Abstract
Particulate matters (PM) are one of the major body burdens leading to diseases. We investigated the capacities of a hydrogen-enriched water (HW) eliminating carbon nanoparticles (CNP) and carbon microparticles (CMP) from the lungs and blood, respectively. In CNP-elimination test, rats were orally administered with purified water (PW) or HW (10 or 30 mL/kg/day) for 10 weeks. At the time point of 4 weeks, the rats were challenged with intratracheal instillation of CNP (4 mg). CNP accumulated in the airways and alveoli, and induced inflammatory lesions. Such pneumoconiosis was markedly improved by feeding HW, while PW was ineffective. CNP-induced pneumoconiosis caused systemic hematological alterations, decreasing major inflammatory cells, but markedly increasing eosinophils, indicative of an allergic reaction, which were attenuated by treatment with HW. Such PM-eliminating and anti-allergic effects of HW reduced body burden as confirmed from the facilitated recovery of body and lung weights. In CMP-clearance test, mice were orally administered with PW or HW for 7 days, and intravenously injected with CMP (300 mg/kg). CMP was rapidly eliminated from the blood in HW-fed mice. Indeed, the phagocytic indices increased to 3.5 and 6.7 folds at 10 and 30 mL/kg of HW, in comparison with a negligible effect of PW. As a mechanism study, only HW significantly inhibited lipid peroxidationin vitro Fenton reaction-mediated ·OH-generating system. Collectively, the results indicate that HW not only effectively eliminated PM from the lungs and blood by enhancing phagocytic activity, but also attenuated the lung injuries by inhibiting lipid peroxidation.
Keywords: particulate matter, pneumoconiosis, hydrogen-enriched water, particle clearance, antioxidation
Introduction
Air pollution is associated with problems to human health as well as loss of quality of life[1–3]. Air pollutants fall into two categories: gases (for example, O3, NO2, SO2, CO, and so on) and particulate matters (PM), with different grain sizes and chemical composition. These pollutants have been linked to adverse health effects even in low concentrations, especially PM, the most responsible for health problems related to the respiratory system[4–5]. The rapid industrialization of the earth has been inducing desertification and environmental pollution, and thereby increasing exposure of humans and wildlife population to a lot of fine particles such as industrial particulates and sands. The fine particle exposure is one of the major burdens of disease attributable to 20 leading risk factors, and especially, it is in higher rank in East Asian countries such as China and Korea[6]. The harmful effect to human health caused by PM depends both on its concentration in the inhaled air and on its granulometry, and chemical composition.
The PM is classified according to its aerodynamic diameter (varies from few nanometers to 100 mm). In addition to sedimentary particles (SP) that cause discomfort, the inhalable PM harmful to health are divided into fine particles smaller in diameter than 10 mm (PM10) and ultrafine particles smaller than 2.5 mm (PM2.5)[5,7]. The harmful effects of PM2.5 occur in the short-term, via direct action on the airways, as well as in the long-term, leading to systemic adverse effects, because the particles can reach the alveoli, enter the bloodstream, and affect other organs besides the lungs[8]. Thus, PM2.5 presents potential health risk even when in relatively low concentrations in the atmosphere; i.e., even when its atmospheric concentration is below the maximum levels of tolerance established by the World Health Organization (WHO) and by the main environmental regulatory agencies of the world[5,8].
Studies show the association between air pollution and incidence of respiratory, cardiovascular, neurological diseases, and several types of cancer[5,7,9]. The association with respiratory diseases is stronger and direct, and the most vulnerable groups are children, elderly people, and those with pre-existing respiratory diseases, especially asthma, chronic bronchitis, and obstructive pulmonary disease[2–3,10–12]. PM exposure triggers a variety of maladaptive signaling pathways in the lungs, blood vessels, liver, and brain that are associated with endoplasmic reticulum (ER) stress, oxidative stress, and inflammatory responses[13–15]. Oxidative stress arises from the strong cellular oxidizing potential of excess reactive oxygen species (ROS), or free radicals[16–17]. The ROS, generated via diverse physiologic and pathological activities, is converted to highly toxic hydroxyl group (·OH) or detoxified by oxidizing and antioxidant enzymes, respectively. High concentration of ·OH is producedvia Fenton reaction mediated by transition metal ions such as Fe2+ and Cu2+. Since there is no known detoxification system for ·OH, scavenging ·OH is a critical antioxidant process[18–19].
Molecular hydrogen (dihydrogen, H2) has been suggested that it acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals[18]. Indeed, hydrogen water has been reported to improve various diseases and tissue injuries through anti-oxidative and anti-inflammatory activities including pulmonary inflammation and asthma[20–24], cerebral infarction (stroke)[18], Alzheimer disease[25], Parkinson disease[26], rheumatoid arthritis (RA)[27], and diabetes[28]. Notably, in addition to tissue-protective effects, hydrogen water attenuated the brain inflammation induced by particulate matters[15]. Such multifaceted beneficial effects of hydrogen water led us to investigate its effectiveness on the fine particle burden in the lungs and blood, and underlying mechanisms.
Materials and methods
Materials
Hydrogen-enriched water (HW) was obtained from Anydoctor Healthcare Co., Ltd. (Cheonan, Korea). To produce HW, filtered purified water (PW) with 99% transmittance was nitrogenized in aluminum cans, and infused with H2 gas under a pressure of 0.2 Mpa using a Hydrogen manufacturing device (Sang-IL Co., Ltd, Geochang, Korea). The can rapidly seamed and sterilized at 75°C (central temperature) for 10 minutes. H2 concentration in HW was confirmed to be 1.2–1.4 ppm (pH 7.3–7.9). Carbon nanoparticles (CNP;≤500 nm, CAS 1333-86-4) and carbon microparticles [CMP (Speedball superblack India ink)≤10 mm] were procured from Sigma-Aldrich (St. Louis, MO, USA) and Homi Art Shop (Seoul, Korea), respectively.
Animals
Six-week-old male Wistar Hannover rats and 6-week-old male ICR mice were obtained from Daehan Biolink (Eumseong, Korea). The animals were housed in a room with a constant temperature (23±2°C), relative humidity (55±10%), and a 12-h light/dark cycle. The animals were fed standard rodent diet (Hanlan #2018) and purified waterad libitum. All experimental procedures were carried out in accordance with the Standard Operation Procedures of Laboratory Animal Center, Chungbuk National University (CBNU), Korea. The protocol was approved by the Institutional Animal Care and Use Committee of CBNU.
Elimination of ultrafine particles from the lungs
After 1-week acclimation to the laboratory environment, the rats (7 weeks old, n = 7/group) were orally administered twice a day with low (10 mL/kg/day) or high (30 mL/kg/day) doses of purified water (PW) or HW for 10 weeks. At time point of 4 weeks from the initial treatment during the 10-week feeding period, the rats in treatment groups were challenged with intratracheal instillation of CNP. In brief, a particle dispenser was made by fixing a 5 cm 4/0 monofilament nylon suture in 2 layers of 5 cm narrow polyethylene tube A (0.61 cm in diameter) and 2 cm wide tube B (1.45 cm in diameter). One end of the aligned 3 layers (nylon suture and tubes A and B) was flame-sealed, and 4 holes were punctured through the outer wide tube B at their flank using a syringe needle. The narrow tube A including nylon suture was inserted into a Sonde needle connected to a syringe containing CNP (Supplementary Fig. 1). CNP (4 mg) was suspended in 150 mL PG-CMC (10% propylene glycol and 1% sodium carboxymethyl cellulose in saline), and instilled into the rat's trachea through the prefixed dispenser guided by a small animal laryngoscope (LS-2-R; Penn-Centry Inc., Wyndmoor, USA).
During the whole experimental period, bodyweights were recorded. At the end of the experiment, the rats were sacrificed and blood samples were collected from the abdominal aorta, and complete blood counts (CBC) were analyzed. Lungs were collected, weighed, and fixed in a neutral formalin solution. Paraffin-embedded lung sections were stained with hematoxylin-eosin, and examined under a light microscope for CNP accumulation and lesions. The accumulation and lesions were scored according to the area and severity with a maximum score of 10.
Clearance of fine particles from the blood
After 1-week acclimation to the laboratory environment, the mice (7 weeks old, n = 5/group) were orally administered twice a day with low (10 mL/kg/day) or high (30 mL/kg/day) doses of purified water (PW) or HW for 7 days. Thirty min after the final treatment, the mice were intravenously injected with CMP. CMP was centrifuged at 5,000 rpm for 15 minutes. Then, the supernatant was diluted 3 folds with sterile 1.5% gelatin in saline to adjust to 30 mg/mL. The diluted carbon suspension was intravenously injected at a volume of 10 mL/kg (300 mg/kg). After 0.5 and 10 minutes of CMP injection, 50 mL blood sample was collected, hemolyzed by adding 1 mL Na2CO3 (0.1%) solution. The absorbance was measured at 600 nm, and the phagocytic index (K) was calculated from an equation: K= 1/t10 – t0.5 X log(C0.5 – C10).
Antioxidation in vitro
Saline-perfused lung tissue was collected, and homogenized in cold 10 mmol/L phosphate-buffered saline (PBS, pH 7.4) to make 10% homogenate. To detect antioxidative capacity, PW and HW (final concentrations of 10% and 20%) were added to PBS containing 10% lung homogenate, and 0.1 mmol/L ferrous perchlorate (0.1 mmol/L). Fenton reaction was initiated by adding H2O2, and incubated at 37°C for 30 minutes. Into the tissue homogenate (250 mL), 250 mL sodium dodecyl sulfate (SDS, 8.1% solution) and 500 mL 20% acetic acid (adjusted to pH 3.5) were added. After adding 250 mL 2-thiobarbituric acid (TBA, 0.75% solution), the mixture was boiled in a glass tube capped with aluminum foil for 30 minutes. Samples were cooled on ice, centrifuged at 13,000g for 10 minutes, and absorbance of the supernatant was read at 532 nm for the quantification of TBA-reactive substances (TBARS).
Statistical analysis
The results are displayed the means±standard error. The significance of differences of all results was analyzed by one-way analysis of variance (ANOVA) followed by the Tukey's (LSD) test, using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set a priority atP<0.05.
Results
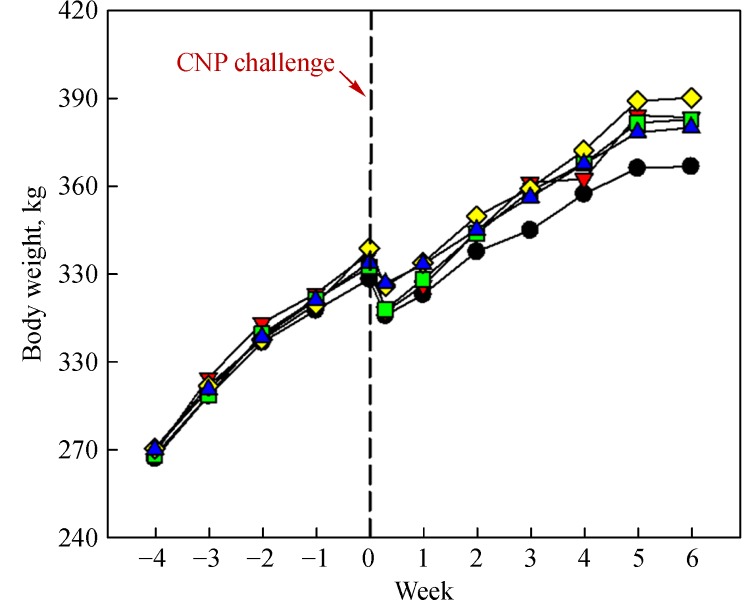
Intratracheal challenge with CNP (4 mg) decreased the rats' bodyweights for 2-3 days, indicative of impact and burden of pulmonary pneumoconiosis (Fig. 1). Notably, daily feeding of PW and HW (10 or 30 mL/kg) facilitated the recovery of bodyweights to some extent. In contrast to the decrease in bodyweights, the absolute and relative lung weights increased following CNP inhalation (Table 1). However, treatment with HW significantly attenuated CNP-induced increase in lung weights, in which HW was superior to PW.
Fig.1.
Time-course of mean bodyweights of rats fed purified water (PW) or hydrogen-enriched water (HW).
Tab.1.
Absolute and relative lung weights of rats fed purified or hydrogen-enriched water 6 weeks after carbon nanoparticle instillation
| Treatment
(mL/kg) |
Body weight
(g) |
Absolute lung weight
(g) |
Relative lung weight
(%) |
|---|---|---|---|
| Normal | 370.1±15.2 | 2.35±0.10 | 0.64±0.03 |
| CNP | 366.8±5.7 | 2.62±0.20 | 0.74±0.06 |
| +PW (10) | 383.5±20.0 | 2.59±0.13 | 0.65±0.03 |
| +PW (30) | 382.9±15.9 | 2.55±0.09 | 0.69±0.03 |
| +HW (10) | 390.3±20.5 | 2.43±0.22 | 0.60±0.03* |
| +HW (30) | 380.0±7.9 | 2.40±0.07 | 0.63±0.01* |
CNP: carbon nanoparticles, PW: purified water, HW: hydrogen-enriched water. *Significantly different from CNP alone (P<0.05).
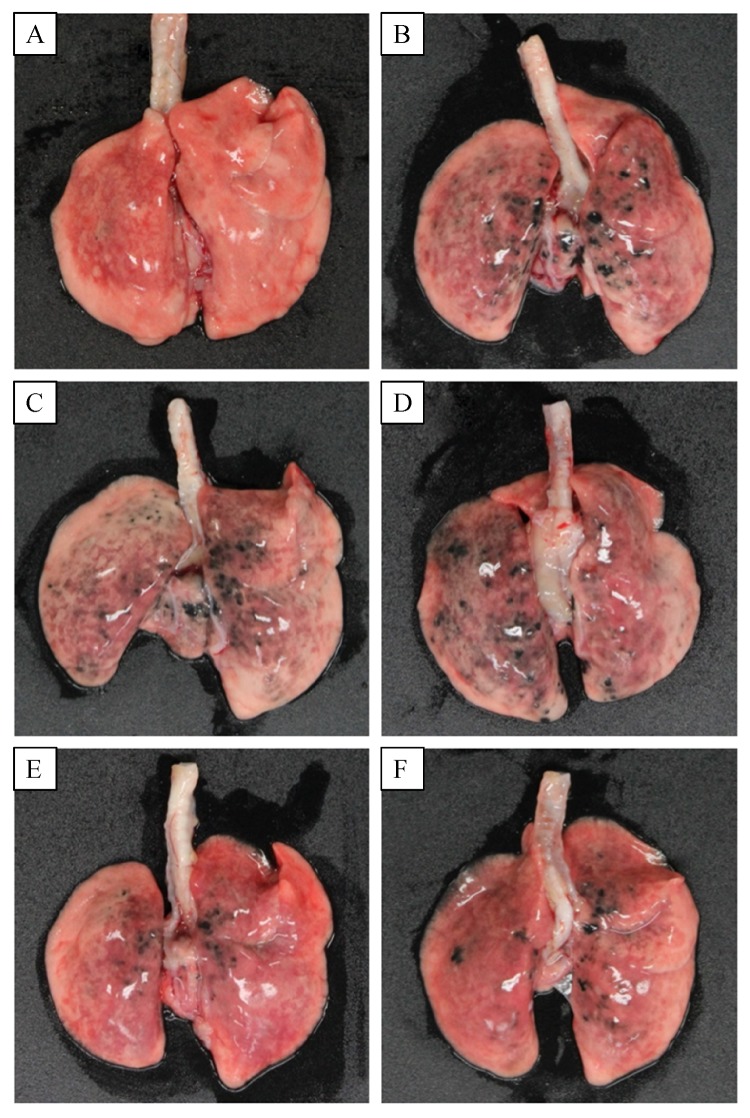
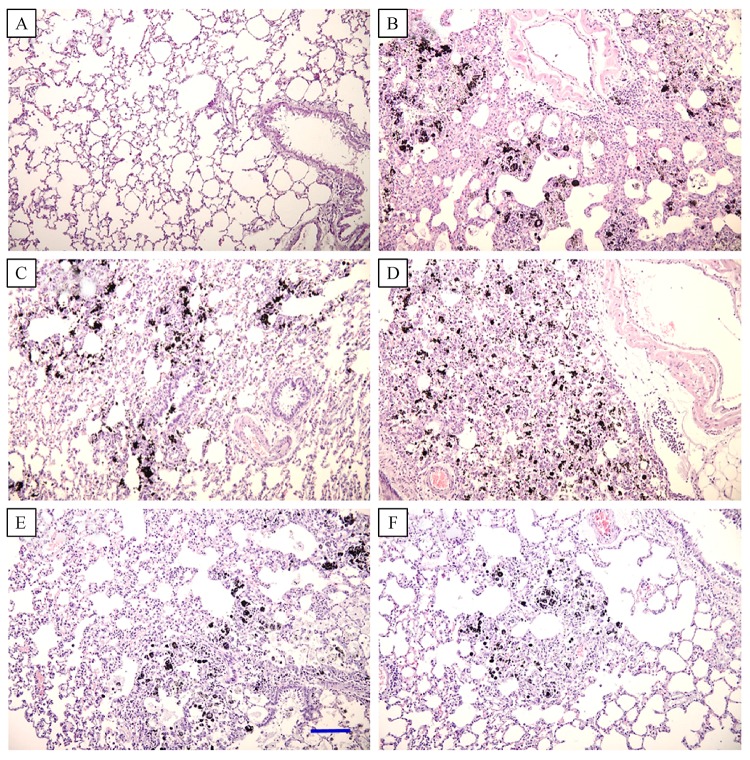
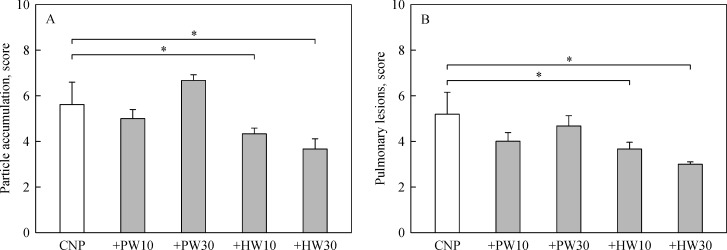
In gross findings, intratracheal instillation of CNP caused serious pneumoconiosis, resulting in overall accumulation of the ultrafine particles in the lungs (Fig. 2). Severe accumulation of CNP in the airways and parenchymal tissues, and ensuing tissue injuries were confirmed in microscopic observations (Fig. 3). Interestingly, the accumulation of carbon particles as well as inflammatory lesions were markedly eliminated by feeding HW in a dose-dependent manner, although the effect of PW was negligible (Fig. 4).
Fig.2.
Gross findings of the lungs of rats fed purified water (PW) or hydrogen-enriched water (HW) 6 weeks after carbon nanoparticle (CNP) instillation.
Fig.3.
Microscopic findings of the lungs of rats fed purified water (PW) or hydrogen-enriched water (HW) 6 weeks after carbon nanoparticle (CNP) instillation.
Fig.4.
Scores of carbon nanoparticle accumulation (A) and pulmonary lesions (B) of the lungs of rats fed purified water (PW) or hydrogen-enriched water (HW) 6 weeks after carbon nanoparticle (CNP) instillation.
CNP-induced pneumoconiosis caused systemic hematological alterations (Table 2). CNP exposure significantly decreased white blood cells (WBC), monocytes, and platelets, but markedly increased eosinophils, indicative of an allergic reaction in spite of general immunosuppression. Notably, PW and HW remarkably attenuated the increase in eosinophils induced by CNP, and restored the number of platelets to some extent, although HW was superior to PW in regulating the eosinophil reaction.
Tab.2.
Hematology of rats fed purified or hydrogen-enriched water 6 weeks after carbon nanoparticle instillation
| Treatment
(mg/kg) |
Normal | CNP | +PW (10) | +PW (30) | +HW (10) | +HW (30) |
|---|---|---|---|---|---|---|
| WBC (10 3/μL) | 5.56±1.52 | 3.95±0.32* | 4.42±0.38 | 5.13±0.62 | 4.43±0.52 | 4.02±0.63 |
| Neutrophils (%) | 11.10±2.72 | 15.40±2.01 | 19.15±1.46 | 14.58±1.14 | 16.40±3.10 | 18.34±2.59 |
| Basophils (%) | 0.11±0.06 | 0.07±0.03 | 0.20±0.07 | 0.13±0.03 | 0.20±0.07 | 0.08±0.04 |
| Eosinophils (%) | 0.96±0.64 | 4.17±1.97* | 3.75±1.18 | 2.30±0.32 | 1.85±0.21 # | 2.04±0.60 # |
| Lymphocytes (%) | 83.49±3.24 | 79.30±2.25 | 75.95±2.58 | 81.93±1.09 | 80.63±3.21 | 78.66±3.16 |
| Monocytes (%) | 1.71±0.61 | 1.07±0.15* | 0.95±0.18 | 1.08±0.24 | 0.93±0.10 | 0.88±0.11 |
| Platelets (10 3/μL) | 1,384.6±137.4 | 735.3±40.5* | 776.0±83.3 | 973.8±25.6 | 789.5±40.0 | 834.8±60.1 |
| RBC (10 6/μL) | 7.90±0.25 | 8.37±0.36 | 8.39±0.24 | 8.40±0.18 | 8.19±0.15 | 8.22±0.47 |
CNP: carbon nanoparticles, PW: purified water, HW: hydrogen-enriched water. *Significantly different from Normal (P<0.05). #Significantly different from CNP alone (P<0.05).
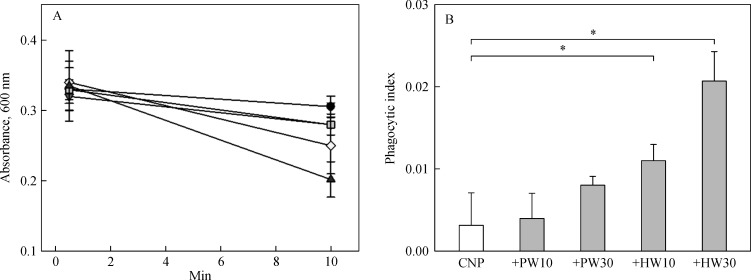
Intravenously injected CMP was slowly eliminated from the blood in normal mice (Fig. 5A). The elimination velocity was slightly increased by pretreatment with PW, doubling the phagocytic index at a high dose (30 mL/kg) (Fig. 5B). Notably, HW was superior to PW, increasing phagocytic indices to 3.5 and 6.7 folds at 10 and 30 mL/kg, implying that HW enhanced phagocytic activity of blood macrophages.
Fig.5.
Clearance of carbon microparticles (CMP) from blood of mice fed purified water (PW) or hydrogen-enriched water (HW) during 10 minutes after carbon CMP injection.
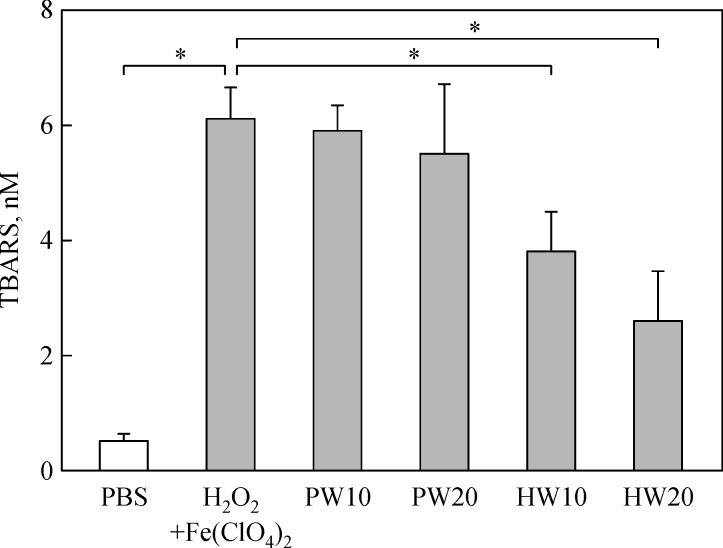
TBARS concentration in the lung tissue greatly increased in a Fenton reaction-mediated ·OH generating condition (Fig. 6). Such lipid peroxidation was significantly lowered by treatment with HW in a concentration-dependent manner, but not by PW, suggestive of the antioxidative capacity of HW.
Fig.6.
Antioxidative activity of purified water (PW) or hydrogen-enriched water (HW) against lipid peroxidation induced by hydrogen peroxide (H2O2) plus ferrous perchlorate [Fe(ClO4)2].
Discussion
It is well known that the types of pneumoconiosis are depending upon the kinds of causative particles such as environmental dusts including Yellow sands, coals and carbons (coal workers' pneumoconiosis), asbestos (asbestosis, malignant mesothelioma, and lung cancer), silica (silicosis), iron (siderosis), etc[29–30]. These particles agglomerate in lung alveolar cells. Coal workers' pneumoconiosis is a chronic occupational and a restrictive lung disease caused by the inhalation of carbon particles into the alveoli. Microorganisms as well as PM including carbon particles in the airways are removed by alveolar macrophages[31], during which proinflammatory cytokines and ROS are released from the activated macrophages[32–33]. However, a heavy burden of PM destroys macrophages, and inflammatory cytokines and ROS have potentials to induce lung tissue injury[31,33].
We observed serious accumulation of CNP in the airways and parenchyma as well as pulmonary lesions following intratracheal instillation of the particles. However, the pneumoconiosis was remarkably attenuated by a long-term feeding of HW, but not by PW. Notably, the CNP was markedly removed by HW, suggestive of an enhanced phagocytic activity of alveolar macrophages. Such facilitated clearance of CNP from the lungs by HW was also confirmed from the blood clearance of CMP: i.e., pretreatment with HW greatly enhanced the phagocytic index of blood macrophages, in comparison with negligible effect of PW. Recent study suggested that liver-resident macrophages, or Kupffer cells, are key cells in the hepatic sequestration of nanoparticles[]. The authors showed that human macrophage phenotype modulates hard nanoparticle uptake, in which “regulatory” M2 phenotype human macrophages, rather than “inflammatory” M1 phenotypes, preferentially took up gold nanoparticles, with a clear hierarchy among the subtypes (M2c > M2 > M2a > M2b > M1). Additional studies are needed to explain mechanism(s) and macrophage-phenotype specificity for the effectiveness of HW in the CNP and CMP clearance.
It is believed that CNP induced pulmonary lesions via direct injury as well as indirect inflammation mediated by cytokines and ROS from activated alveolar macrophages[31–33]. It is of interest to note that in spite of the enhanced phagocytic activity in the lungs and blood following HW feeding, the lung injury induced by CNP instillation was attenuated by HW, which may be due to its strong antioxidative potential as confirmed by the suppressive activity on Fenton reaction-mediated lipid peroxidation. Such antioxidant activity of HW is supported by previous reports demonstrating its suppressive efficacies on paraquat- and irradiation-induced lung injuries[22–23]. In addition, HW displayed anti-inflammatory activities, attenuating cigarette smoke-induced airway mucus production[21] and regulating proinflammatory and anti-inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 macrophages[34]. Interestingly, HW did not inhibit multiple points of the cascade of radical reactions other than the final stage producing ·OH group[18]. Therefore, it is proposed that HW not only eliminates the CNP by activating alveolar macrophages, but also attenuates lung injuryvia direct antioxidative potential masking the activated macrophage-mediated inflammatory stress, in dual manners.
As aforementioned, HW reduced the burden of CNP accumulation in the lungs, and thereby facilitated the recovery of bodyweight and lung weight of the CNP-challenged animals. It is also believed that elimination by HW of CNP from the lungs should block further long-term inflammation and tissue injury. Such enhanced clearance of PM from the lungs and blood might be related to the hematological changes. Actually, CNP challenge decreased the major defensive inflammatory cells (WBC, monocytes, and platelets), but increased eosinophils, indicative of general immune dysfunction and sensitized allergic reaction. In particular, intrapulmonary infusion of CNP in healthy males significantly increased the number of WBC, especially neutrophils, 6 hours later[]. In our long-term (6 weeks) study, there was a light increase in the ratio of neutrophils. The adverse effects of PM on host defense system were in part, especially on eosinophil response, restored by treatment with HW. Such anti-allergic activity of HW was confirmed in asthma, atopic dermatitis, and RA animals[24,27,35–36].
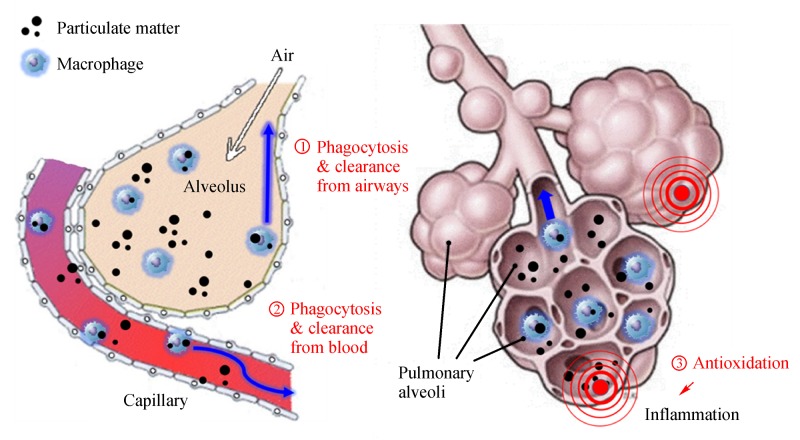
Taken together, HW not only effectively eliminated both the ultrafine and fine particles from the lungs and blood by enhancing phagocytic activity, but also attenuated the lung tissue injuries by inhibiting lipid peroxidation. Such multifaceted activities of HW on the PM elimination and tissue protection was summarized inFig. 7, suggesting enhanced phagocytosis and elimination from the airways (①) and blood (②) and anti-inflammationvia antioxidative potential (③). The results indicate that that a long-term intake of HW might be beneficial for the reduction of bodily burden of PM.
Fig.7.
Schematic summary of the fine particle-eliminating and tissue-protecting efficacies of hydrogen-enriched water
References
- 1. Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter[J]. Atmos Environ, 2012,60:504–526. [Google Scholar]
- 2. Hwang SL, Lin YC, Guo SE, et al. Fine particulate matter on hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in southwestern Taiwan during 2006-2012[J]. Int J Environ Health Res, 2017,27(2):95–105. [DOI] [PubMed] [Google Scholar]
- 3. Nascimento AP, Santos JM, Mill JG, et al. Association between the concentration of fine particles in the atmosphere and acute respiratory diseases in children[J]. Rev Saude Publica, 2017,51(0): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curtis L, Rea W, Smith-Willis P, et al. Adverse health effects of outdoor air pollutants[J]. Environ Int, 2006,32(6):815–830. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO air quality guidelines: global update 2005: particulate matter, ozone, nitrogen dioxide and sulphur dioxide[R]. Geneva: WHO, 2006.
- 6. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010[J]. Lancet, 2012,380(9859):2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dockery DW, Pope CA 3rd, Xu X, et al. An association between air pollution and mortality in six U.S.[J] cities. N Engl J Med, 1993,329(24):1753–1759. [DOI] [PubMed] [Google Scholar]
- 8. Braga ALF, Pereira LAA, Procópio M, et al. Association between air pollution and respiratory and cardiovascular diseases in Itabira, Minas Gerais State, Brazil[J]. Cad Saude Publica, 2007,23(Suppl 4):S570–S578. [DOI] [PubMed] [Google Scholar]
- 9. Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut, 2008,151(2):362–367. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann B, Moebus S, Dragano N, et al. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers[J]. Environ Health Perspect, 2009,117(8):1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kampfrath T, Maiseyeu A, Ying Z, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways[J]. Circ Res, 2011,108(6):716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saldiva PH, Pope CA 3rd, Schwartz J, et al. Air pollution and mortality in elderly people: a time-series study in Sao Paulo, Brazil[J]. Arch Environ Health, 1995,50(2):159–163 [DOI] [PubMed] [Google Scholar]
- 13. Wan G, Rajagopalan S, Sun Q, et al. Real-world exposure of airborne particulate matter triggers oxidative stress in an animal model[J]. Int J Physiol Pathophysiol Pharmacol, 2010,2(1):64–68. [PMC free article] [PubMed] [Google Scholar]
- 14. Laing S, Wang G, Briazova T, et al. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues[J]. Am J Physiol Cell Physiol, 2010,299(4):C736–C749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campbell A, Oldham M, Becaria A, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain[J]. Neurotoxicology, 2005,26(1):133–140 [DOI] [PubMed] [Google Scholar]
- 16. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine[J]. Annu Rev Genet, 2005,39:359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohta S. A multi-functional organelle mitochondrion is involved in cell death, proliferation and disease[J]. Curr Med Chem, 2003,10(23):2485–2494. [DOI] [PubMed] [Google Scholar]
- 18. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals[J]. Nat Med, 2007,13(6):688–694. [DOI] [PubMed] [Google Scholar]
- 19. Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction[J]. Biochim Biophys Acta, 2006;1762:256–265. [DOI] [PubMed] [Google Scholar]
- 20. Chen X, Liu Q, Wang D, et al. Protective effects of hydrogen-rich saline on rats with smoke inhalation injury[J]. Oxid Med Cell Longev, 2015;2015: 106836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ning Y, Shang Y, Huang H, et al. Attenuation of cigarette smoke-induced airway mucus production by hydrogen-rich saline in rats[J]. PLoS One, 2013,8(12):e83429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu S, Liu K, Sun Q, et al. Consumption of hydrogen water reduces paraquat-induced acute lung injury in rats[J]. J Biomed Biotechnol, 2011;2011: 305086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terasaki Y, Ohsawa I, Terasaki M, et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress[J]. Am J Physiol Lung Cell Mol Physiol, 2011,301(4):L415–L426. [DOI] [PubMed] [Google Scholar]
- 24. Xiao M, Zhu T, Wang T, et al. Hydrogen-rich saline reduces airway remodeling via inactivation of NF-B in a murine model of asthma[J]. Eur Rev Med Pharmacol Sci, 2013,17(8):1033–1043. [PubMed] [Google Scholar]
- 25. Li J, Wang C, Zhang JH, et al. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer's disease by reduction of oxidative stress[J]. Brain Res, 2010,1328:152–161. [DOI] [PubMed] [Google Scholar]
- 26. Fujita K, Seike T, Yutsudo N, et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease[J].PLoS One, 2009,4(9):e7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishibashi T, Sato B, Shibata S, et al. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: a randomized, double-blind, placebo-controlled pilot study[J]. Int Immunopharmacol, 2014,21(2):468–473. [DOI] [PubMed] [Google Scholar]
- 28. Kajiyama S, Hasegawa G, Asano M, et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance[J]. Nutr Res, 2008,28(3):137–143. [DOI] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention (CDC). Pneumoconiosis and advanced occupational lung disease among surface coal miners--16 states, 2010-2011[J]. MMWR Morb Mortal Wkly Rep, 2012,61(23):431–434. [PubMed] [Google Scholar]
- 30. Schenker MB, Pinkerton KE, Mitchell D, et al. Pneumoconiosis from agricultural dust exposure among young California farmworkers[J]. Environ Health Perspect, 2009,117(6):988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aberdein JD, Cole J, Bewley MA, et al. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing[J]. Clin Exp Immunol, 2013,174(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanhée D, Gosset P, Boitelle A, et al. Cytokines and cytokine network in silicosis and coal workers' pneumoconiosis[J]. Eur Respir J, 1995,8(5):834–842. [PubMed] [Google Scholar]
- 33. Gordon S. Alternative activation of macrophages[J]. Nat Rev Immunol, 2003,3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 34. MacParland SA, Tsoi KM, Ouyang B, et al. Phenotype determines nanoparticle uptake by human macrophages from liver and blood. ACS Nano, 2017;11(3):2428–2443. [DOI] [PubMed] [Google Scholar]
- 34. Chen HG, Xie KL, Han HZ, et al. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages[J]. Int J Surg, 2013,11(10):1060–1066. [DOI] [PubMed] [Google Scholar]
- 36. Berger M, de Boer JD, Lutter R, et al. Pulmonary challenge with carbon nanoparticles induces a dose-dependent increase in circulating leukocytes in healthy males. BMC Pulm Med, 2017;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ignacio RM, Kwak HS, Yun YU, et al. The drinking effect of hydrogen water on atopic dermatitis induced by Dermatophagoides farinae allergen in NC/Nga mice[J]. Evid Based Complement Alternat Med, 2013;2013: 538673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoon YS, Sajo ME, Ignacio RM, et al. Positive Effects of hydrogen water on 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice[J]. Biol Pharm Bull, 2014,37(9):1480–1485. [DOI] [PubMed] [Google Scholar]