Abstract
Chronic ethanol consumption is associated with changes in the function and structure of the lungs. The aim of this study was to investigate the effect of chronic ethanol exposure on the lungs and whether ginger extract mitigated pulmonary abnormalities induced by ethanol in rats. Male Wistar rats were divided into the control group, the ethanol group, and the ethanol plus ginger extract group. Six weeks of ethanol treatment increased the proliferation of lung cells, and induced fibrosis, inflammation and leukocyte infiltration. A significant rise in the level of 8-hydroxydeoxyguanosine, NADPH oxidase, and oxidized low-density lipoprotein was also observed. Ginger extract significantly ameliorated the above changes. These findings indicate that ethanol induces abnormalities in the lungs by oxidative DNA damage and oxidative stress, and that these effects can be alleviated by ginger, which may function as an antioxidant and anti-inflammatory agent.
Keywords: ethanol, lung, oxidative stress, DNA damage, rat, fibrosis, ginger
Introduction
The association between ethanol consumption and lung abnormalities, such as diffuse alveolar damage, impaired gas exchange, pro-inflammatory cytokines release, as well as predisposing factors that increase the incidence of acute respiratory distress syndrome (ARDS) is well known[1–2]. Despite finding many different abnormalities in the ethanol exposed lungs, the precise mechanism behind the structural and functional changes in ethanol-induced lungs has not yet been completely clarified. A growing body of evidence from recent studies implies that ethanol induces its deleterious effects on different tissues, as well as on lung tissues through oxidative stress and reactive oxygen species (ROS) generation[3–5]. In addition, the beneficial effects of antioxidant therapy during alcohol exposure supports the idea that ethanol may exert its deleterious effects mainlyvia oxidative stress[3–6]. The oxidative nature of ethanol-induced abnormalities, on the one hand, and the ameliorative or protective effect of antioxidant administration, on the other hand, prompted us to re-examine this theory that maybe the deleterious effect of chronic ethanol consumption on the lungs is entirely or partially mediated by oxidative stress. Therefore, in the current study, we investigated the effect of long-term ethanol consumption on oxidative stress indexes, such as 8-hydroxydeoxyguanosine (8-OHdG), nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), oxidized low-density lipoprotein (Ox-LDL) levels, as well as lung cell proliferation and fibrosis in rat lungs. We also sought to investigate whether ginger extract mitigated pulmonary abnormalities induced by ethanol in rats.
Materials and methods
Ginger extract preparation
To prepare ginger extract, a dried ginger rhizome (originally Chinese) was purchased from a local market. A sufficient quantity of rhizome was powdered in an electric grinder. The hydro-alcoholic extract of ginger was prepared by mixing three kg of powder with six liters of ethanol (70% in a suitable container). It was then left for 72 hours at room temperature. Next, the extract was filtered through filter papers and then concentrated using a rotary evaporator. The yield of the extract was kept in a refrigerator until use[6–7]. The control group was treated with vehicle only (tap water).
Animals
All animal studies were performed in strict accordance to the "Principles of Laboratory Animal Care" (NIH publication no. 85–23, revised in 1985) and were approved by the Urmia University of Medical Sciences Animal Care Committee. Twenty-four male Wistar rats were randomly divided into three groups: the control group (Group I), the ethanol group (Group II), and the ethanol plus ginger extract group (Group III). Rats in Group II received ethanol at a dose of 4.5 g/kg bodyweight (Merck KGaA, Darmstadt, Germany) in tap water (20% w/v) once a day by gastric gavage for six weeks. Following previous studies, rats in the Group III received a hydro-alcoholic extract of ginger at a dose of 50 mg/kg bodyweight by gastric gavaged; in addition to their regular daily diet and the same amount of ethanol[7]. Group I was treated with vehicle only (tap water). After six weeks of treatment with ginger exgtract, anesthesia was induced by 10% chloral hydrate (0.5 mL/kg bodyweight, IP), and the depth of anesthesia was probed by pinching a hind paw. At termination, after weighing the animals, the thoracic cavity was opened and the lungs were harvested. The excised lungs were freed of adventitial tissues, fat, and blood clots, and were subsequently washed in ice-cold normal saline and weighed. For analyzing histopathological changes, a part of the lungs was fixed in buffered formalin and embedded in paraffin after standard dehydration steps were taken.
For analyzing oxidative indexes, other parts of the lungs were washed with ice-cold normal saline and then dried on filter papers. An ice-cold extraction buffer (10% w/v), containing a 50 mmol/L phosphate buffer (pH 7.4), was added and subsequently homogenized using Ultra Turrax (T10B, IKA, Germany). The homogenates were then centrifuged at 10,000 ×g for 20 minutes at 4°C. The supernatant was collected and stored at −80°C until analysis.
Biochemical assays
The amount of 8-OHdG was measured by the quantitative sandwich enzyme immunoassay method using a commercial rat 8-hydroxy-desoxyguanosine ELISA kit (Cusabio, China) following the manufacturer's recommended protocol.
Assessment of the level of NADPH oxidase (NOX1) in the lung supernatant was carried out by Rat NADPH Oxidase 1(NOX1) ELISA Kit (Cusabio, China) according to the manufacturer's recommended protocol. The Ox-LDL level of lung tissues was measured using a capture ELISA (also known as a "sandwich" ELISA) kit (Mercodia, Sweden) according to the manufacturer's recommended protocol.
Immunohistochemistry
For histopathological staining, 5- mm thick histological sections from paraffin-embedded lung tissues were used. Proliferating cells were detected by performing immunohistochemistry using an antibody against the proliferation cell nuclear antigen (PCNA). In brief, after taking tissue processing steps, such as deparaffinization, rehydration, and gradient ethanol, sections were stained using monoclonal anti-PCNA antibody (Dako Denmark A/S, Denmark). Optimal results were obtained with the EnVisionTM visualization system (Dako Denmark A/S, Denmark). Furthermore, hematoxylin was adopted as a counterstain. The assessment included proper negative controls. Moreover, two expert pathologists inspected all the slides independently. PCNA-positive indices were regarded as indicators of lung cell proliferation. For assessing the percentage of PCNA-positive indices, four non-overlapping fields of view per section from two-to-three sections (from different regions of the lungs) per animal were analyzed. For each field of view, the number of positively stained cells and the total number of cells were counted. For each animal, the number of positively stained cells was then expressed as a percentage of the total number of cells counted[8]. The criteria set for scoring the quality of PCNA-positive indices were as follows[3]: normal (i.e. PCNA-positive indices are present in less than 5% of the lung cells); mild (i.e. PCNA-positive indices are present in less than 25% of the lung cells), mild to moderate (i.e. PCNA-positive indices are present in 25%–50% of the lung cells); moderate to severe (i.e. PCNA-positive indices are present in 50%–75% of the lung cells); and severe (i.e. PCNA-positive indices are present in 75%–100% of the lung cells)[3]. To evaluate lung tissue fibrosis, 5 µm lung tissue sections were stained using Masson's Trichrome staining in accordance with the manufacturer's instructions (Asiapajohesh, Amol, Iran). The severity of tissue fibrosis was estimated adopting a semiquantitative method as described by Ashcroftet al.[9]. A score ranging from zero (normal lung) to eight (total fibrosis) was set. The criteria set for scoring lung fibrosis were as follows: grade 0= normal lung, grade 1= minimal fibrosis thickening of lung tissue, grade 2 and 3= moderate thickening of lung tissue without obvious damage to the structure of lung tissue, grade 4 and 5= increased fibrosis with definite damage to architecture of the lung and formation of fibrosis bands or small fibrosis masses, grade 6 and 7= severe distortion of structure and large fibrosis areas, and grade 8= total fibrotic obliteration.
For general histological changes of lung tissues, paraffin-embedded sections of the lung tissue were stained with hematoxylin and eosin (H&E).
Statistical analysis
Normal distribution of data within each group was verified performing a Kolmogorov-Smirnov test. By conducting a one-way ANOVA and then the Tukey's post hoc test, statistical differences between the groups were tested. The data obtained from each test were expressed as mean±S.E., andP<0.05 was considered as being statistically significant.
Results
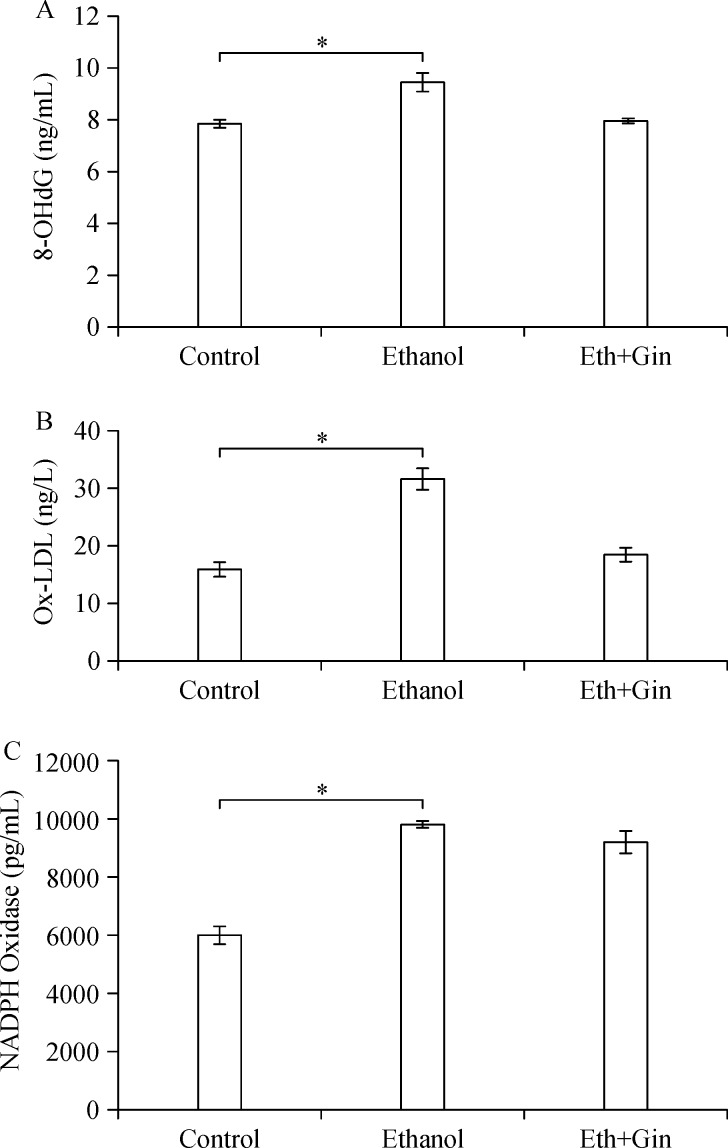
The 8-OHdG levels in the lung tissues in Group II were significantly higher than those of Group II (P<0.002). There was no significant difference in 8-OHdG levels in lung tissues between Group III and Group I (Fig. 1A). The Ox-LDL levels were significantly increased in Group II compared with Group I (P<0.02). There were no significant differences in Ox-LDL levels between Group III and Group I (Fig. 1B). The amount of NADPH oxidase in the lung tissues increased significantly in Group II and Group III compared to Group I (P<0.05) (Fig. 1C).
Fig.1.
Ethanol consumption significantly increases 8-OHdG, Ox-LDL, and NADPH oxidase levels in the lung tissues compared to the control group, and ginger extract administration mitigates ethanol induced increases in 8-OHdG and Ox-LDL levels. *P<0.05.
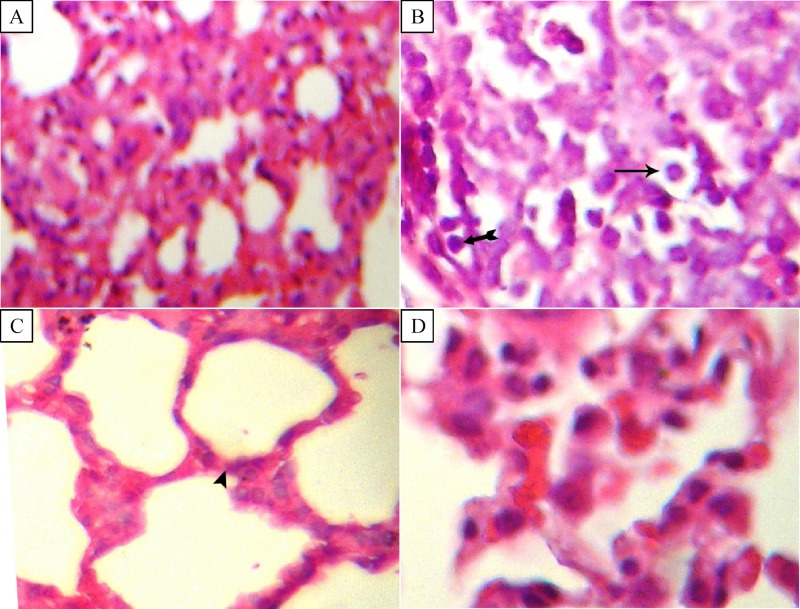
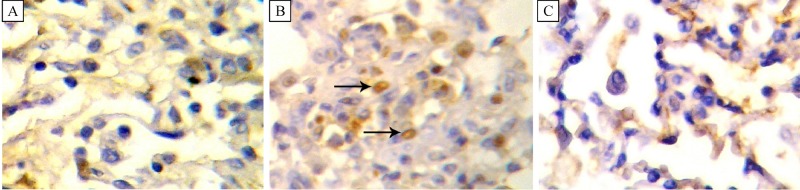
Lung tissue histopathological examination results are given in Figs. 2–4. In tGroup II, several histopathologic changes, such as enlargement and destruction of air spaces, increase of alveolar wall thickness, focal infiltration of polymorphonuclear cells, increase of pneumocytes in the alveolar walls, and vacuolization of cells were observed in different parts of the lung tissue (Fig. 2). No significant differences in lung tissue structure were observed between Group III and Group I. Lung cell proliferation (without considering the type of cells), as detected by the percentage of cells stained positive for PCNA, is depicted inFig. 3. The ratio of proliferated cells in the lung tissues was 1, 40.5±6 and 2 in Group I, II and III, respectively. The PCNA-positive indices were mildly to moderately increased in Group II compared to that of Group I (P<0.05). There were no significant differences between Group I and III.
Fig.2.
Ginger extract attenuates lung tissue histopathological changes induced by ethanol (H&E, *400).
Fig.3.
Immunohistochemical staining of lung tissues by proliferating cell nuclear antigen (PCNA) antibody (magnification *400).
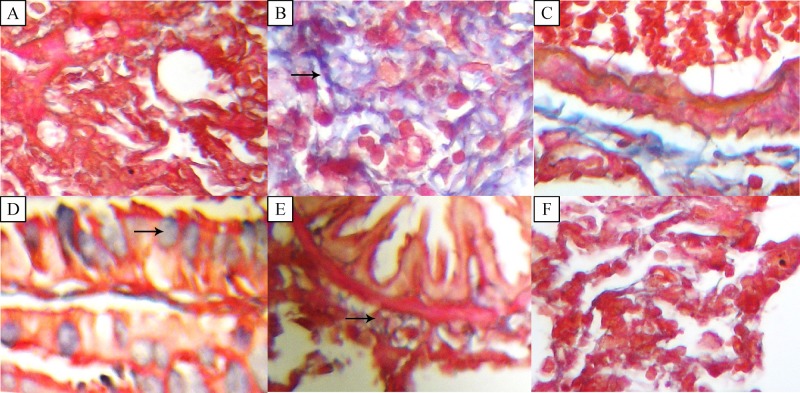
The area of lung tissue was stained for collagen and fibrosis by Masson's Trichrome staining. Fig. 4 shows microscopic fibrosis scores in different parts of the lung tissue, such as the pre-bronch area, pre-vessels and villus hair in different groups. There were no lesion scores in the pre-broch area, pre-vessels and villus in Group I (grade 0). The microscopic lesion score in the pre-bronch area, pre-vessels and villus was 4 to 5, which is an indication of increased fibrosis with definite damage to the lungs architecture and formation of fibrosis bands or small fibrosis masses. There were no significant differences between Group I and III.
Fig.4.
Photomicrograph of lung tissue of rats (Masson's Trichrome staining).
Discussion
The major findings of the current study were that long-term exposure to ethanol led to histopathologic changes, such as enlargement and destruction of the air spaces, an increase in the alveolar wall thickness, focal infiltration of polymorphonuclear cells, an increase in pneumocytes in the alveolar walls, and vacuolization of the lung cells. Moreover, fibrosis with definite damage to the lung architecture and formation of fibrosis bands or small fibrosis masses, and mild to moderate proliferation of lung cells were also observed in the lungs of rats exposed to ethanol compared to the control rats. Furthermore, we also observed oxidative stress manifestations, such as elevations of 8-OHdG, NADPH levels and Ox-LDL in the lung tissues in parallel with structural alterations in rats exposed to ethanol. In addition, giner extract significant ameliorated ethanol induced lung tissue alteration and oxidative stress manifestations in rats. These findings classify redox-sensitive and structural alteration-dependent signaling as novel mechanisms causing ethanol-induced lung abnormalities. Previous studies demonstrated that exposure to ethanol in experimental models led to oxidative stress that was manifested by a marked decrease in antioxidant glutathione in lung tissues and an increase in NADPH oxidase expression and superoxide generation in the lungs through the rennin-angiotensin pathway[10–11]. NADPH oxidase generates superoxide anion which is an important landmark for oxidative stress. In this study, we identified an increase in NADPH oxidase activity, focal polymorphonuclear cell infiltration, as well as fibrosis in rats exposed to ethanol. Based on previous work, cells such as macrophages and neutrophils can induce lung damage through oxidative stress[12]. In normal situations, polymorphonculear leukocytes are essential parts of the lungs' defense system, and these cells can also damage lung tissues by releasing proteolytic enzymes and generating ROS[13]. The activation of infiltrated leukocytes or polymorphonuclear cells release cytotoxic substances, such as ROS, proteolytic enzymes, cytokines, and eicosanoids in the lungs, which in turn initiate a chain of events leading to acute inflammation[14]. Protein kinase C (PKC) is considered as an important possible mechanism for polymorphonuclear cells-induced ROS generation and oxidative stress[15]. Stimulation of the oxidative respiratory burst enzyme (i.e. NADPH oxidase) in polymorphonuclear cells can be regarded as a downstream event of PKC activation[16]. Accordingly, enhancement of NADPH oxidase activity in our study along with infiltration of polymorphonuclear cells in lung tissues, may contribute to the predisposition of lung tissue structural damage such as fibrosis and inflammation by PKC activation of NADPH oxidase. In addition, excessive deposition of the extracellular matrix (ECM) is a future result of fibrosis[17]. Previous studies indicated that ethanol exposure induced lung tissue ECM remodeling by increasing the expression of the matrix protein fibronectin[18]. In the present study, Ox-LDL levels tended to be increased by ethanol exposure. Since Ox-LDL levels are indicative of lipid peroxidation, and consequently of oxidative stress, it is possible that increased fibrosis in the lung tissues in response to ethanol exposure is partly caused by the generation of free radicals during ethanol biotransformation. Ox-LDL is generated during oxidative stress and accumulated in macrophages and other cell types at the site of chronic inflammation[19–20]. A recent study by Greig et al. showed that Ox-LDL triggered activation of several transcription factors, increased chemotaxis of leukocytes, enhanced secretion of chemokines and cytokines, and increased intracellular ROS generation, all of which are involved in the pathogenesis of lungs diseases such as chronic obstr-uctive pulmonary disease[21]. Our recent study has documented the association between Ox-LDL and vascular cell proliferation[22]. Mechanistically, Ox-LDL promotes cell proliferation by generating phospholipase D-related second messengers that modulate mutagenesis[23]. In addition, it stimulates growth via an oxidative mechanism that causes the release of fibroblast growth factore-2 (FGF-2), potentiates the mitogenic effect of angiotensin II, and stimulates mitogen-activated protein kinases (MAPK) activ-ation[23–25]. Moreover, it has been shown by previous studies that Ox-LDL induces expression of proteins known as cell cycle regulatory proteins[23]. Interestingly, the results of our study showed that lung proliferation paralleled the increase in Ox-LDL. In the present study, administration of ethanol caused DNA damage to the lung tissues which was indicated by an increased 8-OHdG level in the lung tissues of rats. To our knowledge, this is the first in vivo study to show that ethanol exposure increases 8-OHdG levels along with lung fibrosis and cell proliferation in rats. The 8-OHdG is one of the predominant forms of free radical-induced lesion of DNA. It results from oxidation of the hydroxyl group being added to the 8th position of the guanine molecule, the oxidatively modified product 8-OHdG[26]. Oxidative stress occurrence in the current study, indicated by increased Ox-LDL and NADPH oxidase levels, on the one hand, and oxidative DNA damage along with tissue fibrosis and cell proliferation, on the other hand, prompted us to speculate that ethanol exerts its hazardous effects on lung tissues through oxidative stress.
The second feature addressed in this study was the mitigated or protective effect of ginger extract against histological alterations and oxidative stress induced by ethanol exposure in the lung tissues. Large bodies of evidence have indicated that plants and their extracts are currently used in medicine and treatments of various diseases. Due to the biological effects of these substances which have antioxidant and anti-inflammation properties, they are of dominant importance in medicine. Our previous work and others also have shown that ginger supplementation increases the total antioxidant capacity and reduces lipid and protein oxidation as two main ROS generating sources in diabetic and ethanol-induced hepatic oxidative stress conditions[7,27–28]. Ginger treatment along with ethanol caused a decrease in the number of PCNA positive cells in the lungs and fibrosis suggesting that ginger extract is an anti-fibrotic and proliferative agent. Accordingly, if ethanol induces some functional and structural abnormalities through oxidative stress, as confirmed by previous studies, the rescue effect of ginger supplementation on these abnormalities may be due to its antioxidant properties. It has also been shown that ginger has anti-inflammatory effects and suppresses pro-inflammatory cytokine expressions such as TNF-α and the arachidonic acid cascade[29–30]. Anti-inflammatory effects of ginger are due to its gingerol and shogaol components that inhibit prostaglandin and leukotriene biosynthesisvia suppression of 5-lipooxygenase synthetase activities[31]. Although various studies have shown the improving effects of ginger supplementation on inflammatory cytokines, such as TNF-α, IL-6, prostaglandins and leukotriene, this is the first in vivo study demonstrating the protective effect of ginger extract against oxidative stress, along with lung tissue fibrosis and lung cell proliferation in the ethanol-treated lungs.
In conclusion, our work launches three points. First, exposure to chronic ethanol leads to lung structural alterations as manifested in the enlargement and destruction of the air spaces, an increase in alveolar wall thickness, focal infiltration of polymorphonuclear cells, an increase in pneumocytes in the alveolar walls, fibrosis in different parts of the lungs, and mild to moderate cellular proliferation. The second point is that ethanol-induced lungs structural alterations correlate with oxidative DNA damage, Ox-LDL, and also with lung tissue NADPH oxidase elevation, and that it provides strong evidence for the occurrence of oxidative stress under ethanol exposure. The third point we have established is that the ingestion of ginger extract concurrent with ethanol nearly eliminates structural changes and oxidative stress induced by ethanol. Our results support the hypothesis that ethanol-related alterations in the lungs structure are caused by oxidative stress along with inflammatory responses.
References
- 1. Moss M, Parsons PE, Steinberg KP, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock[J].Crit Care Med, 2003,31(3):869–877. [DOI] [PubMed] [Google Scholar]
- 2. Nelson S, Kolls JK. Alcohol, host defence and society[J].Nat Rev Immunol, 2002,2(3):205–209. [DOI] [PubMed] [Google Scholar]
- 3. Shirpoor A, Nemati S, Ansari MH, et al. The protective effect of vitamin E against prenatal and early postnatal ethanol treatment–induced heart abnormality in rats: a 3–month follow–up study[J].Int Immunopharmacol, 2015,26(1):72–79. [DOI] [PubMed] [Google Scholar]
- 4. Polikandriotis JA, Rupnow HL, Brown LA, et al. Chronic ethanol ingestion increases nitric oxide production in the lung[J].Alcohol, 2007,41(5):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown LA, Harris FL, Ping XD, et al. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability[J]? Alcohol, 2004,33(3):191–197. [DOI] [PubMed] [Google Scholar]
- 6. Shirpoor A, Rezaei F, Fard AA, et al. Ginger extract protects rat's kidneys against oxidative damage after chronic ethanol administration[J].Biomed Pharmacother, 2016,84:698–704. [DOI] [PubMed] [Google Scholar]
- 7. Ilkhanizadeh B, Shirpoor A, Khadem Ansari MH, et al. Protective effects of ginger (Zingiber officinale) extract against diabetes–induced heart abnormality in rats[J].Diabetes Metab J, 2016,40(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sozo F, O'Day L, Maritz G, et al. Repeated ethanol exposure during late gestation alters the maturation and innate immune status of the ovine fetal lung[J].Am J Physiol Lung Cell Mol Physiol, 2009,296(3):L510–L518. [DOI] [PubMed] [Google Scholar]
- 9. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale[J].J Clin Pathol, 1988,41(4):467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown LA, Harris FL, Bechara R, et al. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator–induced apoptosis[J].Alcohol Clin Exp Res, 2001,25(7):1078–1085. [PubMed] [Google Scholar]
- 11. Polikandriotis JA, Rupnow HL, Elms SC, et al. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung[J].Am J Respir Cell Mol Biol, 2006,34(3):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaid M, Katiyar SK. Grape seed proanthocyanidins inhibit cigarette smoke condensate–induced lung cancer cell migration through inhibition of NADPH oxidase and reduction in the binding of p22(phox) and p47(phox) proteins[J].Mol Carcinog, 2015,54(Suppl 1):E61–E71. [DOI] [PubMed] [Google Scholar]
- 13. Henkels KM, Muppani NR, Gomez–Cambronero J. PLD–specific small–molecule inhibitors decrease tumor–associated macrophages and neutrophils infiltration in breast tumors and lung and liver metastases[J].PLoS One, 2016,11(11):e0166553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee WL, Downey GP. Neutrophil activation and acute lung injury[J].Curr Opin Crit Care, 2001,7(1):1–7. [DOI] [PubMed] [Google Scholar]
- 15. Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications[J].Diabet Med, 2001,18(12):945–959. [DOI] [PubMed] [Google Scholar]
- 16. Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes[J].Diabetes Res Clin Pract, 2007,76(1):44–50. [DOI] [PubMed] [Google Scholar]
- 17. Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis[J].Am J Pathol, 2009,175(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roman J, Ritzenthaler JD, Bechara R, et al. Ethanol stimulates the expression of fibronectin in lung fibroblasts via kinase–dependent signals that activate CREB[J].Am J Physiol Lung Cell Mol Physiol, 2005,288(5):L975–L987. [DOI] [PubMed] [Google Scholar]
- 19. Palanisamy GS, Kirk NM, Ackart DF, et al. Uptake and accumulation of oxidized low–density lipoprotein during Mycobacterium tuberculosis infection in guinea pigs[J].PLoS One, 2012,7(3):e34148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tamada K, Shimozaki K, Chapoval AI, et al. LIGHT, a TNF–like molecule, costimulates T cell proliferation and is required for dendritic cell–mediated allogeneic T cell response[J].J Immunol, 2000,164(8):4105–4110. [DOI] [PubMed] [Google Scholar]
- 21. Greig FH, Kennedy S, Spickett CM. Physiological effects of oxidized phospholipids and their cellular signaling mechanisms in inflammation[J].Free Radic Biol Med, 2012,52(2):266–280. [DOI] [PubMed] [Google Scholar]
- 22. Shirpoor A, Salami S, Khadem Ansari MH, et al. Ethanol promotes rat aortic vascular smooth muscle cell proliferation via increase of homocysteine and oxidized–low–density lipoprotein[J].J Cardiol, 2013,62(6):374–378. [DOI] [PubMed] [Google Scholar]
- 23. Natarajan V, Scribner WM, Hart CM, et al. Oxidized low density lipoprotein–mediated activation of phospholipase D in smooth muscle cells: a possible role in cell proliferation and atherogenesis[J].J Lipid Res, 1995,36(9):2005–2016. [PubMed] [Google Scholar]
- 24. Qin B, Cao Y, Yang H, et al. MicroRNA–221/222 regulate ox–LDL–induced endothelial apoptosis via Ets–1/p21 inhibition[J].Mol Cell Biochem, 2015,405(1–2):115–124. [DOI] [PubMed] [Google Scholar]
- 25. Jiang JX, Zhang SJ, Liu YN, et al. EETs alleviate ox–LDL–induced inflammation by inhibiting LOX–1 receptor expression in rat pulmonary arterial endothelial cells[J].Eur J Pharmacol, 2014,727:43–51. [DOI] [PubMed] [Google Scholar]
- 26. Kasai H. Analysis of a form of oxidative DNA damage, 8–hydroxy–2′–deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis[J].Mutat Res, 1997,387(3):147–163. [DOI] [PubMed] [Google Scholar]
- 27. Mallikarjuna K, Sahitya Chetan P, Sathyavelu Reddy K, et al. Ethanol toxicity: rehabilitation of hepatic antioxidant defense system with dietary ginger[J].Fitoterapia, 2008,79(3):174–178. [DOI] [PubMed] [Google Scholar]
- 28. Nasri H, Nematbakhsh M, Ghobadi S, et al. Preventive and curative effects of ginger extract against histopathologic changes of gentamicin–induced tubular toxicity in rats[J].Int J Prev Med, 2013,4(3):316–321. [PMC free article] [PubMed] [Google Scholar]
- 29. Isa Y, Miyakawa Y, Yanagisawa M, et al. 6–Shogaol and 6–gingerol, the pungent of ginger, inhibit TNF–alpha mediated downregulation of adiponectin expression via different mechanisms in 3T3–L1 adipocytes[J].Biochem Biophys Res Commun, 2008,373(3):429–434. [DOI] [PubMed] [Google Scholar]
- 30. Rafieian–Kopaei M, Nasri H. Ginger and diabetic nephropathy[J].J Renal Inj Prev, 2013,2(1):9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rafieian–Kopaei M, Nasri H. The ameliorative effect of zingiber officinale in diabetic nephropathy[J].Iran Red Crescent Med J, 2014,16(5):e11324. [DOI] [PMC free article] [PubMed] [Google Scholar]