resection and associated with resistance to chemotherapy [21], [22], [23], [24], increased EGFR gene copy number has a prominent correlation with prognosis in mCRC treated with first-line anti-EGFR MoAbs [25], [26], [27], [28]. Primary or acquired resistance to anti-EGFR MoAbs may manifest in previously treated mCRC with mutations in EGFR downstream signaling pathways: PI3K/Akt/mTOR and Ras/Raf/MEK/ERK, which regorafenib targets. The roles of EGFR expression and KRAS status in mCRC previously treated with chemotherapy and anti-EGFR or anti-VEGF MoAbs remain unclear. Thus, the present study aimed to analyze the prognostic value of EGFR expression, KRAS mutation, and tumor sidedness in patients with mCRC treated with regorafenib and FOLFIRI as a third- or fourth-line setting.
Materials and Methods
Participants
This retrospective study recruited 41 patients with progressing mCRC who were previously treated with FOLFOX, FOLFIRI, anti-VEGF MoAb, and anti-EGFR MoAb if KRAS wild-type tumors were identified, between October 2013 and June 2018 in a single institute. The protocol was approved by the institutional ethics committees and conducted in accordance with the 1964 Declaration of Helsinki (2008 revision). The study was registered at clinicaltrials.gov under the identification code ID NCT03698253 (www.clinicaltrials.gov/ct2/show/NCT03698253) and the trial name Metastatic Colorectal Cancer Treated With Regorafenib and FOLFIRI.
Immunohistochemical Analysis for EGFR Expression
Formalin-fixed and paraffin-embedded tissue blocks were cut into 3-μm sections and deparaffinized, rehydrated, and autoclaved at 121°C for 5 minutes in Target Retrieval solution (Dako, Glostrup, Denmark) with pH 6.0 to retrieve antigens. Endogenous peroxidase was blocked using 3% hydrogen peroxide for 5 minutes at room temperature. After the sections were washed with a Tris buffer solution, they were incubated with EGFR for 1 hour at room temperature. Then, the DAKO REAL EnVision Detection System-HRP (DAKO, Glostrup, Denmark) was applied for 30 minutes at room temperature. Finally, the sections were incubated in 3′,3-diaminobenzidine for 5 minutes followed by counterstaining with Mayer's hematoxylin. Dehydration was performed through two changes of 95% ethanol and two changes of 100% ethanol, and the samples were cleared in three changes of xylene and subsequently mounted. Negative controls were obtained by replacing the primary antibody with nonimmune serum. EGFR immunoreactivity was evaluated by two independent researchers who were blind to patient outcome. The expression patterns of EGFR were determined in a semiquantitative manner through light microscopy. EGFR immunoreactivity (membrane staining) was categorized according to the presence of tumor cell staining and staining intensity. The intensity of EGFR immunoreactivity was scored using a three-tier system as follows [29]: 1+ (weak intensity), 2+ (moderate intensity), and 3+ (strong intensity). A negative EGFR expression was defined as the absence of membrane staining above the background in all tumor cells, whereas a positive EGFR expression was defined as complete or incomplete immunohistochemical membrane staining of tumor cells with intensities of 1+, 2+, or 3+.
DNA Extraction and Direct Sequencing of KRAS
Genomic DNA was isolated from frozen primary CRC tissues through proteinase-K (Stratagene, La Jolla, CA) digestion and phenol/chloroform extraction. The designed sequences of oligonucleotide primers for exons 2 and 3 of KRAS and the operational procedure of direct sequencing were based on those of our previous study [30].
Study Design
For each patient, regorafenib and FOLFIRI with dose-adjusted irinotecan according to UGT1A1 genotyping were administered. Because grade ≥3 hand-foot syndrome developed frequently in patients receiving oral regorafenib at 160 mg/day (21 days at a 7-day interval), the dose was adjusted to 120 mg/day. If grade ≥3 regorafenib-induced AEs such as hand-foot syndrome still developed, regorafenib was discontinued until the AEs subsided. Furthermore, according to our previous clinical results [9], patients with UGT1A1*1/*1 and *1/*28 genotypes were initially given a standard dose of 180 mg/m2 irinotecan, and those with the UGT1A1*28/*28 genotype were given 120 mg/m2 of irinotecan. Irinotecan was administered for over 2 hours on day 1 followed by 5-FU (2800 mg/m2 intravenously infused for over 46 hours in a 2-week cycle). For all the patients, the irinotecan dose was increased by 30 mg/m2 up to a maximal dose of 260 mg/m2 every two cycles until grade ≥3 AEs or severe AEs (SAEs) of irinotecan developed, following which the dose was reverted to and maintained at the previously tolerated level.
The treatment response was radiologically assessed every 2 months through computed tomography, magnetic resonance imaging, or positron emission tomography. Objective responses were classified according to the Response Evaluation Criteria in Solid Tumors [31], and optimal treatment responses were recorded. Common Terminology Criteria for Adverse Events version 3.0 [32] was used for evaluating treatment-associated AEs. Treatment with regorafenib plus dose-escalated irinotecan was stopped if progressive disease or grade ≥3 AEs occurred.
Statistical Analysis
Categorical data were compared using the Mann-Whitney U test or Kruskal-Wallis test. The Fisher's exact test was used to compare dichotomous variables, and the Pearson chi-square test was used to analyze nominal variables. The means were compared using the two-sample test and analysis of variance or linear regression, as appropriate. However, for all aforementioned inferential analysis methods, the center effect was not considered in comparing the two treatments. Therefore, analysis of variance incorporating the center effect and Cochran-Mantel-Haenszel test stratified by the center effect were applied to replace the two-sample t test and Fisher's exact test. For efficacy analyses and part of the safety analyses (including laboratory data and vital sign data), considering the effect of baseline data on the endpoints, analysis of covariance was applied to compare the mean of one treatment with that of another, with their respective baseline values as covariates. PFS and OS were calculated using the Kaplan-Meier method. PFS was defined as the time from treatment initiation until the first radiological evidence of progression, whereas OS was defined as the time from treatment initiation until death from any cause. Statistical analyses were conducted using SPSS 20.0 (SPSS, Chicago, IL). A P value less than .05 was considered statistically significant.
Results
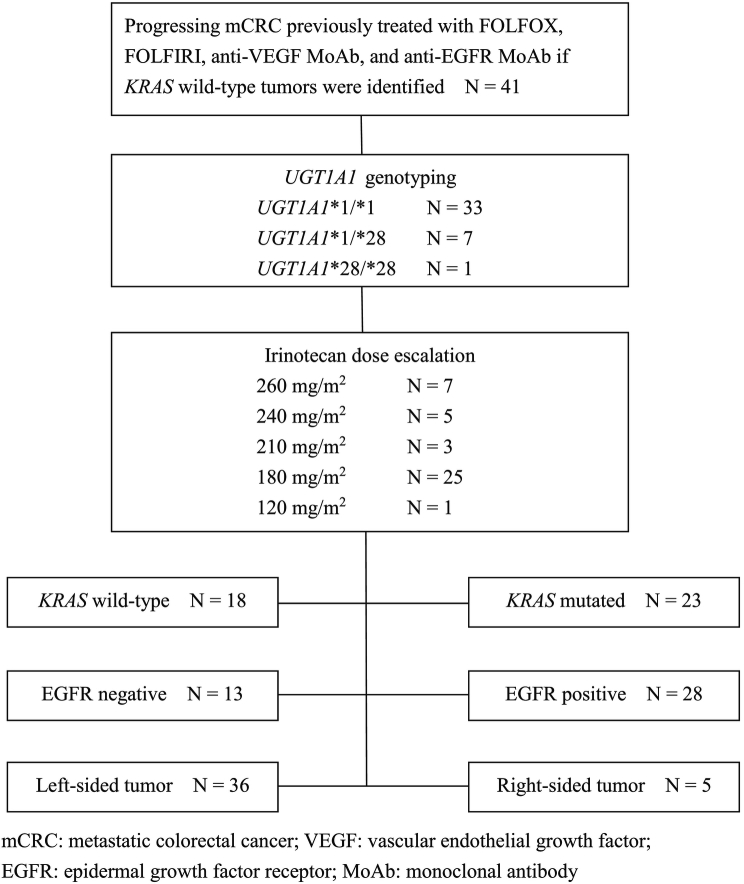
The study scheme is shown in Figure 1. In summary, the median follow-up period was 10.0 months (1.3-23.5 months). The overall disease control rate was 58.5%, whereas the median PFS and OS were 6.0 months and 12.0 months, respectively. KRAS mutations were significantly associated with positive EGFR expression (P = .026). KRAS mutations significantly correlated with a shorter OS than KRAS wild-type (P = .014) but had no significant association with PFS (P = .117). Positive EGFR expression had an inverse correlation with PFS (P = .027) and OS (P = .021). Moreover, left-sided tumors associated with superior PFS (P < .0001) and OS (P < .0001), and tumor sidedness was an independent prognostic factor by the multivariate analysis.
Figure 1.
The study scheme of the present study.
Demographic data of the studied patients are summarized in Table 1. A total of 41 patients were enrolled in the present study, with a median age of 61 years (36-85 years) and male predominance (61.0% vs. 39.0%). The most common site of metastasis was the liver (69.5%) followed by the lung (56.1%), peritoneum (17.1%), brain (2.4%), and neck lymph nodes (2.4%). Of the 41 patients, 17 presented two or more sites of metastasis (41.5%), and the remaining patients presented a single site of metastasis. KRAS mutation was found in 23 patients (56.1%), and positive EGFR expression was noted in 28 patients (68.3%). The number of UGT1A1*1/*1 genotypes was overwhelming in patients with the highest administered irinotecan dose of 260 mg/m2 (180-260 mg/m2). The corresponding irinotecan dose was 120 mg/m2 for the only patient with the UGT1A1*28/*28 genotype. All the patients with KRAS mutated type CRC were previously treated with bevacizumab and FOLFIRI (standard irinotecan dose of 180 mg/m2) in the first-line setting, followed by FOLFOX6 regimen. For patients with KRAS wild-type tumors, cetuximab plus FOLFORI was often first administered in patients with KRAS wild-type tumors; this was followed by FOLFOX6 and becacizumab plus FOLFORI in the third-line setting (irinotecan dose was 180 mg/m2). An additional FOLFOXIRI regimen was administered to two of these KRAS wild-type patients before the reimbursement of regorafenib by Taiwan National Health Insurance as the fourth-line treatment. Consequently, regorafenib plus FOLFIRI with dose-escalated irinotecan served as the third-line salvage treatment for KRAS mutated type tumors and the fourth- or fifth-line treatment for KRAS wild-type tumors. All the treatments were substituted only when diseases progressed or patients were intolerant.
Table 1.
Demographic Data of Study Patients
| Clinical Characteristics | Number of Cases (%) |
|---|---|
| Sex | |
| Male | 25 (61.0) |
| Female | 16 (39.0) |
| Median age (years) | 61.0 (36-85) |
| Site of metastasis | |
| Liver | 27 (65.9) |
| Lung | 23 (56.1) |
| Peritoneum | 7 (17.1) |
| Brain | 1 (2.4) |
| Neck lymph nodes | 1(2.4) |
| Number of sites of metastasis | |
| 1 | 24 (58.5) |
| 2 | 17 (41.5) |
| KRAS status | |
| Wild type | 18 (43.9) |
| Mutated | 23 (56.1) |
| EGFR status | |
| Positive | 28 (68.3) |
| Negative | 13 (31.7) |
| Tumor sidedness | |
| Left sided | 36 (87.8) |
| Right sided | 5 (12.2) |
| UGT1A1 status | |
| *1/*1 | 33 (80.5) |
| *1/*28 | 7 (17.1) |
| *28/*28 | 1 (2.4) |
| Irinotecan dose (mg/m2) | |
| 260 | 7 (17.1) |
| 240 | 5 (12.2) |
| 210 | 3 (7.3) |
| 180 | 25 (61.0) |
| 120 | 1 (2.4) |
| Lines of systemic therapy | |
| 3rd | 23 (56.1) |
| 4th | 16 (39.0) |
| 5th | 2 (4.9) |
| ≥Grade 3 AEs | |
| Hand-foot skin reaction | 13 (31.7) |
| Mucositis | 7 (17.1) |
| Neutropenia | 4 (18.2) |
| Diarrhea | 5 (12.2) |
| Fatigue | 3 (7.3) |
| Best objective response | |
| PR | 4 (9.8) |
| SD | 20 (48.8) |
| PD | 17 (41.5) |
| DCR | 24 (58.5) |
EGFR, epidermal growth factor receptor; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; AEs, adverse events.
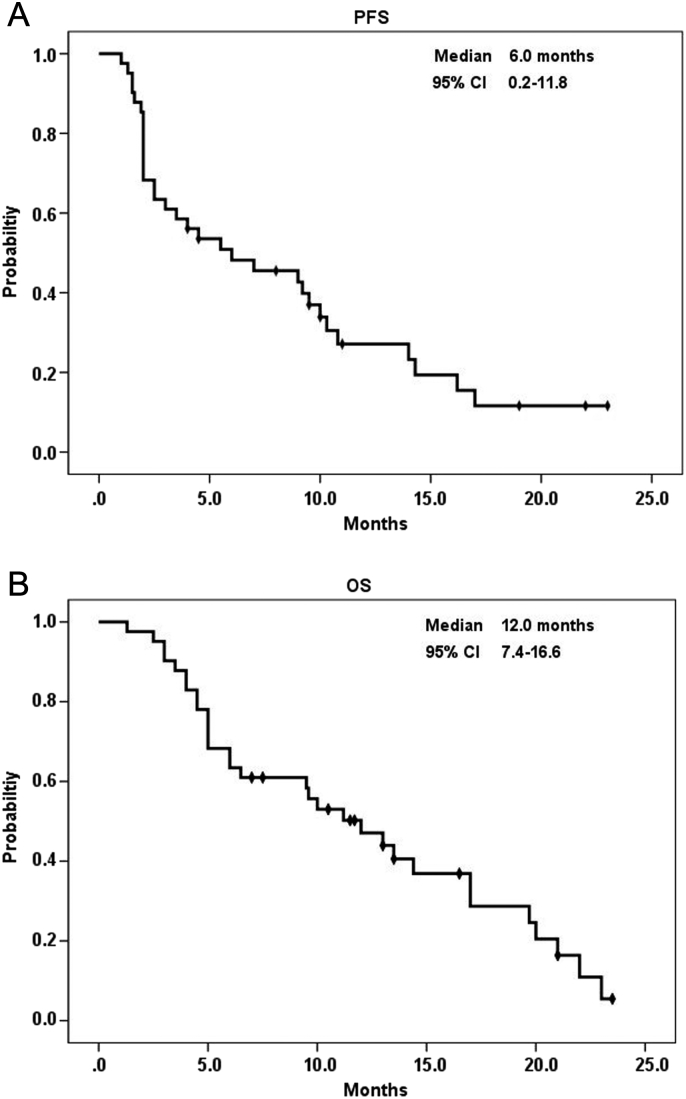
The median follow-up period was 10.0 months (1.3-23.5 months). The patients were followed up until June 2018 or their death. The median duration of regorafenib treatment was 6.0 months (1.3-23.5 months). The most frequently encountered grade ≥3 AE was HFSR (n = 13, 31.7%), followed by mucositis (n = 7, 17.1%), neutropenia (n = 4, 18.2%), diarrhea (n = 5, 12.2%), and fatigue (n = 3, 7.3%). The majority of the patients had stable disease (SD) (n = 20, 48.8%), 4 patients (9.8%) had partial response (PR), and 17 (41.5%) had progressive disease (PD), with an overall DCR of 58.5%. The only patient with the UGT1A1*28/*28 genotype and positive EGFR expression with KRAS mutated cancer received only one cycle of regorafenib plus the initial irinotecan dose of 120 mg/m2; however, she developed grade 3 mucositis and subsequently died. The overall median PFS and OS were 6.0 months (95% CI: 0.2-11.8) and 12.0 months (95% CI: 7.4-16.6), respectively (Figure 2).
Figure 2.
Kaplan-Meier survival analysis of all patients with metastatic colorectal cancer. (A) Progression-free survival and (B) overall survival.
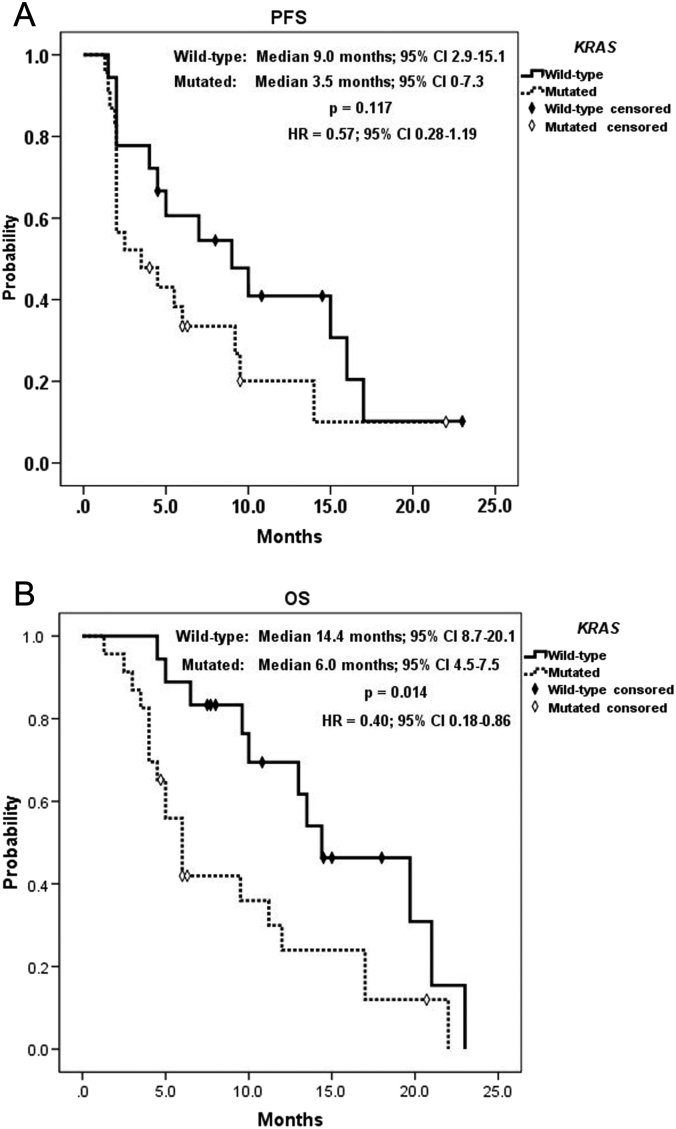
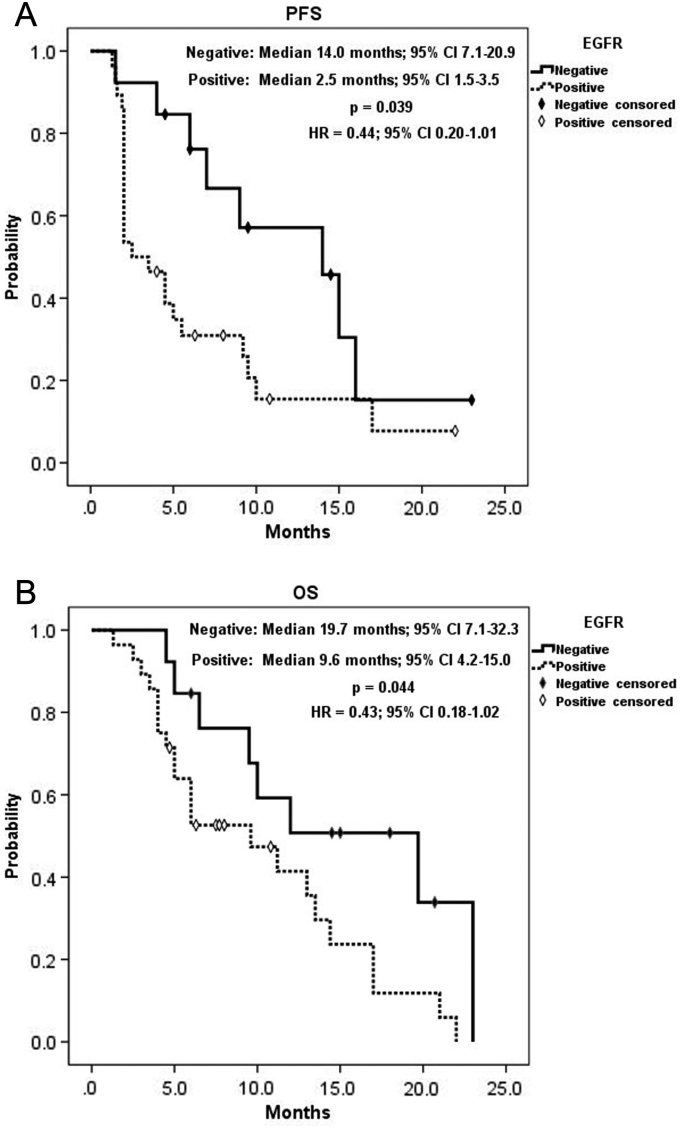
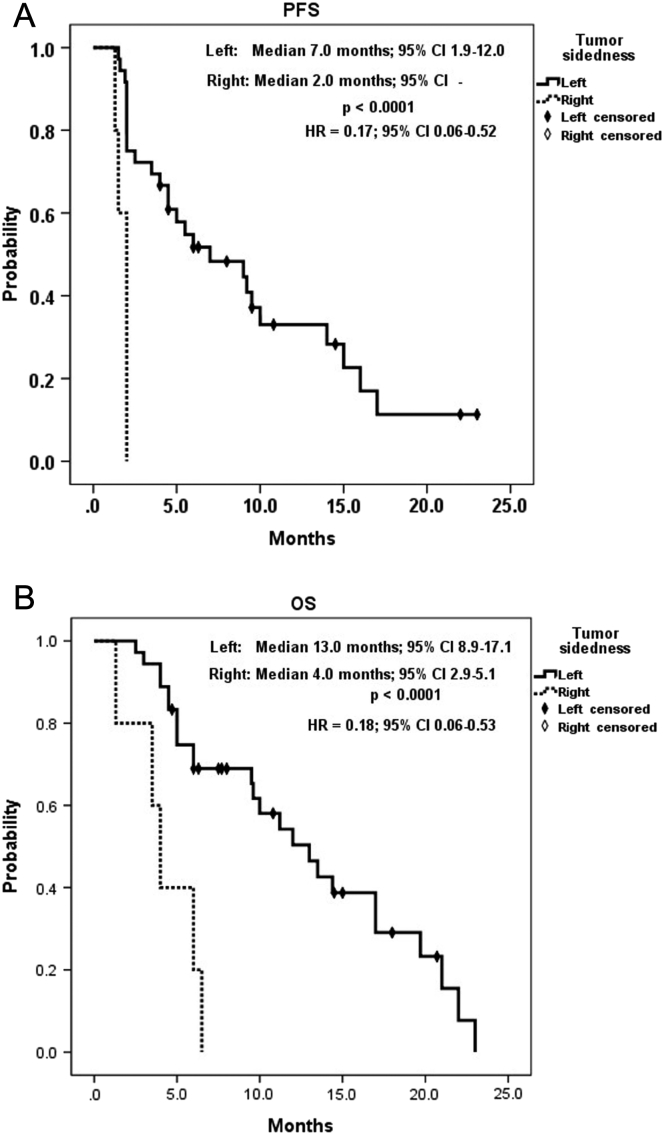
A summary of KRAS status analysis is shown in Table 2. KRAS mutation had a significant correlation with positive EGFR expression (P = .026) and significant inverse correlation with grade ≥3 AEs (P = .019). By contrast, as listed in Table 3, positive EGFR expression was significantly associated with the poorer best objective response (P = .027) and DCR (P = .021). Table 4 shows a summary of analysis for tumor sidedness. Difference of UGT1A1 genotype was associated significantly with tumor sidedness, that the only UGT1A1*28/*28 genotype presented at right-sided tumor, whereas all UGT1A1*1/*28 genotypes located at left-sided tumors (P = .017). Right-sided tumors had significantly worse response (P = .018), and all right-sided tumors were considered progressive disease. Consequently, right-sided tumors had zero DCR compared to 66.7% in left-sided ones (P = .008). KRAS wild-type mCRC was significantly correlated with a longer OS (14.4 vs. 6.0 months, 95% CI: 8.7-20.1 vs. 4.5-7.5, P = .014; HR = 0.40, 95% CI: 0.18-0.86) but had no correlation with PFS (9.0 vs. 3.5 months, 95% CI: 2.9-15.1 vs. 0-7.3, P = .117; HR = 0.57, 95% CI: 0.28-1.19, Figure 3). Moreover, negative EGFR expression had a significant positive effect on both PFS (14.0vs.2.5 months, 95% CI: 7.1-20.9 vs. 1.5-3.5, P = .039; HR = 0.44, 95% CI: 0.20-1.01) and OS (19.7 vs. 9.6 months, 95% CI: 7.1-32.3 vs. 4.2-15.0, P = .044; HR = 0.43, 95% CI: 0.18-1.02, Figure 4). Of utmost important, left-sided tumors were associated with a superior PFS (7.0 vs. 2.0 months, 95% CI: 1.9-12.0 vs. -, P < .0001; HR = 0.17, 95% CI: 0.06-0.52) and OS (13.0 vs. 4.0 months, 95% CI: 2.9-5.1 vs. 8.9-17.1, P < .0001; HR = 0.18, 95% CI: 0.06-0.53) to right-sided tumors as well (Figure 5). Furthermore, tumor sidedness remained an independent prognostic factor in a multivariate analysis adjusted with KRAS and EGFR status for PFS (P = .010, HR = 0.22, 95% CI: 0.07-0.69) and OS (P = .004 HR = 0.20, 95% CI: 0.07-0.59; Table 5, Table 6).
Table 2.
KRAS Status Subgroup Analysis of the Clinical Features of Study Patients
|
KRAS Wild Type (N = 18) |
KRAS Mutated (N = 23) |
P | |
|---|---|---|---|
| Sex, N (%) | .987 | ||
| Male | 11 (61.1) | 14 (60.9) | |
| Female | 7 (38.9) | 9 (30.1) | |
| Median age (range), years | 63 (36-85) | 61 (37-82) | .651 |
| EGFR status, N (%) | .026 | ||
| Positive | 9 (50.0) | 19 (82.6) | |
| Negative | 9 (50.0) | 4 (17.4) | |
| Tumor sidedness | .363 | ||
| Left side | 17 (94.4) | 19 (82.6) | |
| Right side | 1 (5.6) | 4 (17.4) | |
| UGT1A1 status | .421 | ||
| *1/*1 | 16 (88.9) | 17 (73.9) | |
| *1/*28 | 2 (11.1) | 5 (21.7) | |
| *28/*28 | 0 (0) | 1 (4.3) | |
| Irinotecan dose, N (%), mg/m2 | .375 | ||
| 260 | 5 (27.8) | 2 (8.7) | |
| 240 | 3 (16.7) | 2 (8.7) | |
| 210 | 1 (5.6) | 2 (8.7) | |
| 180 | 9 (50.0) | 16 (69.6) | |
| 120 | 0 (0.0) | 1 (4.3) | |
| Best objective response, N (%) | .190 | ||
| PR | 3 (16.7) | 1 (4.3) | |
| SD | 10 (55.6) | 10 (43.5) | |
| PD | 5 (27.8) | 12 (52.2) | |
| DCR, N (%) | 13 (72.2) | 11 (47.8) | .116 |
| AEs | .019 | ||
| Grade ≧3 | 15 (83.3) | 11 (47.8) | |
| Grade <3 | 3 (16.7) | 12 (52.2) |
EGFR, epidermal growth factor receptor; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; AEs, adverse events.
Table 3.
EGFR Status Subgroup Analysis of the Clinical Features of Study Patients
| EGFR Negative (N = 13) |
EGFR Positive (N = 28) |
P | |
|---|---|---|---|
| Sex, N (%) | .460 | ||
| Male | 9 (69.2) | 16 (57.1) | |
| Female | 4 (30.8) | 12 (42.9) | |
| Median age (range), years | 66 (51-85) | 57 (36-82) | .147 |
| KRAS status, N (%) | .026 | ||
| Wild type | 9 (69.2) | 9 (32.1) | |
| Mutated | 4 (30.8) | 19 (67.9) | |
| Tumor sidedness | 1.000 | ||
| Left side | 12 (92.3) | 24 (85.7) | |
| Right side | 1 (7.7) | 4 (14.3) | |
| UGT1A1 status | .765 | ||
| 6/6 | 11 (84.6) | 22 (78.6) | |
| 6/7 | 2 (15.4) | 5 (17.9) | |
| 7/7 | 0 (0.0) | 1 (3.6) | |
| Irinotecan dose, N (%), mg/m2 | .880 | ||
| 260 | 3 (23.1) | 4 (14.3) | |
| 240 | 2 (15.4) | 3 (10.7) | |
| 210 | 1 (7.7) | 2 (7.1) | |
| 180 | 7 (53.8) | 18 (64.3) | |
| 120 | 0 (0.0) | 1 (3.6) | |
| Best objective response, N (%) | .027 | ||
| PR | 3 (23.1) | 1 (3.6) | |
| SD | 8 (61.5) | 12 (42.9) | |
| PD | 2 (15.4) | 15 (53.6) | |
| DCR, N (%) | 11 (84.6) | 13 (46.4) | .021 |
| AEs | .055 | ||
| Grade ≧3 | 11 (84.6) | 15 (46.4) | |
| Grade <3 | 2 (15.4) | 13 (53.6) |
EGFR, epidermal growth factor receptor; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; AEs, adverse events.
Table 4.
Tumor Sidedness Subgroup Analysis of the Clinical Features of Study Patients
| Left side (N = 36) |
Right side (N = 5) |
P | |
|---|---|---|---|
| Sex, N (%) | .362 | ||
| Male | 23 (63.9) | 2 (40.0) | |
| Female | 13 (36.1) | 3 (60.0) | |
| Median age (range), years | 60.5 (36-85) | 64.0 (40-72) | .311 |
| KRAS status, N (%) | .363 | ||
| Wild-type | 17 (47.2) | 1 (20.0) | |
| Mutated | 19 (52.8) | 4 (80.0) | |
| EGFR status, N (%) | 1.000 | ||
| Positive | 24 (66.7) | 4 (80.0) | |
| Negative | 12 (33.3) | 1 (20.0) | |
| UGT1A1 status | .017 | ||
| *1/*1 | 29 (80.6) | 4 (80.0) | |
| *1/*28 | 7 (19.4) | 0 (0) | |
| *28/*28 | 0 (0) | 1 (20.0) | |
| Irinotecan dose, N (%), mg/m2 | .064 | ||
| 260 | 7 (19.4) | 0 (0) | |
| 240 | 4 (11.1) | 1 (20.0) | |
| 210 | 3 (8.3) | 0 (0) | |
| 180 | 22 (61.1) | 3 (60.0) | |
| 120 | 0 (0.0) | 1 (20.0) | |
| Best objective response, N (%) | .018 | ||
| PR | 4 (11.1) | 0 (0) | |
| SD | 20 (55.6) | 0 (0) | |
| PD | 12 (33.3) | 5 (100.0) | |
| DCR, N (%) | 24 (66.7) | 0 (0) | .008 |
| AEs | .336 | ||
| ≧ grade 3 | 24 (66.7) | 2 (40.0) | |
| < grade 3 | 12 (33.3) | 3 (60.0) |
ERFR: epidermal growth factor receptor; PR: partial response; SD: stable disease; PD: progressive disease; DCR: disease control rate; AEs: adverse events.
Figure 3.
Kaplan-Meier survival analysis of KRAS subgroups. (A) Progression-free survival and (B) overall survival.
Figure 4.
Kaplan-Meier survival analysis of EGFR expression subgroups. (A) Progression-free survival and (B) overall survival.
Figure 5.
Kaplan-Meier survival analysis of tumor sidedness subgroups. (A) Progression-free survival and (B) overall survival.
Table 5.
Univariate and Multivariate Progression-free Survival Analysis
| Variable | Univariate (HR) | P | 95% CI | Multivariate (HR) | P | 95% CI |
|---|---|---|---|---|---|---|
| Tumor sidedness (left-sided) | 0.17 | .002 | 0.06-0.52 | 0.22 | .010 | 0.07-0.69 |
| KRAS (wild-type) | 0.57 | .136 | 0.28-1.19 | 0.80 | .591 | 0.36-1.78 |
| EGFR (negative) | 0.44 | .052 | 0.20-1.01 | 0.52 | .144 | 0.22-1.25 |
HR: hazard ratio; CI: confidence interval; ERFR: epidermal growth factor receptor.
Table 6.
Univariate and Multivariate Overall Survival Analysis
| Variable | Univariate (HR) | P | 95% CI | Multivariate (HR) | P | 95% CI |
|---|---|---|---|---|---|---|
| Tumor sidedness (left-sided) | 0.18 | .002 | 0.06-0.53 | 0.20 | .004 | 0.07-0.59 |
| KRAS (wild-type) | 0.40 | .020 | 0.18-0.86 | 0.51 | .117 | 0.22-1.18 |
| EGFR (negative) | 0.43 | .054 | 0.18-1.02 | 0.54 | .182 | 0.22-1.34 |
HR: hazard ratio; CI: confidence interval; ERFR: epidermal growth factor receptor.
Discussion
Irinotecan must be converted by a carboxylesterase to SN-38, which is actively cytotoxic and detoxified by the glucuronidation activity of UGT, primarily by the UGT1A1 isoenzyme; consequently, the UGT1A1 genotype represents the development of drug-associated AEs. Because of the decreased capacity of the UGT1A1*28 allele to metabolize SN-38, patients with homozygous UGT1A1*28/*28 are more vulnerable to irinotecan; thus, a low initial dose of irinotecan should be considered. A genotype-directed dose-determination study on irinotecan dose escalation in first-line FOLFIRI for mCRC conducted by Marcuello et al. reported that homozygous UGT1A1*28/*28 genotype patients developed irinotecan-associated SAEs more frequently [33]. Patients with heterozygous polymorphism of the UGT1A1 promoter (i.e., UGT1A1*1/*28) undergo intermediate glucuronidation activity of UGT1A1 and are considered to be at an increased risk of irinotecan toxicity. On the contrary, clinical presentations in patients with UGT1A1*1/*28 vary individually, but these patients generally tolerate the recommended initial irinotecan dose of 180 mg/m2 [34]. However, patients with the homozygous UGT1A1*1/*1 genotype are more resistant to irinotecan-associated AEs and could tolerate an irinotecan dose as high as 260 mg/m2 in previous observational studies [12], [35]. As the result, for patient safety and treatment efficacy of irinotecan dose-escalated FOLFIRI, UGT1A1 genotyping was carried out before treatment was initiated. In the present and aforementioned studies, an initial irinotecan dose of 180 mg/m2 for the UGT1A1*1/*1 and UGT1A1*1/*28 and 120 mg/m2 for the UGT1A1*28/*28 genotypes was infused and subsequently increased by 30 mg/m2 every two cycles. The irinotecan dose was reverted to the last tolerated dose if any irinotecan-related grade ≥3 AEs or SAEs developed. The final dose of irinotecan could be escalated to 210-260 mg/m2 in 36.6% of the treated patients, and 61.0% of the patients discontinued irinotecan use at an initial dose of 180 mg/m2 because of disease progression and received a substitute treatment. In the present study, the only patient with homozygous UGT1A1*28/*28 tolerated an initial irinotecan dose of 120 mg/m2; however, grade 3 mucositis developed in this patient and might be associated with regorafenib. The irinotecan-associated grade ≥3 AEs that were encountered, such as neutropenia (18.2%) and diarrhea (12.2%), were acceptable; however, Li et al. revealed that the first-line fixed irinotecan dose of 180 mg/m2 for mCRC resulted in a higher incidence of severe diarrhea in patients with single-allele or two-allele variants of UGT1A1*6/*28, without differences in severe neutropenia in patients with different alleles [36]. The UGT1A1*6 allele, with a higher incidence in Asian populations, should be analyzed in a future study in addition to UGT1A1*1 and *28 alleles that may further reduce irinotecan-related AEs through dose adjustment.
Regorafenib is a small molecule that targets and blocks RTKs, including EGFR, VEGF receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor, RET, and KIT, which participate in oncogenesis, angiogenesis, and cell proliferation in the tumor microenvironment, consequently exhibiting antineoplastic capacity [3]. Because regorafenib inhibits multiple pathways, it induces various AEs, including HFSR, hypertension, thrombocytopenia, gastrointestinal bleeding, proteinuria, diarrhea, mucositis, and hepatoxicity. HFSR is the most commonly encountered grade ≥3 AE associated with regorafenib, and it leads to dose reduction or interruption of treatment [4], which may impair the efficacy of regorafenib. Moreover, the incidence of HFSR is more frequent in the Asian population [37], and modification of the dosing schedule from 160 mg once daily for 3 weeks in a 4-week cycle to 120 mg daily for 4 weeks with the same accumulated dose of 3360 mg in a single cycle still yielded high rates of grade 3 HFSR, which recovered earlier than with the original dosing schedule though [12]. A dose reduction from 160 to 120 mg for 3 weeks in a 4-week cycle greatly reduced the occurrence of grade 3 HFSR (75% vs. 16.7%) with comparable DCR (60% vs. 58.3%) in a Japanese study [6]. The incidence of grade 3 HFSR was 31.7%, which generally recovered within 2 weeks after regorafenib stopped, with a dosing schedule of 120 mg daily for 4 weeks combined with FOLFIRI with dose-escalated irinotecan, and the oncological outcomes were favorable. Consequently, adjustment of the irinotecan dose based on UGT1A1 genotyping optimizes the oncological efficacy with balanced toxicities for individuals, and reintroduction of irinotecan with dose escalation may have a synergic effect on regorafenib. Moreover, in a preclinical xenograft study on regorafenib and the potential combination therapy, a combination of regorafeniband irinotecan significantly delayed tumor growth after extended treatment in four xenograft models [38]. Consequently, we intend to conduct a multicenter, 2:1 randomized, controlled clinical trial with two parallel arms to compare regorafenib monotherapy and regorafenib combined with FOLFIRI with dose-escalated irinotecan for previously treated mCRC according to the current results.
KRAS mutation has been well documented as a predictor of tumor resistance to anti-EGFR MoAbs, and anti-EGFR MoAbs are recommended to be administered restrictively for KRAS wild-type mCRC [19], [20]. However, the responses to anti-EGFR MoAbs are variable in KRAS wild-type cancers. Alternations of EGFR and the downstream effectors of EGFR-activated PI3K/Akt/mTOR and Ras/Raf/MEK/ERK pathways contribute to such heterogeneous clinical outcomes. KRAS, NRAS, and BRAF mutations are associated with primary resistance to anti-EGFR MoAbs and are negative prognostic factors [39], [40], [41]. PI3KCA gene mutations play roles in refractory activities; moreover, PIK3CA mutations are significantly correlated with a low response rate to cetuximab plus chemotherapy [42], [43]. In addition, increased EGFR copy number [25], [27], [28], expression of EGFR ligands (amphiregulin, heparin-binding epidermal growth factor, transforming growth factor-alpha, and epiregulin) [26], [44], [45], and expression of phosphatase and tensin homologue (PTEN) [25], [46], [47], which is a downstream inhibitor of the EGFR pathway, are associated with more favorable response and survival rates, whereas HER2/MET overexpression is associated with poorer response and survival in anti-EGFR MoAb therapy [48], [49], [50]. However, most of the aforementioned studies have been conducted in the setting of anti-EGFR MoAbs as first- or second-line treatments, and investigations of biomarkers and regorafenib in anti-EGFR MoAbs refractory cases are rare. The multikinase inhibitory capacities of regorafenib overcome the molecular heterogeneity of the EGFR pathway and salvage those who developed primary or acquired resistance to anti-EGFR MoAbs. In the present study, positive EGFR expression correlated significantly to poorer DCR, PFS, and OS, and EGFR expression was a negative prognostic factor, similar to the role of EGFR expression in CRC not treated with anti-EGFR MoAbs. Moreover, similar PFS and OS in EGFR-positive subgroup with regorafenib monotherapy in CORRECT study reflected poor response to FOLFIRI and limited oncological benefit from FOLFIRI for EGFR-positive tumors. Consistent with the REBECCA study [51], unfavorable OS was significantly associated with KRAS mutations, with no relationship between PFS and KRAS mutations in the present study. Notably, patients with KRAS wild-type tumors presented a higher incidence of grade ≥3 AEs; this finding may be a clinical predictor for survival. EGFR expression with primary or acquired resistance to anti-EGFR MoAbs, in which EGFR-activated PI3K/Akt/mTOR and Ras/Raf/MEK/ERK pathways are not blocked but activated instead, is contributory to response differences in EGFR subgroup. On the other hand, presence of KRAS mutations indicates permanent “turned on” of Ras/Raf/MEK/ERK pathway. Regorafenib targets receptor tyrosine kinases (e.g., vascular endothelial growth factor receptor [VEGFR], platelet-derived growth factor receptor-β [PDGFR-β], fibroblast growth factor receptor [FGFR], and tyrosine kinase with immunoglobulin and epidermal growth factor homology domain [TIE2]) as well as intracellular kinases RAF1 and BRAF. The last two (ie. RAF1 and BRAF) are downstream effectors of RAS and regorafenib therefore blocks Ras/Raf/MEK/ERK pathway with KRAS mutations that results in similar PFS in the KRAS status subgroup though, options of treatments after disease relapse are limited in tumors with KRAS mutations and lead to unfavorable OS consequently.
CRC is a heterogenous disease that right-sided colon differs from left-sided colon and rectum not only in embryological origin and anatomic location and blood supply but also in molecular and morphological alternations. According to the Consensus Molecular Subtype (CMS) Consortium, CMS1 (microsatellite instability [MSI]-immune) tumors were frequently diagnosed in females with right-sided tumors and accounted for 14% of CRC [52]. CMS1 tumors are highlighted by hypermutated, defective DNA mismatch repair, MSI, MLH1 silencing by promoter hypermethylation, CpG island methylation phenotype (CIMP), high BRAF V600E mutation rate, and immune infiltration in the tumor microenvironment. CMS1 tumors were associated with worse survival after relapse, consistent with studies exhibiting poor prognosis in mCRC with MSI and BRAF V600E mutation [53], [54], [55]. Immune checkpoint blockage therapy with immune checkpoint inhibitors to modulate immnogenesity of CMS1 tumors is recently introduced [56], [57]. On the other hand, CMS2 (canonical) subtype was predominant in left-sided lesions [52], [58]. CMS2 tumors are characterized by high frequency of DNA somatic copy number alternation (SCNA), typically initial loss of tumor suppressor APC gene followed by an activating mutation of KRAS and loss of TP53, which in turn activate WNT and MYC pathway. CMS2 subtype of CRC arises from canonical adenoma-carcinoma sequence and had superior survival after relapse and larger proportion of long-term survivors [52]. The prospective, observational CORRELATE study, in which patients with mCRC were treated with regorafenib monotherapy, demonstrated that tumor sidedness had no significant differences in both median PFS (2.8 months, 95% CI: 2.6-2.9 for left-sided tumors vs. 2.7 months, 95% CI: 2.5-3.1 for right-sided tumors) and median OS (7.4 months, 95% CI: 6.7-8.0 for left-sided tumors vs.8.2 months, 95% CI: 6.6-9.3 for right-sided tumors) [59]. In comparison to the present study, regorafenib plus irinotecan dose-escalated FOLFIRI had similar outcomes in right-sided mCRCs, but PFS and OS were much better in left-sided tumors. This finding corresponded to left-sided predominant CMS2 tumors having better survival after relapse. In other words, systemic chemotherapy brought more therapeutic benefits for left-sided or CMS2 mCRCs. Nevertheless, tumor sidedness is still debated and rough for prognosis. More specific and detailed biomarkers and genetic profiles, instead of tumor sidedness, are necessary for analysis of prognostic impact.
According to our review of the relevant literature, this is one of the few studies to evaluate the prognostic values of EGFR expression, KRAS mutations, and tumor sidedness in patients with previously treated mCRC receiving salvage regorafenib plus FOLFIRI with dose-escalated irinotecan. The present study had some limitations. First, this was a retrospective, observational study with a relatively small sample size. We intend to conduct a prospective, randomized large-scale study to confirm the present study findings. Furthermore, extended RAS mutations, gene expression profiling for classification into CMS groups, and other potential regorafenib-related biomarkers should be included in future analyses.
Conclusions
The administration of regorafenib and concomitant reintroduction of FOLFIRI with dose-escalated irinotecan according to UGT1A1 genotyping are clinically feasible and result in tolerable toxicities and favorable oncological outcomes in previously heavily treated mCRC. Positive EGFR expression has a negative effect on response to treatment as well as PFS and OS. KRAS mutations are associated with a poor OS and lower incidence of grade ≥3 AEs, which may serve as a clinical predictor for survival. Tumor sidedness is an independent prognostic factor for both PFS and OS. However, these findings must be validated by a further large-scaled randomized trial to address the roles of EGFR expression, KRAS mutation status, and tumor sidedness in the relevant regorafenib combination therapy.
Acknowledgments
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Conflict of Interests
The authors declare no conflict of interest.
Footnotes
Funding: This work was supported by grants through funding from the Ministry of Science and Technology, Taiwan (MOST107-2321-B-037-003, MOST107-2314-B-037-116, MOST107-2314-B-037-022-MY2, MOST107-2314-B-037-023-MY2), the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123006, MOHW107-TDU-B-212-114026B funded by Health and welfare surcharge of tobacco products), the Kaohsiung Medical Hospital, Taiwan (KMUH102-2M23, KMUH106-6R32, KMUH106-6M28, KMUH106-6M29, KMUH106-6M30, KMUH106-6M31, KMUHS10701, KMUHS10710), and the Kaohsiung Municipal Ta-Tung Hospital, Taiwan (KMTTH104-023). In addition, this study was supported by the grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan, R.O.C.; and grant by the Kaohsiung Medical University, Taiwan (KMU-S105011, KMU-PT10616).
Sources of financial support: none.
Trial registration: The study was retrospectively registered at ClinicalTrials.gov, register number NCT03698253 (www.clinicaltrials.gov/ct2/show/NCT03698253). Date of registration: 5 October 2018.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Arnold D, Stein A. New developments in the second-line treatment of metastatic colorectal cancer: potential place in therapy. Drugs. 2013;73:883–891. doi: 10.1007/s40265-013-0076-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 4.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 6.Osawa H. Response to regorafenib at an initial dose of 120 mg as salvage therapy for metastatic colorectal cancer. Mol Clin Oncol. 2017;6:365–372. doi: 10.3892/mco.2017.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecchin E, Innocenti F, D'Andrea M, Corona G, De Mattia E, Biason P, Buonadonna A, Toffoli G. Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol. 2009;27:2457–2465. doi: 10.1200/JCO.2008.19.0314. [DOI] [PubMed] [Google Scholar]
- 8.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7:2182–2194. [PubMed] [Google Scholar]
- 9.Lu CY, Huang CW, Hu HM, Tsai HL, Huang CM, Yu FJ, Huang MY, Chang SF, Huang ML, Wang JY. Prognostic advantage of irinotecan dose escalation according to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping in patients with metastatic colorectal cancer treated with bevacizumab combined with 5-fluorouracil/leucovorin with irinotecan in a first-line setting. Transl Res. 2014;164:169–176. doi: 10.1016/j.trsl.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Kim KP, Kim HS, Sym SJ, Bae KS, Hong YS, Chang HM, Lee JL, Kang YK, Lee JS, Shin JG. A UGT1A1*28 and *6 genotype-directed phase I dose-escalation trial of irinotecan with fixed-dose capecitabine in Korean patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2013;71:1609–1617. doi: 10.1007/s00280-013-2161-6. [DOI] [PubMed] [Google Scholar]
- 11.Lu CY, Huang CW, Wu IC, Tsai HL, Ma CJ, Yeh YS, Chang SF, Huang ML, Wang JY. Clinical implication of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patients treated with bevacizumab combined with FOLFIRI in the first-line setting. Transl Oncol. 2015;8:474–479. doi: 10.1016/j.tranon.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma CJ, Huang CW, Yeh YS, Tsai HL, Hu HM, Wu IC, Cheng TL, Wang JY. Regorafenib plus FOLFIRI with irinotecan dose escalated according to uridine diphosphate glucuronosyltransferase 1A1 genotyping in patients with metastatic colorectal cancer. Oncol Res. 2017;25:673–679. doi: 10.3727/97818823455816X14786040691928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O'Neil BH, Shaw JE, Atkins JN, Horvath LE, Polite BN. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance) J Clin Oncol. 2016;34:3504. [Google Scholar]
- 14.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 15.Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboue R, Tuech JJ. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–1169. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 17.Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouche O, Landi B, Louvet C. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 18.McBride D. KRAS status predicts response to cetuximab for metastatic colorectal cancer. ONS Connect. 2008;23:25. [PubMed] [Google Scholar]
- 19.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pinter T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 21.Giralt J, de las Heras M, Cerezo L, Eraso A, Hermosilla E, Velez D, Lujan J, Espin E, Rosello J, Majo J. The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: a multicenter, retrospective analysis. Radiother Oncol. 2005;74:101–108. doi: 10.1016/j.radonc.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Galizia G, Lieto E, Ferraraccio F, De Vita F, Castellano P, Orditura M, Imperatore V, La Mura A, La Manna G, Pinto M. Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006;13:823–835. doi: 10.1245/ASO.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 23.Ljuslinder I, Melin B, Henriksson ML, Oberg A, Palmqvist R. Increased epidermal growth factor receptor expression at the invasive margin is a negative prognostic factor in colorectal cancer. Int J Cancer. 2011;128:2031–2037. doi: 10.1002/ijc.25559. [DOI] [PubMed] [Google Scholar]
- 24.Huang CW, Tsai HL, Chen YT, Huang CM, Ma CJ, Lu CY, Kuo CH, Wu DC, Chai CY, Wang JY. The prognostic values of EGFR expression and KRAS mutation in patients with synchronous or metachronous metastatic colorectal cancer. BMC Cancer. 2013;13:599. doi: 10.1186/1471-2407-13-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razis E, Pentheroudakis G, Rigakos G, Bobos M, Kouvatseas G, Tzaida O, Makatsoris T, Papakostas P, Bai M, Goussia A. EGFR gene gain and PTEN protein expression are favorable prognostic factors in patients with KRAS wild-type metastatic colorectal cancer treated with cetuximab. J Cancer Res Clin Oncol. 2014;140:737–748. doi: 10.1007/s00432-014-1626-2. [DOI] [PubMed] [Google Scholar]
- 26.Sunakawa Y, Yang D, Moran M, Astrow SH, Tsuji A, Stephens C, Zhang W, Cao S, Takahashi T, Denda T. Combined assessment of EGFR-related molecules to predict outcome of 1st-line cetuximab-containing chemotherapy for metastatic colorectal cancer. Cancer Biol Ther. 2016;17:751–759. doi: 10.1080/15384047.2016.1178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Algars A, Sundstrom J, Lintunen M, Jokilehto T, Kytola S, Kaare M, Vainionpaa R, Orpana A, Osterlund P, Ristimaki A. EGFR gene copy number predicts response to anti-EGFR treatment in RAS wild type and RAS/BRAF/PIK3CA wild type metastatic colorectal cancer. Int J Cancer. 2017;140:922–929. doi: 10.1002/ijc.30507. [DOI] [PubMed] [Google Scholar]
- 28.Khan SA, Zeng Z, Shia J, Paty PB. EGFR gene amplification and KRAS mutation predict response to combination targeted therapy in metastatic colorectal cancer. Pathol Oncol Res. 2017;23:673–677. doi: 10.1007/s12253-016-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scartozzi M, Bearzi I, Berardi R, Mandolesi A, Fabris G, Cascinu S. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors does not correlate with EGFR expression in related metastatic sites: implications for treatment with EGFR-targeted monoclonal antibodies. J Clin Oncol. 2004;22:4772–4778. doi: 10.1200/JCO.2004.00.117. [DOI] [PubMed] [Google Scholar]
- 30.Yen LC, Uen YH, Wu DC, Lu CY, Yu FJ, Wu IC, Lin SR, Wang JY. Activating KRAS mutations and overexpression of epidermal growth factor receptor as independent predictors in metastatic colorectal cancer patients treated with cetuximab. Ann Surg. 2010;251:254–260. doi: 10.1097/SLA.0b013e3181bc9d96. [DOI] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute http://ctep.cancer.gov [archieved on 31st May 2003]
- 33.Marcuello E, Paez D, Pare L, Salazar J, Sebio A, del Rio E, Baiget M. A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer. 2011;105:53–57. doi: 10.1038/bjc.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 35.Lu CY, Yeh YS, Huang CW, Ma CJ, Yu FJ, Wang JY. FOLFIRI and regorafenib combination therapy with dose escalation of irinotecan as fourth-line treatment for patients with metastatic colon cancer according to UGT1A1 genotyping. Onco Targets Ther. 2014;7:2143–2146. doi: 10.2147/OTT.S69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Wang Z, Guo J, Liu J, Li C, Liu L, Shi H, Liu L, Li H, Xie C. Clinical significance of UGT1A1 gene polymorphisms on irinotecan-based regimens as the treatment in metastatic colorectal cancer. Onco Targets Ther. 2014;7:1653–1661. doi: 10.2147/OTT.S67867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshino T, Komatsu Y, Yamada Y, Yamazaki K, Tsuji A, Ura T, Grothey A, Van Cutsem E, Wagner A, Cihon F. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs. 2015;33:740–750. doi: 10.1007/s10637-014-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmieder R, Hoffmann J, Becker M, Bhargava A, Muller T, Kahmann N, Ellinghaus P, Adams R, Rosenthal A, Thierauch KH. Regorafenib (BAY 73-4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer. 2014;135:1487–1496. doi: 10.1002/ijc.28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 40.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 41.Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, Stintzing S, Graeven U, Arnold D, von Weikersthal LF. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27:1746–1753. doi: 10.1093/annonc/mdw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 43.Mao C, Yang ZY, Hu XF, Chen Q, Tang JL. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2012;23:1518–1525. doi: 10.1093/annonc/mdr464. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida M, Shimura T, Sato M, Ebi M, Nakazawa T, Takeyama H, Joh T. A novel predictive strategy by immunohistochemical analysis of four EGFR ligands in metastatic colorectal cancer treated with anti-EGFR antibodies. J Cancer Res Clin Oncol. 2013;139:367–378. doi: 10.1007/s00432-012-1340-x. [DOI] [PubMed] [Google Scholar]
- 45.Llovet P, Sastre J, Ortega JS, Bando I, Ferrer M, Garcia-Alfonso P, Donnay O, Carrato A, Jimenez A, Aranda E. Prognostic value of BRAF, PI3K, PTEN, EGFR copy number, amphiregulin and epiregulin status in patients with KRAS codon 12 wild-type metastatic colorectal cancer receiving first-line chemotherapy with anti-EGFR therapy. Mol Diagn Ther. 2015;19:397–408. doi: 10.1007/s40291-015-0165-0. [DOI] [PubMed] [Google Scholar]
- 46.Mao C, Liao RY, Chen Q. Loss of PTEN expression predicts resistance to EGFR-targeted monoclonal antibodies in patients with metastatic colorectal cancer. Br J Cancer. 2010;102:940. doi: 10.1038/sj.bjc.6605575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Shi Y, Lin J, Ye YB, Wang XJ, Chen G, Guo ZQ. Combined analysis of EGFR and PTEN status in patients with KRAS wild-type metastatic colorectal cancer. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, Saletti P, De Dosso S, Spitale A, Tejpar S. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer. 2013;108:668–675. doi: 10.1038/bjc.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kishiki T, Ohnishi H, Masaki T, Ohtsuka K, Ohkura Y, Furuse J, Watanabe T, Sugiyama M. Overexpression of MET is a new predictive marker for anti-EGFR therapy in metastatic colorectal cancer with wild-type KRAS. Cancer Chemother Pharmacol. 2014;73:749–757. doi: 10.1007/s00280-014-2401-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietrantonio F, Vernieri C, Siravegna G, Mennitto A, Berenato R, Perrone F, Gloghini A, Tamborini E, Lonardi S, Morano F. Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23:2414–2422. doi: 10.1158/1078-0432.CCR-16-1863. [DOI] [PubMed] [Google Scholar]
- 51.Adenis A, de la Fouchardiere C, Paule B, Burtin P, Tougeron D, Wallet J, Dourthe LM, Etienne PL, Mineur L, Clisant S. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer. 2016;16:412. doi: 10.1186/s12885-016-2440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Q, Hu WG, Song QB, Wei J. BRAF V600E mutation as a predictive factor of anti-EGFR monoclonal antibodies therapeutic effects in metastatic colorectal cancer: a meta-analysis. Chin Med Sci J. 2014;29:197–203. doi: 10.1016/s1001-9294(14)60070-5. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25:1032–1038. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sclafani F. PD-1 inhibition in metastatic dMMR/MSI-H colorectal cancer. Lancet Oncol. 2017;18:1141–1142. doi: 10.1016/S1470-2045(17)30512-0. [DOI] [PubMed] [Google Scholar]
- 57.Lee JJ, Chu E. Recent advances in the clinical development of immune checkpoint blockade therapy for mismatch repair proficient (pMMR)/non-MSI-H metastatic colorectal cancer. Clin Colorectal Cancer. 2018 doi: 10.1016/j.clcc.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mooi JK, Wirapati P, Asher R, Lee CK, Savas PS, Price TJ, Townsend A, Hardingham J, Buchanan D, Williams D. The prognostic impact of consensus molecular subtypes (CMS) and its predictive effects for bevacizumab benefit in metastatic colorectal cancer: molecular analysis of the AGITG MAX clinical trial. Ann Oncol. 2018 doi: 10.1093/annonc/mdy410. [DOI] [PubMed] [Google Scholar]
- 59.Ducreux M, Petersen LN, Öhler L, Bergamo F, Metges JP, de Groot JW, Wang JY, Paredes BG, Dochy E. 2019. Outcomes by tumor location in patients with metastatic colorectal cancer (mCRC) treated with regorafenib (REG): final analysis from the prospective, observational CORRELATE study. [Abstract sumitted to ASCO-GI] [DOI] [PubMed] [Google Scholar]