Abstract
Introduction
Conducting research with children in low/middle-income countries (LMIC) requires consideration of socioeconomic inequalities and cultural and linguistic differences. Our objective was to survey the literature on informed consent in paediatric LMIC research, assessing for practical guidance for culturally and linguistically appropriate procedures.
Methods
We conducted a scoping review on informed consent in paediatric LMIC research searching the PubMed, Web of Science and PsycINFO databases. Eligible articles were published in English, from any date range, of any study design or format.
Results
The search identified 2027 references, of which 50 were included in the analysis following full-text review. Reviewed guidelines emphasised individual, informed and voluntary consent from parents and caregivers. Reviewed articles provided detailed practical guidance on adapting these guiding principles to LMIC settings, including considerations for community engagement, verbal or other alternative consent procedures for low-literacy settings or less commonly spoken languages and guarding against therapeutic misconception by caregivers. There was uncertainty, however, on how to best protect individual autonomy, especially when influenced by gender dynamics, leadership hierarchies or the social status of researchers themselves. There was, furthermore, limited research discussing the special case of research involving adolescents or of procedures for documenting assent by participating children.
Conclusions
A scoping review of paediatric research in LMICs revealed substantial guidance on several features of culturally appropriate informed consent. However, additional research and guidance is needed, especially in the areas of gender imbalances, research with adolescents and children’s own assent to participate in research.
Keywords: ethics, health services research
What is already known on this topic?
Conducting research with children in low/middle-income countries (LMIC) requires careful consideration of socioeconomic inequalities and cultural and linguistic differences.
International standards for the conduct of paediatric research include informed consent, voluntariness and assent, but there is limited guidance on operationalising these concepts in LMICs.
What this study hopes to add?
Highlighting consensus for best practices in community engagement, verbal and alternative consent procedures and guarding against therapeutic misconception in interventional and randomised controlled trial designs.
Demonstrating where additional research i, especially around the protection of the individual autonomy of caregivers and safeguarding children’s own assent to participate in research.
Introduction
Prior to World War II, there was little international consensus on the ethical conduct of human subjects research. The Nuremberg code, developed in 1947 during the Nuremberg war crime trials, was one of the first attempts to articulate basic ethical principles, such as the right to informed consent.1 Subsequently, the World Medical Association’s Declaration of Helsinki in 1964 provided a more definitive consensus statement on the core principles of ethical conduct of research—beneficence, self-determination and informed consent—which is widely considered the foundational international document in modern research ethics.2 Practical guidance on ethical practice is well codified in the joint statements produced by the Council for International Organizations of Medical Sciences (CIOMS) and the WHO.3
Extension of ethical research principles to include considerations appropriate for research in paediatric populations is also important, including guidance on obtaining informed consent from parents or guardians, obtaining assent from children themselves and weighing the balance of risks and benefits of proposed research.3 4 Improvements in the conduct and volume of paediatric clinical trials, which have historically been few in number and of lower quality than corresponding trials in adult subjects, have also recently been advocated.5
However, there still remains uncertainty around how best to implement international ethical principles of paediatric research in some settings. This is especially the case in low/middle-income countries (LMIC), and in research with groups such as indigenous populations, speakers of less common languages or populations with high levels of illiteracy. Practically, we experienced this recently while designing a clinical trial of a nutrition intervention for indigenous Maya children in rural Guatemala, and our experience navigating consent, literacy and translingual adaptation in this population prompted our interest in more formally exploring the topic.6 To this end, here we conduct a scoping review of the existing literature on cultural and contextual considerations for informed consent in the conduct of paediatric research in LMICs. Through this review, we identify evidence for specific culturally and contextually sensitive practices, as well as areas where additional research and guideline development is needed.
Methods
Search and inclusion strategy
To identify articles, we searched the PubMed, Web of Science and PsycINFO databases. We conducted searches using a combination of the following key terms: ‘pediatric’ or ‘children’ or ‘adolescents’; ‘research’ or ‘biomedical research’; ‘consent’ or ‘informed consent’ or ‘ethics’; ‘developing countries’ or ‘low income countries’ or ‘middle income countries’; ‘illiteracy’; ‘culturally competent’. We used no date limits and included all articles published through May 2018. In addition, we visited the websites of international health policy organisations to identify ethics guidelines for the conduct of research in LMICs. We also manually reviewed the reference lists of articles identified using the above methods. For this scoping review we included for analysis any type of study design or format (original research, commentary, case study, review, expert opinion), which addressed the informed consent process specifically for paediatric or adolescent populations in LMICs. Articles not in English were excluded.
Data extraction and synthesis
We exported identified articles into an Excel spreadsheet template which recorded location of study, study type and design, study context, aspects of informed consent examined and key findings. Both authors reviewed the study titles and abstracts. After removal of articles which were deemed not eligible for inclusion, one author (MC) performed a full-text review of all the remaining articles. As a scoping review to assess the patterns of existing literature on informed consent in LMIC paediatric research, assessments of individual study bias and quality were not performed. Data extracted from articles were collated in summary form (table 1–8), and major qualitative findings are presented in the following narrative synthesis.
Table 1.
Summary of selected major guidelines on ethical conduct of research in children
| Guideline | Core principles | Considerations for adapting to low-resource, low-literary and minority language settings |
| World Medical Association, Declaration of Helsinki7 |
|
|
| Council for International Organizations of Medical Sciences3 |
|
|
| Standards for Research (StaR) in Child Health4 |
|
|
| National Bioethics Advisory Commission, Ethical and Policy Issues in International Research8 |
|
|
| European Council and European Parliament Guidelines9 |
|
|
Table 2.
Summary of review and opinion articles on ethical conduct of research in children
| Reference (year) | Study description | Study location | Major findings |
| Ott et al 38 (2018) | Review—Participation of children of minor parents in research | Multiple | Discussion on international research documents and existing laws and practices regarding consent for research for children of minor parents. Few countries have regulations about the subject, which might result in exclusion of those children from research. Authors recommend involving minors in the decision-making about their children and adapting consent procedures so minor parents can participate and their children’s vulnerabilities correctly addressed. |
| Zulu et al 34 (2018) | Review—Ethical challenges of postabortion care research in adolescents in LMICs | Multiple | Authors included 14 articles in their analysis. Regarding the consent process, challenges identified include difficulties in seeking consent from parents/guardians of adolescents who are below the consent age, vulnerability of adolescents compromising ability to make decisions, fear of losing access to healthcare affecting informed consent process and inadequate guidance on how and when to involve communities in the consent process. |
| Regmi et al 39 (2017) | Review—Informed consent in health research in LMICs | Multiple, but focused on Nepal | Authors discuss challenges in adapting informed consent: verbal versus written informed consent in areas of limited literacy; difficulties posed by having to translate consent documents to local languages; issues around the legal age to consent; and how clear threshold ages of consent are not clear in local guidelines. |
| Mandava et al 40 (2012) | Review—Comparison between consent processes in low/middle-income countries and developed countries | Multiple | Authors aimed to compare data about comprehension and voluntariness. In both settings, comprehension of study information varies among participants, and comprehension of randomisation and placebo use is poor. Participants in low/middle-income countries seem to be less likely to say they can refuse participation or withdraw and worry more about the consequences of doing so. Recommendations include developing validated questions to measure comprehension and voluntariness and conducting studies on the impact of cultural norms and sociodemographic characteristics on informed consent. |
| Joseph et al 41 (2016) | Review—Views of stakeholders on aspects of conducting research with children in LMICs | Multiple | Regarding informed consent, stakeholders believe that disempowerment, poor education and difficulty in translating scientific concepts were barriers to informed decision-making. Authors recommend simplifying consent forms and presenting them in culturally and linguistically appropriate format with verification of parental comprehension. Authors discuss that Western ethical principles of consent and child assent, autonomy and individualism need to be contextualised. |
| Morrow et al 42 (2015) | Opinion—Consent for paediatric critical care research in South Africa | South Africa | Authors discuss legal issues in South Africa that create confusion for informed consent for children. They identify barriers to the consent process: impracticability of getting consent when urgent action is needed; the validity of consent in high-stress settings; addressing parents during stressful situations; sociocultural issues and the differences in communication and response to authority figures. The authors discuss alternatives to the prospective informed consent, such as the deferred consent model. |
| MacLeod et al 11 (2015) | Review—Ethical issues of paediatric drug trials in LMICs | Multiple | The review discusses vulnerabilities of paediatric research participants, in particular children in LMICs. Authors discuss characteristics of the consent process, and how socioeconomic status, religious belief and distribution of power affect decisions to participate. They point to the need to consider cultural differences, and the appropriateness of obtaining community consent in some contexts. |
| Swain43 (2014) | Opinion—Barriers to paediatric clinical drug trials in low-resource settings, with emphasis in India | India | The author discusses how the consent process for research can be affected by poverty and lack of education. The author points out that the consent process should be clear and assent should be sought from children 7–18 years old, as per Indian guidelines. Deferred consent for neonatal intensive care studies and other high-acuity settings may reduce caregiver stress and be preferred. |
| Bekker et al 31 (2014) | Review—Ethical issues of HIV research in resource limited countries | Multiple | The authors review ethical issues in HIV research with adolescents in LMICs. They point out best practices for consenting adolescents: auditing ethical-legal requirements for consent; involving adolescents in decision-making; ensuring language, age and cultural appropriateness; and giving sufficient time and resources to consent. |
| Ruiz-Casares44 (2014) | Review—Culturally responsive mental health research | Multiple | Regarding informed consent, the author discusses how to obtain culturally appropriate consent, how to ensure adequate understanding of the consent information, consideration of community structures, documenting informed consent and determination of decision-making capacity. |
| Offringa et al 45 (2013) | Review—Background and summary of Standards for Research (StaR) in Child Health published standards on the conduction of paediatric clinical research | NA | Summary of first six StaR Child Health published standards: (1) consent and recruitment; (2) containing risk of bias; (3) data monitoring committees; (4) determining adequate sample sizes; (5) selection, measurement and reporting of outcomes; and (6) age groups for paediatric trials. |
| Daley et al 46 (2013) | Review—Ethical issues associated with autism spectrum disorders research in low/middle-income countries | Multiple | Authors discuss ethical aspects relevant to the conduct of autism spectrum disorders research in low/middle-income countries. They mention challenges to informed consent such as parents' lack of knowledge about research. |
| Denburg et al 47 (2012) | Review—Ethical aspects and challenges of paediatric oncology research in LMICs | Multiple | Authors conducted a review of ethical issues related to standards of care, trial benefits, ethics review and informed consent. They focused on the ethical implications of drug development and intervention research. Regarding informed consent, they discuss illiteracy, social and political power imbalances, validity of consent in face of ancillary benefits of research, mistrust of foreign investigators by parents and difficulties aligning local perspectives with international norms. |
| Mystakidou et al 48 (2009) | Review—Informed consent in human HIV research in low/middle-income countries | Multiple | In trials involving children and adolescents, authors discuss the process of enrolling subjects, including challenges in getting informed consent from parents or guardians while protecting the privacy of the subjects. Most studies on this topic involve adolescents, and there are limited data about the assent process in younger children. Authors discuss the characteristics that informed consent should have in the context of HIV trials in the developing world, including the need to address cultural differences. |
| Bhutta10 (2004) | Review—Analysis of international guidelines on the subject of informed consent | Multiple | Review and discussion of guidelines for obtaining informed consent. The discussion notes that more focus is put on written documentation of consent and less understanding of the process and adaptation to local contexts, and differences regarding when and how communities should be involved in the consent process. |
| McClure et al 32 (2004) | Review—Challenges to conducting HIV vaccine trials with adolescents, including in low/middle-income countries | Multiple | Authors identified challenges to HIV vaccine trials with adolescents. Adolescents are minors and need parental consent for participating in research. At the same time, their autonomy and privacy need to be respected. The consent process might be affected by less perception of personal risk. |
LMIC, low/middle-income country; NA, not applicable.
Table 3.
Summary of articles discussing social norms, decision-making and autonomy*
| Reference (year) | Study description | Study location | Major findings |
| Kongsholm et al 13 (2018) | Qualitative research—Interviews with researchers and donors about consent experience for genetic research | Pakistan | Researchers report adaptations to consent process including use of elder and oral consent; involving literate witnesses to validate written forms; and disclosure of information adapted to educational level. Challenges include no knowledge about consent process by participants and therapeutic misconception. Donors’ motivations for participating include obtaining direct benefit from their participation and a high level of trust in the research team. |
| Embleton et al 49 (2015) | Case study—Ethical guidelines adaptation for three different studies with street connected youth and children | Kenya | The authors describe processes of consent for street-connected children and youth participating in three research projects. They discuss the importance of guidelines and working with local and international committees, ethicists and the community to identify areas of special concern. Key recommendations include involving the community and working within the local sociocultural context. |
| Millum and Emanuel50 (2007) | Case study—Research with abandoned children | Romania | The authors discuss how research with abandoned children might be constrained by the challenge of getting informed consent. This might result in this vulnerable group not being included in research for reasons of convenience. They argue that vulnerable groups can be protected by enrolling them in studies that pose no or minimal risks. |
| Vreeman et al 51 (2012) | Qualitative research—Analysis of community discussion sessions regarding the participation of orphaned children in research | Kenya | Results showed positive attitudes towards the participation of orphaned children in research, mainly because adults assumed that children would be directly benefited. Consent from parents or guardians was considered necessary but getting assent from children was not. The participation of the community in the consent process was considered appropriate. Authors recommend paying attention to misconceptions about research-related benefits. |
| Molyneux et al 36 (2005) | Qualitative research—Community views regarding the informed consent process, in the context of studies being carried out by KEMRI in Kenya | Kenya | Results show that seeking consent from community elders is necessary but does not substitute the need for individual parental consent. Most respondents suggested males should make the decision to participate and that assent should not be sought from children before ages 10–13. For inpatient studies, respondents identified illness severity, potential risks and parents’ ability to understand as factors influencing the consent process. Results of the study show some therapeutic misconception and discrepancies regarding which interventions need permission. |
*In this and subsequent tables, articles are presented by major thematic groupings. Most articles discuss multiple themes, but are grouped here based on the most prominent or significant theme identified in the review.
KEMRI, Kenya Medical Research Institute.
Table 4.
Summary of articles discussing working in low-literate settings, and with indigenous or less commonly spoken languages
| Reference (year) | Study description | Study location | Major findings |
| Mboizi et al 22 (2017) | Mixed methods research—Recall and decay of consent information among parents using an audiovisual tool | The Gambia | Recall of trial procedures and consent process was evaluated using questionnaires at two points in time. Results show overall good recall of consent when using the Speaking Book audiovisual tool. No differences were found between age, occupation, years of education, religion and family type. |
| Kalabuanga et al 18 (2016) | Case study—Description of the consent process during a malaria clinical trial | Democratic Republic of Congo | Authors identified misunderstanding of the informed consent process among parents. They also identified cases where culturally accepted guardians might not have legal authority to consent for research. They discuss how the use of a witness can impair parents’ autonomy by exerting social pressure. In the context of limited access to care, the ancillary benefits of participating in research may be a strong incentive to participate. |
| Martellet et al 17 (2015) | Case study—Informed consent for a vaccine trial | The Gambia, Mali, India, Senegal, Ghana | Informed consent for a vaccine trial was sought from parents/legal guardians of children aged 1–17 years. Written assent was taken from children aged 12–17. They used literate witnesses when participants/parents were illiterate and translated consent forms to local languages. In some areas, consent was done verbally. Written consent forms were always provided. Some study sites used tools to assess understanding of the research project prior to consent. |
| Tindana et al 52 (2012) | Qualitative—Interviews with research staff and mothers of study participants about the informed consent process for a malaria genetics study | Ghana | The consent process was adapted to include community leaders and groups of women. For individual consent, written forms were used but information was adapted to be more relevant to parents. The timing of consent for inpatient cases was modified to obtain it after children had been stabilised. The provision of medical care and direct benefits to children was identified as a motivation for participating. |
Table 5.
Summary of articles discussing gender
| Reference (year) | Study description | Study location | Major findings |
| Kamuya et al 15 (2015) | Qualitative—Focus groups and interviews conducted with participants of RSV and malaria studies | Kenya | Authors describe the phenomenon of silent refusal. Possible causes include avoiding conflict within households, maintaining a good relationship with the research team and retaining study benefits. For women and young adults, it might be a way to exert agency within the patriarchal system. Authors discuss negotiations that take place during the consent process, and how ethical principles are interpreted and negotiated in a context-specific way. |
| Sarkar et al 29 (2009) | Mixed methods research—Comprehension and recall of informed consent process in a paediatric diarrhoea study | India | Findings showed low recall of study purposes 4 years after enrolment. Most respondents were mothers and mentioned spousal approval and free medical care for their children as main motivations to consent and remain in the study. Educational level was significantly associated with recall of study purpose. Few respondents knew they could leave the study at any time. Authors point out the need for continuous reinforcement of the consent process. |
| Minnies et al 12 (2008) | Mixed methods—Recall of the consent process for a study of immune protection against TB | South Africa | Mothers who had consented for the study then completed a questionnaire about key elements of informed consent, recall and understanding. Most obtained scores greater than 75% for recall and understanding. Seventy-nine per cent were aware of the risks and 64% knew participation was voluntary. A higher level of education and being consented by professional nurses were associated with higher recall. Authors suggest monitoring the quality of consent procedures periodically. |
RSV, respiratory syncytial virus; TB, tuberculosis.
Table 6.
Summary of articles discussing communicating about risks and benefits of research
| Reference (year) | Study description | Study location | Major findings |
| Morris and Wilson53 (2014) | Case study—Research on the use of CPAP in intensive care settings | Ghana | Authors describe how consent was obtained, and express concern about the fact that there were no refusals and that this might reflect that consent was not fully informed or participation was not truly voluntary. The authors do not know to which extent parents understood randomisation, or that CPAP could be used independently of study participation. They discuss how the lack of access for medical care might influence the consent process. |
| Ward et al 54 (2018) | Qualitative research—Interviews with stakeholders about ethical aspects in a paediatric malaria vaccine trial | Ghana and Tanzania | Stakeholders identify the importance of community education and a well-adapted consent process in helping to avoid misconception about trial benefits and healthcare service provision, as well as in preventing undue inducement by clearly stating risks and benefits. |
| Devries et al 55 (2015) | Qualitative research—Experiences of children participating in a cluster RCT of a school-based violence prevention intervention | Uganda | Authors describe the consent process for the RCT and present findings from interviews conducted with children after participating. They found some therapeutic misconception about potential benefits and propose that clearer language in the consent forms might help avoid it. |
| Serce et al 30 (2015) | Quantitative—Questionnaires administered to parents to assess potential participation in research | Turkey | Authors perform univariate and multivariate logistic regression to identify characteristics that might predict participation. Factors associated with willingness to consent include satisfaction with the content of the informed consent and being a business owner. Factors associated with refusal of consent were older age of parents and owning a car. Parents responded that learning more about the trial and its benefits, ensuring health coverage and payment of transport expenses would positively influence consent. |
| Angwenyi et al 28 (2014) | Qualitative—Interviews and group discussions with researchers, community members and parents | Kenya | Authors describe and analyse the community engagement process for the trial. Concerning the consent process, they present results on parents’ understanding of the trial 1 year after recruitment. They report low levels of understanding about the purpose of the trial and the randomisation process. There appeared to be less understanding of the trial where there was less community engagement. |
| Paré Toe et al 27 (2013) | Mixed methods research—Assessment of the relevance of the informed consent procedure in a malaria trial comparing the efficacy of two different treatments | Burkina Faso | Results showed that prior knowledge of the trial was significantly associated with the decision to participate. Common reasons for participating were the perceived aid provided by the trial, better quality of care and better quality of the medication. Information about confidentiality, right to withdraw from the study and potential risks was poorly retained. Randomisation was poorly understood. Authors aim to show that there are other factors besides the information received during the consent process that influence parents' decision to participate in the trial. |
| Rajaraman et al 24 (2011) | Mixed methods research—Analysis of relation between parents’ sociodemographic characteristics and likelihood of asking questions during the consent process | India | The study looked at parents asking questions during the informed consent process. 13.4% of parents asked any questions. There was a high association between asking questions and socioeconomic and educational status, and with presence of both parents. Authors conclude that consent materials should be interactive, to make comprehension easier, and that in paediatric trials effort should be made to get participation of both parents in the consent process. |
| Nabulsi et al 14 (2011) | Qualitative research—Perceptions of Lebanese parents about their children’s participation in research | Lebanon | Fear of potential harm or pain caused to children was identified as a main barrier to parental consent, as were complex consent forms and misunderstanding of randomisation. Perceived direct benefits of participation, trust in the doctor and the institution, financial gains or previous positive experience with research were identified as motivations to participate. Authors recommend improving communication and building trust with parents to enhance recruitment. |
| Oduro et al 56 (2008) | Mixed methods research—Understanding and retention of informed consent process by parents of children participating in a malaria cohort study | Ghana | Findings show overall good recall of procedural aspects of the study. Recall about study benefits was significantly higher than about study risks. Most knew participation was voluntary, but few knew they could withdraw at any time and that information was handled confidentially. Younger parental age was associated with better recall and understanding. Free medical treatment and benefits to the participant were strong motivations for enrolling. |
| Krosin et al 57 (2006) | Quantitative—Parental understanding of the consent process for a malaria vaccine trial | Mali | By using a multiple-choice questionnaire, researchers identified poor comprehension about withdrawal criteria, study side effects and the investigational rather than therapeutic nature of the intervention. Response rate and percentage of correct answers were higher in a more urban setting than in a rural one. |
| Pace et al 26 (2005) | Qualitative—Quality of parental consent in an antimalarial study | Uganda | Most respondents were mothers and had good recall of logistical aspects of the study and study purpose. Comprehension of randomisation was low. The primary reason most respondents gave for enrolling their child was to obtain malaria treatment. Many parents felt pressure to enrol because their child was sick. Only 41% reported they could have refused and 65% knew they could quit. |
| Molyneux et al 58 (2005) | Mixed methods research—Community views about the informed consent process and trust | Kenya | Findings show that trust in the research institution by the community is based on the perceived quality of clinical services it provides, and less on research activities. Trust in the research unit is an important reason behind community members' agreeing to participate in research. Responders valued the informed consent process but thought that low education and being in stressful situations impaired understanding. Authors suggest modifying consent procedures by not giving all information at once and testing to improve comprehension. |
| Leach et al 25 (1999) | Qualitative research—Attitudes of the Gambian people to consent to medical research within the context of a Haemophilus influenzae vaccine trial | The Gambia | Semistructured interviews were conducted with study participants and refusers in urban and rural areas. Results showed that certain points of the trial were recalled well: 90% knew the purpose of the vaccine, but only 10% understood the placebo control design. The main motive for consenting was to receive the vaccine (93%), and for refusing was that the vaccine was experimental (35%) and might have side effects (29%). In all cases, the decision was made by just one of the parents. |
CPAP, continuous positive airway pressure; RCT, randomised controlled trial.
Table 7.
Summary of articles discussing research with adolescents
| Reference (year) | Study description | Study location | Major findings |
| Woollett et al 33 (2017) | Case study—Consent for orphaned adolescents to participate in a mental health study | South Africa | Authors present how consent for research with orphaned adolescents had to be sought from the High Court before approval was granted by academic research committees. The authors discuss how the policy results in excluding vulnerable populations from research and give recommendations for mental health research with adolescents. |
| Joseph et al 59 (2016) | Qualitative research—Stakeholders’ views on international paediatric clinical trials | NA | Regarding the consent process, challenges identified by stakeholders include consent requirements in certain countries that conflict with adolescents' confidentiality rights; impracticality of using long consent forms with multiple required elements; and the need for guidelines to streamline consent form production. |
| Nakkash et al 60 (2009) | Qualitative research—Observation of the consent process for a two-phase preparatory study for an RCT to test the impact of a social skill-building intervention to improve mental health in adolescents | Lebanon | Researchers identified challenges to the consent process: incomplete disclosure of study information; complexity of terms and research design, compounded by low educational levels; issues related to who could provide consent for the child; and social conceptions that youth are not capable of decision-making. The greatest threat to the informed consent process was lack of voluntariness. |
NA, not applicable; RCT, randomised controlled trial.
Table 8.
Summary of articles discussing assent
| Reference (year) | Study description | Study location | Major findings |
| Khabour et al 23 (2017) | Qualitative research—Focus groups to explore parental perceptions about the informed consent and assent process for research | Jordan | Findings show an acceptable understanding of many aspects related to the consent process. However, some parents believed that informed consent is not necessary for questionnaire studies, there were discrepancies regarding the appropriate age for a child’s assent, and some parents said they would force their child to participate regardless of child’s wishes. |
| Vreeman et al 35 (2009) | Case study—Paediatric assent for a study on antiretroviral therapy | Kenya | Authors describe the process of getting review by both US and Kenyan IRBs, mentioning that there is no guideline about how joint review should be conducted. Authors present the differences between the two countries regarding appropriate age for obtaining assent, and discuss local laws, practices and international guidelines. |
IRB, institutional review board.
Results
Results of literature screen
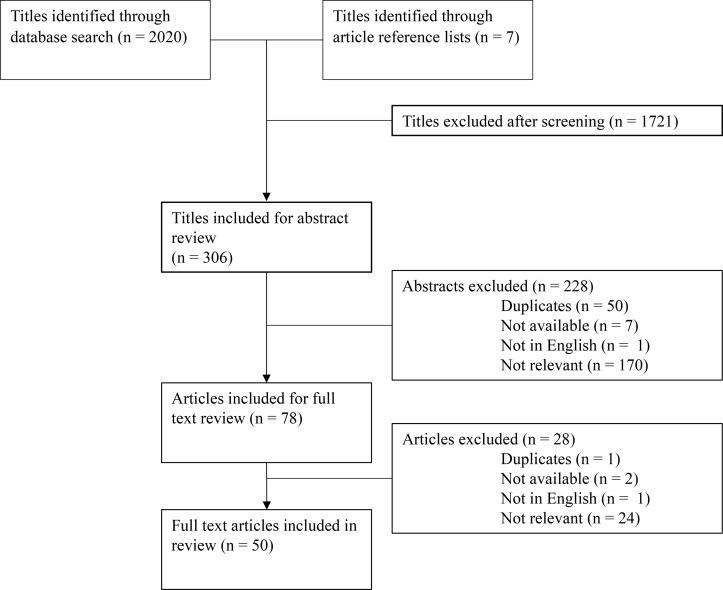
A total of 2027 candidate titles were identified through database searches, supplemented by reference list and website reviews. Of these, 1721 did not meet eligibility criteria, and 306 were included for abstract review. If the abstract was not available but full text was, the title was included for full-text review. After abstract review, 50 duplicates were found, 1 was not in English, 7 were not available (abstract nor full text) and 170 abstracts did not meet inclusion criteria. Seventy-eight articles were selected for full-text review, of which 24 subsequently did not meet inclusion criteria, 1 was in French, 1 was a duplicate and 2 did not have available full text. Therefore, 50 full-text articles were included in this review (figure 1, table 1–8). Of the articles excluded at the abstract and full-text review stages, the most common reasons for exclusion were: no mention of the informed consent process for research with paediatric or adolescent populations; research not taking place in an LMIC; and articles on paediatric research in LMICs that did not discuss the informed consent process.
Figure 1.
Results of literature screen. Flow diagram depicting results of the literature search and review procedure.
Summary of guidelines and commentaries
We identified seven guidelines that addressed issues of informed consent in international settings and in research involving children in our scoping review. Of these, we selected for detailed review five that were most comprehensive, summarising key recommendations in table 1. All guidelines emphasise the importance of obtaining individual, informed and voluntary consent for research.3 4 7–9 Importantly, however, the guidelines do not necessarily specify in detail how best to operationalise these core principles. For example, the Declaration of Helsinki comments only that informed consent requires that a subject be adequately informed of the ‘aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail, post-study provisions and any other relevant aspects of the study’ (Article 26).7 Similarly, on voluntariness, the CIOMS guidelines note only that consent is voluntary if ‘an individual’s decision to participate is free of undue influence’ (p 35).3
Some of the guidelines do suggest modifications appropriate for lower resource settings, such as obtaining witnessed verbal consent when literacy is a barrier.7 9 The US National Bioethics Advisory Commission also acknowledges that oral consent might even be preferable in some circumstances.8 However, as other commentaries note, there is little specificity on how best to operationalise these suggestions, such as how to formally document verbal consent or characteristics of a qualified witness.10 11
Another important consideration of LMIC research addressed in guidelines is an emphasis on the need to at times obtain consent from community stakeholders and leaders, or other key local decision makers. Nevertheless, all guidelines unanimously assert that community-based consent can never replace individual consent. When local cultural practices around community-based consent contradict core principles of the international consensus on the informed consent process, such as the need for voluntary individual consent, researchers are advised to search for culturally sensitive ways of providing all information to potential participants without compromising the substantive ethical standard of informed consent, an adaptive process in which local research ethics committees are expected to place a substantial role.8 10–12
Finally, with respect to children or adolescents not capable of providing informed consent, in addition to obtaining consent from parents or legal representatives, most guidelines also reinforce the need to obtain assent from the child or adolescent in an age-appropriate way.3 4 7 9 The CIOMS guidelines note that assent is ‘a process…not merely the absence of dissent’ and requires ‘meaningful engage[ment] in the research discussion in accordance with…capacities’ (p 67).3 They also note that as adolescents reach the age of maturity, their agreement to participate may be ethically considered as informed consent. However, if they legally remain minors, researchers are cautioned that consent from a parent is still generally needed, but a list is provided of possible situations when parental consent might be waived, such as with legally emancipated adolescents, or under circumstances where obtaining parental consent is not desirable because of the research topic.3
Thematic summary of research on consent in LMIC paediatric research
Existing published work on informed consent in paediatric research in LMICs includes a number of review and opinion articles (table 2) as well as case studies describing the experience of individual research teams and discussing the challenges and solutions used when adapting consent processes to their local context. We summarise several major themes emerging from these studies here in narrative form and provide detailed key findings from the reviewed articles in the accompanying tables.
Understanding social norms around decision-making and protecting individual autonomy
An important principle highlighted in international guidelines on informed consent in LMICs is appropriate and early engagement with existing local leadership structures (such as a council of elders) balanced against respect for the autonomy of individual children or their caregivers.3 8 In practice, this can be a delicate balance to maintain (table 3). Kongsholm and colleagues, for example, describe consent processes in rural Pakistan, where family structures are patriarchal and hierarchical. In this setting, consent procedures involved first seeking consent from an elder, who provided initial consent for the entire family. However, under this approach, the voluntariness of individual participants may be undermined, and it is unclear how best to ensure that individuals still retain an ‘opt out’ mechanism or, conversely, the right to participate in research if they wish to do so but the elder declines.13
Another important consideration explored by some studies is understanding how not all potential consenting caregivers may feel empowered to decline participating in research. Consent procedures administered by local research personnel or by individuals with high social status, such as physicians, may inspire trust.13 14 However, it may also make them reluctant to decline participation, or to resist active participation. For example, in one study in Kenya, explicit refusals to participate were often considered to be impolite. Here researchers found that caregivers expressed their unwillingness to participate by delaying the consent process, or by participating inconsistently in research procedures even after initially having consented to the study.15
Adapting consent procedures to low-literate settings
There is strong consensus in international ethics guidelines that written, informed consent is preferred when conducting research (table 4). In the case of paediatric research, this typically involves obtaining written consent from one or both primary caregivers.4 9 16 However, in many LMIC settings, literacy may be low or a high value may be placed on oral interactions, and lack of alternative consent procedures may violate another core ethics principle, namely the equitable distribution of research benefits and burdens across populations.3 14 17 Some of the studies we reviewed described these procedures, with verbal consent commonly being obtained, most often in the presence of a literate witness who is able to read available consent documents.13 14 17 18 In one very thoughtful piece, Kalabuanga and colleagues note, however, that witnesses may often impose their views on the consenting caregiver and their child, rather than encouraging dialogue and acting as a safeguard, especially since they are often recruited in an ad hoc fashion (eg, other literate patients or ancillary hospital staff).18 Kalabuanga et al go on to suggest that these challenges may be mitigated by careful vetting and training of independent witnesses or, alternatively, by allowing potential consenting caregivers to use a trusted relative or friend as their witness.18
Another issue identified in the review is that of emerging mandates in some LMICs to document consent procedures. For example, in India, audiovisual documentation of obtaining informed consent is now required for most clinical trials if participants are low literate. This introduced significant new logistical challenges and costs related to obtaining and archiving recordings, and it may also pose a barrier to potential research subjects who may distrust or refuse to be recorded.19
Working in indigenous or less commonly spoken languages
International ethics guidelines emphasise that research information should be provided to consenting caregivers in a local language understandable to the individual (table 4).7 8 16 However, this is most commonly understood to be a working lingua franca, and the issue of provisioning consent processes in an indigenous language is largely unaddressed in LMICs.20 This is an important consideration, given that a substantial proportion of the potential paediatric research population in LMICs are from populations that speak indigenous or less commonly spoken languages.21 In an interesting review of lessons learnt in a paediatric vaccine trial in West Africa, Martellet and colleagues noted challenges in preparing consent procedures in some of the less common language groups included in the trial, where use of the written form was uncommon, where substantial need to rely on metaphor and paraphrase made back-translation difficult and where written documents were perceived as not being dynamic enough in cultures which valued interactivity and person-to-person exchange. They describe alternative procedures, such as the preparation of recordings of consent scripts in local languages and extensive practice sessions with research staff obtaining consent in local languages.17 Similarly, another vaccine trial in The Gambia described the successful use of Speaking Book audiovisual tools in local less common languages to consent caregivers.22
Gender dynamics in caregiver consent
Local gender dynamics and decision-making procedures when consenting male and female caregivers for research are an important consideration (table 5). For example, when consenting with caregiving couples or within an extended family unit, instances are discussed where a female caregiver wishes to allow her child to participate, but is unable to do so because her husband or another male authority figure refuses.13 The opposite may also occur, if a research study is consented by a male figure, but requires significant participatory effort from the primary female for study-related activities, leading the woman to express their refusal through procedural delay or inconsistent participation.15 Given concerns about gender power imbalance and potential repercussions for consenting female caregivers, some studies discussed working to routinely involve fathers or male authority figures in the consent process for more complex or higher risk research interventions.15 23 In one interesting study based in India, Rajaraman and colleagues found that caregivers were more likely to actively participate in the consent process when both were present. They also observed, however, that this factor may have been due to the fact that most study staff obtaining consent were male, and they call for more research on how the gender of research staff impacts the consent process.24
It is important to note that most discussions of gender dynamics that we reviewed were limited in nuance, tending to focus on instances of overt over-riding of female decision-making by male authorities. A broader consideration of the range of ways in which female caregivers communicate, influence and negotiate decision-making with male family members and other community authorities is an obvious point for future investigation.
Disclosing potential benefits and risks of participation in research
Participation in some research studies, particularly those with a randomised controlled design or those with differing intervention arms, may not result in direct benefit to all participants. Several studies report difficulties explaining to caregivers that medical research procedures may not result in direct benefit to their children, and in verifying that caregivers comprehended randomisation or control procedures (table 6).25–28 Others noted the need to address issues of information recall and retention, particularly with complex study procedures or consent forms, and to emphasise the right of study withdrawal and the ongoing reaffirmation of consent throughout a study.26–29 Furthermore, other reports discussed how therapeutic misconception—the perception by research subjects that participation in any component of a multiple-arm, controlled trial will result in therapeutic benefits—might be hard to avoid in certain contexts, as it might be affected by factors like educational level and cultural and religious beliefs about disease.13 18
At the same time, care must be given to a culturally appropriate degree of information disclosure. For example, in several studies, caregivers—especially those of higher socioeconomic or educational status—were more likely to participate when provided with detailed and in-depth information about the study processes and given opportunities to ask questions.12 23 24 30 At the same time, other case studies point out how overdetailed discussion of study procedures or scientific rationale may provoke unneeded reserve or suspicion where such detailed disclosures by health professionals are not culturally customary.13
Finally, in settings where access to healthcare and other important social goods may be limited, even basic diagnostic or ancillary procedures that occur as part of research studies may be better than the local standard of care, leading to an undue inducement or highly compelling incentives for caregivers to enrol their children in research, even after being informed about the experimental nature of studies and the risk-benefit balance.11 13 18 These considerations highlight the importance of considering the socioeconomic and cultural background of study settings well before beginning research and making plans to incorporate appropriate early, equitable benefit-sharing measures when possible, such as using study resources to improve community-level care, not just care for eligible trial participants.18
Adolescents
Adolescents constitute a special population with vulnerabilities different from those of adults and younger children, and they should be included in research that addresses their specific needs (table 7). However, as legal minors they often cannot give informed consent for research.16 In research in LMICs, regulations vary significantly from country to country regarding when adolescents can provide legal consent for research.31 For example, even when legal frameworks allow adolescents to seek contraception services without parental permission, they cannot necessarily provide consent for research on that theme.32 33 In a scoping review of postabortion care research, Zulu and coauthors discuss how the need to balance adolescents’ privacy needs and the demand for parental consent poses difficulties for researchers in this field.34 Woollett and colleagues describe an interesting case study where they sought consent from a High Court in South Africa for research involving orphaned HIV-positive adolescents. In that study, they provide detailed recommendations for consent involving adolescents, including training staff about confidentiality requirements; recognising immature decision-making by adolescents and developing appropriate methods for probing comprehension and consent; and using methods that promote active participation in research, such as mobile phones.33
Assent
Paediatric research guidelines are unanimous on the need to obtain age-appropriate assent from children and adolescents who do not provide their own informed consent (table 1). However, we found little explicit discussion or description of procedures for obtaining assent in the research reports we reviewed (table 8).35 36 One interesting qualitative study on parental perceptions of assent in Jordan revealed considerable variability in caregivers’ perspectives about at what age assent should be solicited or if assent should even be obtained and dissent respected in all cases.23
Discussion
Children in low-resource settings are highly vulnerable to exploitation in research, because of circumstances including socioeconomic inequalities, limited access to healthcare and high burden of illness.37 In addition, even where international consensus exists around core ethical principles for providing protections to children as research subjects, it may be unclear how best to operationalise those principles in many low-resource settings, where gender norms, literacy, unfamiliarity with scientific research and language barriers may all be important adaptive barriers.10 11
Through a scoping review of research reports and case studies from LMICs, we identified, however, several core areas where existing research reports provided considerable insight and operational guidance which could be used to guide informed consent design processes in additional LMIC settings. These included: (1) careful consideration of community hierarchy, where consent for research may first proceed through a council of elders or other authority figure, prior to approaching individual caregivers; (2) guidance on developing verbal consent procedures in settings where caregivers have low literacy levels; (3) recognition of the challenges of consent in indigenous or less commonly spoken languages, particularly when that language is not commonly written and where alternative procedures, such as audio recordings in the language, must be employed; and (4) careful consideration of the possibility of therapeutic misconception and of developing consent procedures that ensure caregivers’ comprehension of the potential benefits (or lack thereof) and risks of research procedures for their children.
However, within these four broad thematic areas, we also noted the need for additional careful investigation. In particular, there is considerable uncertainty on how to ensure the principle of subsequent individual informed consent when community leaders or other authorities are approached first. This is especially the case when gender power imbalance is at play, and female caregivers may be either unempowered to consent or to opt out of a research decision made by a male authority. In addition, the social status of individuals administering or witnessing consent procedures may unduly influence the decision-making of caregivers, and research is needed to better understand and accommodate for the interpersonal dynamics of obtaining consent.
Finally, two thematic topics seem to be particularly under-represented in the literature on paediatric LMIC research, and more work is urgently needed. First, despite extensive discussions about the difficulties of conducting research with adolescents, we found only few studies with practical discussions or guidance on how to navigate these difficulties. More investigation of the ethical conduct of research with adolescents is needed, with a broader representation of health conditions, research designs and geographic regions. Second, despite strong representation of the principle of assent in international guidelines on research with children and adolescents, we found little research on cultural and regional differences around notions of assent and virtually no discussion of the mechanics of assessing assent in research studies. Additional research into the topic of assent for research among children in LMICs should be an important priority.
Our review has two important limitations that must be considered. First, we included only articles published in English in major indexing databases. We believe this approach is justified, given our desire to provide a high-level overview of the topic without focusing specifically on any geographic region. Nevertheless, our review has undoubtedly missed resources in other languages or within the grey literature, which could be taken up in more detailed region-specific work on this topic. Second, given the diversity and heterogeneity of the literature reviewed, it was not possible to detail many of the practical insights and tips given in the individual articles. Nevertheless, given the annotation and thematic organisation provided in the tables, we are confident that readers will be able to identify areas of particular interest for more in-depth examination.
Supplementary Material
Acknowledgments
We thank the research subjects and clinical staff at Maya Health Alliance for inspiring our interest in this important topic.
Footnotes
Contributors: MC designed the search strategy, extracted data from articles and wrote the first draft of the manuscript. PR conceived the study, reviewed abstracts and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Weindling P. The origins of informed consent: the International Scientific Commission on Medical War Crimes, and the Nuremburg code. Bull Hist Med 2001;75:37–71. 10.1353/bhm.2001.0049 [DOI] [PubMed] [Google Scholar]
- 2. Rickham PP. Human experimentation. code of ethics of the world medical association. declaration of helsinki. Br Med J 1964;2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Council for International Organizations of Medical Sciences (CIOMS). International ethical guidelines for health-related research involving humans. 4 edn Geneva, Switzerland: CIOMS, 2016. [Google Scholar]
- 4. Caldwell PH, Dans L, de Vries MC, et al. Standard 1: consent and recruitment. Pediatrics 2012;129(Suppl 3):S118–S123. 10.1542/peds.2012-0055D [DOI] [PubMed] [Google Scholar]
- 5. Klassen TP, Hartling L, Hamm M, et al. StaR Child Health: an initiative for RCTs in children. Lancet 2009;374:1310–2. 10.1016/S0140-6736(09)61803-1 [DOI] [PubMed] [Google Scholar]
- 6. Martinez B, Webb MF, Gonzalez A, et al. Complementary feeding intervention on stunted Guatemalan children: a randomised controlled trial. BMJ Paediatr Open 2018;2:e000213–8. 10.1136/bmjpo-2017-000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Medical Association. WMA Declaration of Helsinki - ethical principles for medical research involving human subjects. 1964. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
- 8. National Bioethics Advisory Commission. Ethical and policy issues research: clinical trials in developing countries. 2001;1:59 http://bioethics.georgetown.edu/nbac/clinical/Vol1.pdf [Google Scholar]
- 9. European Commission. EU Directive 2001/20/EC. Off J Eur Communities 2001:1–15. [Google Scholar]
- 10. Bhutta ZA. Beyond informed consent. Bull World Health Organ 2004;82:771–7. [PMC free article] [PubMed] [Google Scholar]
- 11. MacLeod SM, Knoppert DC, Stanton-Jean M, et al. Pediatric clinical drug trials in low-income countries: key ethical issues. Paediatr Drugs 2015;17:83–90. 10.1007/s40272-014-0103-3 [DOI] [PubMed] [Google Scholar]
- 12. Minnies D, Hawkridge T, Hanekom W, et al. Evaluation of the quality of informed consent in a vaccine field trial in a developing country setting. BMC Med Ethics 2008;9:1–9. 10.1186/1472-6939-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kongsholm NCH, Lassen J, Sandøe P. "I didn’t have anything to decide, I wanted to help my kids"-An interview-based study of consent procedures for sampling human biological material for genetic research in rural Pakistan. AJOB Empir Bioeth 2018;2018:1–35. 10.1080/23294515.2018.1472148 [DOI] [PubMed] [Google Scholar]
- 14. Nabulsi M, Khalil Y, Makhoul J. Parental attitudes towards and perceptions of their children’s participation in clinical research: a developing-country perspective. J Med Ethics 2011;37:420–3. 10.1136/jme.2010.035899 [DOI] [PubMed] [Google Scholar]
- 15. Kamuya DM, Theobald SJ, Marsh V, et al. "The one who chases you away does not tell you go": silent refusals and complex power relations in research consent processes in Coastal Kenya. PLoS One 2015;10:e0126671 10.1371/journal.pone.0126671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macrae DJ. The Council for International Organizations and Medical Sciences (CIOMS) guidelines on ethics of clinical trials. Proc Am Thorac Soc 2007;4:176–9. 10.1513/pats.200701-011GC [DOI] [PubMed] [Google Scholar]
- 17. Martellet L, Sow SO, Diallo A, et al. Ethical challenges and lessons learned during the clinical development of a group a meningococcal conjugate vaccine. Clin Infect Dis 2015;61:S422–7. 10.1093/cid/civ598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalabuanga M, Ravinetto R, Maketa V, et al. The challenges of research informed consent in socio-economically vulnerable populations: a viewpoint from the democratic republic of Congo. Dev World Bioeth 2016;16:64–9. 10.1111/dewb.12090 [DOI] [PubMed] [Google Scholar]
- 19. Shetty PA, Maurya MR, Figer BH, et al. Audiovisual recording of the consenting process in clinical research: Experiences from a tertiary referral center. Perspect Clin Res 2018;9:44–7. 10.4103/picr.PICR_172_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fitzpatrick EF, Martiniuk AL, D’Antoine H, et al. Seeking consent for research with indigenous communities: a systematic review. BMC Med Ethics 2016;17:65 10.1186/s12910-016-0139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flood D, Rohloff P. Indigenous languages and global health. Lancet Glob Health 2018;6:e134–e135. 10.1016/S2214-109X(17)30493-X [DOI] [PubMed] [Google Scholar]
- 22. Mboizi RB, Afolabi MO, Okoye M, et al. Recall and decay of consent information among parents of infants participating in a randomized controlled clinical trial using an audio-visual tool in The Gambia. Hum Vaccin Immunother 2017;13:2185–91. 10.1080/21645515.2017.1320624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khabour OF, Alomari MA, Al-Sheyab NA. Parental perceptions about informed consent/assent in pediatric research in Jordan. J Empir Res Hum Res Ethics 2017;12:261–8. 10.1177/1556264617718937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rajaraman D, Jesuraj N, Geiter L, et al. How participatory is parental consent in low literacy rural settings in low income countries? Lessons learned from a community based study of infants in South India. BMC Med Ethics 2011;12:3 10.1186/1472-6939-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leach A, Hilton S, Greenwood BM, et al. An evaluation of the informed consent procedure used during a trial of a Haemophilus influenzae type B conjugate vaccine undertaken in The Gambia, West Africa. Soc Sci Med 1999;48:139–48. 10.1016/S0277-9536(98)00317-7 [DOI] [PubMed] [Google Scholar]
- 26. Pace C, Talisuna A, Wendler D, et al. Quality of parental consent in a Ugandan malaria study. Am J Public Health 2005;95:1184–9. 10.2105/AJPH.2004.053082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paré Toe L, Ravinetto RM, Dierickx S, et al. Could the decision of trial participation precede the informed consent process? Evidence from Burkina Faso. PLoS One 2013;8:e80800–10. 10.1371/journal.pone.0080800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Angwenyi V, Kamuya D, Mwachiro D, et al. Complex realities: community engagement for a paediatric randomized controlled malaria vaccine trial in Kilifi, Kenya. Trials 2014;15:65 10.1186/1745-6215-15-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarkar R, Grandin EW, Gladstone BP, et al. Comprehension and recall of informed consent among participating families in a birth cohort study on diarrhoeal disease. Public Health Ethics 2009;2:37–44. 10.1093/phe/phn040 [DOI] [Google Scholar]
- 30. Serce O, Gonen I, Bakir M. Factors influencing parental consent in a hypothetical pediatric vaccine trial in a developing country setting: a questionnaire study. Account Res 2015;22:1–13. 10.1080/08989621.2014.882779 [DOI] [PubMed] [Google Scholar]
- 31. Bekker LG, Slack C, Lee S, et al. Ethical issues in adolescent HIV research in resource-limited countries. J Acquir Immune Defic Syndr 2014;65:S24–8. 10.1097/QAI.0000000000000036 [DOI] [PubMed] [Google Scholar]
- 32. McClure CA, Gray G, Rybczyk GK, et al. Challenges to conducting HIV preventative vaccine trials with adolescents. J Acquir Immune Defic Syndr 2004;36:726–33. 10.1097/00126334-200406010-00010 [DOI] [PubMed] [Google Scholar]
- 33. Woollett N, Peter J, Cluver L, et al. Enrolling HIV-positive adolescents in mental health research: a case study reflecting on legal and ethical complexities. S Afr Med J 2017;107:679–83. 10.7196/SAMJ.2017.v107i8.12409 [DOI] [PubMed] [Google Scholar]
- 34. Zulu JM, Ali J, Hallez K, et al. Ethics challenges and guidance related to research involving adolescent post-abortion care: a scoping review. Reprod Health 2018;15:1–10. 10.1186/s12978-018-0515-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vreeman RC, Nyandiko WM, Meslin EM. Pediatric assent for a study of antiretroviral therapy dosing for children in Western Kenya: a case study in international research collaboration. J Empir Res Hum Res Ethics 2009;4:3–16. 10.1525/jer.2009.4.1.3 [DOI] [PubMed] [Google Scholar]
- 36. Molyneux CS, Wassenaar DR, Peshu N, et al. ’Even if they ask you to stand by a tree all day, you will have to do it (laughter)!': community voices on the notion and practice of informed consent for biomedical research in developing countries. Soc Sci Med 2005;61:443–54. 10.1016/j.socscimed.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 37. Initiative for Vaccine Research of the Department of Vaccines and Biologicals World Health Organization. Ethical considerations arising from vaccine trials conducted in paediatric populations with high disease burden in developing countries WHO/IRV ethics meeting, November 26-28, 2002. Accra, Ghana, 2002. www.who.int/vaccines-documents [Google Scholar]
- 38. Ott MA, Crawley FP, Sáez-Llorens X, et al. Ethical considerations for the participation of children of minor parents in clinical trials. Paediatr Drugs 2018;20:215–22. 10.1007/s40272-017-0280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Regmi PR, Aryal N, Kurmi O, et al. Informed consent in health research: challenges and barriers in low-and middle-income countries with specific reference to Nepal. Dev World Bioeth 2017;17:84–9. 10.1111/dewb.12123 [DOI] [PubMed] [Google Scholar]
- 40. Mandava A, Pace C, Campbell B, et al. The quality of informed consent: mapping the landscape. A review of empirical data from developing and developed countries. J Med Ethics 2012;38:356–65. 10.1136/medethics-2011-100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joseph PD, Caldwell PH, Tong A, et al. Stakeholder views of clinical trials in low- and middle-income countries: a systematic review. Pediatrics 2016;137:e20152800 10.1542/peds.2015-2800 [DOI] [PubMed] [Google Scholar]
- 42. Morrow BM, Argent AC, Kling S. Informed consent in paediatric critical care research--a South African perspective. BMC Med Ethics 2015;16:62 10.1186/s12910-015-0052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swain TR. Clinical trials for children: some concerns. Indian J Pharmacol 2014;46:145–6. 10.4103/0253-7613.129300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruiz-Casares M. Research ethics in global mental health: advancing culturally responsive mental health research. Transcult Psychiatry 2014;51:790–805. 10.1177/1363461514527491 [DOI] [PubMed] [Google Scholar]
- 45. Offringa M, Needham AC, Chan WW. StaR Child Health: improving global standards for child health research. Early Hum Dev 2013;89:861–4. 10.1016/j.earlhumdev.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 46. Daley TC, Singhal N, Krishnamurthy V. Ethical considerations in conducting research on autism spectrum disorders in low and middle income countries. J Autism Dev Disord 2013;43:2002–14. 10.1007/s10803-012-1750-2 [DOI] [PubMed] [Google Scholar]
- 47. Denburg AE, Joffe S, Gupta S, et al. Pediatric oncology research in low income countries: ethical concepts and challenges. Pediatr Blood Cancer 2012;58:492–7. 10.1002/pbc.23419 [DOI] [PubMed] [Google Scholar]
- 48. Mystakidou K, Panagiotou I, Katsaragakis S, et al. Ethical and practical challenges in implementing informed consent in HIV/AIDS clinical trials in developing or resource-limited countries. SAHARA-J: Journal of Social Aspects of HIV/AIDS 2009;6:46–57. 10.1080/17290376.2009.9724930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Embleton L, Ott MA, Wachira J, et al. Adapting ethical guidelines for adolescent health research to street-connected children and youth in low- and middle-income countries: a case study from western Kenya. BMC Med Ethics 2015;16:89 10.1186/s12910-015-0084-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Millum J, Emanuel EJ. Ethics. The ethics of international research with abandoned children. Science 2007;318:30–7. 10.1126/science.1153822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vreeman R, Kamaara E, Kamanda A, et al. Community perspectives on research consent involving vulnerable children in Western Kenya. J Empir Res Hum Res Ethics 2012;7:44–55. 10.1525/jer.2012.7.4.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tindana P, Bull S, Amenga-Etego L, et al. Seeking consent to genetic and genomic research in a rural Ghanaian setting: a qualitative study of the MalariaGEN experience. BMC Med Ethics 2012;13:1 10.1186/1472-6939-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morris MC, Wilson PT. Medical device research in resource-poor settings: a pediatric case study in Ghana. IRB 2014;36:1–7. [PubMed] [Google Scholar]
- 54. Ward CL, Shaw D, Anane-Sarpong E, et al. The ethics of health care delivery in a pediatric malaria vaccine trial: the perspectives of stakeholders from ghana and Tanzania. J Empir Res Hum Res Ethics 2018;13:26–41. 10.1177/1556264617742236 [DOI] [PubMed] [Google Scholar]
- 55. Devries KM, Child JC, Elbourne D, et al. "I never expected that it would happen, coming to ask me such questions": ethical aspects of asking children about violence in resource poor settings. Trials 2015;16:516 10.1186/s13063-015-1004-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oduro AR, Aborigo RA, Amugsi D, et al. Understanding and retention of the informed consent process among parents in rural northern Ghana. BMC Med Ethics 2008;9:1–9. 10.1186/1472-6939-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krosin MT, Klitzman R, Levin B, et al. Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clin Trials 2006;3:306–13. 10.1191/1740774506cn150oa [DOI] [PubMed] [Google Scholar]
- 58. Molyneux CS, Peshu N, Marsh K. Trust and informed consent: insights from community members on the Kenyan coast. Soc Sci Med 2005;61:1463–73. 10.1016/j.socscimed.2004.11.073 [DOI] [PubMed] [Google Scholar]
- 59. Joseph PD, Craig JC, Tong A, et al. Researchers', regulators', and sponsors' views on pediatric clinical trials: a multinational study. Pediatrics 2016;138:e20161171 10.1542/peds.2016-1171 [DOI] [PubMed] [Google Scholar]
- 60. Nakkash R, Makhoul J, Afifi R. Obtaining informed consent: observations from community research with refugee and impoverished youth. J Med Ethics 2009;35:638–43. 10.1136/jme.2008.028936 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.