Abstract
Objective
This study aimed to investigate the correlation between the face scale and heart rate (HR), exercise load and oxygen uptake (V̇O2) during cardiopulmonary exercise testing.
Methods
This was a prospective, observational study of face scale rating of perceived exertion (RPE) and HR, exercise load and V̇O2 during cardiopulmonary exercise testing. A total of 30 healthy college men and 21 healthy college women were included. Subjects performed a cardiopulmonary exercise test with ramps and an increment increase in workload of 20 W/min. We recorded the responses of subjects using a face scale for RPE, HR, exercise load and V̇O2 every minute during the cardiopulmonary exercise test.
Results
In men, there was a significant positive correlation between the face scale RPE and HR (ρ=0.856, p<0.01), exercise load (ρ=0.888, p<0.01) and V̇O2 (ρ=0.878, p<0.01) during the cardiopulmonary exercise test. Similarly, in women, there was a significant positive correlation between the face scale RPE and HR (ρ=0.885, p<0.01), exercise load (ρ=0.908, p<0.01) and V̇O2 (ρ=0.895, p<0.01) during the cardiopulmonary exercise tests.
Conclusion
The face scale proposed in this study was related to physiological parameters, which suggests that it may be used to determine the intensity of exercise in healthy adults.
Keywords: exercise test, heart rate, adult, oxygen, perceived exertion, face scale
What are the new findings?
We found that the face scale rate of perceived exertion (RPE) tended to increase with exercise load.
The face scale RPE is easy to understand perceived exertion during exercise; furthermore, this scale was usable to determine the intensity of exercise.
How might it impact on clinical practice in the near future?
Face scale RPE may be was preferred over the Borg scale to determine exercise load for elderly and children subjects.
Because this scale has the face illustration, it may be easily to understand for elderly and children subjects.
Introduction
Aerobic exercise is a low-intensity to high-intensity exercise that depends primarily on the aerobic energy-generating process.1 It includes activities that increase breathing and heart rate (HR) such as walking, jogging, swimming and biking exercises.2 Aerobic exercise can be used in patients with various diseases such as cardiovascular disease,3 chronic obstructive pulmonary disease,4 stroke,5 Parkinson’s disease,6 cancer7 and diabetes.8 It helps maintain cardiorespiratory and muscular fitness in addition to flexibility in healthy adults.9 Since there is a positive correlation between HR and exercise intensity in individuals performing aerobic exercise using HR monitoring devices,10 11 HR is often used to determine the intensity of aerobic exercise. In addition, ratings of perceived exertion (RPE), such as the Borg scale, are often used as an alternative to HR to determine aerobic exercise intensity.12–14 However, the Borg scale can be challenging for the elderly and children who may have difficulty understanding numbers and instrumental words such as ‘somewhat strong’ for perceived exertion. In contrast, the face scale was used to assess pain in patients15 using a set of six faces that express the various levels of overt distress.16 Since it employs figures to rate perceived pain levels, it is easy to understand and is therefore often used to assess pain in elderly15 and children17 subjects. However, to our knowledge, no reports investigated the validity and feasibility of using the face scale instead of the RPE to determine exercise intensity. Hence, we used the face scale as a measure of the RPE to determine exercise load. This study aimed to investigate the correlation between the face scale and HR, intensity of exercise and oxygen uptake (V̇O2) during cardiopulmonary exercise.
Methods
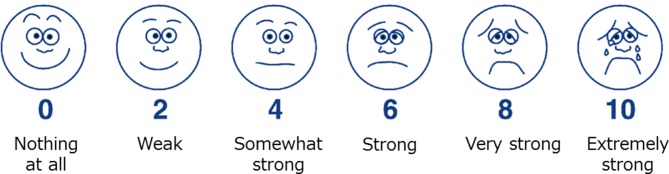
This was a prospective, observational study to determine the correlation between the face scale RPE and HR, exercise load and V̇O2 during cardiopulmonary exercise. A total of 30 healthy college men and 21 healthy college were included. Subjects performed cardiopulmonary exercise tests with ramp exercise protocols to determine the V̇O2.18 Each participant provided written informed consent after receiving information regarding the potential risks, study objectives, measurement techniques and benefits associated with the study. Our protocol consisted of a 4 min rest, 4 min warm-up, cardiopulmonary exercise and 2 min cool-down. A ramp programme with an incremental increase in workload of 20 W/min was employed using stationary bicycles (Aerobike 75XLIII; Konami, Tokyo, Japan) with ECG (DS-7520, Fukuda Denshi, Tokyo, Japan), and an exhaled gas analyzer (AE-310S; Minato Medical Science, Osaka, Japan). All subjects were instructed to maintain a cadence of 50 rotations per minute (rpm) during the cardiopulmonary exercise test. Exhaustion was defined as follows19 (1): a plateau in oxygen consumption (VO2); (2) respiratory exchange ratio >1.1; (3) HR values near the age-predicted maximal heart rate, calculated as 220 – (0.65×age); and (4) a decrease in the cycling cadence to <50 rpm, despite strong verbal encouragement. The highest value obtained for V̇O2 was considered the V̇O2 peak. We evaluated HR using ECG, exercise load (watts) and V̇O2 using an exhaled gas analyzer every minute during cardiopulmonary exercise test and at the end of the exercise test. All subjects were asked ‘how hard you feel you are working’ using the face scale RPE and their responses were recorded (figure 1). Additionally, we determined anaerobic thresholds (ATs) using the V-slope method during the cardiopulmonary exercise tests.20
Figure 1.
Face scale rate of perceived exertion.
The outcomes were reported as a mean and SD or median. Spearman’s rank correlation coefficients (ρ) were calculated to evaluate the correlation between the face scale RPE and HR, watts, and V̇O2 every minute during the cardiopulmonary exercise tests. Statistical analyses were performed using SPSS V.19.0J. P values<0.05 were considered statistically significant.
Results
All subjects performed the cardiopulmonary exercise test. Table 1 shows characteristics of subjects stratified by sex, results of the cardiopulmonary exercise tests at rest, AT and at the end.
Table 1.
Characteristics of subjects (n=51)
| Characteristics | Men (n=30) | Women (n=21) |
| No | No | |
| Age, years | ||
| Mean (SD) | 21 (1.1) | 20.9 (0.3) |
| Median | 21 | 21 |
| Height, cm | ||
| Mean (SD) | 171.9 (5.7) | 158.8 (4.9) |
| Body weight, kg | ||
| Mean (SD) | 63.2 (5.7) | 50.8 (4.9) |
| Cardiopulmonary exercise testing | ||
| At rest | ||
| Face scale RPE | 0.3 (0.8) | 0.3 (0.7) |
| HR (beat/min) | 82.9 (11.9) | 88.5 (13.7) |
| Exercise load (W) | 0 | 0 |
| VO2 (mL/kg/min) | 4.1 (0.5) | 3.9 (0.3) |
| Anaerobic threshold | ||
| Face scale RPE | 3.5 (1.6) | 4.4 (1.5) |
| HR (beat/min) | 128.8 (13.0) | 139.2 (20.2) |
| Exercise load (W) | 97.7 (14.4) | 75.7 (13.3) |
| VO2 (mL/kg/min) | 18.0 (2.9) | 16.9 (3.1) |
| End of test (maximum) | ||
| Face scale RPE | 9.1 (1.1) | 8.3 (1.5) |
| HR (beat/min) | 184 (12.2) | 174.7 (13.5) |
| Exercise load (W) | 192.3 (79.1) | 125.6 (20.6) |
| VO2 (mL/kg/min) | 37.9 (5.3) | 26.4 (4.1) |
VO2, oxygen uptakeHR, heart rate; RPE, rate of perceived exertion.
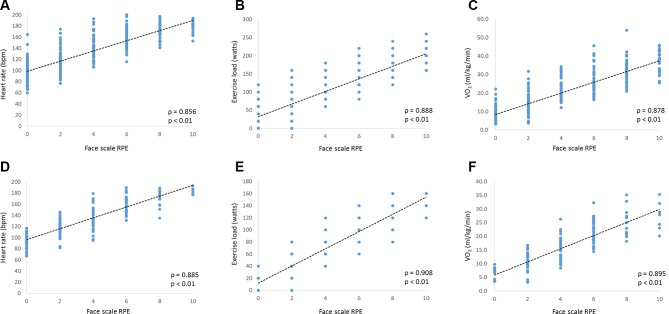
In men, there was a significant positive correlation between the face scale RPE and HR (ρ=0.856, p<0.01), exercise load (ρ=0.888, p<0.01) and V̇O2 (ρ=0.878, p<0.01) during the cardiopulmonary exercise testing figure 2. Similarly, in women, there was a significant positive correlation between the face scale RPE and HR (ρ=0.885, p<0.01), exercise load (ρ=0.908, p<0.01) and V̇O2 (ρ=0.895, p<0.01) during cardiopulmonary exercise testing.
Figure 2.
Correlation between the face scale rate of perceived exertion and physiological outcomes during the cardiopulmonary exercise tests The scatterplots illustrate the correlation between the face scale rate of perceived exertion and physiological outcomes during cardiopulmonary exercise tests. (A)–(C) show the correlation between the face scale rate of perceived exertion and heart rate, exercise load and oxygen uptake (VO2) in men, respectively.(D)–(F) show the correlation between the face scale rate of perceived exertion and heart rate, exercise load and oxygen uptake (VO2) in women, respectively. RPE, ratings of perceived exertion.
Discussion
This study showed that in both sexes of healthy college subjects there was a positive correlation between the face scale RPE and physiological outcomes during cardiopulmonary exercise testing. Limited data are available on the use of face scale RPE in cardiopulmonary exercise tests. Similar to our results, a previous study that compared the RPE scale using facial images and the Borg scale during exercise in both young adults and children21 using five pedalling workload levels (20%, 40%, 60%, 80% and 100%) reported that facial RPE was positively correlated to exercise load and HR in both groups. Our study reported that face scale RPE during cardiopulmonary exercise testing was positively correlated to HR and exercise load; in addition, face scale RPE was positively correlated to V̇O2 during cardiopulmonary exercise in young adults. In our study, the correlation coefficient (ρ) between the face scale and HR, exercise load and V̇O2 were 0.8–0.9 during cardiopulmonary exercise, which indicates a high positive correlation.22 The face scale RPE may be correlated to oxygen uptake not only HR and exercise load during cardiopulmonary exercise testing. The OMNI scale is often used to assess perceived exertion in children during exercise.23 It was initially developed for children who have difficulty understanding the association between written words and exercise intensity. However, it comprises pictures that are displayed on a line with a 20–25° slope.23 In contrast, the face scale RPE used in this study is easy to understand and highly correlated to physiological exercise parameters. Thus, it may be used to determine the exercise load.
This study was limited to investigating the correlation between the face scale RPE and HR, exercise load and V̇O2 in healthy adults and did not include elderly or children subjects. Thus future studies are warranted for investigating the correlation between the face scale and physiological parameters during cardiopulmonary exercise in elderly and children subjects.
Conclusion
In this study, we investigated the correlation between the face scale RPE and HR, exercise load and V̇O2 during cardiopulmonary exercise in healthy adults. Our results showed that there was a significant positive correlation between the face scale RPE and HR, exercise load and V̇O2. This suggests that it may be used to determine the intensity of physical exercise in healthy adults.
Perspective
We found that the face scale rate of perceived exertion tended to increase with exercise load. The face scale rate of perceived exertion was usable in both men and women to determine the intensity of exercise. The face scale rate of perceived exertion may be used as an alternative to heart rate to determine exercise load. Face scale may be was preferred over the Borg scale24 to determine exercise load for elderly and children subjects. Because this scale has the face illustration, it may be easily to understand for elderly and children subjects.
Acknowledgments
The authors are grateful to the study participants and physical therapy students Sho Kojima and Yuta Inagaki for helping in the measuring data.
Footnotes
Presented at: This study was partially presented as a poster presentation at the 12th International Society of Physical and Rehabilitation Medicine World Congress, July 10, 2018, Paris, France.
Contributors: SM, AT and HO were responsible for the data collection and data analysis. SN and JBF interpreted the data and wrote the first draft of the paper. All authors contributed to the final paper.
Funding: This study was partly supported by a Grant-in-Aid for Niigata University of Health and Welfare as well as the M.D. Anderson Cancer Center support grant CA 016672.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Ethics Committee of Niigata University of Health and Welfare (approval no. 17912-171107) and conformed to the standards outlined in the Declaration of Helsinki.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: Data are available by contacting the corresponding author.
References
- 1. Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136:493–503. [DOI] [PubMed] [Google Scholar]
- 2. Artal R, O'Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med 2003;37:6–12. 10.1136/bjsm.37.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hill AM, Buckley JD, Murphy KJ, et al. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr 2007;85:1267–74. 10.1093/ajcn/85.5.1267 [DOI] [PubMed] [Google Scholar]
- 4. Borghi-Silva A, Arena R, Castello V, et al. Aerobic exercise training improves autonomic nervous control in patients with COPD. Respir Med 2009;103:1503–10. 10.1016/j.rmed.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 5. Stoller O, de Bruin ED, Knols RH, et al. Effects of cardiovascular exercise early after stroke: systematic review and meta-analysis. BMC Neurol 2012;12:45 10.1186/1471-2377-12-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2008;23:631–40. 10.1002/mds.21922 [DOI] [PubMed] [Google Scholar]
- 7. Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 2009;27:344–51. 10.1200/JCO.2007.15.4963 [DOI] [PubMed] [Google Scholar]
- 8. Dixit S, Maiya A, Shastry B. Effect of aerobic exercise on quality of life in population with diabetic peripheral neuropathy in type 2 diabetes: a single blind, randomized controlled trial. Qual Life Res 2014;23:1629–40. 10.1007/s11136-013-0602-7 [DOI] [PubMed] [Google Scholar]
- 9. Zheng G, Xia R, Zhou W, et al. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2016;50:1443–50. 10.1136/bjsports-2015-095699 [DOI] [PubMed] [Google Scholar]
- 10. Gillinov S, Etiwy M, Wang R, et al. Variable accuracy of wearable heart rate monitors during aerobic exercise. Med Sci Sports Exerc 2017;49:1697–703. 10.1249/MSS.0000000000001284 [DOI] [PubMed] [Google Scholar]
- 11. Emerenziani GP, Gallotta MC, Meucci M, et al. Effects of aerobic exercise based upon heart rate at aerobic threshold in obese elderly subjects with type 2 diabetes. Int J Endocrinol 2015;2015:1–7. 10.1155/2015/695297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Astokorki AH, Mauger AR. Tolerance of exercise-induced pain at a fixed rating of perceived exertion predicts time trial cycling performance. Scand J Med Sci Sports 2017;27:309–17. 10.1111/sms.12659 [DOI] [PubMed] [Google Scholar]
- 13. Eston RG, Davies BL, Williams JG. Use of perceived effort ratings to control exercise intensity in young healthy adults. Eur J Appl Physiol Occup Physiol 1987;56:222–4. 10.1007/BF00640648 [DOI] [PubMed] [Google Scholar]
- 14. Weston M, Batterham AM, Tew GA, et al. Patients awaiting surgical repair for large abdominal aortic aneurysms can exercise at moderate to hard intensities with a low risk of adverse events. Front Physiol 2016;7 10.3389/fphys.2016.00684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herr KA, Mobily PR, Kohout FJ, et al. Evaluation of the faces pain scale for use with the elderly. Clin J Pain 1998;14:29–38. 10.1097/00002508-199803000-00005 [DOI] [PubMed] [Google Scholar]
- 16. McGrath PA, Seifert CE, Speechley KN, et al. A new analogue scale for assessing children's pain: an initial validation study. Pain 1996;64:435–43. 10.1016/0304-3959(95)00171-9 [DOI] [PubMed] [Google Scholar]
- 17. Wong DL, Baker CM. Pain in children: comparison of assessment scales. Okla Nurse 1988;33:8. [PubMed] [Google Scholar]
- 18. Myers J, Bellin D. Ramp exercise protocols for clinical and cardiopulmonary exercise testing. Sports Med 2000;30:23–9. 10.2165/00007256-200030010-00003 [DOI] [PubMed] [Google Scholar]
- 19. Rupp T, Thomas R, Perrey S, et al. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur J Appl Physiol 2008;102:153–63. 10.1007/s00421-007-0568-7 [DOI] [PubMed] [Google Scholar]
- 20. Hopker JG, Jobson SA, Pandit JJ. Controversies in the physiological basis of the 'anaerobic threshold' and their implications for clinical cardiopulmonary exercise testing. Anaesthesia 2011;66:111–23. 10.1111/j.1365-2044.2010.06604.x [DOI] [PubMed] [Google Scholar]
- 21. Chen YL, Chiou WK, Tzeng YT, et al. A rating of perceived exertion scale using facial expressions for conveying exercise intensity for children and young adults. J Sci Med Sport 2017;20:66–9. 10.1016/j.jsams.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 22. Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 23. Robertson RJ, Moyna NM, Sward KL, et al. Gender comparison of RPE at absolute and relative physiological criteria. Med Sci Sports Exerc 2000;32:2120–9. 10.1097/00005768-200012000-00024 [DOI] [PubMed] [Google Scholar]
- 24. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]