Abstract
Background and objective
Paediatric intestinal failure (IF) is a disease entity characterised by gut insufficiency often related to short bowel syndrome. It is commonly caused by surgical removal of a large section of the small intestine in association with necrotising enterocolitis (NEC), which usually affects premature infants. This study investigated the incidence and risk of IF in preterm infants with or without NEC.
Design
A matched cohort study to investigate the incidence and risk factors for IF in a population-based setting in Sweden from 1987 to 2009 using the Swedish Patient Register.
Participants
Infants with a diagnosis of NEC (n=720) were matched for gestational age and year of birth with reference individuals without NEC (n=3656). The study cohort was censored at death, IF or at end of follow-up (2 years of age). We calculated HRs with 95%CIs for IF using Cox regression, adjusting for pertinent perinatal factors.
Results
IF was 15 times more common in the infants with NEC compared with the reference infants (HR=7.2, with 95% CI 3.7 to 14.0). Other risk factors for IF were small for gestational age, extreme preterm birth and abdominal surgery. Neonatal mortality in infants with NEC decreased from 20.6% in 1987–1993 to 10.4% in 2007–2009.
Conclusion
IF was more common in the infants with NEC but was also linked to extreme preterm birth, a history of abdominal surgery and small for gestational age. IF was more common at the end of the study period, indicating that it increases when more preterm infants with NEC survive the neonatal period.
Keywords: neonatology, gastroenterology, infant feeding, nutrition, paediatric surgery
What is already known on this topic?
Intestinal failure (IF) is more common in necrotising enterocolitis (NEC) infants with a severe stage of the disease and who are treated with surgery. Intrauterine growth restriction and extreme prematurity are other established cases of this condition.
The risk of IF was about seven times higher in the infants with NEC than in the reference group of infants. Previous NEC, abdominal surgery, extreme preterm birth and small for gestational age were risk factors in a Swedish nationwide study. It was also more common with IF at the end of the study period, potentially associated with a reduction in neonatal mortality in NEC infants.
What this study hopes to add?
The risk of IF was about seven times higher in the infants with NEC than in the reference group of infants. Previous NEC, abdominal surgery, extreme preterm birth and small for gestational age were risk factors in a Swedish nationwide study. It was also more common with IF at the end of the study period, potentially associated with a reduction in neonatal mortality in NEC infants.
Better knowledge of predisposing factors for IF may improve early intervention and detection of the condition. Our findings may help informing patients and families of complications following NEC and surgical procedures.
Introduction
Paediatric intestinal failure (IF) is a malabsorption disorder, often affecting those preterm infants surviving severe necrotising enterocolitis (NEC), a serious condition causing long-term dependency on parenteral nutrition. NEC is characterised by an exaggerated gastrointestinal inflammatory response from the highly immune-reactive and immature mucosa.1 2 NEC can progress quickly from onset to severe disease with bowel necrosis, perforation and even death.2–4
In severe NEC, there is often a need for bowel resection due to bowel necrosis and perforation leading to shortening of the bowel length. This condition, combined with malabsorption, is a cause of short bowel syndrome.5 6 The European Society for Clinical Nutrition and Metabolism has defined IF for adults as the ‘reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, such that intravenous supplementation is required…’.7
IF has largely been studied in infants affected by NEC. The incidence of IF varies from 2% in medically treated infants to 42% in surgically treated infants.8 In a Canadian study, IF, defined as the need for prolonged parenteral nutrition, occurred in up to 42% of the patients.9 Direct comparisons between populations for risk factors and incidence are difficult because no scientific body has formulated a uniform definition and classification of IF that is being used in research. In addition to the definitional issue, most studies lacked a control or comparison group.
Therefore, this study sought to investigate the incidence of IF and its risk factors in a cohort with NEC compared with a large reference cohort without NEC.
Material and methods
Study design
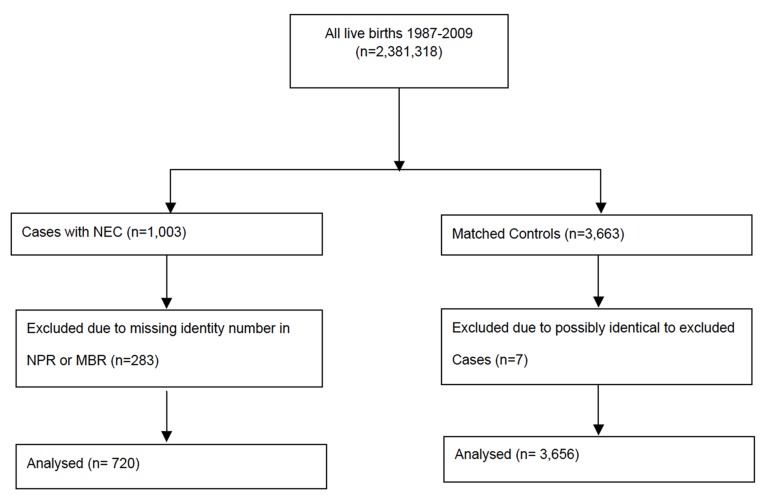
We performed a matched cohort study that included preterm infants with NEC and reference preterm infants (controls) as the study population (figure 1). The study design and data collection process were described in detail in a previous nationwide observational study on NEC.10 The present study population was followed until occurrence of IF, death or the end of study period (2 years of attained age), whichever occurred first. The individuals with NEC and the reference individuals without NEC were matched for gestational age (GA) in days (categorised as degree of prematurity) and birth year.
Figure 1.
Flow chart describing selection and exclusion of cases and controls. First, NEC cases were identified, cleaned of any multiple registrations and excluded if they did not meet inclusion criteria. Then, controls were randomly selected for each NEC case. *Personal identify number. MBR, Medical Birth Register; NEC, necrotising enterocolitis; NPR, National Patient Register.
Data collection
Relevant data were obtained from the following health data registries11 12 held by the Swedish National Board of Health and Welfare: the National Patient Register (NPR),13 the Medical Birth Register (MBR) and the Cause of Death Register (CDR). We used the NPR to obtain data on healthcare. The NPR contains nationwide information on International Statistical Classification of Diseases and Related Health Problems (ICD) diagnosis on all inpatient care from 1987 and on all outpatient care from 2001 and beyond. The register does not contain data on primary care.
All children born between 1987 and 2009 with a diagnosis of NEC in health data registers were identified. The diagnosis of NEC was based on ICD codes: ICD-9 (Ninth Revision) (code 777F) or ICD-10 (Tenth Revision) (code P779). The MBR includes antenatal and perinatal data on more than 98% of all births in Sweden since 1973.14 GA, weight at birth and other perinatal data were obtained by linkage with the MBR based on the children’s unique national personal identity number assigned to 99.9% of all Swedish citizens or permanent residents.15 Further details of this cohort have been reported elsewhere.10 For each NEC case, up to six reference individuals matched for calendar year and GA were randomly selected. Because of an insufficient number of available individuals, especially among the most preterm infants, the final number of matched reference individuals varied between one and six per index individual, which also resulted in a slightly uneven distribution of infants with or without NEC across GA and birth weight categories.
Definition of IF
The study population was followed by linking it with the NPR from 1987 to 2011, including follow-up data to catch all episodes of IF, defined as the presence of one of the ICD-9 or ICD-10 discharge diagnoses or procedural codes presented in table 1 within 14 days to 2 years of follow-up since each individual’s birth date. IF was defined as readmission registered in the NPR. Diagnosis suggesting IF within 14 days since day of birth was disregarded because many preterm infants receive total parenteral nutrition during a brief period after birth as standard care before reaching full enteral feeding. The study population was followed until the incidence of IF or death (censoring events).
Table 1.
List of ICD-9 and ICD-10 diagnoses for intestinal failure
| Diagnosis/procedure | ICD-9 | ICD-10 |
| Intestinal malabsorption |
579W
579X |
K904 K908/9 |
| Postoperative malabsorption/short bowel syndrome | 579D | K912 |
| Parenteral nutrition | 9915 | DV055 |
579 C was excluded because of inconsistencies between ICD-9/ICD-10 classifications.
ICD-9/10, International Classification of Disease Codes and Related Health Problems – Ninth Revision/Tenth Revision.
Variables
Neonatal variables
Data on neonatal factors were collected via the MBR. Birth weight was divided into two categories:<1500 g and ≥1500 g. GA was divided into extremely preterm (<28 weeks), very preterm (28–31 weeks), moderately preterm (32–36 weeks) and full term (37–42 weeks). Moreover, the relative size in comparison with GA was grouped into appropriate for GA (AGA), large for GA (LGA) and small for GA (SGA), all based on the information in the MBR. Missing information on SGA was supplemented with data from the NPR (ICD-9 codes 764A and 764B and ICD-10 code P050). Perinatal asphyxia was defined as having an Apgar score <7 at ≥5 min. Children were also divided according to area of birth (Stockholm vs the rest of the country), which was done because of previously noted regional differences in NEC incidence.10 To achieve balanced categories, the study period was divided into birth cohorts: 1987–1993, 1993–2001, 2002–2006 and 2007–2009 (quartiles of the study population). Evidence of death was collected from the MBR and cross-checked with the CDR. Neonatal mortality was defined as death occurring <28 days after birth.
Abdominal surgery
Online supplementary appendix 1 shows all ICD-9 and ICD-10 procedural codes used to define surgical procedures. The most common procedure was laparotomy with or without intestinal resection.
bmjpo-2018-000316supp002.docx (44.8KB, docx)
Maternal factors
Data on prepregnancy maternal smoking and at week 32 (for mothers whose pregnancy lasted there was additional registration of smoking status) were obtained from the MBR and divided into smokers and non-smokers. Maternal education was divided into ≤12 years or >12 years of formal education (secondary school vs tertiary level education).
Statistical analysis
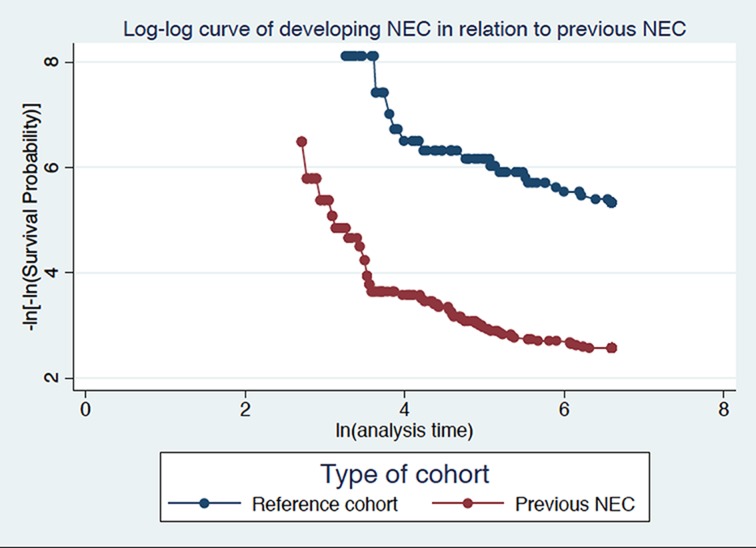
Differences in categorical variables were analysed using a two-sided χ2 test or Fisher’s exact test (whenever appropriate), with the significance level set at p<0.05. The cumulative incidence of IF was defined as the number of new cases of IF divided by the number of NEC individuals and reference individuals without NEC during 2 years of follow-up. Table 3 presents the distribution of potential risk factors by NEC individuals and reference individuals without NEC. Cox regression analysis was performed to examine potential risk factors for IF in the study population. Data were censored at death, occurrence of IF within 2 years of follow-up or at the end of study period (maximum 730 days), whichever came first. The proportional hazards assumption was tested graphically using log-minus-log curves (see figure 2) Based on our a priori hypothesis, sex and presence of growth restriction (SGA vs LGA/AGA) were also included in the regression model.9 Additional variables in the regression model were evaluated separately. Variables achieving a p value <0.10 were retained in the full multivariable regression model using a forward selection procedure. Overlapping variables, that is, the degree of prematurity and birth weight category, were assessed; however, only the degree of prematurity was kept in the final model to avoid adjusting for overlapping variables. A likelihood ratio test was performed to assess the models.
Table 3.
Perinatal variables in individuals with NEC divided by status of intestinal failure
| NEC individuals, without IF | NEC individuals, with IF | Fisher’s exact test* | |
| N (%) | N (%) | P values | |
| Total | 677 (100) | 43 (100) | |
| Variable | |||
| Category | |||
| Gender | |||
| Male | 371 (54.8) | 23 (53.5) | 0.88 |
| Female | 306 (45.2) | 20 (46.5) | |
| Degree of prematurity (gestational weeks)† | |||
| 32–42 | 246 (36.3) | 9 (20.9) | 0.07 |
| 28–31 | 184 (27.2) | 12 (27.9) | |
| <28 | 247 (36.5) | 21 (51.2) | |
| Size relative to gestational age (GA)‡ | |||
| LGA and AGA (3) | 520 (76.8) | 24 (55.8) | <0.01 |
| SGA | 157 (23.2) | 19 (44.2) | |
| Weight category | |||
| ≥1500 g | 272 (40.1) | 10 (23.3) | 0.05 |
| <1500 | 387 (57.2) | 33 (76.7) | |
| Missing | 18 (2.7) | 0 (0) | |
| Perinatal asphyxia§ | |||
| No | 499 (73.7) | 33 (76.7) | 0.85 |
| Yes | 134 (19.8) | 7 (16.3) | |
| Missing | 44 (6.5) | 3 (7.0) | |
| Abdominal surgery¶ | |||
| No | 544 (80.4) | 19 (44.2) | <0.01 |
| Yes | 133 (19.7) | 24 (55.8) | |
| Birth cohort (year)** | |||
| 1987–1993 | 188 (27.8) | 1 (2.3) | <0.01 |
| 1994–2001 | 159 (23.5) | 3 (7.0) | |
| 2002–2006 | 184 (27.2) | 12 (27.9) | |
| 2007–2009 | 146 (21.6) | 27 (62.8) | |
| Maternal smoking | |||
| No | 574 (84.8) | 40 (93.0) | 0.18 |
| Yes | 103 (15.2) | 3 (7.0) | |
| Missing | |||
| Region in Sweden | |||
| Rest of Sweden | 440 (65.0) | 25 (58.1) | 0.36 |
| Stockholm county | 237 (35.0) | 18 (41.9) | |
*A p value <0.05 was significant.
†GA in weeks.
‡Size in relation to GA: LGA or AGA and SGA.
§Apgar <7 at or more than 5 min.
¶Defined by International Classification of Disease Codes and Related Health Problems codes versions 9 and 10.
**Based on infant’s birth year.
AGA, appropriate for GA; LGA, large for GA; NEC, necrotising enterocolitis; SGA, small for GA.
Figure 2.
Log-log curve showing proportional hazard assumption divided in NEC and reference cohort. Survival time over time is plotted in the NEC versus reference cohort. NEC, necrotising enterocolitis.
Finally, a multivariable Cox regression model was constructed to include the following variables: gender, degree of prematurity, presence of growth restriction study period (birth cohort in quartiles), previous abdominal surgery and history of NEC. Table 5 summarises the results of the Cox regression analyses, estimating the relative risk of IF following NEC expressed as HRs with 95% CIs.
Table 5.
Risk of intestinal failure in the study population after adjusting for potential confounding variables
| Variable | Univariable model* | Multivariable model† | ||
| Category | HR | 95% CI | HR | 95% CI |
| History of NEC | ||||
| No | Reference | 1.0 | Reference | 1.0 |
| Yes | 15.9 | 9.0 to 28.3 | 7.2 | 3.7 to 14.0 |
| Sex | ||||
| Male | Reference | 1.0 | Reference | 1.0 |
| Female | 1.2 | 0.7 to 1.9 | 1.0 | 0.6 to 1.7 |
| Degree of prematurity (gestational weeks)‡ | ||||
| 32–42 | Reference | 1.0 | Reference | 1.0 |
| 28–31 | 2.0 | 1.0 to 4.3 | 1.2 | 0.5 to 2.5 |
| <28 | 4.5 | 2.3 to 8.8 | 1.9 | 1.0 to 3.9 |
| Size in relation to gestational age (GA)§ | ||||
| LGA and AGA | Reference | 1.0 | Reference | 1.0 |
| SGA | 4.0 | 2.4 to 6.7 | 3.0 | 1.7 to 5.0 |
| Perinatal asphyxia¶ | ||||
| No | Reference | 1.0 | N/A | N/A |
| Yes | 1.5 | 0.7 to 2.9 | ||
| Abdominal surgery** | ||||
| No | Reference | 1.0 | Reference | 1.0 |
| Yes | 18.9 | 11.3 to 31.5 | 6.2 | 3.4 to 11.3 |
| Maternal smoking | ||||
| No | Reference | 1.0 | N/A | N/A |
| Yes | 0.5 | 0.2 to 1.1 | ||
| Birth cohort (year)†† | ||||
| 1987–2001 | Reference | 1.0 | Reference | 1.0 |
| 1994–2001 | 0.9 | 0.2 to 4.2 | 0.7 | 0.2 to 3.1 |
| 2002–2006 | 4.2 | 1.4 to 12.5 | 3.4 | 1.1 to 10.2 |
| 2007–2009 | 11.0 | 3.9 to 30.8 | 8.0 | 2.8 to 23.1 |
| Region in Sweden | ||||
| Rest of Sweden | Reference | 1.0 | N/A | N/A |
| Stockholm county | 1.7 | 1.0 to 2.9 | ||
*Adjusted for each variable, one by one.
†Adjusted for gender, degree of prematurity, intrauterine growth restriction (LGA/AGA vs SGA), study period, previous abdominal surgery and history of NEC.
‡NEC, necrotising enterocolitis.
‡GA in weeks.
§Size in relation to GA: LGA or AGA, and SGA.
¶Apgar <7 at or more than 5 min.
**Defined by International Classification of Disease Codes and Related Health Problems codes versions 9 and 10.
††Based on infant’s birth year.
AGA, appropriate for GA; LGA, large for GA; NEC, necrotising enterocolitis; SGA, small for GA.
Stata V.14 was used to conduct all analyses.
Results
Selection of case and controls cohort
One thousand and three registrations of hospital discharges of patients with a NEC diagnosis were identified in the MBR or NPR. Some 283 of these discharge registrations lacked complete identity information in the MBR or NPR. These registrations were excluded. The total number of excluded individuals may be fewer, as some of these registrations may refer to the same patients. After this exclusion, there remained 720 identified separate patients with NEC that constitute the NEC cohort. Moreover, 3664 matched controls without NEC were identified. Seven of them were excluded because they could not be convincingly be separated from the 283 excluded NEC registrations. The reason for this was the lacking identity information and the limited number of eligible controls in the children with the shortest GA group. Figure 1 outlines the details of how the study population was collected. Thus, the final sample comprised 3656 infants as the reference group without NEC. Table 2 shows neonatal and demographic characteristics of the study population.
Table 2.
Neonatal characteristics of the study population, divided by history of NEC
| Reference individuals | NEC individuals | χ2 test* | |
| Total, n (%) | 3656 (100) | 720 (100) | |
| Variable | |||
| Category | |||
| Gender | |||
| Male | 2006 (54.9) | 394 (54.7) | 0.94 |
| Female | 1650 (45.1) | 326 (45.3) | |
| Degree of prematurity (gestational weeks)† | |||
| 32–42 | 1529 (41.8) | 255 (35.4) | <0.01 |
| 28–31 | 1065 (29.1) | 196 (27.2) | |
| <28 | 1062 (29.1) | 269 (37.4) | |
| Mean (weeks) | 31.3 | 30.5 | |
| Median | 30 | 29 | |
| Birth weight category (g) | |||
| ≥1500 | 1843 (50.4) | 282 (39.2) | <0.01 |
| <1500 | 1771 (48.4) | 420 (58.3) | |
| Missing | 42 (1.1) | 18 (2.5) | |
| Size relative to gestational age (GA)‡ | |||
| LGA and AGA | 3060 (83.7) | 544 (75.6) | <0.01 |
| SGA | 596 (16.3) | 176 (24.4) | |
| Perinatal asphyxia§ | |||
| No | 2916 (79.8) | 532 (73.9) | <0.01 |
| Yes | 590 (16.1) | 141 (19.6) | |
| Missing | 150 (4.1) | 47 (6.5) | |
| Abdominal surgery¶ | |||
| No | 3574 (97.8) | 563 (78.2) | <0.01 |
| Yes | 82 (2.2) | 157 (21.8) | |
| Birth cohort (year)** | |||
| 1987–1993 | 1052 (28.8) | 189 (26.3) | 0.34 |
| 1994–2001 | 806 (22.1) | 162 (22.5) | |
| 2002–2006 | 1015 (27.8) | 196 (27.2) | |
| 2007–2009 | 783 (21.4) | 173 (24.0) | |
| Region in Sweden | |||
| Rest of Sweden | 2852 (78.0) | 465 (64.6) | <0.01 |
| Stockholm county | 804 (22.0) | 255 (35.4) | |
| Presence of intestinal failure¶ | |||
| No | 3640 (99.6) | 677 (94.0) | <0.01 |
| Yes | 16 (0.44) | 43 (6.0) | |
| Presence of neonatal death†† | |||
| No | 3351 (91.7) | 613 (85.1) | <0.01 |
| 305 (8.3) | 107 (14.9) |
*P value <0.05 was considered significant.
†GA in weeks.
‡Size in relation to GA: LGA or AGA, and SGA.
§Apgar <7 at or more than 5 min.
¶) Defined by International Statistical Classification of Diseases and Related Health Problems codes versions 9 and 10.
**Based on infant’s birth year.
††Registered in the Causes of Death Register, during the neonatal period (≤28 days of age).
AGA, appropriate for GA; LGA, large for GA; NEC, necrotising enterocolitis; SGA, small for GA.
Characteristics of the study population
Table 2 shows the neonatal and demographic characteristics of the study population of individuals with or without NEC. The male–female ratio was close to one. More individuals with NEC belonged to the lowest GA category (37.4% vs 29.1% of reference individuals, p<0.01). About 58.3% of the NEC individuals had a birth weight <1500 g compared with 48.4% of the reference individuals (p<0.01) (table 2). NEC was more common in the individuals born in Stockholm County than in the rest of the country (35.4% vs 22.0%, p<0.01) (table 2).
There were no significant differences in maternal characteristics such as age, tertiary education and maternal smoking according to NEC status (online supplementary table A).
bmjpo-2018-000316supp001.docx (17KB, docx)
The neonatal mortality (≤28 days) was higher in NEC individuals (14.9%) than in reference individuals (8.3%), p<0.001 (table 2).
Individuals with NEC more frequently underwent abdominal surgery (21.8%, n=157 compared with reference individuals (2.2%, n=82, p<0.01) (table 2).
Incidence and distribution of risk factors for IF in the study cohort
The cumulative incidence of IF within the cohort of NEC individuals was 6.0% (43/720 individuals) compared with 0.4% or 16/3656 of the reference individuals without NEC (table 2), corresponding to a univariable HR of 15.9 with 95% CI (9.0 to 28.3) and a multivariable HR of 7.2 with 95% CI (3.7 to 14.0) (table 5).
In table 3, the distribution of risk factors in NEC individuals by presence of IF is presented. Notably, individuals with IF were over-represented in the extremely preterm NEC (≤28 weeks) category (51.2% for those with IF vs 36.5% for those without IF), although the difference was not statistically significant (p=0.07). Furthermore, within the NEC cohort, SGA was more common in those who developed IF (44.2%) compared with those without IF (23.2%) (p<0.01). About two-thirds of the IF cases (62.8%) were found in the birth cohort 2007–2009. The neonatal characteristics of the reference individuals without NEC for IF are shown in table 4
Table 4.
Perinatal variables in reference individuals divided by status of intestinal failure (IF)
| Reference individuals, without IF | Reference individuals, with IF | Fisher’s exact test | |
| N (%) | N (%) | P values* | |
| Total | 3640 (100) | 16 (100) | |
| Variable | |||
| Category | |||
| Gender | |||
| Male | 1999 (54.9) | 7 (43.8) | 0.45 |
| Female | 1641 (45.1) | 9 (56.3) | |
| Degree of prematurity (gestational weeks)† | |||
| 32–42 | 1526 (41.9) | 3 (18.8) | 0.05 |
| 28–31 | 1061 (29.2) | 4 (26.7) | |
| <28 | 1053 (28.9) | 9 (56.3) | |
| Size relative to gestational age (GA)‡ | |||
| LGA and AGA | 3051 (83.8) | 9 (56.3) | <0.01 |
| SGA | 589 (16.2) | 7 (43.8) | |
| Weight category (g) | |||
| ≥1500 | 1838 (50.5) | 5 (31.3) | 0.28 |
| <1500 | 1760 (48.4) | 11 (68.9) | |
| Missing | 42 (1.2) | 0 (0) | |
| Perinatal asphyxia§ | |||
| No | 2903 (79.8) | 13 (81.2) | 0.87 |
| Yes | 587 (16.1) | 3 (18.8) | |
| Missing | 150 (4.1) | 0 (0) | |
| Abdominal surgery¶ | |||
| No | 3564 (97.9) | 10 (62.5) | <0.01 |
| Yes | 76 (2.1) | 6 (37.5) | |
| Birth cohort (year)** | |||
| 1987–1993 | 1049 (28.8) | 3 (18.8) | 0.02 |
| 1994–2001 | 806 (22.1) | 0 (0) | |
| 2002–2006 | 1010 (27.8) | 5 (31.3) | |
| 2007–2009 | 775 (21.3) | 8 (50.0) | |
| Maternal smoking | |||
| No | 3004 (82.5) | 14 (87.5) | 1.00 |
| Yes | 636 (17.5) | 2 (12.5) | |
| Region in Sweden | |||
| Rest of Sweden | 2839 (78.0) | 13 (81.3) | |
| Stockholm county | 801 (22.0) | 3 (18.8) | 0.75 |
*A p value of <0.05 was significant.
†GA in weeks.
‡Size in relation to GA: LGA or AGA, and SGA.
§Apgar <7 at or more than 5 min.
¶According to ICD-9 and ICD-10 procedural codes.
**Based on infant’s birth year.
AGA, appropriate for GA; ICD-9, International Classification of Disease Codes and Related Health Problems, Ninth Revision; ICD-10, International Classification of Disease Codes and Related Health Problems, Tenth Revision; LGA, large for GA; SGA, small for GA.
The most common main diagnosis in IF patients without previous history of NEC was very and extreme prematurity (P072-3; n=9); very/extreme low birth weight (P07.0/1; n=11) and light for GA (P05x; n=9). These are shown categorised in table 4. Other diagnoses included neonatal skin infection (P39.4; n=1) and haematemesis (K92.0; n=1).
The risk of developing IF in the study cohort
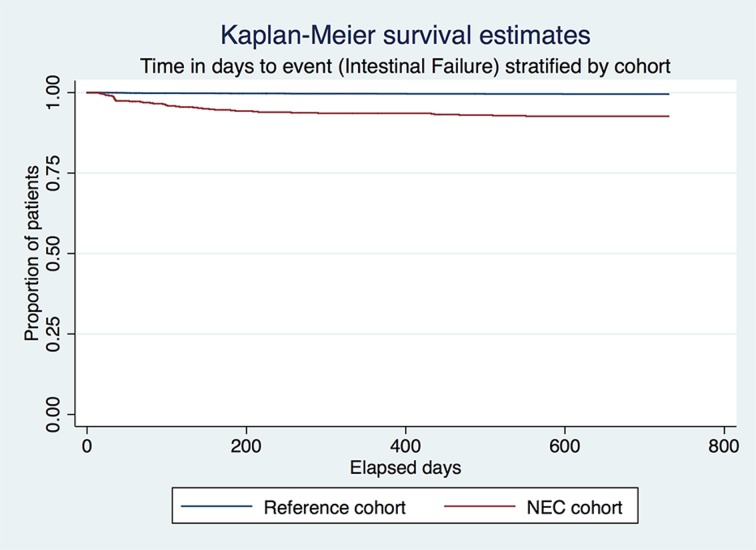
Table 5 lists the HR of developing IF. There were significant associations between the risk of IF and history of NEC and abdominal surgery, degree of prematurity, presence of growth restriction (SGA) and birth cohort. Maternal smoking rendered a non-significant negative risk estimate for IF in univariable analysis (HR=0.5 with 95% CI 0.2 to 1.1). The strongest predictors of IF were history of NEC (HR=7.2 with 95% CI 3.7 to 14.0) and birth cohort 2007–2009 using 1987–1993 as a reference, corresponding to a HR=8.0, 95% CI 2.8 to 23.1. As shown in figure 3, there is a clear difference in incidence of IF in between the NEC and reference cohort and that most cases occur within the first year of follow-up from study start.
Figure 3.
Kaplan-Meier table showing survival (time-to event; intestinal failure) stratified on type of cohort, NEC versus reference cohort. NEC, necrotising enterocolitis.
The number of admissions among cases with IF varies depending on whether they had NEC or belonged to the reference group; that is, there was a mean of nine admissions (range 2–41) among the reference cohort compared with six (range 1–28) in the NEC cohort.
Discussion
We found that 6% of the NEC individuals developed IF compared with 0.4% of the reference individuals, corresponding to a sevenfold increased risk after adjustment for perinatal factors. We also demonstrated that extreme preterm birth, SGA and previous abdominal surgery increased the risk of IF. However, neither sex nor maternal smoking influenced the risk of IF. Moreover, the risk of IF varied over time, with the highest risk occurring for infants born during 2007–2009, a finding that is yet to be explained. One reason could be increased registration of Total parental nutrition (TPN) as a procedural code and/or increased usage of TPN. The mean GA in weeks among infants born in 1987–2003 was 32.6 (median of 32 weeks) compared with 29.7 (median of 28 weeks) in 2007–2009. Thus, the population is indeed more premature and more prone to complications such as NEC and secondary IF. Moreover, the birth weight differs significantly depending on birth cohort, for example, 17% of infants born between 2007 and 2009 had a birth weight less than 750 g compared with 6.5% in 1987–1993. The proportion of infants with a birth weight more than 2500 g was 16.5% in 2007–2009 compared with 39% in 1987–1993 (data not shown).
Possibly, treatment of NEC has been more successful over time in increasing survival but at the same time causing long-term complications such as IF. In fact, neonatal mortality dropped from 20.6% from 1987 to 1997 to 10.4% for the birth cohort in 2007–2009, supporting this notion (table 2). We also examined the impact of being born in Stockholm County, where an over-representation of NEC was observed.10 This analysis showed an increased risk of IF in the univariable analysis. However, when we adjusted for history of NEC, being born in Stockholm County did not increase the risk of IF.
We examined maternal smoking to elucidate whether a protective or causative association exists. Other types of gastrointestinal inflammation (eg, ulcerative colitis) have a protective relationship with smoking. In contrast, increased risk of Crohn’s disease and NEC per se is seen in maternal smokers.16 However, our data failed to show a distinct relationship with the development of IF.
Comparison with other studies
IF was found in 6.0% of the NEC cohort and needs to be related to the stage of NEC and previous treatment (including surgery). For instance, Duro and colleagues8 demonstrated in a US multi-centre cohort study that IF is more prevalent in surgically treated NEC patients (42%), largely correlating with a more severe form of disease,17 than in those with medically treated NEC in which only 2% developed IF. Another US study reported an IF proportion approaching 14% in NEC-afflicted patients with a severe disease.18 In our study, 21% of the surgically treated infants with NEC developed IF. The reasons for the varying incidence of IF could be several, but the inconsistent definition of IF likely contributes to this discrepancy.
Strengths and weaknesses
Unlike other studies, we incorporated a matched reference group (‘control group’) that allowed us to assess risk factors compared with children born at the same GA without developing NEC. Such a manipulation avoids potential bias from preterm birth. Other studies have investigated IF in individuals with NEC without a control group, focusing exclusively on NEC survivors.8 9 Other main strengths of the study are the long study period and the population-based approach with nationwide coverage. Furthermore, the use of high quality, prospectively recorded and validated health data minimises recall bias.
The intention of the matching procedure was to ensure a similar proportion of individuals based on GA (table 2). However, there was a lack of eligible reference individuals in this category of extreme preterm infants, resulting in more individuals with NEC in the extreme preterm category. Nevertheless, we have adjusted for the in the multivariable regression analysis and thus the risk estimate for IF should not be materially affected.
Because NEC is a rare disease and because IF is an uncommon complication, statistical power has been an issue in earlier research and is a potential limitation in our study as well. However, any misclassification of outcome should be equally distributed regarding NEC status and thus only dilute our risk estimates. Although our study population was relatively large and the follow-up extended over 24 years, the limited number of patients with IF restricted our exploration of uncommon risk factors. Another limitation is our definition of IF. No standard definition of this diagnosis exists in Swedish registers and we were therefore forced to use proxy variables (such as intestinal/postoperative malabsorption and parenteral nutrition) to detect cases of IF. Ideally, the best course would have been to manually review all medical charts indicating IF to validate and reduce misclassification of the IF outcome. Our definition may suffer from both over and under ascertainment of IF, rendering incidence comparisons between populations difficult. In general, an earlier validation study found that 85%–95% of all register diagnoses were correct.13 The definition of IF often depends on the use of parenteral nutrition and therefore the time limit used for diagnosis determines the frequency of IF. As an example, the proportion of infants suffering from IF after severe NEC varied from 42% when using 42 days of parenteral nutrition to 13% when using at least 90 days of parenteral nutrition.9
In addition, to assess the true incidence of IF, we would have needed to conduct a prospective cohort study with continuous data collection, similar for all participants, and knowledge of potential confounding factors; that is, other risk factors that could explain the increased risk in the NEC cohort. In our study, we lacked direct access to the patients’ charts, which made the outcome assessment more difficult and less accurate. Instead, we used register-based data with a high internal validity in general.12
Conclusion
In determining the course of action when caring for NEC-afflicted children, the knowledge of long-term complications is important to guide therapy. This study found a sevenfold increased risk of IF in patients with NEC. The incidence of IF in Swedish patients with NEC is otherwise comparable with that in other studies. Regrettably, limited power restricted our exploration of risk factors for IF in patients with NEC. IF was more common in the infants born at the end of the study period, possibly explained by better neonatal survival. In view of this, increased efforts are needed to alleviate post-NEC complications such as IF.
Supplementary Material
Footnotes
Contributors: TSB contributed to the study design, performed the statistical analyses and drafted the manuscript. MA conceived the study design, performed the data acquisition and participated in the data analysis. MA performed critical revision of the manuscript. AE carried out critical revision of the manuscript and aided in the interpretation of the results. OB helped with the statistical analyses and in drafting the manuscript. OB participated in the interpretation of the results. JFL provided critical revision of the manuscript and interpretation of the data. REA conceived the study design, participated in the data acquisition and the data analysis and interpretation of the results. REA performed critical revision of the manuscript. All authors approved the final version of the manuscript.
Funding: The Stockholm County Council provided funding for TSB’s clinical postdoctoral appointment. AE was funded by grants from the ALF agreement between the Swedish government and Sahlgrenska University Hospital. MA and REA were funded by the Region of Östergötland, Sweden; Futurum, the Academy of Health Care, Jönköping County Council, Jönköping, Sweden, and the Medical Research Council of Southeast Sweden.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study was approved by the Regional Ethical Review Board in Linköping (permit number 2010/405–32).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: According to Swedish Law, the data used in this study cannot be placed in a publicly available repository. However, researchers can, after ethical approval, apply for the data from Statistics Sweden and the Swedish National Board of Health and Welfare.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand RJ, Leaphart CL, Mollen KP, et al. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock 2007;27:124–33. 10.1097/01.shk.0000239774.02904.65 [DOI] [PubMed] [Google Scholar]
- 3.Eaton S, Rees CM, Hall NJ. Current research in necrotizing enterocolitis. Early Hum Dev 2016;97:33–9. 10.1016/j.earlhumdev.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 4.Clark RH, Gordon P, Walker WM, et al. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol 2012;32:199–204. 10.1038/jp.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pironi L. Definitions of intestinal failure and the short bowel syndrome. Best Pract Res Clin Gastroenterol 2016;30:173–85. 10.1016/j.bpg.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 6.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology 1997;113:1767–78. 10.1053/gast.1997.v113.pm9352883 [DOI] [PubMed] [Google Scholar]
- 7.Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr 2015;34:171–80. 10.1016/j.clnu.2014.08.017 [DOI] [PubMed] [Google Scholar]
- 8.Duro D, Kalish LA, Johnston P, et al. Risk factors for intestinal failure in infants with necrotizing enterocolitis: a Glaser Pediatric Research Network study. J Pediatr 2010;157:203–8. 10.1016/j.jpeds.2010.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elfvin A, Dinsdale E, Wales PW, et al. Low birthweight, gestational age, need for surgical intervention and gram-negative bacteraemia predict intestinal failure following necrotising enterocolitis. Acta Paediatr 2015;104:771–6. 10.1111/apa.12997 [DOI] [PubMed] [Google Scholar]
- 10.Ahle M, Drott P, Andersson RE. Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987-2009. Pediatrics 2013;132:e443–e451. 10.1542/peds.2012-3847 [DOI] [PubMed] [Google Scholar]
- 11.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–36. 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 12.Rosén M. National Health Data Registers: a Nordic heritage to public health. Scand J Public Health 2002;30:81–5. 10.1177/14034948020300020101 [DOI] [PubMed] [Google Scholar]
- 13.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welfare TNBoHa. The Swedish Medical Birth Register. Volume 2016;2016. [Google Scholar]
- 15.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659–67. 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downard CD, Grant SN, Maki AC, et al. Maternal cigarette smoking and the development of necrotizing enterocolitis. Pediatrics 2012;130:78–82. 10.1542/peds.2011-3808 [DOI] [PubMed] [Google Scholar]
- 17.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. 10.1016/S0031-3955(16)34975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelleher J, Mallick H, Soltau TD, et al. Mortality and intestinal failure in surgical necrotizing enterocolitis. J Pediatr Surg 2013;48:568–72. 10.1016/j.jpedsurg.2012.11.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2018-000316supp002.docx (44.8KB, docx)
bmjpo-2018-000316supp001.docx (17KB, docx)