Abstract
In 2009, a National Confidential Enquiry into Patient Outcome and Death report detailed significant shortcomings in recognition and management of patients with acute kidney injury (AKI). As part of a national collaborative to reduce harm from AKI, the Scottish Patient Safety Programme developed two care bundles to improve response (‘SHOUT’) and review (‘BUMP’) of AKI.
Baseline data from eight patients with AKI on the acute medical unit (AMU) in Ninewells Hospital showed 62% compliance with SHOUT. However, most patients were transferred from AMU within 24 hours so BUMP could not be assessed. Our aim was to achieve >95% compliance with SHOUT on AMU within 2 months. The content of the SHOUT bundle was condensed onto a sticker for the case notes, which was implemented using Plan-Do-Study-Act cycles. Compliance was assessed weekly and feedback obtained from stakeholders concerning their opinion of the sticker, SHOUT bundle and care bundles in general.
Use of the sticker was 27% in week 1 but fell to 5% by week 4. Compliance with the bundle varied from 45% to 60% and was only slightly improved by use of the sticker (OR 1.58, 95% CI 0.39 to 6.42). Staff found the sticker burdensome and did not agree that all elements of SHOUT were equally important. This opinion was supported by finding that their compliance with sepsis and hypovolaemia recommendations was 91%–100% throughout, whereas urinalysis was documented in only 55%–63% of patients. Several staff mentioned ‘bundle fatigue’ and on one day we identified 22 other care bundles or structured improvement forms in AMU.
We concluded that the AMU staff had legitimate concerns about the SHOUT care bundle and that our intervention was demotivating. Overcoming bundle fatigue will not be a simple task. We plan to work with staff on integrating AKI into patient safety huddles and on using modelling and recognition of good practice to improve motivation.
Keywords: pdsa, quality improvement, checklists
Problem
Acute kidney injury (AKI) has consistently been identified as being poorly managed in hospital, and a common cause of inpatient mortality. In a recent UK study, 48% of patients who sustained in-hospital AKI died within 1 year postdischarge, compared with 12% in patients without AKI.1 A UK national audit by National Confidential Enquiry into Patient Outcome and Death in 2009 concluded that only 50% of patients who died in hospital with an AKI received an overall standard of care considered to be good.2
In response to the growing concern surrounding AKI, the Scottish Patient Safety Programme (SPSP) initiated an 18-month collaborative in August 2017 aiming to reduce harm from AKI in NHS Scotland. A primary driver identified was to improve the response and review for people with AKI.3 Data from NHS Tayside identified 1713 cases of AKI in hospital inpatients between 1 October 2016 and 22 January 2017. The acute medical unit (AMU) at Ninewells Hospital had the highest rate of AKI (303 cases, 19 per week, 18% total). The AMU is a 31-bed unit where 16 861 adult f patients were assessed, investigated and treated for urgent medical problems in the year from March 2017 to February 2018. Patients generally remain on AMU <24 hours before being discharged or transferred to continuing care wards. There are around 50–60 acute medical admissions per day. The aim of this project was to achieve 95% compliance with the SPSP AKI response and review care bundles3 in the AMU within 2 months.
Background
A care bundle is designed to be a structured method of improving care processes and outcomes. It contains a small (up to five) set of evidence-based practices intended for a defined population and care setting.4 A care bundle for AKI could improve the quality of care5 and there is evidence that their implementation improves reliability of care.6–10 Moreover, two studies at one UK hospital found that compliance with an AKI care bundle was associated with reduced mortality and progression of AKI.11 12 The SPSP developed two care bundles as part of their AKI programme3: SHOUT defines the immediate response on detection of an AKI, whereas BUMP concerns the review of patients in the following days. Elements included in the SHOUT bundle include ‘sepsis’, ‘hypovolaemia’, ‘obstruction’, ‘urinalysis’ and ‘toxins’. BUMP includes aspects of review such as blood review, consideration for renal referral, medicine review and fluid maintenance. The bundles were developed further through baseline testing on urology and acute surgical receiving wards in Ninewells Hospital, with input from renal physicians, foundation and specialty trainees, nursing staff, consultant physicians and the patient safety team. This contributed to the adaptation of the bundle into a practical and more effective format, being refined into two small stickers that would be easy to add to patient notes. For this intervention we focused on the SHOUT bundle (see online supplementary appendix 1) because patients do not generally stay on the AMU for >24 hours.
bmjoq-2018-000392supp001.docx (15.1KB, docx)
A number of interventions had been implemented within NHS Tayside to improve identification and awareness of AKI before the start of this project. An electronic alert (e-alert) system based on changes in serum creatinine (SrC) from baseline was introduced in 2015 to improve recognition of AKI. In addition, the AKI management guidelines were redeveloped with an educational video. This is an 11 min video developed with a cartoonist covering recognition and management of AKI, which is aimed at junior doctors and nursing staff and available on YouTube. The aim of this video was to provide an accessible and engaging educational resource. An educational lanyard card (see online supplementary appendix 2) was developed to provide an easily accessible reminder about risk factors, essential tests and management steps for AKI. Both the video and lanyard card were used at junior doctor induction and as part of AKI awareness week.13
bmjoq-2018-000392supp002.docx (2MB, docx)
Measurement
Cases of AKI were identified from the e-alert system, which uses the NHS England algorithm for detecting increase in SrC.14 Baseline data were analysed from eight patients with AKI who were admitted to AMU between 30 October 2017 and 5 November 2017. All eight patients were assessed for possible sepsis and hypovolaemia with full documentation. However, the other three bundle elements (assessment for obstruction, urinalysis documented, high-risk medicines withheld) were each documented in five (62%) patients and only three (37%) patients had all five SHOUT bundle elements completed.
Process measures assessed compliance with each different component of the bundle in all patients with AKI, irrespective of whether the intervention sticker was used or not. Compliance was only recorded if the patient notes confirmed the step was completed. Data were collected daily on Monday to Friday by one investigator (RL). Data from over the weekend were collected on the Monday. Bundle compliance was assessed weekly (over 7 days).
Achieving a balanced accounting of the impact of an intervention requires careful consideration of expected undesirable effects (potential trade-offs) from the outset, and more consideration of unexpected effects after implementation.15 16 We sought information about staff concerns with the changes during Plan-Do-Study-Act (PDSA) cycles and from additional verbal feedback throughout the project. We deliberately paused implementation after 4 weeks to evaluate any pleasant or unpleasant surprises.15
Design
We used the Capability, Opportunity, Motivation-Behaviour to consider how to facilitate behaviour change in the AMU.17 18 The NHS Tayside AKI video was designed to enhance capability through education about management of AKI.13 We used environmental restructuring (reminder lanyards, stickers) to enhance opportunity and feedback of data over time with support from AMU consultants to improve motivation to apply the SHOUT bundle and hence improve the reliability of care in response to AKI.
Following the start of the intervention we used PDSA cycles to identify barriers to change. PDSA cycles allow for rapid assessment of small-scale changes, and enable continuous improvement and adaptation of an intervention.19 We used the Theoretical Domains Framework to understand barriers to implementation.20 There was continuous input from the Tayside AKI collaborative group consisting of a renal consultant, AMU consultant, patient safety advisor, a medical student and a consultant biochemist.
We planned to review the consequences of the intervention after 4 weeks and consider the need for adaptation.16
Strategy
Five PDSA cycles (see online supplementary appendix 3) were used to test the introduction of the SHOUT AKI bundle, increase awareness and assess barriers to compliance. Further changes to sticker implementation were made based on previous PDSA results, which also helped identify other areas for improvement. Feedback received over the course of the project is summarised in table 1.
Table 1.
Feedback received over the course of the project
| Baseline |
|
| Week 1 |
|
| Week 2 |
|
| Week 3 |
|
| Week 4 |
|
bmjoq-2018-000392supp003.docx (71.8KB, docx)
PDSA 1: preintervention, gathering feedback and process mapping
In PDSA 1, we engaged with key stakeholders to gather feedback before testing the sticker. This also involved testing the sticker in its original format with one patient and doctor to assess how it worked in practice. Additionally, we planned to observe practice on AMU to process map a patient from admission to discharge. We were able to engage well with the medical team as one of the AMU consultants was part of the improvement team. We also arranged meetings with the nurse educator, senior charge nurse and head of nursing to engage nursing staff. Changes were recommended for the bundle, which we took into consideration.
PDSA 2: roll-out of the care bundle
In PDSA 2, we aimed to introduce the sticker for use on all patients with AKI. A senior AMU consultant sought to engage the team by distributing a reminder email about the trial the day before it was introduced and verbal reminders were given to staff throughout the first week. We gathered feedback from clinicians using the sticker but unsurprisingly, compliance was poor in the first week at only 27%. After assessing compliance with the sticker in the first week, we planned to increase awareness through informative posters, placards and further staff engagement.
PDSA 3: increasing awareness
To address compliance, we designed a poster which detailed the purpose of the project and where to find the stickers. Placards reminding the doctors to use the sticker were also attached to the computer. Midway through week 2, a concern was raised regarding the gentamicin prescribing advice contained within the sticker. Due to this unforeseen discrepancy the stickers were not used from the second half of week 2, making any impact of the posters and placards on compliance difficult to assess.
PDSA 4: aligning care bundle with gentamicin prescribing guidelines
In PDSA 4, we amended the sticker due to concerns surrounding gentamicin prescribing advice (table 1). Following discussion with the renal team, the ‘Toxins’ section of the bundle was revised to reflect AMU gentamicin guidelines. However, compliance dropped further despite results showing certain steps are often missed. We fed back the results of testing to the team and increased engagement with nursing staff as a result.
PDSA 5: further feedback
In PDSA 5, we planned to intensify efforts to increase awareness through further reminder emails and engagement with the nursing staff, with details of the project being added to the daily email circulated among the nursing team. However, results indicated that compliance was continuing to fall over time (figure 1). While data collected demonstrated that AKI is often not managed appropriately, most of the junior doctors maintained that introducing a sticker would not resolve the issue.
Figure 1.
Use of SHOUT bundle sticker over time. AMU, acute medical unit; AKI, acute kidney injury; PDSA, Plan-Do-Study-Act.
Results
The SHOUT sticker was tested between 6 November 2017 and 3 December 2017, with uptake being disappointingly low (figure 1). After 4 weeks of testing, compliance with the SHOUT sticker dropped from 27% in the first week to 5% in the last week, with compliance just 16% overall (figure 1). Junior doctors told us that finding and completing stickers was adding unnecessary paperwork and increasing the time taken to complete essential tasks.
We assessed the impact of introducing the sticker on management of AKI patients, including the patients who did not have a sticker used. We identified 64 patients with AKI postintervention but were only able to collect complete data about bundle compliance from 51 patients. Of these, 36 (61%) had stage 1, 15 (25%) had stage 2 and 8 (14%) had stage 3 AKI. Bundle compliance was only slightly improved by use of the sticker (6/10, 60% with sticker vs 20/41, 49% without sticker; OR 1.58, 95% CI 0.39 to 6.42).
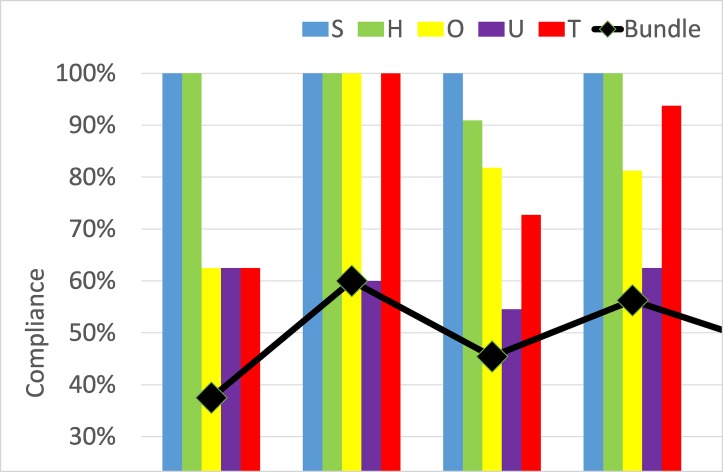
We gave weekly feedback to AMU staff about compliance with the SHOUT bundle. The format for feedback was a run chart with a single line that showed the percentage of patients that were compliant with all five bundle elements, which varied from 45% to 60%. Staff questioned this presentation of results because they did not agree that all bundle elements were equally important. After 4 weeks of the intervention we analysed compliance with each bundle element, revealing consistent differences throughout the 5 weeks of data collection (figure 2). Management of sepsis and hypovolaemia was documented reliably in 91%–100% of patients with AKI. Management of obstruction and decisions about high-risk medicines were documented in 68%–100% of patients but urinalysis was documented in only 55%–63% of patients (figure 2). Staff told us the ‘all or none’ format for feedback on the AKI bundle was demotivating and devalued their care for hypovolaemia and sepsis.
Figure 2.
Compliance with the SHOUT care bundle and its components before (8 patients) and after (51 patients) introduction of bundle stickers. The line shows compliance with the whole bundle, the bars show compliance with S Screen for sepsis, H Hypovolaemia, O Obstruction, U Urinalysis, T Toxins (high-risk medicines).
Staff repeatedly mentioned ‘bundle fatigue’. A brief check on AMU on 1 day identified 22 other care bundles or structured management forms, which could be divided into four categories. First were risk assessments for screening on admission to AMU (eg, for delirium or falls). Second were bundles for care processes common to all patients (eg, a safe transfer bundle). Third were bundles for invasive procedures (eg, insertion and maintenance bundles for both peripheral venous cannulas and urinary catheters). Fourth were structured pathways to be used for patients with specific problems (eg, alcohol withdrawal, decompensated liver cirrhosis, deep venous thrombosis, diabetic ketoacidosis, Parkinson’s disease, sepsis). A concern for senior AMU staff was the time and attention required to document compliance with the AKI care bundle could lead to a decrease in compliance with another care bundle or policy. In PDSA 4, we had identified and resolved conflicting recommendations between the SPSP AKI bundle and the NHS Tayside sepsis policy. However, in PDSA 5 we became aware of concerns about unnecessary ordering of renal ultrasound to investigate AKI on the AMU. The lead consultant (who is also a consultant in renal medicine) issued guidance saying that tests in general on AMU should not be ordered if they are unlikely to affect management in the next 24 hours and that lower tract obstruction is easily found using bedside bladder scan. However, the guidance identified three specific indications for renal tract ultrasound on AMU, which included one indication for urgent investigation, within 4 hour of recognition of AKI. This guidance demonstrated the level of clinical detail required to interpret the SHOUT bundle measure ‘bladder scan and/or ultrasound if obstruction suspected’.
An unexpected desirable consequence (pleasant surprise)15 16 from the AMU AKI bundle intervention was the introduction of automated measurement of bicarbonate for patients with AKI. We found only 27% of patients with an AKI had their bicarbonate measured on AMU. This is not part of the SHOUTAKI response bundle but is included in the BUMP AKI review bundle (see online supplementary appendix 1). The AMU team agreed this was an important test to carry out in AMU given the high risk of metabolic acidosis with AKI21 and the need to modify fluid replacement for patients at high risk.5 After discussions between the renal, patient safety and laboratory team, a process was introduced into the laboratory biochemistry system that automatically requests a bicarbonate measurement for all patients with AKI 2 or 3.
Lessons and limitations
Enabling success for quality improvement work often stems from appropriate engagement with the teams involved. A multidisciplinary approach where all stakeholders are involved in the process promotes the concept of collective ownership of the project, which influences how willing people are to assist in the improvement process.22 The high throughput of very sick patients and staffing by changing multidisciplinary teams in an AMU make this a very challenging environment.23 Despite having a clear, shared goal and multidisciplinary approach, we encountered significant resistance to change (table 2). Barriers arising from lack of awareness of the sticker were easy to address but this did not improve use of the sticker (figure 1) because of additional barriers to motivation (table 2). The junior doctors did not believe the sticker altered how they managed a patient with AKI because the sticker content did not add anything that the AKI lanyard card did not already cover (online supplementary appendix 2). This opinion was supported by the results, because there was very little difference in compliance with the bundle elements between patients who did or did not have a sticker. A second important motivation barrier was that the doctors did not agree that all elements of the bundle were equally important. Consequently, we found consistent differences in compliance between the five bundle elements (figure 2).
Table 2.
Barriers to change identified through PDSA cycles classified with the Theoretical Domains Framework and the COM-B model17 20
| Barrier | Theoretical Domain | Definition | COM-B |
| Lack of awareness | Environmental context and resources | Any circumstance of a person’s situation or environment that discourages or encourages the development of skills and abilities, independence, social competence and adaptive behaviour | Opportunity Physical |
| Finding and completing stickers perceived as unnecessary duplication | Goals | Mental representations of outcomes or end states that an individual wants to achieve | Motivation Reflective |
| Bundle fatigue: 22 other bundles and checklists already in use on the AMU | Optimism | The confidence that things will happen for the best or that desired goals will be attained | Motivation Reflective |
| Conflict with sepsis and antibiotic policies and with guidance on renal ultrasound in AMU | Beliefs about consequences | Acceptance of the truth, reality or validity about outcomes of a behaviour in a given situation | Motivation Reflective |
| Compliance with some bundle elements already perceived as good | Beliefs about capabilities | Acceptance of the truth, reality or validity about an ability, talent or facility that a person can put to constructive use | Motivation Reflective |
| Not all bundle elements perceived as equally important | Intentions | A conscious decision to perform a behaviour or a resolve to act in a certain way | Motivation Reflective |
AMU, acute medical unit; COM-B, Capability, Opportunity, Motivation-Behaviour; PDSA, Plan-Do-Study-Act.
The Institute for Healthcare Improvement (IHI) recommend that care bundles should include no more than five ‘evidence-based interventions for a defined patient segment/population and care setting that, when implemented together, will result in significantly better outcomes than when implemented individually’.4 Published studies about AKI bundles address the same issues but the number of elements in each bundle varies from 5 to 11.9 Moreover, the definition of compliance with each element is variable. For example, criteria for compliance on Obstruction varies from ‘Exclude obstruction’7 to requiring bladder scan6 or renal ultrasound8 for all patients within 24 hours. The Obstruction component of SHOUT includes two different components (appropriate use of bladder scan and ultrasound). In order to bring about change, problems need to be defined in behavioural terms: what is the behaviour, where does it occur and who is involved in performing the behaviour?17 The guidance on renal tract ultrasound requesting that was issued by the AMU lead consultant in November 2017 made it clear that only selected patients required investigation to be undertaken on the AMU within 24 hours and that the person responsible for ordering the test was dependent on the clinical indication. Criteria for bundle compliance on high-risk medicines vary from ‘Address medications’7 through ‘stop nephrotoxic drugs’6 to instructions to stop specific drugs (non-steroidal anti-inflammatory drugs, ACE inhibitors, angiotensin receptor blockers, potassium sparing diuretics) and initiate pharmacy review within 24 hours.8 The SHOUT bundle includes ‘avoid gentamicin’ but we found this statement conflicted with local antibiotic and sepsis guidelines, which recommend continuing gentamicin for patients with AKI stages 1 or 2. These policies have been associated with significant reduction in risk of Clostridium difficile in acute medicine in NHS Tayside.24 Managing competing risks is a constant challenge in the real world of unscheduled care and it is difficult to evaluate complex decisions with a simple yes/no tick box.25
IHI attributed the success of their central line and ventilator bundles to the fact that ‘participating clinicians agreed that there was sufficient medical evidence supporting each individual element in the bundle to recommend that it be applied to most, if not all patients’.4 Two studies from the Royal Derby Hospital have shown that compliance with an AKI bundle was associated with reduced mortality and progression of renal failure.11 12 However, only a minority of patients had the bundle completed: 12.2% in the first study12 and 25.6% in the second study.11 Moreover, the definition of bundle compliance was unclear because urinalysis was one of the required AKI bundle elements but was documented in <12% of ‘bundle compliant’ patients in both studies.11 12 According to IHI, patients should be scored as non-compliant if any single element of a care bundle is missing.4 This evidence does not support inclusion of urinalysis in an AKI bundle and it falls a long way short of showing that all the remaining elements should be applied to most, if not all patients.
Care bundles are an example of the measure and manage approach to risk.26 This approach usually focuses on hazards that have led to harm or near-misses and the response is often to create a protocol, care pathway or bundle.27 However, this approach is less suitable when care is very complex, and ignores the fact that most risk does not cause harm because clinicians, patients and carers work together to mitigate risks.28 29 The complex interactions between people and technology in healthcare settings mean that there are individual situations where strict adherence to a protocol is not the safest way to proceed in a given situation.28 30 Furthermore, while adverse events are not uncommon, most risk in healthcare is prevented from causing harm by professionals and patients working to mitigate that risk.31 Newer approaches to improving safety therefore aim to understand positive features of everyday care and teamwork that promote safety, as well as the adjustments and trade-offs made by professionals when balancing safety with the need to deal with very high workloads.25 27 32 Healthcare improvers are increasingly interested in making this underlying expertise more visible so that teams can learn from successes as well as failures.33 A promising approach to examining this underlying expertise is video reflexive ethnography (VRE), which combines observation (ethnography) of everyday practice with structured feedback to clinical teams using video to prompt reflection and improve care.34–36 The AMU is working with researchers from Dundee and Maastricht on application of VRE to improving interprofessional team working through in-depth interviews, ethnographic observation and video filming of interprofessional collaborative routines (eg, ward rounds, handovers, safety huddles).37
Management of AKI in AMU needs to be addressed within a holistic approach to the deteriorating patient. A crucial barrier is that AKI is rarely part of the patient’s presenting health problem. To address this, we need to provide information about rates and outcomes of AKI on a regular basis. We will use the e-alerts to enable live reporting of AKI rates for AMU on a weekly basis and test the effect of including these reports in the AMU safety huddles. This information will enhance capability by making AKI part of regular discussions about safety and creating opportunities to develop relevant skills such as goal-setting, monitoring, providing feedback and developing specific plans to change.17 We plan to enhance motivation through incentivisation and modelling. Incentivisation is defined as creating an expectation of reward17 and will be achieved through feedback of information in a format that celebrates good practice. Modelling is defined as providing an example for people to aspire to or imitate.17 These intervention functions are coherent with appreciative inquiry into patient safety.31–33 The AMU has joined the Learning from Excellence community,38 which aims to identify, appreciate, study and learn from episodes of excellence in frontline healthcare. We plan to use case studies about management of deteriorating patients as the content for workplace based, simulated patient education sessions, which will enable management of AKI to be discussed in the broader context of deteriorating patients on AMU.
Limitations
We only had a short timeframe for the project and more time may have allowed us to gather further evidence about AKI management on the AMU and focus on what the needs of the team were to ensure best care for patients. However, even with more time the ability to tailor the intervention to the needs of the AMU team would still have been limited by the intervention itself.
The SHOUT and BUMP care bundles were designed by SPSP in conjunction with experts in quality improvement, patient safety and nephrology, among others. However, the bundle was not unique to individual care units and while there was flexibility to modify aspects of the bundle, some content was relatively set to allow SPSP to collect data on specific elements of bundle compliance that could be compared across different health-boards participating in the AKI collaborative. An AMU consultant was involved in the collaborative team, but most of the bundle design occurred prior to the launch of the collaborative so there was no direct involvement of the AMU team at the point of intervention design. This is an important limitation as it may have been that more active involvement of the AMU team at the point of design would have allowed an intervention to be developed that worked for the team as well as patients.
Another important limitation is lack of sustainability. Given the limited enthusiasm for continuing with the intervention while staff were being actively reminded about it, there is no possibility that they would have continued with testing when active reminders were removed. As we did not have the resources for semi-structured interviews with staff, our analysis of barriers to implementation was limited. However, while the bundle itself was not sustainable, this experience highlighted the nature in which AKI is frequently dismissed as less significant compared with other comorbidities which has encouraged us to pursue reporting AKI rates and outcomes more regularly as part of safety huddles. This could emphasise the high rates and poorer outcomes associated with AKI to staff, which could make teams more willing to engage with future improvement efforts.
We only had a small sample of patients and clinicians in which we were able to test the bundle and it is difficult to know whether this sample was reflective of common practice. Furthermore, we were only able to complete data collection on bundle compliance in 51 (80%) of 64 patients with AKI during the study period. It is possible that missing data biased the study results.
Conclusions
The SPSP AKI bundles were part of a change package intended to reduce harm to people in NHS Scotland from AKI by December 2017.3 Our experience in AMU builds on testing of the SHOUT and BUMP bundles in the urology ward and acute surgical receiving unit in Ninewells Hospital. We concluded that clinical staff had legitimate concerns about the bundles. Overcoming bundle fatigue will not be a simple task. We plan to work with staff on integrating AKI into patient safety huddles and on using modelling and recognition of good practice to improve capability and motivation. We have advised SPSP against further implementation of the AKI bundles because there is insufficient evidence that each individual element in the bundles should be applied to most, if not all patients, in all contexts.
Acknowledgments
The authors would like to thank Dr Bill Bartlett for enabling automated measurement of bicarbonate for all patients with AKI 2 and 3.
Footnotes
Patient consent for publication: Not required.
Contributors: RL was involved in the collection of data, developing the care bundle and liaising with the AMU team throughout the improvement project. She was also responsible for writing the first draft of the manuscript. SB was responsible for approving and developing the care bundle, liaising with the Scottish Patient Safety Programme, AMU and renal team and was the clinical supervisor for RL throughout the project. Middle authors are in alphabetical order by surname and all made equally important contributions to interpretation of results and to writing subsequent drafts of the manuscript. PD was the academic supervisor for RL and took a lead role in balanced accounting of the intervention and interpreting results about barriers to change. AD and VT were responsible for assisting with developing a sound quality improvement methodology for implementing the intervention. AD also liaised with SPSP throughout the project. SG is leading research on Video Reflexive Ethnography on the AMU and was responsible for interpreting the results in the context of understanding complex adaptive systems. AV acted as the primary contact for the AMU team and conveyed their feedback throughout the project, as well as providing assistance for implementing the intervention. All authors critically revised the manuscript for important intellectual content, gave their final approval of the version to be published and agree to be accountable for all aspects of the work. RL conducted this work as part of a BMSc in Healthcare Improvement at the University of Dundee.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jurawan N, Pankhurst T, Ferro C, et al. . Hospital acquired Acute Kidney Injury is associated with increased mortality but not increased readmission rates in a UK acute hospital. BMC Nephrol 2017;18:1–11. 10.1186/s12882-017-0729-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Confidential Enquiry into Patient Outcome and Death (NCEPOD). Adding Insult to Injury. A review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure, 2009. [Google Scholar]
- 3.Scottish Patient Safety Programme Acute Adult. Acute Kidney Injury. AKI reduction driver diagram and change package. http://ihub.scot/acute-kidney-injury/resources-downloads/.
- 4.Resar R, Fa g CH, Tw N. Using care bundles to improve health care quality. IHI White Papers 2012:1–14 http://www.ihi.org/knowledge/Pages/IHIWhitePapers/UsingCareBundles.aspx%5Cnfile:///C:/Users/Rie Johansen/Downloads/IHIUsingCareBundlesWhitePaper2012 (1).pdf. [Google Scholar]
- 5.Bagshaw SM. Acute kidney injury care bundles. Nephron 2016;131:247–51. 10.1159/000437152 [DOI] [PubMed] [Google Scholar]
- 6.Bhagwanani A, Carpenter R, Yusuf A. Improving the management of acute kidney injury in a district general hospital: introduction of the donut bundle. BMJ Qual Improv Rep 2013;2:u202650.w1235 10.1136/bmjquality.u202650.w1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forde C, McCaughan J, Leonard N. Acute Kidney Injury: It’s as easy as ABCDE. BMJ Qual Improv Rep 2012;1:u200370.w326 10.1136/bmjquality.u200370.w326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joslin J, Wilson H, Zubli D, et al. . Recognition and management of acute kidney injury in hospitalised patients can be partially improved with the use of a care bundle. Clin Med 2015;15:431–6. 10.7861/clinmedicine.15-5-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selby NM, Kolhe NV. Care Bundles for Acute Kidney Injury: Do They Work? Nephron 2016;134:195–9. 10.1159/000447758 [DOI] [PubMed] [Google Scholar]
- 10.Tsui A, Rajani C, Doshi R, et al. . Improving recognition and management of acute kidney injury. Acute Med 2014;13:108–12. [PubMed] [Google Scholar]
- 11.Kolhe NV, Reilly T, Leung J, et al. . A simple care bundle for use in acute kidney injury: a propensity score-matched cohort study. Nephrol Dial Transplant 2016;31:1846–54. 10.1093/ndt/gfw087 [DOI] [PubMed] [Google Scholar]
- 12.Kolhe NV, Staples D, Reilly T, et al. . Impact of compliance with a care bundle on acute kidney injury outcomes: a prospective observational study. PLoS One 2015;10:e0132279–12. 10.1371/journal.pone.0132279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHS Tayside. Acute kidney injury. 2017. https://www.youtube.com/watch?v=gW0pgXrIdgo.
- 14.NHS England. Acute kidney injury (aki) algorithm. 2014. https://www.england.nhs.uk/akiprogramme/aki-algorithm/.
- 15.Toma M, Dreischulte T, Gray NM, et al. . Balancing measures or a balanced accounting of improvement impact: a qualitative analysis of individual and focus group interviews with improvement experts in Scotland. BMJ Qual Saf 2018;27:547–56. 10.1136/bmjqs-2017-006554 [DOI] [PubMed] [Google Scholar]
- 16.Toma M, Davey PG, Marwick CA, et al. . A framework for ensuring a balanced accounting of the impact of antimicrobial stewardship interventions. J Antimicrob Chemother 2017;72:3223–31. 10.1093/jac/dkx312 [DOI] [PubMed] [Google Scholar]
- 17.Michie S, Atkins L, West R. The Behaviour Change Wheel: A Guide to Designing Interventions. London: Silverback Publishing, 2014. [Google Scholar]
- 18.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor MJ, McNicholas C, Nicolay C, et al. . Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf 2014;23:290–8. 10.1136/bmjqs-2013-001862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atkins L, Francis J, Islam R, et al. . A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci 2017;12:1–18. 10.1186/s13012-017-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. The Lancet 2012;380:756–66. 10.1016/S0140-6736(11)61454-2 [DOI] [PubMed] [Google Scholar]
- 22.Dixon-Woods M, Leslie M, Tarrant C, et al. . Explaining Matching Michigan: an ethnographic study of a patient safety program. Implement Sci 2013;8:70 10.1186/1748-5908-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid LE, Dinesen LC, Jones MC, et al. . The effectiveness and variation of acute medical units: a systematic review. Int J Qual Health Care 2016;28:433–46. 10.1093/intqhc/mzw056 [DOI] [PubMed] [Google Scholar]
- 24.Patton A, Davey P, Harbarth S, et al. . Impact of antimicrobial stewardship interventions on Clostridium difficile infection and clinical outcomes: segmented regression analyses. J Antimicrob Chemother 2018;73:517–26. 10.1093/jac/dkx413 [DOI] [PubMed] [Google Scholar]
- 25.Vincent C, Amalberti R. Safer Healthcare. Strategies for the Real World. London: Springer, 2015. [PubMed] [Google Scholar]
- 26.NPSA. Seven steps to patient safety. National Patient Safety Agency: An overview guide for NHS staff, 2004. [Google Scholar]
- 27.Health Foundation. A framework for measuring and monitoring safety: A practical guide to using a new framework for measuring and monitoring safety in the NHS, 2014. [Google Scholar]
- 28.Braithwaite J, Runciman WB, Merry AF. Towards safer, better healthcare: harnessing the natural properties of complex sociotechnical systems. Qual Saf Health Care 2009;18:37–41. 10.1136/qshc.2007.023317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iedema RA, Jorm C, Braithwaite J, et al. . A root cause analysis of clinical error: confronting the disjunction between formal rules and situated clinical activity. Soc Sci Med 2006;63:1201–12. 10.1016/j.socscimed.2006.03.035 [DOI] [PubMed] [Google Scholar]
- 30.Health Foundation. Evidence Scan: Complex adaptive systems, 2010. [Google Scholar]
- 31.Braithwaite J, Wears RL, Hollnagel E. Resilient health care: turning patient safety on its head. Int J Qual Health Care 2015;27:418–20. 10.1093/intqhc/mzv063 [DOI] [PubMed] [Google Scholar]
- 32.Hollnagel E, Wears R, Braithwaite J. From safety-I to safety-II: a white paper, 2015:1–32. [Google Scholar]
- 33.McNab D, Bowie P, Morrison J, et al. . Understanding patient safety performance and educational needs using the ‘Safety-II’ approach for complex systems. Educ Prim Care 2016;27:443–50. 10.1080/14739879.2016.1246068 [DOI] [PubMed] [Google Scholar]
- 34.Carroll K, Iedema R, Kerridge R. Reshaping ICU ward round practices using video-reflexive ethnography. Qual Health Res 2008;18:380–90. 10.1177/1049732307313430 [DOI] [PubMed] [Google Scholar]
- 35.Collier A, Sorensen R, Iedema R. Patients’ and families’ perspectives of patient safety at the end of life: a video-reflexive ethnography study. Int J Qual Health Care 2016;28:66–73. 10.1093/intqhc/mzv095 [DOI] [PubMed] [Google Scholar]
- 36.Iedema R, Mesman J, Carroll K. Visualising Health Care Improvement: Innovation from Within: Radcliffe Publishing Ltd, 2013. [Google Scholar]
- 37.Grant S, Guthrie B, Mesman J. Improving the safety of inter-professional collaboration in an Acute Medical Unit: an examination of the feasibility and implementation of video reflexive ethnography (VRE) in UK healthcare. Tenovus Scotland Major Research Grant 2018. [Google Scholar]
- 38.Birmingham Children’s Hospital. Learning from Excellence. A call to learn from what goes well in healthcare. https://learningfromexcellence.com/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjoq-2018-000392supp001.docx (15.1KB, docx)
bmjoq-2018-000392supp002.docx (2MB, docx)
bmjoq-2018-000392supp003.docx (71.8KB, docx)