Introduction
The production of biofilms is a common strategy used by many microorganisms during infection. Exopolysaccharides are a major component of the extracellular biofilm matrix serving to anchor organisms to surfaces, forming the structural scaffold of the biofilm, and protecting organisms from damage by hostile factors such as antibiotics and host immune defenses (Fig 1). Biochemical and genetic studies of biofilm exopolysaccharide synthesis have revealed that production of N-acetyl hexosamine-containing exopolysaccharides is one strategy used by diverse pathogens to facilitate biofilm formation and virulence. Following polymerization and extracellular extrusion by membrane embedded glycosyl transferases ([HexNAc]n + nucleotide-HexNac → [HexNAc]n+1 + nucleotide), these glycans then undergo postsynthetic enzymatic deacetylation ([HexNAc)n → HexN-[HexNAc]n-1 + acetyl group) to render them cationic. Deacetylation is critical for the function of these glycans in biofilm formation and host–pathogen interactions. This Pearl explores the role of these deacetylated cationic exopolysaccharides within the biofilm matrix in microbial pathogenesis and resistance to antimicrobial agents, and their potential as antibiofilm therapeutic targets.
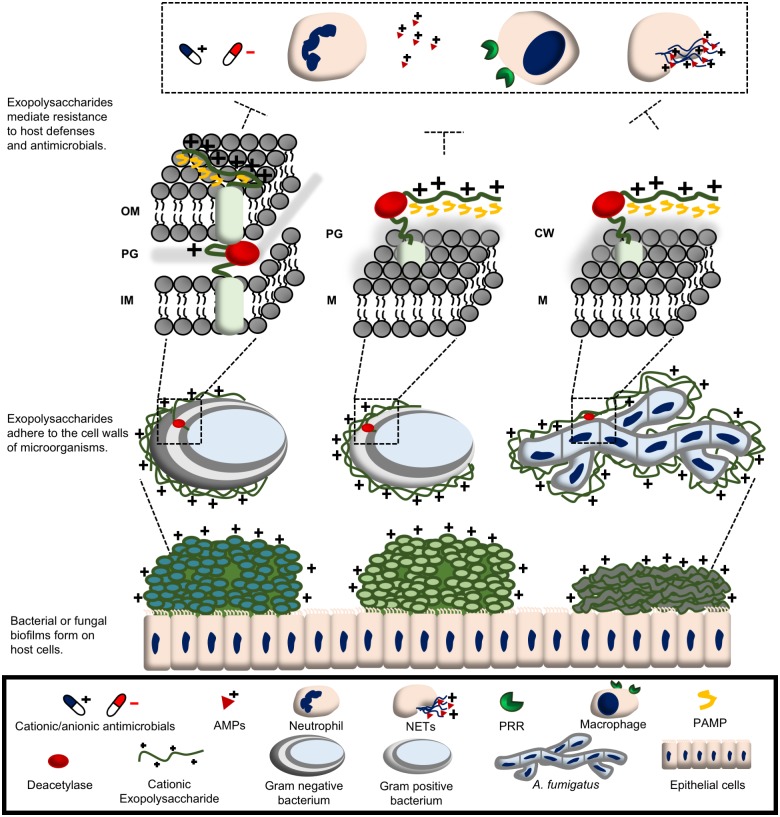
Fig 1. Cationic exopolysaccharides play a role in microbial pathogenesis.
Deacetylated cationic exopolysaccharides mediate biofilm formation and adhere to microbial surfaces to protect microorganisms from host defenses and antimicrobials as detailed in the text. AMP, antimicrobial peptide; CW, cell wall; IM, inner membrane; M, membrane; NET, neutrophil extracellular trap; OM, outer membrane; PAMP, pathogen-associated molecular pattern; PG, peptidoglycan; PRR, pattern recognition receptor.
Partially deacetylated, cationic hexosamine polymers are common in biofilm forming microorganisms
A wide range of medically important microbial species produce and secrete hexosamine-rich exopolysaccharides into their self-produced extracellular biofilm matrices (Table 1). The best studied example of these glycans is poly-β-1,6-N-acetylglucosamine (PNAG), a homopolymer of N-acetylglucosamine (GlcNAc) residues produced by a wide range of gram-positive and gram-negative pathogenic bacteria, including Staphylococcus spp., Yersinia pestis, Bordetella spp., and Escherichia coli [1–4]. The gram-negative opportunistic pathogen Pseudomonas aeruginosa produces several biofilm-associated exopolysaccharides, including the linear heteropolymer Pel, composed of GlcNAc and N-acetyl galactosamine (GalNAc), whereas the gram-positive organism Listeria monocytogenes produces a β-1,4-linked N-acetylmannosamine polysaccharide decorated with terminal α-1,6-linked galactose (Gal) residues [5,6]. More recently, biofilm formation by the opportunistic filamentous fungal pathogen Aspergillus fumigatus was found to be dependent on galactosaminogalactan (GAG), a heteropolymer composed of α-1,4-linked GalNAc and Gal residues [7].
Table 1. Functions of hexosamine-rich biofilm exopolysaccharides of medically important organisms.
| Organism | Polysaccharide | Deacetylase | Phenotype of deacetylase deletion strain or functional mutant strain |
|---|---|---|---|
| Staphylococcus epidermidis | PNAGa | IcaB | Devoid of cell-wall–associated PNAG [1] Impaired biofilm formation [1] Phagocytosed by neutrophils [1] Susceptible to antimicrobials [9] Susceptible to AMPs [1] Attenuated virulence in animal model [1] |
| S. aureus | PNAGa | IcaB | Devoid of cell-wall–associated PNAG [10] |
| Escherichia coli | PNAGa | PgaBEc | Impaired polymer export [11] Attenuated virulence in animal model [3] |
| Yersinia pestis | PNAGa | PgaBYpb | Devoid of cell-wall–associated PNAG [12] Impaired biofilm formation [12] |
| Bordetella bronchiseptica | PNAGa | PgaBBbc | Forms immature biofilm [2] |
| B. pertussis | PNAGa | PgaBBbc | N/Ad |
| Klebsiella pneumoniae | PNAGa | PgaBKp | N/Ad |
| Aspergillus fumigatus | GAG | Agd3 | Devoid of cell-wall–associated GAG [8] Impaired biofilm formation [8] Detection by PRR [8] NET-induced damage [13] Increased susceptibility to antimicrobials [14] Attenuated virulence in animal model [7] |
| Pseudomonas aeruginosa | Pel | PelA | Devoid of cell-wall–associated Pel [15] Impaired biofilm formation [15] Increased susceptibility to antimicrobials [16] |
| Listeria monocytogenes | EPS | PssB | Devoid of cell-wall–associated EPS [6] |
aPreviously called polysaccharide intercellular adhesion.
bPreviously called HmsF.
cPreviously called BpsB.
dFunction of deacetylase remains to be studied.
Abbreviations: AMP, antimicrobial peptide; Bb, Bordetella bronchiseptica; Ec, Escherichia coli; EPS, exopolysaccharide; GAG, galactosaminogalactan; Kp, Klebsiella pneumoniae; N/A, not applicable; NET, neutrophil extracellular trap; PNAG, poly- β-1,6-N-acetylglucosamine; PRR, pattern recognition receptor; Yp, Yersinia pestis.
Biochemical and genetic studies have shown that, for many of these polymers, their hexosamine residues are partially deacetylated after synthesis by polysaccharide deacetylases [8]. Deacetylation of these exopolysaccharides results in a change in their physical properties. In relatively acidic environments, the exposed primary amino groups are protonated, thereby rendering the polysaccharide polycationic [1]. The polycationic nature of these exopolysaccharides is critical for the function of these glycans in microbial pathogenesis as detailed below.
Deacetylation of hexosamine-containing exopolysaccharides underlies their adhesive properties
Deacetylation plays an important role in the synthesis, transport, and localization of many of these exopolysaccharides. Mutants of E. coli, L. monocytogenes, P. aeruginosa, Y. pestis, Staphylococcus spp., Bordetella bronchiseptica, and A. fumigatus deficient in their respective exopolysaccharide deacetylase were found to lack detectable cell-wall–associated polysaccharide [6,8,10–12,15]. In the case of L. monocytogenes and P. aeruginosa, deacetylation of their polymers seems to be required for polymer synthesis, whereas in E. coli, loss of deacetylase activity results in retention of immature polymer in the periplasm [6,11,17]. Studies of Staphylococcus epidermidis and A. fumigatus have demonstrated that the loss of exopolysaccharide deacetylation in these species results in the production of a fully acetylated glycan that is shed in the culture supernatant and does not adhere to the cell wall. This observation suggests that cationic glycans likely adhere to anionic components of the bacterial and fungal cell wall through charge.
The ability of deacetylated exopolysaccharides to adhere to microbial surfaces likely contributes to their ability to support the formation of biofilms and other microbial aggregates. Aggregate formation by L. monocytogenes and Pel-producing P. aeruginosa depends on the presence of deacetylated exopolysaccharides [6,18]. In B. bronchiseptica and likely Klebsiella pneumoniae, cationic glycan is necessary for the formation of the complex three-dimensional architecture of mature biofilms [2,19]. Deacetylation of exopolysaccharides also supports biofilm formation by enhancing the ability of microorganisms to adhere to host cells or abiotic surfaces. Production of deacetylated GAG and PNAG by A. fumigatus, and S. epidermidis and Staphylococcus aureus, respectively, has been found to enhance the adherence of these organisms to the negatively charged cell membranes of host epithelial cells. Similarly, loss of polysaccharide deacetylation impairs biofilm formation on anionic inorganic substrates, such as plastic and glass, by these organisms [1,8]. These observations strongly suggest the cationic charge of deacetylated polymers enhances adherence to anionic surfaces to support the formation of biofilms.
Production of deacetylated exopolysaccharides mediates resistance to host defenses
Mutant organisms deficient in the production of GAG and PNAG exhibit attenuated virulence in mouse or invertebrate models of infection [1,3,7,10,19,20]. These experiments suggest that the production of cationic, adhesive exopolysaccharides also provides protection against detection and elimination by elements of the host immune system. Adhesion of exopolysaccharides to the microbial cell wall can conceal cell-surface pathogen-associated molecular patterns from immune recognition by the innate immune system. This phenomenon has been best studied in A. fumigatus, for which deletion of the deacetylase Agd3 leads to greater recognition of β-glucans on the surface of hyphae by pattern recognition receptor Dectin-1 [8]. Similar findings were observed with the loss of PNAG deacetylation in S. epidermidis, resulting in a mutant prone to being avidly phagocytosed by human neutrophils [1]. PNAG has also been shown to inhibit complement deposition on the cell surface of Bordetella pertussis [20]. The presence of Pel in P. aeruginosa biofilms provides protection from killing by the leukocyte-like cell line HL-60, although the mechanism by which this occurs has not been defined [16].
Cationic exopolysaccharides can also provide direct protection from charged antimicrobial peptides (AMPs) or other antimicrobial molecules through electrostatic interactions. Partially deacetylated PNAG of S. epidermidis enhances resistance to the microbicidal effects of the cationic LL-37 and β-defensin 3 AMPs, presumably through electrostatic repulsion [1]. Similar observations were made with Aspergillus spp., in which the production of cell wall GAG reduced the binding of neutrophil extracellular traps (NETs) to hyphae and protected the organism from NET-induced damage [13]. Deacetylation of GlcNAc residues on the cell wall surfaces of L. monocytogenes and Streptococcus pneumoniae renders these pathogens resistant to the positively charged lysozyme enzyme from exerting the bacteriolytic activity of cleaving bonds in the peptidoglycan layer between N-acetylmuramic acid and GlcNAc [21,22]. Cationic polysaccharides can also bind to and sequester anionic molecules within the biofilm matrix to prevent their access to deeper cellular structures. For example, S. epidermidis PNAG protects the integrity of biofilms from the bactericidal activity of the anionic human AMP dermcidin [23]. Deacetylated exopolysaccharides, as demonstrated in these observations, contribute to actively protecting organisms against components of the host defense.
Deacetylated exopolysaccharides increase resistance to antimicrobials
Cationic exopolysaccharides can also mediate resistance to antimicrobial agents through repulsion or sequestration of these molecules. PNAG production increases the biofilm resistance of Aggregatibacter actinomycetemcomitans to the cationic detergent cetylpyridinium chloride and protects S. epidermidis against microbicidal action of glycopeptide antibiotics, such as vancomycin [9,24]. The production of GAG by A. fumigatus limits intracellular penetration of the hydrophobic antifungal posaconazole and reduces its activity [14]. Degrading Pel polysaccharide within P. aeruginosa biofilms enhanced susceptibility to colistin, and disrupting the Pel operon resulted in enhanced susceptibility of P. aeruginosa to the aminoglycosides tobramycin and gentamicin [16,18]. The ability of Pel to enhance antimicrobial resistance may be strain or condition dependent, however, because other studies have reported that disruption of the PelA deacetylase failed to alter susceptibility to tobramycin or ciprofloxacin [25]. It has been suggested that Pel polysaccharide may enhance antimicrobial resistance through interacting with and anchoring other macromolecules such as anionic extracellular DNA (eDNA) within P. aeruginosa biofilms, which in turn can act to sequester or repel antimicrobials and thereby prevent access to intracellular targets [5]. Although similar interactions of other cationic polysaccharides with eDNA have not been reported, it is likely for these cationic exopolysaccharides to have similar roles within their respective biofilms. Together, these observations suggest that the deacetylation of polymers also actively contributes to protection of their respective organisms against antimicrobials.
Development of therapeutics that target deacetylated exopolysaccharides
Studies of the biosynthetic pathways governing the production of deacetylated exopolysaccharides have suggested that these pathogens produce hydrolytic enzymes specific for cleavage of these polymers. These enzymes include Sph3 to cleave A. fumigatus GAG, the hydrolase domain of PelA to cleave P. aeruginosa Pel, and PssZ to cleave L. monocytogenes exopolysaccharide (EPS) [6,14,26]. Cross-species activity of hydrolytic enzymes has also been demonstrated. Dispersin B (DspB) of A. actinomycetemcomitans and NghA of Y. pseudotuberculosis cleave PNAG β-1,6-linked GlcNAc polymers and residues, respectively, and the P. aeruginosa PelA hydrolase cleaves fungal GAG [14,27,28].
Recent studies have highlighted the potential for the use of these enzymes as therapeutic agents to target biofilm formation by their respective organisms, as well as other organisms producing polymers with shared composition and linkages. DspB has been shown to sensitize biofilms of Actinobacillus actinomycetemcomitans to the bactericidal effect of cationic detergent cetylpyridinium chloride, as well as anionic detergent sodium dodecyl sulfate and Actinobacillus pleuropneumoniae to ampicillin [24,29]. The hydrolase domain of B. bronchiseptica PgaB (PgaBBb) from B. bronchiseptica and DspB both potentiate the killing of S. epidermidis and E. coli by gentamicin [30]. Treatment with the GAG-specific hydrolase Sph3 as well as the P. aeruginosa PelA hydrolase enhanced the antifungal activity of posaconazole, amphotericin B, and caspofungin against A. fumigatus [14]. Targeting biofilms with hydrolase enzymes also enhances the susceptibility of organisms to host defenses, because treatment of P. aeruginosa with PelA disrupted Pel-dependent biofilms and enhanced susceptibility of this organism to the antibiotic colistin as well as killing by the leukocyte-like HL-60 cell line [16]. Intratracheal treatment with recombinant Sph3 is well tolerated and attenuates virulence of A. fumigatus in an immunocompromised mouse model of invasive aspergillosis [14]. However, a recent study reported that treatment of P. aeruginosa biofilms with enzymes targeting cell wall polysaccharides resulted in dissemination of bacteria due to biofilm dispersion [31]. More work is therefore required to establish the long-term safety of these enzymes and identify the optimal organism–enzyme combinations that improve outcomes during infection.
The development of inhibitors of exopolysaccharide deacetylases is another promising therapeutic strategy. Although this work remains in its infancy, several metal chelating inhibitors have been synthesized to inhibit E.coli PgaB (PgaBEc) deacetylation of PNAG [32–34].
Future directions
Although our understanding of the biosynthetic pathways governing the synthesis of deacetylated exopolysaccharides has expanded greatly, there are many unanswered questions about their functions in host–pathogen interactions. For example, studies of A. fumigatus GAG suggest that purified fractions of this glycan can directly modulate host immune responses, although the receptors and signaling pathways governing this process remain unknown [35,36]. These and similar studies in other organisms await robust protocols for the purification of deacetylated exopolysaccharides or the production of synthetic oligosaccharides derived from these glycans. In addition, the function of deacetylated exopolysaccharides has been largely studied in the context of single-species biofilms. However, as these organisms typically exist in polymicrobial environments, the potential for exopolysaccharides to play cooperative roles in multispecies biofilms also needs to be explored.
Funding Statement
Research described in this paper is supported by grants from the Canadian Institutes of Health Research (#81361 to DCS and PLH; #123306 to DCS, #43998, #13337, #FDN154327 to PLH); Cystic Fibrosis Canada and GlycoNET (to DCS and PLH); and the National Institutes of Health (4R33AI119116 to PLH). DCS is supported by a Chercheur-Boursier Award from the Fonds de Recherche Quebec Santé. PLH is the recipient of a Canadian Research Chair. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, DeLeo FR, et al. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem 2004. December 24;279(52):54881–54886. 10.1074/jbc.M411374200 [DOI] [PubMed] [Google Scholar]
- 2.Little DJ, Milek S, Bamford NC, Ganguly T, DiFrancesco BR, Nitz M, et al. The protein BpsB is a poly-beta-1,6-N-acetyl-D-glucosamine deacetylase required for biofilm formation in Bordetella bronchiseptica. J Biol Chem 2015. September 11;290(37):22827–22840. 10.1074/jbc.M115.672469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subashchandrabose S, Smith SN, Spurbeck RR, Kole MM, Mobley HL. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog 2013;9(12):e1003788 10.1371/journal.ppat.1003788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobrov AG, Kirillina O, Forman S, Mack D, Perry RD. Insights into Yersinia pestis biofilm development: topology and co-interaction of Hms inner membrane proteins involved in exopolysaccharide production. Environ Microbiol 2008. June;10(6):1419–1432. 10.1111/j.1462-2920.2007.01554.x [DOI] [PubMed] [Google Scholar]
- 5.Jennings LK, Storek KM, Ledvina HE, Coulon C, Marmont LS, Sadovskaya I, et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Natl Acad Sci U S A 2015. September 8;112(36):11353–11358. 10.1073/pnas.1503058112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koseoglu VK, Heiss C, Azadi P, Topchiy E, Guvener ZT, Lehmann TE, et al. Listeria monocytogenes exopolysaccharide: origin, structure, biosynthetic machinery and c-di-GMP-dependent regulation. Mol Microbiol 2015. May;96(4):728–743. 10.1111/mmi.12966 [DOI] [PubMed] [Google Scholar]
- 7.Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, et al. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS Pathog 2013;9(8):e1003575 10.1371/journal.ppat.1003575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee MJ, Geller AM, Bamford NC, Liu H, Gravelat FN, Snarr BD, et al. Deacetylation of Fungal Exopolysaccharide Mediates Adhesion and Biofilm Formation. MBio 2016. April 5;7(2): 10.1128/mBio.00252-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farber BF, Kaplan MH, Clogston AG. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis 1990. January;161(1):37–40. [DOI] [PubMed] [Google Scholar]
- 10.Cerca N, Jefferson KK, Maira-Litran T, Pier DB, Kelly-Quintos C, Goldmann DA, et al. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-beta-(1–6)-glucosamine. Infect Immun 2007. July;75(7):3406–3413. 10.1128/IAI.00078-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh Y, Rice JD, Goller C, Pannuri A, Taylor J, Meisner J, et al. Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-beta-1,6-N-acetyl-D-glucosamine. J Bacteriol 2008. May;190(10):3670–3680. 10.1128/JB.01920-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman S, Bobrov AG, Kirillina O, Craig SK, Abney J, Fetherston JD, et al. Identification of critical amino acid residues in the plague biofilm Hms proteins. Microbiology 2006. November;152(Pt 11):3399–3410. 10.1099/mic.0.29224-0 [DOI] [PubMed] [Google Scholar]
- 13.Lee MJ, Liu H, Barker BM, Snarr BD, Gravelat FN, Al Abdallah Q, et al. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS Pathog 2015. October 22;11(10):e1005187 10.1371/journal.ppat.1005187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snarr BD, Baker P, Bamford NC, Sato Y, Liu H, Lehoux M, et al. Microbial glycoside hydrolases as antibiofilm agents with cross-kingdom activity. Proc Natl Acad Sci U S A 2017. July 3;114(27):7124–7129. 10.1073/pnas.1702798114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colvin KM, Alnabelseya N, Baker P, Whitney JC, Howell PL, Parsek MR. PelA deacetylase activity is required for Pel polysaccharide synthesis in Pseudomonas aeruginosa. J Bacteriol 2013. May;195(10):2329–2339. 10.1128/JB.02150-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker P, Hill PJ, Snarr BD, Alnabelseya N, Pestrak MJ, Lee MJ, et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci Adv 2016. May 20;2(5):e1501632 10.1126/sciadv.1501632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmont LS, Whitfield GB, Rich JD, Yip P, Giesbrecht LB, Stremick CA, et al. PelA and PelB proteins form a modification and secretion complex essential for Pel polysaccharide-dependent biofilm formation in Pseudomonas aeruginosa. J Biol Chem 2017. November 24;292(47):19411–19422. 10.1074/jbc.M117.812842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GC, et al. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog 2011. January 27;7(1):e1001264 10.1371/journal.ppat.1001264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen KM, Chiang MK, Wang M, Ho HC, Lu MC, Lai YC. The role of pgaC in Klebsiella pneumoniae virulence and biofilm formation. Microb Pathog 2014. December;77:89–99. 10.1016/j.micpath.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 20.Ganguly T, Johnson JB, Kock ND, Parks GD, Deora R. The Bordetella pertussis Bps polysaccharide enhances lung colonization by conferring protection from complement-mediated killing. Cell Microbiol 2014. July;16(7):1105–1118. 10.1111/cmi.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollmer W, Tomasz A. Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect Immun 2002. December;70(12):7176–7178. 10.1128/IAI.70.12.7176-7178.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boneca IG, Dussurget O, Cabanes D, Nahori MA, Sousa S, Lecuit M, et al. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci U S A 2007. January 16;104(3):997–1002. 10.1073/pnas.0609672104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 2004. March;6(3):269–275. [DOI] [PubMed] [Google Scholar]
- 24.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol 2008. January;74(2):470–476. 10.1128/AEM.02073-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan W, Bernier SP, Kuchma SL, Hammond JH, Hasan F, O'Toole GA. Aminoglycoside resistance of Pseudomonas aeruginosa biofilms modulated by extracellular polysaccharide. Int Microbiol 2010. December;13(4):207–212. 10.2436/20.1501.01.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamford NC, Snarr BD, Gravelat FN, Little DJ, Lee MJ, Zacharias CA, et al. Sph3 Is a Glycoside Hydrolase Required for the Biosynthesis of Galactosaminogalactan in Aspergillus fumigatus. J Biol Chem 2015. November 13;290(46):27438–27450. 10.1074/jbc.M115.679050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson DL, Jarrett CO, Callison JA, Fischer ER, Hinnebusch BJ. Loss of a biofilm-inhibiting glycosyl hydrolase during the emergence of Yersinia pestis. J Bacteriol 2008. December;190(24):8163–8170. 10.1128/JB.01181-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh Y, Wang X, Hinnebusch BJ, Preston JF 3rd, Romeo T. Depolymerization of beta-1,6-N-acetyl-D-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol 2005. January;187(1):382–387. 10.1128/JB.187.1.382-387.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izano EA, Sadovskaya I, Vinogradov E, Mulks MH, Velliyagounder K, Ragunath C, et al. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathog 2007. July;43(1):1–9. 10.1016/j.micpath.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little DJ, Pfoh R, Le Mauff F, Bamford NC, Notte C, Baker P, et al. PgaB orthologues contain a glycoside hydrolase domain that cleaves deacetylated poly-beta(1,6)-N-acetylglucosamine and can disrupt bacterial biofilms. PLoS Pathog 2018. April 23;14(4):e1006998 10.1371/journal.ppat.1006998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming D, Rumbaugh K. The Consequences of Biofilm Dispersal on the Host. Sci Rep 2018. July 16;8(1):10738-018-29121-2. 10.1038/s41598-018-29121-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chibba A, Poloczek J, Little DJ, Howell PL, Nitz M. Synthesis and evaluation of inhibitors of E. coli PgaB, a polysaccharide de-N-acetylase involved in biofilm formation. Org Biomol Chem 2012. September 21;10(35):7103–7107. 10.1039/c2ob26105g [DOI] [PubMed] [Google Scholar]
- 33.Rouffet M, Cohen SM. Emerging trends in metalloprotein inhibition. Dalton Trans 2011. April 14;40(14):3445–3454. 10.1039/c0dt01743d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, Nagano Y, Kouketsu A, Matsuura A, Maruyama S, Kurotaki M, et al. Novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxamates. J Med Chem 2005. February 24;48(4):1019–1032. 10.1021/jm049207j [DOI] [PubMed] [Google Scholar]
- 35.Gresnigt MS, Bozza S, Becker KL, Joosten LA, Abdollahi-Roodsaz S, van der Berg WB, et al. A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog 2014. March 6;10(3):e1003936 10.1371/journal.ppat.1003936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine T, Delangle A, Simenel C, Coddeville B, van Vliet SJ, van Kooyk Y, et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog 2011. November;7(11):e1002372 10.1371/journal.ppat.1002372 [DOI] [PMC free article] [PubMed] [Google Scholar]