Abstract
Ductal carcinoma in situ (DCIS) is a highly heterogeneous disease. It presents in a variety of ways and may or may not progress to invasive cancer, which poses challenges for both diagnosis and treatment. On May 15, 2017, the Dana-Farber/Harvard Cancer Center hosted a retreat for over 80 breast specialists including medical oncologists, surgical oncologists, radiation oncologists, radiologists, pathologists, physician assistants, nurses, nurse practitioners, researchers, and patient advocates to discuss the state of the science, treatment challenges, and key questions relating to DCIS. Speakers and attendees were encouraged to explore opportunities for future collaboration and research to improve our understanding and clinical management of this disease. Participants were from Dana-Farber Cancer Institute, Brigham and Women’s Hospital, Massachusetts General Hospital, Beth Israel Deaconess Medical Center, Duke University Medical Center, and MD Anderson Cancer Center. The discussion focused on three main themes: epidemiology, detection, and pathology; state of the science including the biology of DCIS and potential novel treatment approaches; and risk perceptions, communication, and decision-making. Here we summarize the proceedings from this event.
Approximately 50 000 cases of ductal carcinoma in situ (DCIS) are diagnosed in the United States each year (1). The term “DCIS” encompasses a highly heterogeneous group of lesions that differ in their clinical presentation, histologic and biologic features, and outcome. Although DCIS is considered to be a precursor to invasive breast cancer, 14–53% of DCIS will not progress to invasive breast cancer, and thus it is considered a nonobligate precursor (2–6). As a result, there remains considerable uncertainty about optimal clinical management at the individual patient level.
In May 2017, the Dana-Farber/Harvard Cancer Center brought together clinicians and researchers from multiple institutions to discuss the current trends and identify and address the key questions (Supplemental Table 1, available online) that must be answered to improve our understanding and clinical management of DCIS.
Part I: Current Trends, Outcomes, Detection, and Pathology
Treatment Outcomes and Trends
Surgery is the standard of care for DCIS; however, until recently there were limited data examining the impact of surgery, with or without radiation, on survival by grade or size of DCIS. Data were also limited on the use of adjuvant therapies for DCIS nationally and whether these therapies truly improve outcomes. To address this gap, Sagara and colleagues conducted a study to determine the survival benefit of surgical treatment by nuclear grade in patients with DCIS (7). Using data from the Surveillance, Epidemiology, and End Results (SEER) database, they analyzed a cohort of women diagnosed with DCIS by biopsy only. Overall, the study showed no breast cancer-specific survival benefit of surgery for women with low-grade disease; however, intermediate- or high-grade DCIS patients did experience a survival benefit from surgery.
The Early Breast Cancer Trialists’ Collaborative Group examined the efficacy of radiotherapy (RT) following breast-conserving surgery (BCS) for DCIS in a meta-analysis of four randomized controlled trials (8), and reported that RT reduced the rate of local recurrence by ∼50%, irrespective of tumor type and patient characteristics. However, RT did not improve overall survival or breast cancer-specific survival. In contrast, Sagara et al used SEER data along with patient prognositic score to investigate the benefit of RT among patients treated between 1988 and 2007 (9). Propensity score analysis of the overall cohort demonstrated a small yet statistically significant survival benefit (0.3%) associated with the receipt of radiation (9), reflecting the power of a large population-based analysis and suggesting that RT may modestly improve survival for some patients with DCIS.
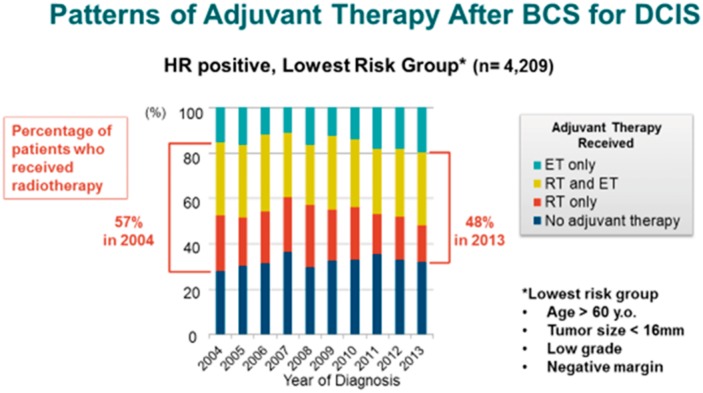
Using the American College of Surgeons’ National Cancer Database, Sagara et al also investigated factors associated with the use of adjuvant RT and/or endocrine therapy (ET) following BCS in women with DCIS (10). This study demonstrated that both clinico-pathologic and demographic factors influence the use of adjuvant therapy. For hormone receptor (HR)-positive DCIS treated with BCS, there has been a shift towards decreasing use of RT and increasing use of ET alone or ET in combination with RT after BCS. In contrast, for HR-negative DCIS, the proportion of women receiving RT after BCS has increased over the last decade. Further, in a low-risk cohort—defined as patients older than 60 years of age with low-grade HR-positive DCIS lesions, less than 16 mm in size, excised to negative margins—the use of RT and/or RT with ET has decreased (Figure 1). These national trends suggest that there is a DCIS patient population and their physicians who are receptive to de-escalating therapy.
Figure 1.
Patterns of adjuvant therapy use after breast-conserving surgery for ductal carcinoma DCIS. Reprinted with permission from Springer Nature (10).
Controversies in Imaging in DCIS
Prior to 1985, DCIS represented 2% of all breast cancer. When mammography screening began, incidence increased and, based on SEER data from 2007–2013, one-third of breast cancers detected by screening mammography in current practice are DCIS. Some see this increased detection as a problem, leading to overtreatment for DCIS patients, and call for reduced screening mammography (11). In recent years, breast imaging has shifted from 2 D mammography to 3 D mammography and magnetic resonance imaging (MRI). On 2 D mammography, breast imagers were looking for patterns suggestive of cancer, and a focus was on calcifications. With the introduction of 3 D mammography, or tomosynthesis, the overall cancer detection rate has not varied dramatically, but there has been a lower percentage of DCIS diagnoses and a higher percentage of invasive cancers detected compared with 2D (12–14). This suggests that tomosynthesis may be less sensitive for detecting DCIS and/or that mammographers are now focusing more attention on identifying imaging features that are more likely to be associated with invasive cancers and possibly less attention to microcalcifications. Despite this, we have not seen a significant decrease in interval cancers with tomosynthesis, suggesting that this shift in detection patterns may not be leading to improved clinical outcomes.
Several studies of mammography, MRI, and ultrasound confirm that MRI has the highest sensitivity for detecting DCIS (15,16). Kuhl et al. calculated the performance of imaging modalities based on DCIS grades (16). When compared with mammography, MRI was superior in detecting both low- and high-grade DCIS, but MRI sensitivity was especially evident in high-grade DCIS (low-grade = 80% MRI detection/60% mammography detection vs high-grade = 98%/52%). However, the type of mammograpy used in this study for three of the five years was film-screen, known to have inferior sensitivity for DCIS detection to full-field digital mammography, which is now in widespread use.
The ECOG-ACRIN DCIS 4112 study aims to identify women with DCIS who may be managed less aggressively without sacrificing excellent outcomes. This study investigates the use of preoperative breast MRI and its impact on surgical treatment decisions. Early findings suggest that conversion to mastectomy was common, with nearly one in five patients ultimately undergoing mastectomy, and that breast MRI findings accounted for less than one-half of these conversions (Table 1). Patient preference is a strong factor for converting to mastectomy for a wide range of reasons. For patients who remained candidates for wide local excision after MRI, 96.4% achieve successful wide local excision as the final surgical procedure. MRI also highlights other lesions, of which more than one-half are benign (17). These false positive findings require careful management with effective communication between the radiologist, surgeon, and patient to reduce anxiety and avoid unnecessary mastectomies.
Table 1.
Mastectomy in ECOG-ACRIN DCIS 4112*
| Reasons for mastectomy | No. (%) |
|---|---|
| Based on MRI findings | 25 (39.1) |
| Contralateral MRI findings | 3 (12.0) |
| Lesion size on MRI too large for breast conservation | 15 (60.0) |
| Multi-centricity on MRI | 7 (28.0) |
| Patient preference | 24 (37.5) |
| After WLE attempt | 10 (15.6) |
| Positive margin | 9 (60.0) |
| Unknown | 1 (40.0) |
| Other | 5 (7.8) |
| Genetic history | 3 (60.0) |
| Contraindications to RT | 2 (40.0) |
| Total | 64 (100.0) |
*DCIS = ductal carcinoma in situ; MRI = magnetic resonance imaging; RT = radiation therapy.
It is also important to note that modern imaging has the potential not only to identify lesions that may be cancer, but also to evaluate information beyond the lesions. For example, features of the breast tissue around the DCIS may be potential biomarkers for risk of recurrence or invasive disease. Future research must determine how and in what context we can use this type of information clinically.
Risk Prediction and Uncertainty
After decades of research, it is still not possible to reproducibly identify which DCIS lesions will progress to invasive disease and which are unlikely to progress and, correspondingly, which patients can be managed safely with excision alone or no treatment beyond the diagnostic biopsy. Young age has been shown to be a risk factor for local recurrence in patients with DCIS. There appear to be no differences in distribution of pathology-related factors (grade or necrosis) according to age, though younger patients are more likely to have greater extent of disease and present with a palpable mass lesion (18). Additionally, women with symptomatic presentation (ie, palpable mass in breast) are more likely to progress to invasive breast cancer (19).
The association between specific genetic changes and grade could provide insights into the biology of DCIS that influences pathologic classification and clinical management. All of the intrinsic molecular subtypes identified in invasive cancers are also seen in DCIS (20). However, among patients with DCIS, a slightly greater proportion are classified as HER2 enriched. Two groups have investigated whether certain DCIS phenotypes are associated with an increased risk of invasive cancer. Williams et al. (21) concluded that all phenotypes were associated with an increased risk compared with the luminal A phenotype, and the Cancer Research Network showed only the HER2-enriched group was at increased risk (Laurel A. Habel, Ninah Achacoso, Stuart J. Schnitt, Laura C. Collins, Monica Morrow, Reina Haque, Larissa Nekhlyudov, Suzanne W. Fletcher, Allen M. Gown, Lynn Goldstein, Charles P. Quesenberry, Jr., unpublished data). Many studies have shown a relationship between various histologic features of DCIS and clinical outcome following BCS; however, the findings are often conflicting and thus the relative importance of the various histologic features is still not well defined (19,22,23). Risk stratification based on pathologic factors remains elusive and this confounds communication of risk to patients.
The USC/Van Nuys Prognostic index combines tumor size, grade, and margin status with patient age to estimate local recurrence risk and benefit from RT. Yet, this tool is challenging to apply in clinical practice due to the stringent sampling requirements, making it useful in only a minority of cases. Silverstein et al. argue that if the lesion is adequately excised (margin width >10 mm), a patient’s risk of local recurrence is unaffected by nuclear grade, presence of comedo necrosis, lesion size, or addition of RT (24). However, given the surgical and cosmetic implications of such wide margin widths, this is difficult to achieve for all patients. A Memorial Sloan Kettering Cancer Center study shows that patients with a higher volume of disease near the margin derive a greater benefit from the addition of RT (25). Other data suggest patients with small, low-grade lesions may be adequately treated with wide excision only (26,27).
The Memorial Sloan Kettering Cancer Center nomogram is an another tool that combines 10 clinico-pathologic factors to generate 5- and 10-year risks of ipsilateral breast cancer recurrence. This validated tool estimates outcomes with or without RT and/or ET. This score has a tendency to overpredict recurrence in patients treated with RT and underpredict recurrence in patients treated with BCS alone (28–30).
The Oncotype DCIS score is a commercial risk assessment test that is prognostic only. However, in two studies that have assessed the prognostic value of this score, patients in the low-risk group still have local recurrence rates over 10% at 10 years (31–33). More recent data suggest that integrating the DCIS score with tumor size and patient age helps further refine risk assessement. The DCISionRT test has also been recently developed to predict ipsilateral breast events after DCIS and benefit from RT (34). This test is currently undergoing further validation in two ongoing prospective clinical studies.
In summary, currently no histopathologic features of DCIS consistently provide accurate risk prediction for progression to invasive carcinoma. Several challenges were noted during the group discussion: that large databases with adequate outcomes including clinical and pathologic details have not been widely available, and DCIS outcomes are not the same today as in the older studies included in the prospective randomized trials. Several potential research directions were also discussed and are detailed in Supplemental Table 2 (available online).
Part II: State of the Science
Biology of DCIS and the Role of the Microenvironment
Little progess has been made over the last 20 years in reproducibly distinguishing biologically favorable from unfavorable DCIS. Given that few differences have been found at the genomic and transcriptomic levels between the cells of DCIS and invasive breast cancers of equivalent grade, it is possible that studying the DCIS microenvironment may be the key to understanding the biological progression from DCIS to invasive cancer.
By definition, the neoplastic cells in DCIS are present within the mammary ductal-lobular system and have not spread outside these sites. In the early stages of progression, immune cells may help to eliminate the cancer cells. In the equilibrium stage, the tumor is still somewhat controlled by the immune system; invasion/progression occurs when the tumor cells escape from the immune system. Understanding the mechanisms of escape in DCIS, which might be immune-mediated, may lead to more options for prevention.
Myoepithelial cells, which produce the basement membrane of the ducts and have a tumor suppressor function, are still present in DCIS (these cells are not present in invasive cancer) but the gene expression profiles and immunophenotype of myoepithelial cells in DCIS differ from myoepithelial cells in normal breast tissue (35). Myoepithelial cells prevent invasive progression both due to forming a structural barrier and also by expressing many anti-invasive and tumor suppressive genes (35–37). Therefore, myoepithelial cells can be viewed as “gatekeepers” of invasive progression, and alterations in myoepithelial cells seen in DCIS could predict risk of invasive recurrence (38). As many of the genes differentially expressed between DCIS-associated and normal myoepithelial cells encode for secreted proteins, it is possible that the myoepithelial cells orchestrate the microenvironmental changes, including changes in the immune microenvironment, that are present in DCIS compared with normal breast tissue.
When comparing normal breast tissue to DCIS and invasive cancers, the presence of myoepithelial cells, leukocytes, macrophages, cytotoxic T cells, and helper T cells in the microenvironment are different. Interestingly, Gil Del Alcazar and colleagues reported a decrease in activated cytotoxic T cells in invasive tumors compared with DCIS and at the same time also observed an increase in the expression of immune checkpoint proteins such as PD-L1 and CTLA4 with invasive progression (39). In the case of PD-L1 this included a selection for cancer cells that have amplification of the gene encoding for PD-L1 (39).
The microenvironment is diverse and this diversity shifts as the cancer progresses. There are measurable differences between these factors when comparing normal tissue, tissue from patients with DCIS and tissue from patients with invasive disease, as well as tissue from low-grade DCIS patients versus high-grade DCIS patients. If we can pinpoint the significance of the biologic changes in this environment, we may be able to distinguish a DCIS that will progress to invasive cancer from a DCIS that will not progress and guide patient management accordingly.
Vaccines and Prevention
Historically, vaccines were evaluated in patients with metastatic disease. Although early-phase trials showed that vaccination could generate antigen-specific immune responses, there was minimal evidence of clinically meaningful activity (40). Vaccinating patients with metastatic disease is a challenge due to extent of disease burden and the immunosuppressive microenvironment, which hampers T cell activity. To address these limitations, investigators have put forward the hypothesis that cancer vaccines may be more effective in a minimal disease setting to prevent disease recurrence after standard therapy or for primary prevention.
The goal of vaccinating in the adjuvant setting is to elicit a memory immune response that could be reactivated if cancer cells are detected in order to eliminate those cells before they can become established as recurrent disease. While a simple vaccination strategy may not be effective as secondary prevention, researchers have questioned whether it may be possible to administer a vaccine for primary prevention. This strategy is currently being used with the administration of the human papillomavirus vaccine to prevent human papillomavirus-associated malignancies. However, targeting tumor antigens rather than a virus is complex. Initially, it was thought that nonviral, nonmutated tumor antigens are too similar to self-antigens and targeting them may lead to autoimmunity. However, studies based largely on melanoma antigens have implied that autoimmunity is required for antitumor effect. In addition, epidemiologic data suggest that an immune response against epithelial antigens stimulated early in life either through the development of a childhood disease such as chicken pox or mumps, due to febrile illnesses, or via childhood vaccination, may decrease the risk of developing a malignancy (41–45). Therefore, it is possible that vaccines would be successful in preventing progression in patients with premalignant lesions (43).
Vaccination may be most effective in DCIS and atypical ductal hyperplasia before tumor cells are genetically unstable and rapidly dividing. Czerniecki and colleagues conducted a neoadjuvant study using a HER2-targeted dendritic cell vaccine administered to patients with a biopsy diagnosis of DCIS before surgery (46,47). Compared with pretreatment biopsies, surgical specimens showed an increase in CD4+ and CD8+ T cells after vaccination and a decrease in HER2 expression, suggesting the possibility of using vaccination to elicit an antigen-specific, anti-tumor immune response at the earliest stages of disease. Another trial, VADIS (NCT02636582), is a phase II trial investigating the E75+GM-CSF peptide vaccine administered three times before surgery in DCIS patients (48). The primary endpoint is the generation of E75-CTL T cells in vaccinated patients. Secondary endpoints include toxicity, epitope spreading, T cell functional capacity, and histologic response. Evaluating vaccines in patients with DCIS is an initial step toward developing a truly preventive breast cancer vaccine.
Prevention of Invasive Breast Cancer: New Opportunities
The main limitation of current prevention studies is that outcomes may be rare, and time to events can be prolonged; thus it may take many years for definitive conclusions to be drawn from these studies. Therefore, researchers are investigating surrogate outcomes (eg, changes in mammographic density, or molecular and immune biomarkers) for prevention. Investigators have conducted studies with agents that are unlikely to cause unwanted side effects, but these agents are also less likely to lead to large differences in risk (ie, dietary components such as flaxseed). By studying surrogate outcomes, the hope is to identify prevention agents that are tolerable and can be scaled.
Vitamin D, a steroid hormone (Alliance 70806) (49), and metformin, an antidiabetic drug (Alliance A2211102) (50), are potentially promising agents for breast cancer prevention. Many women already take vitamin D for bone health and it is well tolerated; previous studies suggest the effectiveness of metformin to improve metabolic factors (51) and regulate levels of pAKT and pAMPK (52).
Given the results of the IBIS trial (53), which showed a long duration of benefit and significant breast cancer risk reduction for women who take tamoxifen, there is interest in finding new ways to use tamoxifen as a prevention agent. The Afimoxifene in Reducing the Risk of Breast Cancer in Women With Mammographically Dense Breasts study (NCI) (54) is a randomized, phase II trial investigating the efficacy of tamoxifen topical gel applied to the breast area. Preliminary analysis reveals women were more willing to try transdermal tamoxifen than oral tamoxifen (55). In BRCA1 mutation carriers, there are ongoing prevention studies investigating bezadioxyzine an an estrogen receptor degrader (56) and denosumab as a RANK ligand inhibitor (57).
Challenges identified during our group discussion included the heterogeneity in DCIS samples, which raises many issues related to the use of limited core biopsy samples and the need for fresh tissue to continue studying the underlying biology of progression of DCIS. Further, prevention trials have many challenges including historically low accrual, need for long-term treatment and follow-up, and well-studied but not lucrative drugs leading to high clinical trial costs. However, evaluation of these drugs as well as alternative approaches to prevention such as cryotherapy or intraductal injection of anti-tumor agents to elicit immediate and long-term immune rejection of lesions may yield important insights into DCIS prevention and treatment.
Part III: Risk Perceptions, Communication, and Decision-Making
Risk Perceptions, Communication, and Decision-Making for DCIS
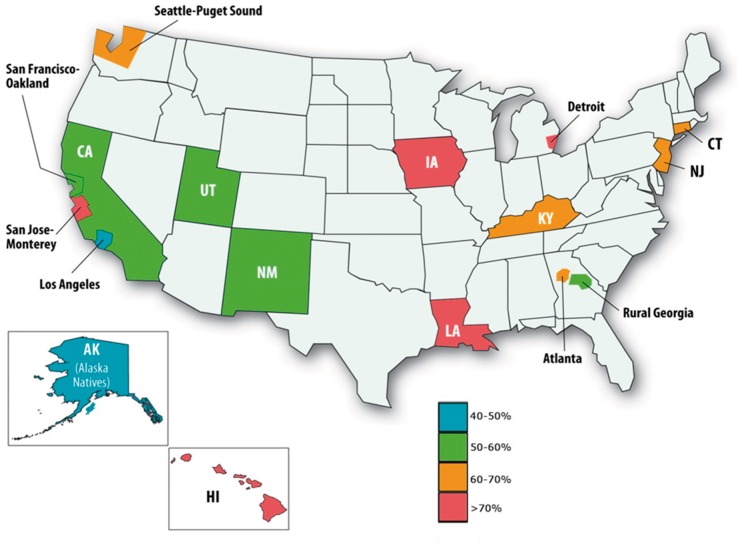
The use of BCS as an alternative to mastectomy for DCIS gained acceptance in the 1990s (58). Randomized trials have demonstrated that adding RT after BCS reduces both the risk of having an invasive recurrence and a DCIS recurrence (59–62); however, a meta-analysis of these trials reveals that the use of RT does not improve survival (8). Differences in the interpretation of these data or values associated with local versus survival benefits have led to large regional variation in the use of RT for DCIS (Figure 2) (63,64).
Figure 2.
Regional variation in use of radiation therapy after excision for DCIS by SEER area. Reprinted with permission from Oxford University Press (64).
Many attempts have been made to identify patients at low risk of local recurrence after BCS alone. However, there is little consensus on how best to combine classical clinico-pathologic characteristics in these analyses. As noted previously, the Oncotype DCIS score was developed to provide a gene expression assay for potentially improved characterization of risk of local recurrence, but how best to incorporate this assay in clinical practice remains uncertain. Raldow et al. sought to determine the cost-effectiveness of different treatment strategies using the Oncotype DCIS score (65). The authors used a Markov model to simulate 10-year outcomes for 60-year-old women eligible for the ECOG E5194 study (32) and determined the cost-effectiveness of employing genomic testing after BCS for all women versus treating all women with excision alone or excision with RT (66). None of the treatment strategies employing the genomic test or strategies treating all women with RT was cost-effective relative to excision alone. Sensitivity analyses revealed that the most cost-effective strategy was highly sensitive to the utility of being without disease after excision alone or after excision and RT. This finding highlights the importance of engaging patient preferences in the treatment decision process.
Physicians note that decision-making about treatment for DCIS is very difficult for patients, who have a tendency to overestimate their risk of recurrence (67). In one study, more than 25% of DCIS patients believe that there is at least a moderate likelihood of DCIS spreading to other places in their body (68). These data underscore the need for patient education about treatment outcomes to improve the quality of decision-making about treatment. Decision-making is further complicated in that the outcome most important to an individual patient or stakeholder group may vary. Invasive breast cancer diagnosis is only one such outcome—other important outcomes include the likelihood of undergoing further breast biopsy or surgery, breast preservation, chemotherapy, and financial costs.
To help inform patients of the risks associated with different treatment options for DCIS, Punglia et al. have created a web-based decision aid: www.onlineDeCISion.org (69). Ongoing modifications to the tool will allow physicians to view outcomes by patient age and to tailor results by other treatment and risk factors if they are available (eg, age, grade, ER status) (70). One important message of the decision aid is that survival outcomes are essentially the same whichever treatment is chosen. With this information, patients may be able to better evaluate treatments based on their preferences and their tolerance for recurrence, versus the inconvenience or side effects of treatment, and improve the quality of their DCIS decisions.
Are We Ready for De-Escalation of Treatment for DCIS?
Ryser and colleagues conducted a usual care versus active surveillance analysis using SEER data on women with pure DCIS (Supplemental Figure 1, available online) (71). The main outcome measure was the probability of breast cancer death at 10 years. Under active surveillance, younger women had a higher risk of dying from breast cancer when compared with older women. However, by age 70 years, patients have much higher competing causes of mortality regardless of DCIS treatment choice. This does suggest that there may be a group of patients where active surveillance may be reasonable. Nevertheless, there is the concern of unrecognized invasive cancer, which has been reported in approximately 25% of patients diagnosed with DCIS on core needle biopsy (72). Therefore, patients can choose between the standard treatment options for DCIS (BCS, mastectomy, RT, and ET) or living with an increased risk of breast cancer-specific mortality ranging from 0.2% to 2.6% at 10 years. It is possible that some patients may consider this level of risk small and would prefer to forgo aggressive treatment, particularly in the face of preexisting comorbidities. Patient participation in evaluation of these trade-offs is crucial.
Currently there are three randomized controlled trials of active surveillance open to low-risk DCIS patients: LOw Risk DCIS (LORD), LOw RISk DCIS (LORIS) and Comparison of Operative to Monitoring and Endocrine Therapy (COMET) (Table 2) (73–77). In all three trials, patients are randomized between two treatment arms: 1) standard treatment regimen, or 2) no intervention, but with close monitoring with mammography. The primary outcome for LORD and LORIS is ipsilateral invasive cancer-free survival; the primary endpoints for COMET are invasive cancer diagnosis, overall survival, disease-specific survival, quality of life, fear of cancer recurrence, and body image. Active surveillance is not encouraged outside of a clinical trial context, and this treatment strategy is not appropriate for patients with high-grade or extensive DCIS, palpable disease, mass on imaging, or other specific breast signs or symptoms.
Table 2.
Inclusion and exclusion criteria for the COMET, LORIS, and LORD trials (75)*
| CRITERIA | COMET | LORIS | LORD |
|---|---|---|---|
| Inclusion criteria | |||
| Age, y | ≥40 | ≥46 | ≥45 |
| Nuclear grade | Low and intermediate | Low and intermediate | Low |
| Morphology | Calcifications only | Calcifications only | Calcifications only |
| Hormone receptor status | ER and/or PR positive, plus HER2 negative if performed | N/A | N/A |
| Exclusion criteria | |||
| History of cancer | Exclude if invasive breast cancer | Exclude if invasive breast cancer or ipsilateral DCIS | Exclude if any cancer except in situ of the cervix or basal carcinoma of the skin |
| Symptomatic | Exclude | Exclude | Exclude |
| Comedonecrosis | Exclude* | Exclude | N/A |
| Synchronous invasive cancer | Exclude | Exclude | Exclude |
| Bilateral DCIS at presentation | Include | Include | Exclude |
| High risk | Include | Exclude if high risk per NICE guidelines (76) | Exclude if family with BRCA 1/2 |
| History of chemoprevention | Exclude | N/A | N/A |
*Criteria deemed not applicable (N/A) are not mentioned in the inclusion or exclusion criteria of the study protocols. The table reports the data included in reference (75) in regards to the COMET trial exclusion critera; however, the criteria were recently updated. Comedonecrosis no longer an exclusion criteria. The trial now allows any patients with low or intermediate grade DCIS. COMET = Comparing Operative to Medical Endocrine Therapy for low-risk DCIS; DCIS = ductal carcinoma in situ; LORD = LOw Risk DCIS; LORIS = LOw RISk DCIS; NICE = National Institute for Health and Care Excellence.
The many challenges that remain also include how patient treatment decisions, and associated trial accrual, may be affected by the language used by health care professionals when discussing DCIS and the difficulty in conveying uncertainty in outcome estimates to patients. Moreover, does randomization to active surveillance put a woman at risk for needing more extensive surgery later that may be more deforming than BCS? This is an important endpoint that will be collected in the active surveillance studies.
Future Research Directions
Patients with DCIS have excellent breast cancer-specific survival, irrespective of their choice for local therapy. Patients are not generally dying from the disease, so the question remains: how best do we treat each individual patient? We identified several take-aways from the retreat. First and foremost, patient preferences are paramount in treatment decisions regarding DCIS. We must strive to better educate our patients about DCIS, its heterogeneity, the benefits and risks of all the treatment options, and present the data in an unbiased way to help each patient make a decision that is right for her. We also must better understand the biology of the disease to make meaningful strides in how we manage the disease clinically. This requires access to well-annotated biospecimens for research. Moreover, encouraging the development and characterization of induct transplanted models, transgenic, and knockout mice models of DCIS may allow study of in situ cancer at all stages of progression to invasive cancer.
During the discussion sessions, it became clear that we must develop a common, standardized language to communicate about DCIS. Some debate exists as to whether or not “carcinoma” should be included in the naming of DCIS. If different clinicians speak about DCIS using different terms and with different perceptions of risks, then how do we expect our patients to have a clear understanding of their disease? We need to ensure that we are presenting the disease using a shared language. This language will be informed by the biological advances as they come, but in the meantime, it is important to remain consistent in our presentation and treatment discussions.
Finally, continuing to seek out and participate in regional, national, and international collaborations is another crucial step to furthering our understanding and our patients’ understanding of DCIS. There are a number of DCIS collaborative group clinical trials available, and we should encourage patients to consider these options. The trials are a result of institutional collaborations both nationally and internationally, and results from these trials have the potential to change the way we treat people with DCIS. Unraveling the biological drivers of cancer progression in DCIS and applying this knowledge to refine patient care exemplifies the goal of precision therapy. Combining biologic insights with improved strategies to elicit a patients’ treatment preferences will allow for the mitigation of both overtreatment and undertreatment. It is hoped that the lessons learned in DCIS research will inform an overall framework of how to rationally address the issues posed by cancer screening and early detection, not only for breast cancer but for other screen-detected cancers.
Funding
Funding for the data presented was obtained from NIH grants R35CA197623 (KP), U01 CA143233 (KP), and the Breast Cancer Research Foundation (KP and MG); research support from General Elective (CL); AHRQ (HHSA290200500161) and PCORI (CE-1211-4173) to RP; and NIH R01 CA185138-01, PCORI (1503-29572 and 1505-30497) and a Department of Defense Breakthrough Award (BC132057) to SH.
Notes
Affiliations of authors: Radiation Oncology (RSP) and Medical Oncology (KB, KP, JG, AHP) and Surgical Oncology, Division of Breast Surgery, Department of Surgery, (MG, EM, TAK), Dana-Farber/Brigham and Women’s Cancer Center, Boston, MA; Department of Radiology, Massachusetts General Hospital, Boston, MA (CL); Department of Pathology, Beth Israel Deaconess Medical Center, Boston, MA (LC); Department of Breast Surgical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX (EM); Department of Surgery, Duke University Medical Center, Durham, NC (SEH); Department of Pathology, Brigham and Women’s Hospital, Boston, MA (SJS); Dana-Farber/Brigham and Women’s Cancer Center, Boston, MA (SJS).
Author disclosures: Member of the health care advisory board of General Electric (CL) and speaker for Genomic Health (TK).
Supplementary Material
References
- 1. Ward EM, DeSantis CE, Lin CC, et al. Cancer statistics: breast cancer in situ. CA Cancer J Clin. 2015;65(6):481–495. [DOI] [PubMed] [Google Scholar]
- 2. Allred DC, Mohsin SK, Fuqua SA.. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8(1):47–61. [DOI] [PubMed] [Google Scholar]
- 3. Jones JL. Overdiagnosis and overtreatment of breast cancer: progression of ductal carcinoma in situ: the pathological perspective. Breast Cancer Res. 2006;8(2):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15(6):e234–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burstein HJ, Polyak K, Wong JS, et al. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350(14):1430–1441. [DOI] [PubMed] [Google Scholar]
- 6. Erbas B, Provenzano E, Armes J, et al. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97(2):135–144. [DOI] [PubMed] [Google Scholar]
- 7. Sagara Y, Mallory MA, Wong S, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg. 2015;150(8):739–745. [DOI] [PubMed] [Google Scholar]
- 8. Early Breast Cancer Trialists’ Collaborative G, Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sagara Y, Freedman RA, Vaz-Luis I, et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: a population-based longitudinal cohort study. J Clin Oncol. 2016;34(11):1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sagara Y, Freedman RA, Wong SM, et al. Trends in adjuvant therapies after breast-conserving surgery for hormone receptor positive ductal carcinoma in situ: findings from the National Cancer Database, 2004–2013. Breast Cancer Res Treat. 2017;166(2):583–592. [DOI] [PubMed] [Google Scholar]
- 11. Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the breast cancer surveillance consortium. Radiology. 2017;283(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56. [DOI] [PubMed] [Google Scholar]
- 13. Rose SL, Tidwell AL, Bujnoch LJ, et al. Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol. 2013;200(6):1401–1408. [DOI] [PubMed] [Google Scholar]
- 14. Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507. [DOI] [PubMed] [Google Scholar]
- 15. Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–849. [DOI] [PubMed] [Google Scholar]
- 16. Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370(9586):485–492. [DOI] [PubMed] [Google Scholar]
- 17. Kuhl CK, Keulers A, Strobel K, et al. Not all false positive diagnoses are equal: on the prognostic implications of false-positive diagnoses made in breast MRI versus in mammography/digital tomosynthesis screening. Breast Cancer Res. 2018;20(1):13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collins LC, Achacoso N, Nekhlyudov L, et al. Relationship between clinical and pathologic features of ductal carcinoma in situ and patient age: an analysis of 657 patients. Am J Surg Pathol. 2009;33(12):1802–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins LC, Achacoso N, Haque R, et al. Risk factors for non-invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast Cancer Res Treat. 2013;139(2):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tamimi RM, Baer HJ, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10(4):R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams KE, Barnes NL, Cramer A, et al. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann Oncol. 2015;26(5):1019–1025. [DOI] [PubMed] [Google Scholar]
- 22. Boyages J, Delaney G, Taylor R.. Predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Cancer. 1999;85(3):616–628. [PubMed] [Google Scholar]
- 23. Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31(32):4054–4059. [DOI] [PubMed] [Google Scholar]
- 24. Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340(19):1455–1461. [DOI] [PubMed] [Google Scholar]
- 25. Rudloff U, Brogi E, Reiner AS, et al. The influence of margin width and volume of disease near margin on benefit of radiation therapy for women with DCIS treated with breast-conserving therapy. Ann Surg. 2010;251(4):583–591. [DOI] [PubMed] [Google Scholar]
- 26. Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS). Breast Cancer Res Treat. 2014;143(2):343–350. [DOI] [PubMed] [Google Scholar]
- 27. Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27(32):5319–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins LC, Achacoso N, Haque R, et al. Risk prediction for local breast cancer recurrence among women with DCIS treated in a community practice: a nested, case-control study. Ann Surg Oncol. 2015;22(S3):502–508. [DOI] [PubMed] [Google Scholar]
- 29. Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28(23):3762–3769. [DOI] [PubMed] [Google Scholar]
- 30. Yi M, Meric-Bernstam F, Kuerer HM, et al. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30(6):600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rakovitch E, Nofech-Mozes S, Hanna W, et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152(2):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rakovitch E, Gray RJ, Baehner FL, et al. Refined estimates of local recurrence risks and the impact of the DCIS score adjusting for clinico-pathological features: meta-analysis of E5194 and Ontario DCIS cohort studies. J Clin Oncol. 2017;35(15_suppl):528. [Google Scholar]
- 34. Bremer T, Whitworth P, Patel R, et al. A biologic signature for breast ductal carcinoma in situ to predict radiation therapy (RT) benefit and assess recurrence risk. Clin Cancer Res. 2018; doi:10.1158/1078-0432.CCR-18-0842. [DOI] [PubMed] [Google Scholar]
- 35. Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. [DOI] [PubMed] [Google Scholar]
- 36. Hu M, Yao J, Cai L, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37(8):899–905. [DOI] [PubMed] [Google Scholar]
- 37. Hu M, Yao J, Carroll DK, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13(5):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Polyak K, Hu M.. Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia. 2005;10(3):231–247. [DOI] [PubMed] [Google Scholar]
- 39. Gil Del Alcazar CR, Huh SJ, Ekram MB, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7(10):1098–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenberg SA, Yang JC, Restifo NP.. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cramer DW, Titus-Ernstoff L, McKolanis JR, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1125–1131. [DOI] [PubMed] [Google Scholar]
- 42. Pinheiro SP, Hankinson SE, Tworoger SS, et al. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer. Epidemiol Biomarkers Prev. 2010;19(6):1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Finn OJ. Vaccines for cancer prevention: a practical and feasible approach to the cancer epidemic. Cancer Immunol Res. 2014;2(8):708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abel U, Becker N, Angerer R, et al. Common infections in the history of cancer patients and controls. J Cancer Res Clin Oncol. 1991;117(4):339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krone B, Kolmel KF, Grange JM, et al. Impact of vaccinations and infectious diseases on the risk of melanoma—evaluation of an EORTC case-control study. Eur J Cancer. 2003;39(16):2372–2378. [DOI] [PubMed] [Google Scholar]
- 46. Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. [DOI] [PubMed] [Google Scholar]
- 47. Lowenfeld L, Mick R, Datta J, et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2pos DCIS independent of route: results of randomized selection design trial. Clin Cancer Res. 2017;23(12):2961–2971. [DOI] [PubMed] [Google Scholar]
- 48.Nelipepimut-S Plus GM-CSF Vaccine Therapy in Treating Patients with Breast Cancer. https://clinicaltrials.gov/ct2/show/NCT02636582.
- 49.Vitamin D and Breast Cancer Biomarkers in Female Patients. https://clinicaltrials.gov/ct2/show/NCT01224678. Accessed August 6, 2018.
- 50.Metformin Hydrochloride in Preventing Breast Cancer in Patients with Atypical Hyperplasia or In Situ Breast Cancer. https://cancer.osu.edu/cancer-specialties/clinical-trials/find-a-clinical-trial/a211102-testing-for-atypia-in-random-periareolar. Accessed August 6, 2018.
- 51. Goodwin PJ, Parulekar WR, Gelmon KA, et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst. 2015;107(3):djv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hadad SM, Coates P, Jordan LB, et al. Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res Treat. 2015;150(1):149–155. [DOI] [PubMed] [Google Scholar]
- 53. Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Afimoxifene in Reducing the Risk of Breast Cancer in Women With Mammographically Dense Breast https://clinicaltrials.gov/ct2/show/NCT03063619. Accessed August 6, 2018.
- 55. Karavites LC, Allu S, Khan SA, Kaiser K. Awareness of preventive medication among women at high risk for breast cancer and their willingness to consider transdermal or oral tamoxifen: a focus group study. BMC Cancer. 2015;15:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ethun KF, Wood CE, Register TC, et al. Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys. Menopause. 2012;19(11):1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nolan E, Vaillant F, Branstetter D, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med. 2016;22(8):933–939. [DOI] [PubMed] [Google Scholar]
- 58. Sumner WE 3rd, Koniaris LG, Snell SE, et al. Results of 23, 810 cases of ductal carcinoma-in-situ. Ann Surg Oncol. 2007;14(5):1638–1643. [DOI] [PubMed] [Google Scholar]
- 59. Group EBCC, Group ER, Bijker N, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853—a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. [DOI] [PubMed] [Google Scholar]
- 60. Fisher B, Land S, Mamounas E, et al. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–418. [DOI] [PubMed] [Google Scholar]
- 61. Emdin SO, Granstrand B, Ringberg A, et al. SweDCIS: radiotherapy after sector resection for ductal carcinoma in situ of the breast. Results of a randomised trial in a population offered mammography screening. Acta Oncol. 2006;45(5):536–543. [DOI] [PubMed] [Google Scholar]
- 62. Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102. [DOI] [PubMed] [Google Scholar]
- 63. Dodwell D, Clements K, Lawrence G, et al. Radiotherapy following breast-conserving surgery for screen-detected ductal carcinoma in situ: indications and utilisation in the UK. Interim findings from the Sloane Project. Br J Cancer. 2007;97(6):725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Punglia RS, Schnitt SJ, Weeks JC.. Treatment of ductal carcinoma in situ after excision: would a prophylactic paradigm be more appropriate? J Natl Cancer Inst. 2013;105(20):1527–1533. [DOI] [PubMed] [Google Scholar]
- 65. Raldow AC, Sher D, Chen AB, et al. Cost Effectiveness of the oncotype DX DCIS score for guiding treatment of patients with ductal carcinoma in situ. J Clin Oncol. 2016;34(33):3963–3968. [DOI] [PubMed] [Google Scholar]
- 66. Hayman JA, Kabeto MU, Schipper MJ, et al. Assessing the benefit of radiation therapy after breast-conserving surgery for ductal carcinoma-in-situ. J Clin Oncol. 2005;23(22):5171–5177. [DOI] [PubMed] [Google Scholar]
- 67. Partridge A, Winer JP, Golshan M, et al. Perceptions and management approaches of physicians who care for women with ductal carcinoma in situ. Clin Breast Cancer. 2008;8(3):275–280. [DOI] [PubMed] [Google Scholar]
- 68. Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100(4):243–251. [DOI] [PubMed] [Google Scholar]
- 69.Welcome to onlineDeCISion.org. www.onlineDeCISion.org. Accessed August 6, 2018.
- 70. Ozanne EM, Schneider KH, Soeteman D, et al. onlineDeCISion.org: a web-based decision aid for DCIS treatment. Breast Cancer Res Treat. 2015;154(1):181–190. [DOI] [PubMed] [Google Scholar]
- 71. Ryser MD, Worni M, Turner EL, et al. Outcomes of active surveillance for ductal carcinoma in situ: a computational risk analysis. J Natl Cancer Inst. 2016;108(5):djv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260(1):119–128. [DOI] [PubMed] [Google Scholar]
- 73.Management of Low-Risk DCIS (LORD). https://clinicaltrials.gov/ct2/show/NCT02492607. Accessed August 6, 2018.
- 74.A Trial Comparing Surgery with Active Monitoring for Low Risk DCIS (LORIS). http://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-comparing-surgery-with-active-monitoring-for-low-risk-dcis-loris. Accessed August 6, 2018.
- 75.Comparison of Operative to Monitoring and Endocrine Therapy (COMET) Trial for Low Risk DCIS (COMET). https://clinicaltrials.gov/ct2/show/NCT02926911. Accessed August 6, 2018.
- 76. Grimm LJ, Shelley Hwang E.. Active surveillance for DCIS: the importance of selection criteria and monitoring. Ann Surg Oncol. 2016;23(13):4134–4136. [DOI] [PubMed] [Google Scholar]
- 77.Familial Breast Cancer: Classification, Care and Managing Breast Cancer and Related Risks in People with a Family History of Breast Cancer. https://www.nice.org.uk/guidance/CG164. Accessed August, 2015. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.