Abstract
A 49-year-old Caucasian woman presented with subacute headache and right eye pain associated with scotoma, blurred vision and photophobia. MRI was suggestive of optic neuritis of the right optic nerve and she was treated with steroids. Due to persistent symptoms, a lumbar puncture was performed and cerebrospinal fluid analysis was positive for venereal disease research laboratory and rapid plasma reagin titres. On further history, she recalled experiencing an illness associated with diffuse rash, likely secondary syphilis, 1–2 months prior. She tested negative for HIV. She was treated with intravenous penicillin for 2 weeks following which she experienced improvement in symptoms.
Keywords: infectious diseases, infection (neurology), retina, neuroopthalmology
Background
Optic neuritis (ON) is an inflammatory disease of the optic nerve that causes acute or subacute vision loss and pain with eye movement. ON has an estimated lifetime prevalence of 0.6/1000 people.1 Typical ON (T-ON) is an idiopathic inflammatory demyelination of the optic nerve occurring either alone or in association with multiple sclerosis (MS). In contrast, atypical ON (A-ON) is associated with conditions such as neuromyelitis optica, sarcoidosis, vasculitis, systemic lupus erythematosus or infection (eg, HIV, syphilis, Lyme disease, bartonella, etc).2 3 Because treatment is targeted towards the underlying aetiology, it is imperative to differentiate between T-ON and A-ON.1
Syphilis is a sexually transmitted disease caused by the spirochete Treponema pallidum. In 2013, the rates of primary and secondary syphilis in the USA increased by 15.1% and 5.1%, respectively. From 2000 to 2014, the increase was mainly seen among men who have sex with men (MSM), but in recent years there has been an increasing incidence in women.4 Ocular manifestations, which are considered to be part of the spectrum of neurosyphilis, were documented in less than 1% of syphilis cases.5 6 Most reported cases of ocular syphilis were among MSM, half of which were HIV infected. Isolated ON as the manifestation of syphilis in HIV-negative patients is uncommon and is often overlooked due to the frequency of other aetiologies of ON.7 We describe a case of syphilitic ON in an HIV-negative woman who was initially diagnosed as T-ON.
Case presentation
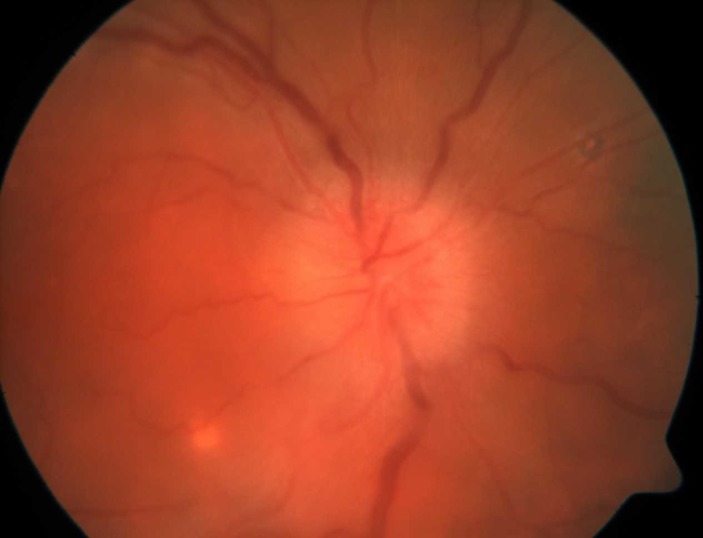
A 49-year-old Caucasian woman with medical history of anxiety and treated hepatitis C virus infection presented with a 2-week history of headache, right eye pain and blurred vision associated with a scintillating scotoma and photophobia. She reported a history of tension-type headaches typically relieved with non-steroidal anti-inflammatory drugs (NSAIDs), but had recently developed new headache symptoms unrelieved by NSAIDs. She reported that 1 month prior she had experienced malaise, night sweats and a generalised rash that self-resolved. Neurological examination demonstrated a diminished visual acuity in the right eye, right relative afferent pupillary defect, dyschromatopsia and photopsia. Extraocular movements were normal, as was the rest of her physical and neurological examination. Ophthalmological examination revealed visual acuity of 20/70 oculus dexter (OD) and 20/20 oculus sinister (OS). Funduscopy of the right eye showed a hyperaemic optic disc with elevation and blurring of the disc margin. There was retinal vein engorgement without peripapillary haemorrhages. There were also chorioretinal scars consistent with a history of ocular histoplasmosis as she was from an endemic region. Gadolinium-enhanced MRI of the brain and orbits demonstrated non-specific white matter changes in the subcortical and periventricular regions. There was a T2 hyperintense signal within the right optic nerve, suggestive of ON. She was treated with a 3-day course of intravenous corticosteroids for an assumed diagnosis of T-ON. At a 2-week clinic follow-up, the patient reported worsening of vision symptoms. Further investigations were pursued, including lumbar puncture and ophthalmology consultation. Slit lamp examination now showed interval development of anterior chamber cells and vitreous cells in both eyes. Her optic disc oedema remained unchanged in the right eye (figure 1) and early optic disc oedema of the left eye was noted.
Figure 1.
Optic disc photo of right eye demonstrating hyperaemia and blurring of the optic disc margin consistent with optic disc oedema. The image also demonstrates chorioretinal scars from ocular histoplasmosis, which is endemic to the region where she resided.
Investigations
Her erythrocyte sedimentation rate was 76 mm/hour and C reactive protein was 1.9 mg/dL. Cerebrospinal fluid (CSF) studies were significant for a white cell cell (WCC) count of 0.08×109/L (10% neutrophils, 84% lymphocytes and 6% monocytes/macrophages/histiocytes), protein of 100 mg/dL and glucose of 46 mg/dL. CSF albumin was elevated at 62.4 mg/dL and CSF IgG was elevated at 18.9, giving a CSF IgG to CSF albumin ratio of 0.3. The ratio of CSF IgG to serum IgG was 0.77, which is within normal limits per the reference lab used. There were five oligoclonal bands. A CSF venereal disease research laboratory (VDRL) titre was positive at 1:4. Follow-up serum rapid plasma reagin (RPR) was reactive (titre of 1:128) and T. pallidum particle agglutination) test was positive. HIV combined antigen and antibody test was negative. Aquaporin 4 antibody testing was negative. Antinuclear antibodies were elevated at a titre of 1:1280 without a specific auto antibody identified. PCR testing for Borrelia burgdorferi, Borrelia afzelii, Borrelia mayonii and Borrelia garinii was negative.
Treatment
Following the diagnosis of neurosyphilis, the patient was treated with a 14-day course of daily intravenous penicillin, 24 MU, as a continuous infusion.
Outcome and follow-up
After 1 week of treatment, ocular findings on funduscopy had improved. By 2 weeks, her vision started improving, and funduscopy showed resolving disc oedema and mild subretinal fluid. Five weeks later, she reported near-resolution of ocular symptoms. On examination, her disc oedema had resolved, and residual subretinal fluid was minimal (figure 2). Four months after treatment, she had a visual acuity of 20/25 in the right eye and 20/20 in the left eye. Her anterior chamber and vitreous cells had resolved. She had repeat serum RPR titre of 1:8; 2 months later, it was 1:4. Approximately 8 months later, she had repeat lumbar puncture with CSF demonstrating no leucocytes and a negative VDRL.
Figure 2.
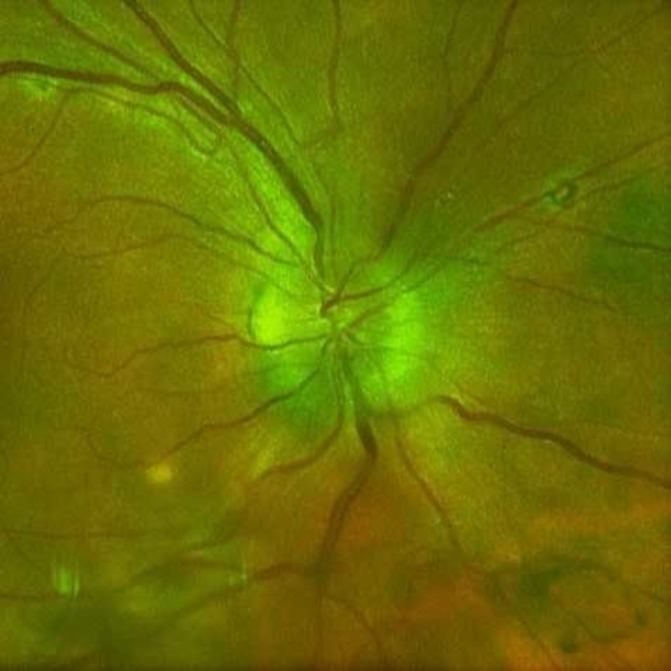
Optic disc photo of right eye (different imaging device) approximately 5 weeks after initiation of penicillin therapy. There is a sharp disc margin and improvement of disc hyperaemia.
Discussion
T-ON presents with an acute or subacute onset of vision loss, alterations of colour vision and ocular pain.2 The Optic Neuritis Treatment Trial (ONTT), which compared outcomes of T-ON among groups that received either intravenous methylprednisolone followed by oral prednisone versus oral prednisone alone versus placebo, found that most patients in all groups recovered within 2 weeks from symptom onset, but recovery was hastened in those who received methylprednisolone.8 A-ON is generally suspected in cases with red-flag symptoms and signs, such as severe optic disc swelling with retinal exudates, haemorrhages and profound visual loss.3 Based on the ONTT, the patient’s lack of symptom improvement following 2 weeks of therapy was an indicator that suggested A-ON. Additionally, the CSF findings were not consistent with T-ON due to demyelination. Although elevations in CSF leucocytes can be seen in T-ON, the elevation is generally mild,9 as opposed to in patients with an infectious aetiology, who tend to have CSF WCC count >50 cells/mm3.10 Our patient’s CSF WCC count was 82 cells/mm3, suggestive of infection.
Although our patient had elevated IgG oligoclonal bands (5 CSF bands, 0 serum bands), our patient’s CSF IgG index was not elevated. A pathological increase in CSF IgG proteins can be due to a blood–CSF barrier dysfunction, as seen in an infectious aetiology, or due to intrathecal IgG synthesis because of a chronic inflammatory condition, such as MS.11 However, the diagnosis of MS does not hinge solely on the presence of elevated CSF IgG proteins, there should also be a demonstration of radiographic lesions disseminated in time and space along with exclusion of alternative diagnoses. Although our patient had some T2/fluid attenuated inverse recovery (FLAIR) periventricular hyperintensities, they did not have the appearance of typical MS lesions which are usually larger in size (3–8 mm), ovoid and located perpendicular to the ventricle.12 In this patient with CSF leucocytosis, a positive CSF-VDRL and a positive RPR, the clinical picture is thus most congruent with a diagnosis of syphilitic ON.
Humans are the only known natural host of T. pallidum. Syphilis is re-emerging in the USA despite public health efforts, well-established screening tests and access to curative treatment.13 Transmission occurs across mucous membranes and skin surfaces during intimate contact. Following inoculation, T. pallidum multiply in the subepithelium and within weeks cause the typical chancre of primary syphilis, which is a painless, deep ulcer with a clean base and raised borders. When this chancre occurs on mucosal surfaces, such as intravaginal areas, it may go unrecognised compared with other commonly exposed locations (eg, external male genitalia, oral mucosa or labia).14 15 Our patient did not recall prior genital or oral lesions.
Primary syphilis is followed by wide-spread haematogenous and lymphatic dissemination resulting in secondary syphilis, characterised by malaise, fever, myalgia/arthralgia, lymphadenopathy and a generalised polymorphic maculopapular rash that often involves the palms and soles.16 This stage can progress into tertiary syphilis (commonly described as neurosyphilis and cardiovascular syphilis), which typically follows an asymptomatic period called latent syphilis. It is, however, imprecise to consider neurosyphilis to be just a late manifestation. Dissemination of T. pallidum to the central nervous system can occur as early as the primary or secondary phases of infection.17 18 Centers for Disease Control and Prevention (CDC) surveillance data show that most cases of suspected ocular syphilis occurred in the early phases (primary, secondary and early latent).6 Abnormal CSF studies and a reactive CSF-VDRL qualify for a diagnosis of neurosyphilis regardless of the approximation to inoculation.19 Our patient had documented negative RPR testing approximately 9 months prior to developing ocular symptoms. The generalised macular rash, malaise and night sweats that developed 1–2 months before onset of ocular symptoms suggests that our patient most likely had central nervous system involvement during the secondary stage of syphilis. As per the sexually transmitted diseases guidelines from the CDC, all patients with syphilis and particularly ocular syphilis should have concomitant HIV testing, as almost half of documented cases were found to be HIV positive.6 Fortunately, our patient tested negative for HIV. Ocular syphilis is treated the same as neurosyphilis, with penicillin G 18–24 MU as continuous intravenous infusion daily for 10–14 days.
In summary, syphilis has re-emerged as the ‘great masquerader’ with changing epidemiology in the USA. ON can be one of the presenting features of syphilis without other classical manifestations. CSF studies and syphilis testing must be considered early when red-flag signs are noted on funduscopy or illness progression (ie, lack of resolution within 2 weeks or lack of improvement with therapy). Early identification of syphilis is important in neurosyphilis as outcomes are better with penicillin therapy.
Learning points.
Panuveitis and lack of improvement in vision despite steroid therapy should prompt workup for atypical causes of optic neuritis.
Syphilis is an important and emerging cause of optic neuritis, which can sometimes be the only presenting symptom of syphilis infection.
Ocular syphilis is a manifestation of neurosyphilis. Dissemination of Treponema pallidum to the central nervous system can occur as early as primary or secondary syphilis.
Clinical outcomes are better with intravenous penicillin therapy in syphilitic optic neuritis. Duration of therapy is 2 weeks, which is the same as in other manifestations of neurosyphilis.
Footnotes
Patient consent for publication: Obtained.
Contributors: All authors participated in the care of the patient as well as the conception and authorship of the manuscript. NSN and LEG performed literature review and authored the manuscript in regard to syphilis infection and treatment. MAH provided expertise and authorship in regard to optic neuritis. AE and HR provided critical insight and editing to the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jenkins TM, Toosy AT. Optic neuritis: the eye as a window to the brain. Curr Opin Neurol 2017;30:61–6. 10.1097/WCO.0000000000000414 [DOI] [PubMed] [Google Scholar]
- 2.Voss E, Raab P, Trebst C, et al. Clinical approach to optic neuritis: pitfalls, red flags and differential diagnosis. Ther Adv Neurol Disord 2011;4:123–34. 10.1177/1756285611398702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren FA. Atypical optic neuritis. J Neuroophthalmol 2014;34:e12–3. 10.1097/WNO.0000000000000180 [DOI] [PubMed] [Google Scholar]
- 4.Patton ME, Jr S, Nelson R, et al. Primary and secondary syphilis-United States, 2005-2013. MMWR Morbidity and mortality weekly report 2014;63:402–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Apinyawasisuk S, Poonyathalang A, Preechawat P, et al. Syphilitic optic neuropathy: re-emerging cases over a 2-year period. Neuroophthalmology 2016;40:69–73. 10.3109/01658107.2015.1134586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver SE, Aubin M, Atwell L, et al. Ocular Syphilis - Eight Jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep 2016;65:1185–8. 10.15585/mmwr.mm6543a2 [DOI] [PubMed] [Google Scholar]
- 7.Pless ML, Kroshinsky D, LaRocque RC, et al. Case records of the Massachusetts General Hospital. Case 26-2010. A 54-year-old man with loss of vision and a rash. N Engl J Med 2010;363:865–74. 10.1056/NEJMcpc1000968 [DOI] [PubMed] [Google Scholar]
- 8.Beck RW, Gal RL. Treatment of acute optic neuritis: a summary of findings from the optic neuritis treatment trial. Arch Ophthalmol 2008;126:994–5. 10.1001/archopht.126.7.994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolak LA, Beck RW, Paty DW, et al. Cerebrospinal fluid in acute optic neuritis: experience of the optic neuritis treatment trial. Neurology 1996;46:368–72. 10.1212/WNL.46.2.368 [DOI] [PubMed] [Google Scholar]
- 10.Horwitz H, Friis T, Modvig S, et al. Differential diagnoses to MS: experiences from an optic neuritis clinic. J Neurol 2014;261:98–105. 10.1007/s00415-013-7166-x [DOI] [PubMed] [Google Scholar]
- 11.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001;184:101–22. 10.1016/S0022-510X(00)00501-3 [DOI] [PubMed] [Google Scholar]
- 12.Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev 2014;13(4-5):518–24. 10.1016/j.autrev.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 13.Patton ME, Su JR, Nelson R, et al. Primary and secondary syphilis--United States, 2005-2013. MMWR Morb Mortal Wkly Rep 2014;63:402–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Fiumara NJ. Treatment of primary and secondary syphilis. Serological response. JAMA 1980;243:2500–2. [PubMed] [Google Scholar]
- 15.Chapel TA. The variability of syphilitic chancres. Sex Transm Dis 1978;5:68–70. 10.1097/00007435-197804000-00009 [DOI] [PubMed] [Google Scholar]
- 16.Syphilis LSA, et al. : Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, Harrison’s Principles of Internal Medicine. 19th edn New York, NY: McGraw-Hill Education, 2015. [Google Scholar]
- 17.Lukehart SA, Hook EW, Baker-Zander SA, et al. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med 1988;109:855–62. 10.7326/0003-4819-109-11-855 [DOI] [PubMed] [Google Scholar]
- 18.Rolfs RT, Joesoef MR, Hendershot EF, et al. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group. N Engl J Med 1997;337:307–14. 10.1056/NEJM199707313370504 [DOI] [PubMed] [Google Scholar]
- 19.Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev 2006;19:29–49. 10.1128/CMR.19.1.29-49.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]