Abstract
Plants interact with a wide variety of fungi in a mutualistic, parasitic or neutral way. The associations formed depend on the exchange of nutrients and signalling molecules between the partners. This includes a diverse set of protein classes involved in defence, nutrient uptake or establishing a symbiotic relationship. Here, we have analysed the secretomes of the mutualistic, root-endophytic fungus Piriformospora indica and Arabidopsis thaliana when cultivated alone or in a co-culture. More than one hundred proteins were identified as differentially secreted, including proteins associated with growth, development, abiotic and biotic stress response and mucilage. While some of the proteins have been associated before to be involved in plant-microbial interaction, other proteins are newly described in this context. One plant protein found in the co-culture is PLAT1 (Polycystin, Lipoxygenase, Alpha-toxin and Triacylglycerol lipase). PLAT1 has not been associated with plant-fungal-interaction and is known to play a role in abiotic stress responses. In colonised roots PLAT1 shows an altered gene expression in a stage specific manner and plat1 knock-out plants are colonised stronger. It co-localises with Brassicaceae-specific endoplasmic reticulum bodies (ER-bodies) which are involved in the formation of the defence compound scopolin. We observed degraded ER-bodies in infected Arabidopsis roots and a change in the scopolin level in response to the presence of the fungus.
Introduction
The roots of more than 80% of all land plants form mycorrhizal interaction [1]. The interactions can shift from mutualism to parasitism depending on environmental conditions and the communication between the organisms [2]. The communication between the symbionts is driven by molecules released into the rhizosphere by both partners including diverse protein classes [3–6]. Based on genome analyses, it has been estimated that the proportion of released fungal proteins represent about 3% to 10% of the corresponding total proteome [6]. Furthermore, the size of the predicted secretomes correlates with the lifestyle of a fungus. For example, pathogenic fungi appear to have more secretory proteins compared to saprophytic and mutualistic species [6, 7]. Proteomic-based technologies and bioinformatic tools identified multiple fungal proteins which determine the mode of interaction and reprogram the root cells [8–10]. Depending on the organism and strain colonising the plant, the number and amount of the secreted proteins from both partners can vary, as shown during the symbiotic interaction of different fungi with Pisum sativum, Medicago truncatula and Nicotiana benthamiana [11–14]. Mutualistic fungi secrete also various effector proteins that repress plant defence responses. After entering the plant cell, some effector proteins are transported to the plant nucleus, where they can interact with transcription factors and repress plant defence [15]. This is well studied for the small secreted protein 7 (SP7) from Rhizophagus irregularis and MISSP7 (mycorrhiza-induced small secreted protein-7) from Laccaria bicolor [16–18].
Similar to the fungal secretome, between 6% to 17% of plant proteins are estimated to be secreted [19, 20]. These proteins have multiple functions including growth regulation, communication, nutrient uptake and defence responses. However, most of them are uncharacterised [21, 22]. Among the better studied are germin-like proteins which can produce reactive oxygen species in response to stress and during plant growth. They have multiple roles outside the cell, particularly in defence and development [23, 24]. Furthermore, small peptides, such as members of the CLE (clavata3/embryo-surrounding region) family, have been implicated in development, inter-plant communication, defence and in the formation of new symbiosis related organs such as nodules [21, 25, 26].
Exudate proteins are often identified by bioinformatic tools based on the prediction of their N-terminal secretion sequence which directs them into the endoplasmic reticulum (ER) from where they are secreted via vesicle fusion with the plasma membrane [16, 27, 28]. However, only ~ 50% of the known secreted plant proteins possess such a signal [11, 22] whereas others utilise unconventional mechanisms of secretion, which resemble those of animal exosomes [29] or include exocyst-like structures [30]. For example, between 50% to 80% of proteins found in the secretome of Arabidopsis after treatment with salicylic acid or pathogens do not possess a N-terminal secretional sequence [11, 31, 32]. Although some of these proteins can also be contaminations, e.g. due to cell damage, untargeted secretome studies are valuable addition to bioinformatic-based studies. While A. thaliana does not form mycorrhizal associations, the plant can be colonised by endophytes, such as Piriformospora indica. This fungus of the order Sebacinales was isolated from roots of plants growing in the Indian Thar desert [33]. The fungus has a saprophytic lifestyle and colonises the roots of many plant species. Colonised plants produce more biomass, in particular under unfavourable conditions, and are more resistant to abiotic and biotic stresses [34–36]. Based on expression profiles during the colonisation of Arabidopsis [37] and barley roots [5], the interaction can roughly be split into three stages, which corresponds to the saprophytic and mutualistic traits of P. indica [38]. The initial contact is characterised by the down-regulation of plant defence mechanisms, followed by a phase in which the fungus reduces the expression of genes associated with saprophism [38]. During the last phase, a long-lasting beneficial interaction is established [5, 12, 37].
To balance beneficial and non-beneficial traits in a symbiosis, the propagation of microbes in the roots must be controlled by host defence compounds. One of them is the coumarin scopoletin, which accumulates in the ER-body [39]. The ER-bodies derive as subdomains from the ER network and have specialised functions in glucosinolate-based defence [39–41]. ER-bodies harbour high levels of β-glucosidases including PYK10, the most abundant enzyme of the organelle [42]. When ER-bodies are disrupted during pathogen attack or damage, PYK10 and other enzymes are released into the cytoplasm. PYK10 is involved in establishing the interaction between P. indica and A. thaliana roots [43]. The roots of mutant lines with reduced PYK10 level are overcolonised by P. indica, although PYK10 expression is unaffected by P. indica in wild type (WT) seedlings [43, 44]. PYK10 hydrolyses multiple indole glucosinolates and scopoletin to scopolin [44, 45]. Scopoletin, and its glycone scopolin, accumulates in roots and helps plants to fine tune their defence responses by scavenging of reactive oxygen species [46, 47]. Scopoletin acts as antifungal component against Alternaria alternata in tobacco and is strongly suppressed in the beneficial symbiosis of tomato with R. irregularis [48, 49]. A key enzyme for scopoletin biosynthesis is the feruloyl-CoA 6'-hydroxylase 1 (F6'H1), and the accumulation of this secondary metabolite is severely reduced in the corresponding knock-out line [46]. Interestingly, biosynthesis of scopoletin can also be stimulated by ectopic expression of the Arabidopsis PLAT1 and -2 (Polycystin, Lipoxygenase, Alpha-toxin and Triacylglycerol lipases) proteins in tobacco [50]. It has been hypothesized that the PLAT domain provides a docking platform for proteins to regulate their catalytic activities [51–53]. AtPLAT1 and -2 proteins are functionally and physically associated with ER-bodies [51]. This suggests an interaction with PYK10, although the exact function of the PLAT domain is still enigmatic.
In this study, we identified proteins which are found in the growth medium of A. thaliana, P. indica or P. indica co-cultivated with A. thaliana. Proteins which are only found in the co-culture or disappear from it, are considered symbiosis-specific and are mainly involved in growth and defence-related processes. Many enzymes belonging to the latter category are implicated in the production or release of secondary metabolites. The role of one of these proteins, PLAT1, was investigated in more detail and its postulated function in the formation of the defence compound scopolin was related to the degradation of ER-bodies and root colonisation.
Results
Secretome analysis of P. indica and A. thaliana reveal distinct changes in the co-culture
To identify symbiosis-specific proteins that are secreted during the early phase of the interaction between P. indica and A. thaliana, we co-cultivated both organisms in liquid plant nutrition media (PNM) for three days and compared them with cultures of the plant and fungus grown separately (S1 Fig). The spent media were filtered and proteins were isolated by solid phase extraction followed by LC-MS/MS (liquid chromatography–tandem mass spectrometry) analysis. The resulting MS/MS spectra were searched against the UniProt database (http://www.uniprot.org/) [54] where a total of 590 different A. thaliana and 164 P. indica proteins were identified in three independent biological replicates (S1 Table). In the single cultures of Arabidopsis 199, 316 and 117 proteins were observed within three replicates, while the number of identified proteins in the single fungal cultures were 39, 76 and 76. In the co-culture 140, 311 and 85 plant proteins and 120, 108 and 84 fungal proteins were observed per replicate. The Venn diagram in S2A Fig shows the overlap of Arabidopsis proteins in the co-culture among all replicates. 57 plant proteins were common to all three replicates, which represent 16% of all identified proteins. In total, 33% of proteins were found in at least 2 replicates (S2A Fig). When comparing the replicates of the plant and fungal single cultures, similar percentages were found.
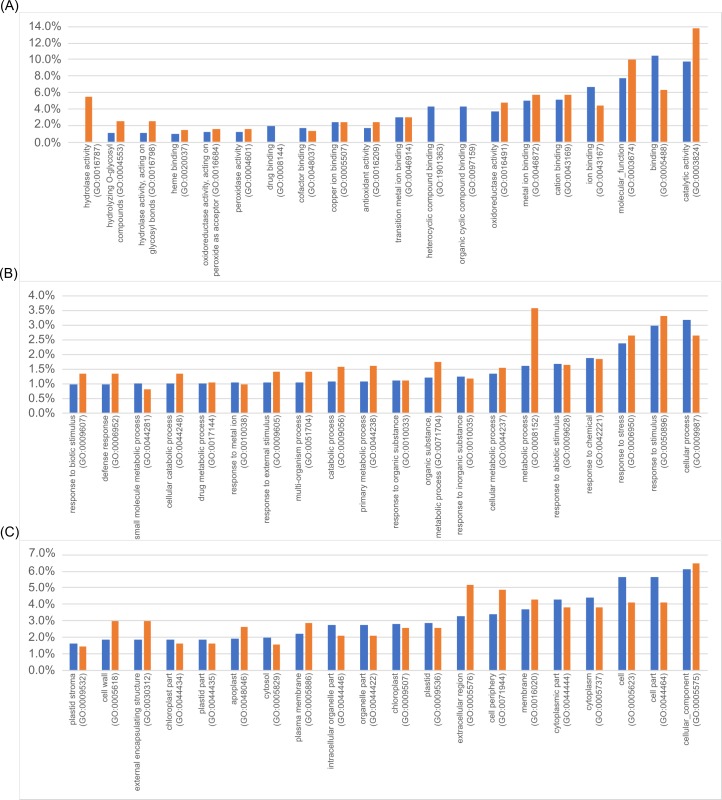
For further investigations, we took all identified plant proteins and classified them (S2 Table) according to their GO (Gene ontology) terms using AmiGO (http://www.geneontology.org, version 1.8) [55–57]. Due to the absence of a functional annotation for a high number of fungal proteins, no GO terms have been captured for P. indica. The GO terms annotated to plant proteins were sorted within the categories “molecular functions”, “biological processes” and “cellular components” (Fig 1A–1C). For each category, the 20 most abundant terms are shown in Fig 1A–1C. The functional categorisation revealed that secreted plant proteins in both cultures belong mainly to identical GO terms, however, the number of protein entries varies for each term. As shown in Fig 1A, the most frequent term (10%) related to proteins found in the single culture of Arabidopsis is “binding of different compounds” (GO:0005488), whereas the most abundant term related to proteins from the co-culture (12%) is “catalytic activity” (GO:0003824). As another example, about 5% of the proteins in the co-culture play a role in hydrolysis (GO:00016787), while proteins associated with this GO term were not found in the single culture. Among the terms of the category “biological processes” (Fig 1B), slight differences between the single and co-culture were observed. For instance, proteins with the GO term “metabolic process” (GO:0008152) were found twice as often in the co-culture. Furthermore, the terms “defence”, “external stimuli”, “response to biotic stimulus” and “catabolic process” were found more often for co-cultures. The category “cellular component” (Fig 1C) included notably more proteins associated with the GO terms “extracellular region” and “cell periphery” in the co-culture. Contrary, GO terms associated with “cellular processes” or “organelles” were more frequently found in the single culture.
Fig 1. GO enrichment analysis of the secretomes.
All identified plant proteins were analysed using the GO term analysis tool using AmiGO (http://www.geneontology.org, version 1.8) and sorted according to the categories of biological processes (A), molecular functions (B) and cellular components (C). The 20 most abundant GO terms are presented here (in percentage). Blue bars indicate the frequency of this GO term in single cultures, orange bars indicate the frequency of the respective GO term in co-cultures.
A. thaliana responds to the presence of P. indica by an altered secretion of proteins associated with defence and growth
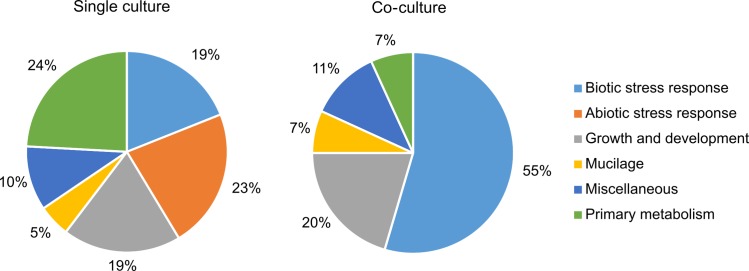
To better understand changes in the composition of secreted proteins, we looked for specific differences between the co-culture and single culture (S2 Table). The analysis considered only proteins found in two or more root exudates and these proteins were classified based on their function. We observed 102 proteins to be differentially secreted (Fig 2). The most enriched proteins in the single culture are associated with the functions “primary metabolism”, “abiotic stress response”, “growth and development” and “biotic stress response” (~20% each). In the co-cultures, 55% of the proteins play a role in defence against biotic stresses, 20% have been described in the context of growth and development, 11% belong to the primary metabolism and 7% proteins are related to abiotic stress responses. Furthermore, 11% proteins are classified as miscellaneous. Interestingly, 7% of the differentially secreted proteins are associated with mucilage.
Fig 2. Differentially secreted proteins.
Arabidopsis proteins that were found in at least two replicates and with different frequencies in the single and co-culture (S2 Table) were sorted according to their primary function. Numbers are given in %.
Among proteins, which were involved in development and growth and which were predominantly detected in the co-culture, are the ribonuclease T2 (At1G14220), the lipid recognition protein At5G23840 and the pectin methylesterase inhibitor At1G23205. Examples related to biotic stress responses include the pathogenesis-related protein 5 (At1G75040), which was strongly induced by the fungus. In Arabidopsis, it has been implicated in systemic acquired resistance [58]. Furthermore, the two functionally distinct germin like proteins (GLPs), GLP2 (At1G02335) and GLP4 (At1G18970) appear to be secreted into the co-culture upon interaction with the fungus. Additionally, PLAT1 (At4G39730) was found in the medium of the co-culture.
The single cultures contain a different set of proteins. The list includes several proteins associated with abiotic stress responses, particularly some proteins involved in cadmium stress such as At1G11860 (aminomethyl transferase), At1G48030 (dihydrolipoyl dehydrogenase 1) and At3G23990 (chaperonin CPN60) [59]. The single culture also contains ABA (abscisic acid)-inducible proteins such as At1G76180 (dehydrin ERD14), At1G20440 (dehydrin COR47), At5G15970 (stress-induced protein KIN2), the glycine-rich protein At1G04800 and the peroxidase 69 (At5G64100) [60–69]. Surprisingly, the well-known defence compound remorin 3 (At2G45820), which was shown to be associated with plasma membranes and to bind proteins derived from pathogens [70–72], was only detected in the single cultures. Interestingly, the analyses also detected six mucilage-related Arabidopsis proteins as potential targets for P. indica (S2 Table). In the co-culture, the mucilage-related proteins aspartic protease 1 (At3G18490), responsive to dehydration 21A (At1G47128) and its homologue 21B (At5G43060) were identified [73]. In contrast, the xyloglucan endotransglucosylase 6 (At5G65730) was only found in the single culture. A homologue of endotransglucosylase 6 was found in the mucilage of maize roots [74]. Also, the oxalate-CoA ligase At3G48990, which has a role in seed mucilage, and the subtilisin-like protease 1.8 (SBT1.8, At2G05920) were only found in the single culture [73, 75]. The latter is highly homologues to SBT1.7 (At5G67360), a key protein for mucilage formation, which is present in both the single and the co-culture (S1 Table). Other mucilage-associated proteins such as fasciclin-like arabinogalactan protein 10 (At3G60900), auxin-induced in root cultures protein 12 (At3G07390) and non-specific lipid-transfer protein 6 (At3G08770) were found in both fractions (S1 Table) [73].
All differentially enriched proteins were also examined individually to assess whether they possess an N-terminal secretion sequence using available bioinformatics tools TargetP 1.1 (http://www.cbs.dtu.dk/services/TargetP/), SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) and Predotar 1.3 (https://urgi.versailles.inra.fr/Tools/Predotar) [76, 77]. The results showed that 36 out of 44 proteins in the co-culture and 18 out of 58 proteins in the single culture possess a predicted N-terminal secretion sequence (S2 Table).
Symbiosis reprograms the P. indica secretome
A total of 164 P. indica proteins were found in all experiments (S1 Table): 36 of them were differentially enriched between the treatments (S2 Table). Among these proteins, two were found predominantly in the single fungal culture (S2 Table): PIIN_00867, an uncharacterised protein, -and PIIN_06517 related to glyoxal oxidase. 34 proteins were present only in the co-culture. Generally, many of the fungal proteins identified in this study are uncharacterised or possess homology to degrading enzymes. For instance, PIIN_01733, which was found in the co-cultures, is a putative beta-mannanase with homology to hydrolases from two saprophytic fungi [78]. Additionally, we analysed fungal proteins for subcellular localization using available bioinformatics tools: 21 contained an N-terminal secretory signal peptide (S2 Table). One of them is a indole-3-acetaldehyde dehydrogenase (PIIN_04899) which has been suggested to be involved in the formation of auxin [79].
P. indica-root colonisation influences PLAT1 and leads to changes in scopolin production
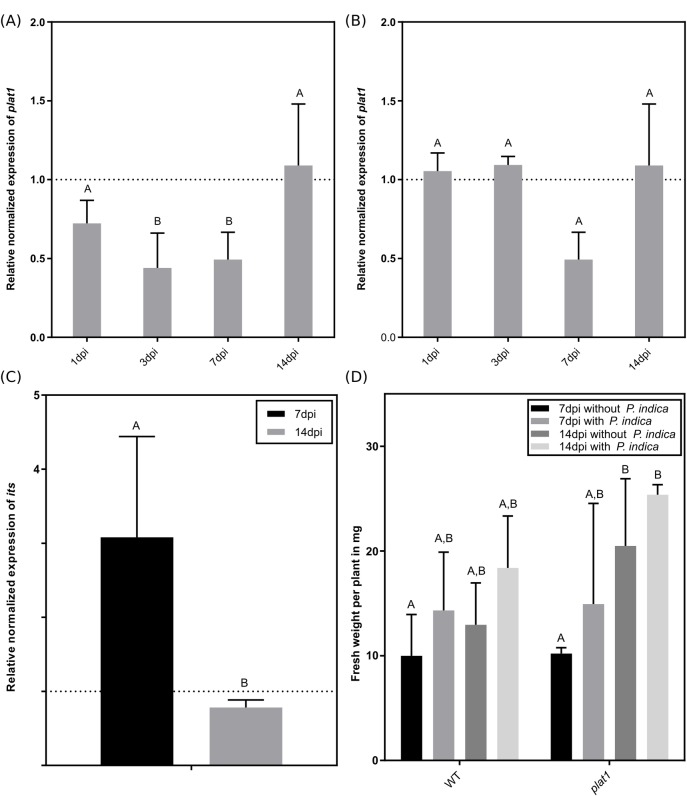
As a case example to study the secretome, we further analysed the role of the Polycystin, Lipoxygenase, Alpha-toxin and Triacylglycerol lipases 1 (PLAT1). This protein was found in the medium of the co-culture and has not been reported in context with P. indica before. We checked the expression level of PLAT1 in colonised WT roots and observed a significant lower level of plat1 mRNA at three and seven days of co-cultivation. After two weeks the level of plat1 mRNA was comparable to uncolonised plants (Fig 3A). In shoots no regulation was observed (Fig 3B). A weak but not significant downregulation was observed for the homologous plat2 mRNA, whereas for the putative pseudogene plat3 no regulation was observed. Interestingly, a ten-time higher expression in leafs compared to roots was observed for plat3. Nevertheless, for all tested time points and plant parts the expression level of plat3 was extremely low (S2B and S2C Fig). Since P. indica had the strongest effect on PLAT1 expression, we took a closer look at the colonisation of plat1 mutant seedlings by the fungus. After one week, we found a three times higher amount of fungal RNA in the plat1 mutant compared to the WT seedlings, whereas after two weeks no differences in colonisation between plat1 mutants and WT plants was observed (Fig 3C). This stronger colonisation did not have an impact on the total fresh weight of the plat1 mutants (Fig 3D).
Fig 3. Characterisation of plat1 mutant and plat1 gene expression.
Gene expression of PLAT1 in WT roots (A) and leaves (B) was determined between one day and 14 days of colonisation. The dashed line represents levels of mRNA in uncolonised WT roots. (C) Colonisation of P. indica in WT and plat1 roots determined by RT-qPCR. The dashed line represents the level of fungal internal transcribed spacer (its) mRNA in colonised WT roots after one and two weeks, respectively. At 7dpi (days post inoculation) the plat1 mutant was significant stronger colonised compared to WT plants. (D) Effect of P. indica on fresh weight in WT and different mutant A. thaliana plants after one and two weeks of colonisation. All experiments were repeated three to six times with at least ten plants each. Error bars represent standard deviation. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction).
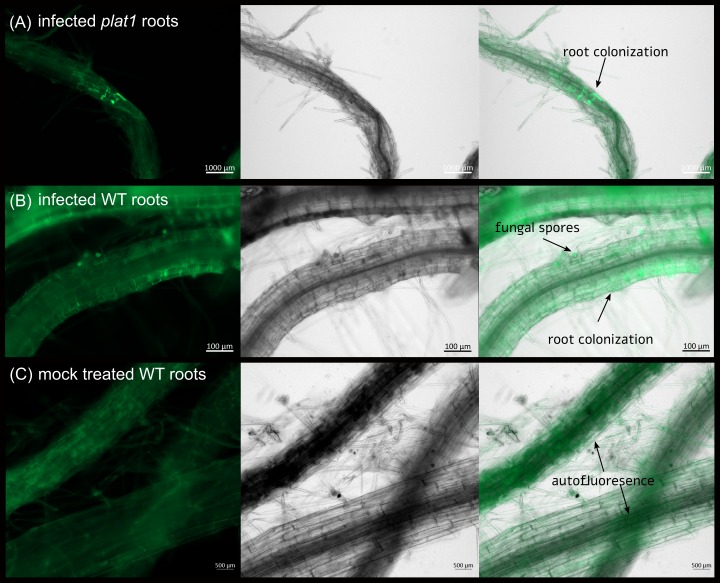
These results could be confirmed by microscopy (Fig 4): after seven days we observed a stronger colonisation in the plat1 mutant (Fig 4A) compared to the WT (Fig 4B), where almost no fungus could be detected.
Fig 4. Root colonisation of Arabidopsis WT and plat1 by P. indica at 7 dpi.
Colonisation of Arabidopsis plat1 (A) and WT roots (B) was imaged using fluorescence microscopy at 7 dpi. Uncolonised roots are shown at (C). The first pictures show the autofluorescence of the root and GFP (green fluorescent protein) fluorescence of P. indica. The second pictures show the bright field image. The last pictures show the overlay of all images for each row.
Since AtPLAT1, which was identified in the co-culture, promotes scopoletin biosynthesis [50], and an upregulation of scopoletin in colonised A. thaliana roots has been described before [12], we analysed the connection between PLAT1, scopoletin and P. indica colonisation.
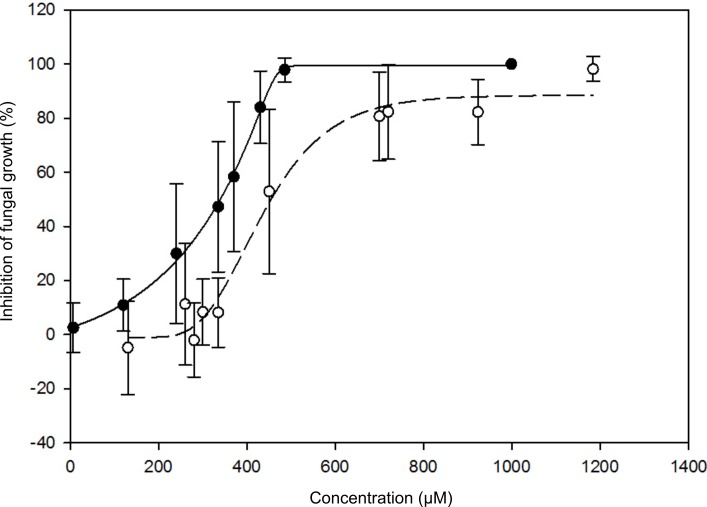
In a first step we examined the influence of scopolin and scopoletin on P. indica in vitro and found that both chemicals inhibit the growth of P. indica with a half maximal inhibitory concentrations (IC50) of 0.34 mM and 0.45 mM, respectively (Fig 5).
Fig 5. Growth inhibition of P. indica by scopoletin / scopolin.
Sigmoidal regression line of the growth effect of scopoletin (full dots) and scopolin (hollow dots) on growth of P. indica. Error bars represent standard deviation. The experiment was repeated in three biological replicates with two technical replicates each.
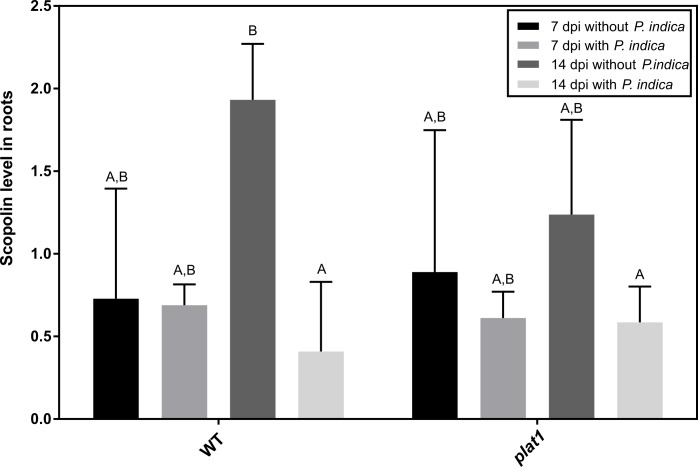
Scopolin is much more abundant than scopoletin and can easily be measured in high quantities, in Arabidopsis roots [80]. Therefore, we studied the influence of P. indica on scopolin production in Arabidopsis roots in vivo. After two weeks, the mock treated WT roots showed a four-time upregulation of scopolin compared to the other time points, whereas in colonised roots the scopolin level was not altered. Instead, the same level of scopolin was observed for all time points and treatments in the plat1 mutant (Fig 6).
Fig 6. Characterisation of plat1 mutant and plat1 gene expression.
First, we determined the scopolin levels in root of WT and plat1, and the peak areas of HPLC chromatograms were normalised by an internal standard. All experiments were repeated four to five times with at least ten plants each. Error bars represent standard deviation. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction).
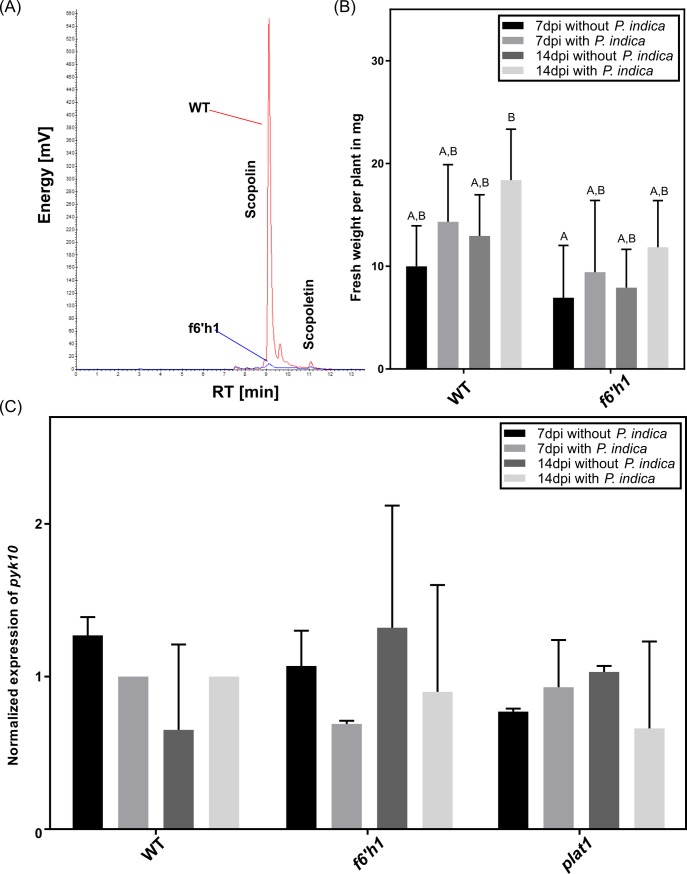
To examine whether scopolin alone is responsible for the altered colonisation in the plat1 mutant, we investigated the performance of the fungus in the f6’h1 Arabidopsis mutant line where scopolin and scopoletin production is severely reduced (Fig 7A). When comparing the fresh weight between WT and f6’h1 plants, we observed, similar to plat1 (Fig 3D), that the mutant lines are not influenced by the presence of the fungi. Furthermore, the mutant lines were smaller than WT plants (Fig 7B). Since PYK10 can be involved in scopolin formation, we checked its expression: pyk10 is not influenced by the fungus neither in the WT plants nor in the plat1 or the f6’h1 mutant background (Fig 7C). Other genes, which are also associated with scopolin formation, are not influenced by the fungus in f6’h1 or WT plants as well (S3 Fig).
Fig 7. Characterisation of the f6’h1 mutant.
(A) Fluorescence (336/438 nm) HPLC chromatograms of root extracts from WT (red) and f6'h1 (B) Growth promotion of WT and f6’h1 by P. indica after 7 and 14 days of co-cultivation. At 14 dpi a slightly higher growth-stimulating effect was visible for f6’h1 compared to the WT. (C) Expression of PYK10 in the roots of WT and f6'h1 mutants 14 days after co-cultivation with or without P. indica. All experiments were repeated three to four times with at least ten plants each. Error bars represent standard deviation. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction).
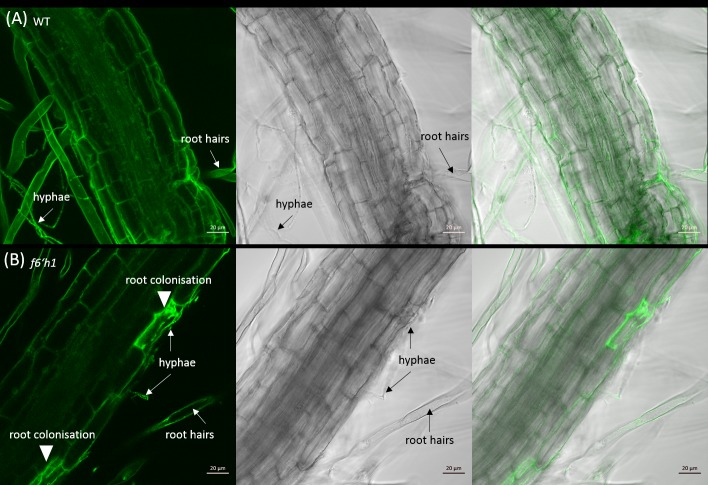
We further analysed the colonisation of Arabidopsis roots by microscopy. Seven days after co-cultivation most of the hyphae associated with WT roots were detected on the root surface between the epidermal and subepidermal root cells and barely detectable within root cells, whereas in f6’h1 mutant roots, hyphae could be detected around and within root cells. This effect was even stronger 14 and 19 days after co-cultivation (Fig 8).
Fig 8. Root colonisation of Arabidopsis WT and f6’h1 by P. indica at 14 dpi.
Arabidopsis WT (A) and f6’h1 (B) were imaged using confocal microscopy. The first pictures show the autofluorescence of the root and GFP fluorescence of P. indica. The second pictures show the bright field image. The last pictures show the overlay of all images for each row.
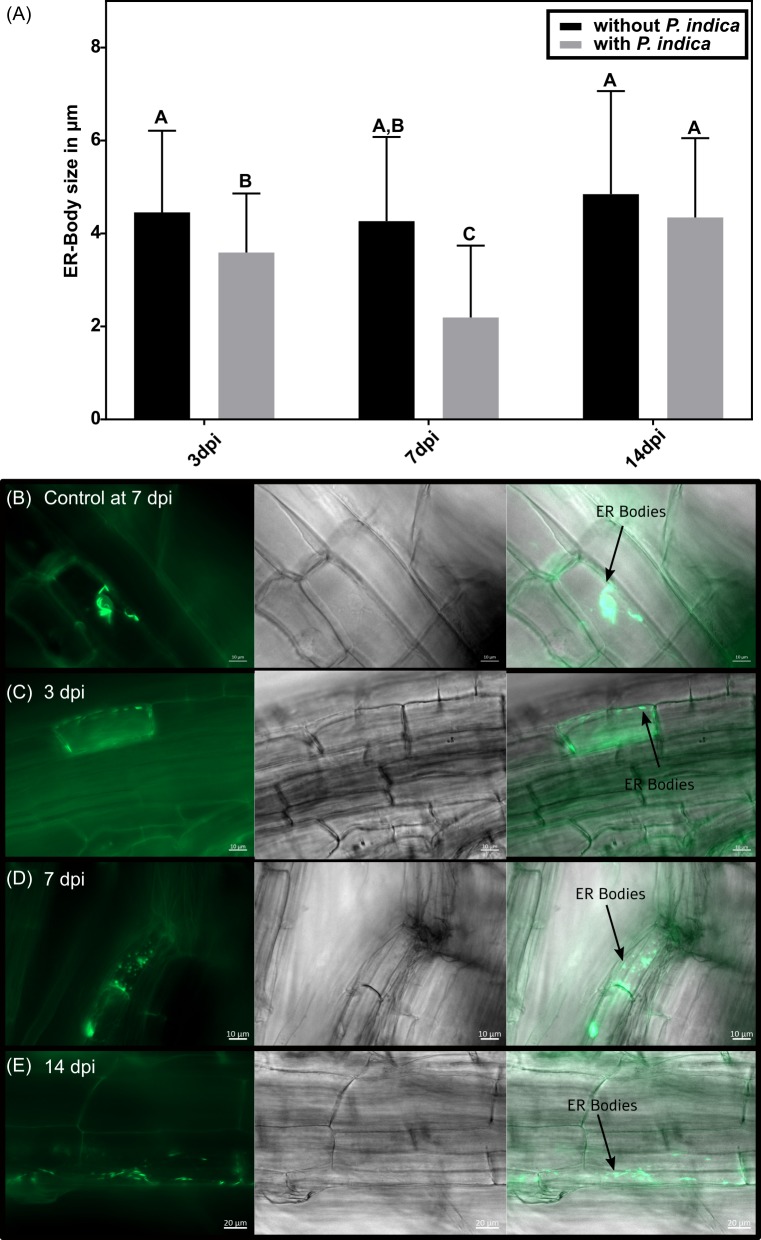
P. indica-root colonisation causes degradation of ER-bodies
The Arabidopsis lipases PLAT1 and -2 are functionally associated and co-localise with ER-bodies in vivo [51]. Thus, we analysed the fate of ER-bodies during the establishment of symbiosis (Fig 9). The size of ER-bodies in uncolonised roots is not altered during the development of the plant but decreases during the interaction with the fungus (Fig 9A). After three days, the ER-bodies in the WT have the normal rod-shaped structure (Fig 9B) but are slightly smaller in the colonised roots (Fig 9C). After seven days, the ER-bodies in the fungal treated roots have a significant reduced size and, moreover, a diffuse signal was observed (Fig 9D). This effect diminishes after two weeks and the size and structure of the ER-bodies was again comparable to the mock treated samples (Fig 9E).
Fig 9. Effect of P. indica on ER-body size.
(A) Comparison of the ER-body length in Arabidopsis root cells without and with P. indica at 3 dpi, 7 dpi and 14 dpi. Error bars represent standard deviation. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction). Per time point and treatment four roots were used and the size of 20–100 ER-bodies per root was measured. (B) Fluorescence microscopy of ER-bodies in A. thaliana root without P. indica at 7 dpi and with P. indica at 3 dpi (C), 7 dpi (D) and 14 dpi (E).
We further determined the size of ER-bodies in the liquid culture at the time point of the secretome study. No difference in structure was observed between the mock treated and colonised roots (S4 Fig). But when comparing the liquid culture with the solid culture, a slight size-reduction was observed for the ER-bodies in untreated roots at 3 dpi (S5 Fig).
Discussion
P. indica alters the secretome of colonised plants
The availability of the complete genome sequence and a system of hydroponic cultivation makes P. indica an interesting model endophyte for studying the molecular basis of beneficial symbiotic interactions. In this study, P. indica and A. thaliana were cultivated alone and together in hydroponic media and the secreted proteins were compared after three days of co-cultivation. This approach allowed us to identify symbiosis-relevant proteins secreted during the early stage of interaction. At the time point of this study, the fungus and the plant have not established a mutualistic symbiosis and the fate of the interaction is still undetermined [12, 38, 81]. Our results demonstrate that 102 plant proteins were differentially enriched the presence of the fungus (S2 Table and Fig 2). The most severe alterations in the level of plant proteins affect biotic and abiotic stress responses, the primary metabolism and mucilage synthesis.
In the single culture, proteins belonging to the primary metabolism (24%), abiotic stress (23%), growth and development (19%) and biotic stress (19%) were present with a similar frequency. On the contrary, in the co-culture proteins involved in biotic stress responses (55%) as well as growth and development (20%) were more abundant. The high proportion of proteins associated with these GO terms is not surprising, as P. indica influences many aspects of plant growth and defence mechanisms [5, 34, 37, 81, 82].
Abiotic stress response was one of the most dominant categories in the single culture but not the co-culture. Interestingly, we identified several proteins associated with cadmium stress in the single culture. It is unclear whether these proteins play a direct role in cadmium stress responses or whether they have additional function, but intriguingly, for tobacco and sunflower it was reported that P. indica alleviate cadmium specific stress [83].
While abiotic stress, such as osmotic stress, can be expected in a liquid cultivation system, we were surprised to find almost no proteins which play a role in this category in the co-culture. As exception, the abiotic stress-related cysteine proteinase RD21A, the Aspartic Protease in Guard Cell 1 and PLAT1 were identified in the co-culture [73]. PLAT1 is induced by ABA and plays a role in abiotic stress response, but our and previous findings indicate an additional role of PLAT1 in plant-microbial interaction [50]. ABA plays a vital role in the interaction of plants with P. indica. It accumulates stage-specific in colonised roots, which is required for successful colonisation [81, 84]. Thus, an altered level of ABA induced by the fungus may be involved in or responsible for the observed differences in PLAT1 accumulation.
Besides PLAT1, we identified 34 differentially regulated proteins with a role in biotic stress. Some of the proteins have already been described in the context of P. indica colonisation before. For example, the polygalacturonase inhibitor 1, which restricts programmed cell death of the host [85], was found in the co-culture. A homologue of this protein was recently identified as a target of P. indica in Chinese cabbage [86]. Another protein from the co-culture, the pectin methylesterase inhibitor At1G23205 is induced by volatiles from a plant growth-promoting Bacillus subtilis strain [87].
In a recent proteome study, Brassica napus was co-cultivated with P. indica. Similar to our study, proteins associated with biotic stress as well as growth were identified. Another investigation showed that a GLP was downregulated in rape by the fungus after 28 days of co-cultivation [88]. In our short-time assay, homologues of this protein, GLP2 and GLP4, were found in the medium of the co-culture. While GLP4 has an important part in controlling Blumeria graminis infection in wheat and barley [89, 90], GLP2 plays a role in root development, possibly by controlling the distribution of carbohydrates between different root parts [91]. These findings are not surprising since the roots are in contact with different fungal cell wall components and the fungus might manipulate the carbohydrate metabolism in roots to stimulate plant growth [92]. Furthermore, the beginning of interaction is characterised by a stressful biotrophic-like phase [81]. At the early time point studied here, the fungus establishes the colonisation, which is accompanied by the balanced regulation of defence compounds. Interestingly, we found that the salicylic acid level is reduced while jasmonate, indolic glucosinolates and flavonoids are increased [12, 37]. Apparently, after 3 days of co-culture, jasmonate-dependent defence strategies dominate in the co-culture which further support the concept that a beneficial interaction phase has not yet been reached.
Another observation was that proteins of the primary metabolism were found predominantly in the single culture and not the co-culture (24% and 7%, respectively). Since secreted proteins were analysed, this observation is difficult to explain. Based on the results discussed above, one would expect that the fungus represses the primary metabolism to shift metabolites towards defence during the early time point investigated in this study. Under this assumption the amount of both cellular and exudated proteins involved in the plant primary metabolism could be reduced when P. indica is present. The strong reduction of these proteins in the exudate preparations might be due to their easier accessibility for fungal proteases. The high number of putative degrading enzymes in the P. indica secretome supports this hypothesis. However, whether plant proteins involved in the primary metabolism are specifically degraded, is unclear. An interpretation of these results requires more information about the targets of the fungal proteases, which is still poor.
We also found proteins associated with mucilage in the media of the different cultures. Recent evidence points to mucilage function in seed germination, root tip growth and the formation of arbuscular mycorrhiza [93–95]. The importance of mucilage for arbuscular mycorrhiza has been shown for several plant symbioses including the interaction of maize roots with Gigaspora gigantea in which secreted plant components induce hyphal branching [96]. The function of mucilage during the interaction of Arabidopsis with microbes, such as P. indica, has not been studied before even though mucilage was investigated in Arabidopsis seeds [73]. Proteins associated with mucilage were identified in the single culture, in the co-culture or in both treatments (S2 Table). For example, the subtilisin-like protease 1.7 (SBT1.7) was present in both cultures (S1 Table), while its homologue SBT1.8 was only detected in the single culture. These quite specific effects suggest that the mucilage-related proteins have specific functions in the symbiosis and are differentially regulated by the fungus. Barely anything is known about the role of mucilage in controlling hyphae entry into root cells, the regulation of plant genes and proteins involved in their synthesis, although it is obvious that they might be an important barrier for fungal invasion, besides other functions which can be envisioned in symbiotic interactions cf. [93–96].
Secretome from P. indica
Even though the genome of P. indica is sequenced, the function of many proteins is still unknown. In our study, most of the differentially regulated proteins were found in the medium of the co-culture. For one of them (PIIN_04899, a putative indole-3-acetaldehyde dehydrogenase), Hilbert and colleagues (2012) showed an induction of this gene by arabinose, a cell wall component of A. thaliana [97, 98]. For another protein (PIIN_04899), a homologous protein from the Pseudomonas syringae strain DC3000 was described. This protein produces indole acetic acid (IAA) via indole-3-acetaldehyde dehydrogenase (ALDA) that catalyses the formation of IAA from indole-3-acetaldehyde. Disruption of aldA and a close homolog, aldB, leads to reduced IAA production and virulence of DC3000 on A. thaliana. McClerklin and colleagues (2017) further showed that the IAA produced by this pathway in DC3000 suppresses salicylic acid-mediated defences in Arabidopsis [99]. P. indica can produce IAA in vitro and its growth promoting effect in Arabidopsis can be imitated by this hormone [79]. In the interaction of P. indica with barley, IAA is required to establish a symbiotic interaction, but not for the growth promotion effect [97]. Hence, the identified fungal protein might trigger the IAA synthesis and, in turn, confer beneficial traits to Arabidopsis.
Role of PLAT1, ER-bodies and scopolin in the colonisation of P. indica
We found PLAT1 in the supernatant of the co-culture. PLAT1 functions as positive regulator of abiotic stress tolerance and promotes plant growth [51]. Furthermore, PLAT1 expression is regulated by the ABA-dependent transcription factors 1–4 [51] which prompted us to have a closer look at the function of the protein in the interaction. In the beginning of the colonisation the expression of this gene is reduced in colonised roots; in confirmation with this results plat1 mutants are stronger colonised by the fungus after seven days. After two weeks, no difference to the wild-type can be detected. PLAT domains are found in different membrane- or lipid-associated proteins [52, 100–103] and exhibit similarity to the C2 domain involved in protein–protein and protein-membrane interactions [52, 104]. Therefore, it has been hypothesised that the PLAT domain provides a docking platform for proteins to regulate their catalytic activities and that the proteins per se do not have enzymatic activities [51–53]. Hyun and colleagues (2014) further observed a co-localisation of PLAT1 with ER-bodies [51]. Since PYK10, which is found in ER-bodies, is involved in the establishment of symbiosis between P. indica and A. thaliana [43], one could speculate that besides PYK10, also PLAT1 might play an important role, by regulating defence processes during early phases of the symbiotic interaction. This is supported by the stronger colonisation of plat1 mutants seven days after colonisation and by the altered level of scopolin in plat1 roots. Furthermore, the scopolin mutant f6’h1 plants are stronger colonised by the fungus. This indicates that F6’H1 or its product scopolin restricts the entry of P. indica hyphae into roots cells. In agreement with these results, scopolin inhibits growth of P. indica and other fungi in vitro [39]. Downregulation of the plat1 mRNA in the infected roots seem to be associated with the observed degradation of the ER-bodies, whereas it is not clear whether the expression of PLAT1 in the nucleus or the ER-bodies in the cytoplasm, or both are the primary target of the fungus in Arabidopsis roots, and whether both effects are directly connected to each other. The appearance of the PLAT1 protein in the exudate fraction might be caused by the disruption of cells, which occurs when the ER-bodies become gradually smaller due to the activation of defence processes against P. indica. Ultimately, smaller ER-bodies in colonised roots might restrict the liberation of defence compounds such as scopolin from its precursor scopoletin, allowing a more efficient colonisation of roots by the fungus.
Apparently, the early phase of interaction between A. thaliana and P. indica includes an arms-race in which P. indica tries to restrict the plant defence by reducing the amount and function of ER-bodies. At later time points, when a beneficial interaction starts and the fungus is recognized as a friend, the defence compounds PLAT1 and scopolin appear to be less important. These processes can be triggered by altered phytohormone levels. For example, Camehl and colleagues (2010) showed that ethylene can inhibit the fungus and is required for balancing the interaction between P. indica and A. thaliana [104]. Other defence-related phytohormones have been reported to be activated during different phases of the symbiotic interaction. Although they may explain alterations in the expression of genes involved in scopolin/scopoletin biosynthesis after exposure of the roots to P. indica, the role of PLAT1 and the coumarins in this scenario require more investigation.
Conclusion
In this study, we analysed the secretion of proteins during the early interaction between A. thaliana and P. indica. The identified fungal proteins demonstrate that root colonisation results in an almost complete alteration in the fungal exudation profile. Unfortunately, barely anything is known about the function of the secreted fungal proteins, making it difficult to draw meaningful conclusions.
Several of the identified Arabidopsis proteins are differentially regulated. They function in defence and might be required to restrict entry of the hyphae into the host cells. This includes remorin 3, germin like protein 4, osmotin like protein 34 or pathogenesis-related protein 5. For other defence-related proteins, a clear function has not yet been described. Our analysis demonstrated that multiple peroxidases and proteins with a role in growth processes, such as patellin 1 and 2 as wells lipid transfer protein 8, were influenced by the fungus. Furthermore, proteins related to mucilage formation, such as RD21A, RD21B or aspartic protease 1, respond to the fungus. The quite unique regulation of the individual mucilage-related proteins in response to the fungus suggests that they play an important and so far, little investigated role, and that symbiosis-specific signals manipulate mucilage formation.
The establishment of symbiosis requires a reprograming of fungal and plant proteins. Benefits for the host only become visible after successful root colonisation and the recognition of the microbe as mutualistic symbiont. Several identified proteins in the single and co-culture suggest that this phase has not yet been reached three days after infection, and that our analyses was performed at a time point when the arms-race between the two symbionts is ongoing.
ER-bodies are involved in defence and the degradation of ER-bodies in response to fungal colonisation might play a role in balancing defence and growth responses. The downregulation of plat1 by the fungus seems to cause a downregulation of the defence compound scopolin and thus allowing a better colonisation. However, other enzymes and proteins with not well-defined functions are present in the ER-bodies and lower PLAT1 and scopolin levels after root colonisation may only be one aspect in the arms-race that involves ER-body functions.
Methods and material
Cultivation of A. thaliana and P. indica
Cultivation of A. thaliana and P. indica was performed as described before [105]. Briefly, A. thaliana wild type (Col-0) and mutant seeds (SALK_112728c for plat1, cs69080 for GFP-labelled ER-bodies and SALK_132418C for f6’h1) were surface-sterilised with a solution containing sterile distilled water (dH2O), sodium lauroyl sarcosinate and Clorix (64%, 4%, 32%; v/v/v) for eight minutes under constant shaking, followed by six rinses with dH2O. Surface-sterile seeds were sown on a 0.75% (w/v) agar plate. P. indica (P. indica-JE1) was cultured as described on solid Kaefer medium (KM) (1% (w/v) agar). Arabidopsis seeds were obtained from the Nottingham Arabidopsis Stock Centre [106].
Cultivation of A. thaliana with P. indica for secretome study
Seeds were planted on MS medium (Murashige and Skoog) [107] in Petri dishes closed with micropore band. After vernalisation at 4°C for two days the plates were kept under an 8 h light (60 μmol photons*m- 2*s- 1)/ 16 h dark period with a constant temperature of 21°C for 12 days. For the experiment ten petri dishes were filled with 20 ml liquid PNM without sugar for each treatment. For the P. indica single culture and the co-culture, a fungal plaque with 1 cm diameter was added to the plates and cultivated at room temperature for three days, whereas for the single A. thaliana culture and the mock control (medium without fungus or plant) a KM plaque with the same diameter was added. After three days, four plants were added to the petri dishes of the A. thaliana single culture and the co-culture. To the P. indica single culture and the mock control, a plaque of MS media was added instead. The mock treatment was performed to check for contaminations. After three days, the supernatant was collected and used for the solid phase extraction.
Co-cultivation of A. thaliana with P. indica for expression and growth promotion studies
For the gene expression study, A. thaliana and P. indica were cultivated as described before [105]. A plaque with one cm diameter of P. indica and KM, respectively, was transferred to petri dishes with PNM covered by a sterilised nylon membrane. After seven days, four twelve-day old seedlings were transferred to the fungal plate and the control plate, respectively. For all experiments, WT and mutant plants were grown in parallel. Roots and leaves were harvested and snap frozen in liquid nitrogen prior further analysis.
Analysis of secreted proteins
Solid phase extraction
Supernatant of liquid spent culture medium was filtered (0.22 μm PES (polyethersulfone) Corning bottle top filter Sigma-Aldrich, Munich, Germany) and treated with a protease inhibitor cocktail (1 tablet cOmplete Ultra, Mini, EDTA (ethylenediaminetetraacetic acid)-free, Easypack (Roche, Basel, Switzerland) per 100 ml medium). Trifluoroacetic acid (TFA) was added to gain a final concentration of 0.1% TFA. Chromabond C4 SPE (solid phase extraction) cartridges (Macherey-Nagel, Düren, Germany) were reconstituted with 3 ml acetonitrile (ACN) and 3 ml 0.1% TFA. Each 20 ml spent medium of 10 biological replicates of plant, fungus, co-culture or mock samples were aspirated (vacuum-driven) through the SPE cartridges. Subsequently, the cartridges were washed with 3 ml 5% MeOH (methanol), 0.1% TFA, and the enriched proteins were eluted from the C4 resin with 3 ml 0.1% TFA in ACN/H2O (80%/ 20%, v/v). The eluates were freeze-dried in a Christ alpha 2–4 lyophilizer (Christ, Osterode am Harz, Germany). The lyophilised powder was resolubilised in 100 μl denaturation buffer, i.e. 50 mM TEAB (triethylammonium bicarbonate) in 50/50 trifluoroethanol (TFE)/H2O (v/v) by ultrasonification in the water bath for 15 min and heat treatment at 90°C for 10 min. For reduction of oxidised cysteine thiol residues 4 μl 500 mM TCEP (tris(2-carboxyethyl)phosphine) in 100 mM TEAB was added for 1 h incubation at 55°C. Cysteine thiol alkylation was performed by addition of 4 μl 625 mM iodoacetamide in 100 mM TEAB and incubation at room temperature in the dark for 30 min. Proteins were cleaned-up by MeOH/H2O/chloroform precipitation using the protocol of Wessel and Flügge (1984) [108] and the precipitate was resolubilised in 100 μl of 100 mM TEAB by 15 min ultrasonic bath treatment. Total protein concentration was determined by the Merck Millipore Direct Detect system (Merck, Darmstadt, Germany) according to the manufacturer’s instructions. Trypsin and LysC protease mix (Promega Cat. # V5072) was added to the samples at a ratio of 1:25 protease mix to protein. Proteins were digested for 18 h at 37°C. Reactions were stopped by addition of 10 μl 10% HCOOH, and the samples were dried in a SpeedVac (Thermo Fisher Scientific, Waltham, MA, USA). Tryptic peptides were resolubilised in 25 μl 0.05% TFA, 2% ACN by 15 min ultrasonic bath treatment. Finally, samples were filtered through 10 kDa PES molecular weight cut-off centrifugal filters (VWR International, Radnor, PA, USA) for 15 min at 16000 g transferred into HPLC (high-performance liquid chromatography) vials and stored at -80°C until LC-MS/MS analysis.
LC-MS/MS analysis
LC-MS/MS analysis was carried out on an Ultimate 3000 RSLC Nano system coupled to a QExactive HF mass spectrometer (both Thermo Fisher Scientific). Peptides were enriched online using a nano trap column (Acclaim Pep Map 100, 2 cm x 75 μm, 3 μm, Thermo Fisher) at a flow rate of 5 μl/min. Further peptide separation on an analytical column was performed on an Acclaim Pep Map RSLC nano column (50 cm x 75 μm, 2μm) (Thermo Fisher Scientific). The mobile phase consisted of eluent (A) 0.1% (v/v) formic acid in H2O and eluent (B) 0.1% (v/v) formic acid in ACN/H2O (90%/ 10%, v/v). Gradient elution was performed as follows: 0 min at 4% B, 5 min at 6% B, 25 min at 8% B, 65 min at 20% B, 80 min at 30% B, 90 min at 50% B, 95–100 min at 96% B, 100.1–120 min at 4% B.
A stainless-steel emitter in the Nanospray Flex Ion Source (Thermo Fisher Scientific) was used to generate positively charged ions at a spray voltage of 2.2 kV. The quadrupole/orbitrap instrument was operated in Full MS / data-dependent MS2 (Top15) mode. Precursor ions were monitored at m/z 300–1500 at a resolution of 120k FWHM using a maximum injection time (ITmax) of 100 ms and an AGC (automatic gain control) target of 1e6. HCD fragmentation at 30% normalised collision energy (NCE) generated MS2 ions, which were scanned at 15k FWHM using a maximum ITmax of 100 ms and an AGC target of 2e5. Dynamic exclusion of precursor ions was set to 30 s. The LC-MS/MS instrument was controlled by Chromeleon 7.2, QExactive HF Tune 2.8 and Xcalibur 4.0 software (Thermo Fisher Scientific).
Protein database search
Tandem mass spectra were searched against the UniProt database of A. thaliana and P. indica (2017/02/05) using Proteome Discoverer (PD) 2.1 (Thermo Fisher Scientific) and the algorithms of Mascot 2.4 (Matrix Science, London, UK), Sequest HT (integral version of PD 2.1) and MS Amanda 1.0. Two missed cleavages were allowed for trypsin digestion. The precursor mass tolerance was set to 10 ppm and the fragment mass tolerance was set to 0.02 Da. Modifications were defined as dynamic methionine oxidation and static cystheine carbamidomethylation. At least 2 peptides per protein and a strict false discovery rate (FDR) < 1% were required for positive protein hits. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [109] partner repository with the dataset identifier PXD009563.
For the GO-Term analysis, all identified proteins were searched against the GO consortium database using AmiGO (http://www.geneontology.org, version 1.8) [55–57]. The frequency (in %) of a specific GO-Term was determined individually for each treatment and biological replicate. The mean frequency for the different treatments was calculated and the 20 most abundant terms were used for further analysis.
The algorithms TargetP 1.1 (http://www.cbs.dtu.dk/services/TargetP/), SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) and Predotar 1.3 (https://urgi.versailles.inra.fr/Tools/Predotar) were used for predicting subcellular localisation [76, 77].
Additional analysis
Gene expression studies
The co-culture of P. indica and A. thaliana was performed as described before [105]. Roots and leaves were collected for RNA extraction and cDNA (complementary Deoxyribonucleic acid) synthesis followed by RT-qPCR (reverse transcriptase quantitative polymerase chain reaction) as described in [81]. All experiments were repeated in three to four biological and three technical replicates. Primers are given in S3 Table. The efficiency of expression was manually determined by LinRegPCR 2014 [110] followed by the ΔΔ Ct (threshold cyclce) method [111]. Expression levels of genes were normalised by glyceraldehyde 3-phosphate dehydrogenase (GAPDH), whereas the relative normalised expression depends on the respective control as stated in the figures.
Microscopy
Three, seven and 14 days after infection of A. thaliana with GFP-labelled P. indica (gift from Prof. P. Schäfer, University Warwick) root colonisation was imaged using an LSM (laser scanning microscope) 880 (Zeiss Microscopy GmbH, Jena, Germany), with the 488 nm laser line of an argon multiline laser. Images were taken with a 40x objective (Plan-Apochromat 40x/0.8). Pictures for quantification of ER-bodies were taken with an Axiolmager M2 microscope (Zeiss). Digital images were processed using ZEN (ZEISS Efficient Navigation) software.
Extraction of scopolin/ scopoletin from plant material for HPLC analysis
The procedure is based on Döll [112]. For 100 mg frozen plant material, 400 μl methanol and 2mM trans-cinnamic acid (Sigma-Aldrich) per sample as internal standard was added and homogenized for 60 seconds at full speed by an electric homogenizer (RZR 2102 Control (Heidolph, Schwabach, Germany). The samples were transferred to a centrifuge tube and centrifuged at 15.000 rpm and 4°C for 10 min. The supernatant was transferred to a new tube and the pellet resolved in 400 μl methanol. After a second centrifugation step, the supernatants of identical samples were mixed and centrifuged again for 10 min. Finally, the samples were filtered through a 20 μm filter into a glass vial.
HPLC measurement
For the measurement a JASCO HPLC 900 system (JASCO Germany GmbH, Gross-Umstadt, Germany) was used. It was equipped with a DG-2080-53 degaser, a LG-980-02 solvent mixer, a PU-980 vacuum pump, an AS-950-10 autosampler and a MD-910 multiwave length detector. We used C18 column (Kromasil C18 HPLC column 5μm particle size, pore size 100 Å, length 150 mm, inner diameter 4.6 mm, K08670356, Sigma-Aldrich) at 20°C with a flowrate of 0.6 ml/min. The device was coupled via a JASCO LC-Net II/ADC interface with the computer controlled by ChromPass/Galaxie 1.10.0.5590 from JASCO. Purging was performed for 1 min at a flow rate of 5 ml/min with 0.1% (v/v) formic acid in acetonitrile. This was followed by degassing with 0.1% (v/v) formic acid in water for 1 min at the same flowrate. The column was washed with 0.1% (v/v) formic acid in acetonitrile for ten minutes. For equilibration 2% (v/v) acetonitrile + 0.1% (v/v) formic acid in 98% (v/v) water + 0.1% formic acid (v/v) was used until constant pressure was reached. 10 μl of sample were injected and eluted over 27 min. In the beginning the acetonitrile content in the mobile phase was 2% (v/v) increasing to 100% over 15 min kept at 100% for 5 min, decreased to 2% over 1 min and kept for 6 min at 2%. As an authentic standard 10 μl of 0.1 mM scopolin and 10 μl of 0.625 mM scopoletin (Phytolab, Vestenbergsgreuth, Germany) were used. The amount of scopolin and scopoletin in the sample was determined by the peak area normalised with the peak for trans-cinnamic acid (Sigma-Aldrich).
Growth inhibition experiment of P. indica by scopoletin and scopolin
The procedure was performed as described by [113]. Scopolin and scopoletin was purchased from Sigma-Aldrich and diluted in methanol. Scopolin, scopoletin and methanol (for the control) were added to six-well plates. After evaporation of the solvent, the wells were filled with 1.5 ml liquid KM medium. 4.5x105 fungal spores were added per well. After 11 days of growth, P. indica was harvested and dried (60°C, overnight) to determine the dry weight for calculation of the inhibition of fungal growth in %. Bars represent standard deviation. The experiment was repeated in three biological replicates.
Statistics and data deposition
All statistic was performed by Sigma Plot 13.0.083 (Systat Software, Erkrath, Germany). Details are given in the figure legends.
The MS proteomics data in this paper have been deposited via ProteomeXchange with identifier PXD009563.
Supporting information
Selected proteins were identified by at least two unique peptides and a false discovery rate of <1%. The frequency of occurrence is given as Low (1 out of 3), Medium (2 out of 3) or High (3 out of 3 replicates) based on the frequency of detection in all replicates.
(XLSX)
Identified proteins were considered differentially enriched, when they were found in at least two replicates in one treatment and not in the other treatment. Furthermore, proteins that were found with a high frequency in one treatment and with a low frequency in the other treatment, were considered differentially secreted as well. Information about the function was obtained using the BLAST algorithm for homology searches against the UniProt database [54, 114]. The location was predicted using TargetP, SignalP and Predotar algorithms.
(XLSX)
(XLSX)
Arabidopsis roots in liquid (A) and solid media (B) were imaged using fluorescence microscopy. In both cultures, only little fungus was found in and around the root. The first pictures show the autofluorescence of the root and GFP fluorescence of P. indica. The second pictures show the bright field image. The last pictures show the overlay of all images for each row. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method.
(TIF)
(A) Venn diagram of A. thaliana proteins found in three replicates in the co-culture (generated with http://bioinformatics.psb.ugent.be/webtools/Venn/). (B) Profile for plat2 expression in WT roots and leaves. (C) Profile for plat3 expression in WT roots and leaves. For plat3, CT values were extremely low around 35–40 whereas for all other genes CT values were between 25 and 35. (D) Primer used in this study for RT-qPCR.
Dashed line represents the expression in the untreated WT plants. Error bars represent standard deviation calculated from four biological replicates. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction).
(TIF)
Expression of PYK10 and other key genes of the scopolin pathway in roots of WT and F6’H1 at 7 dpi (A) and 14 dpi (B). Error bars represent standard deviation calculated from four biological replicates. The asterisks shows the significant difference, as described in Fig 3A.
(TIF)
Fluorescence microscopy of ER-bodies in roots without and with P. indica. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method. Mock treatment in liquid culture (A) and in solid culture (B) and colonised roots at 3 dpi in liquid culture (C) and in solid culture (D) are shown. The first pictures show the autofluorescence of the root ER-Bodies. The second pictures show the bright field image. The last pictures show the overlay of all images for each row. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method.
(TIF)
Comparison of the ER-body length in Arabidopsis root cells without and with P. indica at 3 dpi. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method. Error bars represents standard deviation. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction). Per time point and treatment four roots were used and the size of 20–100 ER-Bodies per root was measured.
(TIF)
Acknowledgments
We would like to thank Anatoli Ludwig, Claudia Röppischer and Sarah Mußbach for their technical assistance during the cultivation of A. thaliana and P. indica
Data Availability
All relevant data are within the paper and its Supporting Information files with the exception of the mass spectrometry proteomics data. They have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD009563 (Reviewer account details: Username: reviewer95832@ebi.ac.uk; Password: zHk3U002).
Funding Statement
This research was funded by Deutsche Forschungsgemeinschaft (CRC1127 – ChemBioSys to JT, AB, and RO), (CRC/Transregio 124 – FungiNet (project Z2) to TK and OK), and the Jena School of Microbial Communication. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wang B, Qiu Y-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza. 2006; 16: 299–363. 10.1007/s00572-005-0033-6 [DOI] [PubMed] [Google Scholar]
- 2.Johnson NC, Graham Jh, Smith Fa. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997; 135: 575–585. 10.1046/j.1469-8137.1997.00729.x [DOI] [Google Scholar]
- 3.Bonfante P, Genre A. Arbuscular mycorrhizal dialogues: do you speak 'plantish' or 'fungish'. Trends Plant Sci. 2015; 20: 150–154. 10.1016/j.tplants.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Lanfranco L, Fiorilli V, Gutjahr C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol. 2018. 10.1111/nph.15230 [DOI] [PubMed] [Google Scholar]
- 5.Zuccaro A, Lahrmann U, Güldener U, Langen G, Pfiffi S, Biedenkopf D, et al. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 2011; 7: e1002290 10.1371/journal.ppat.1002290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellegrin C, Morin E, Martin FM, Veneault-Fourrey C. Comparative analysis of secretomes from ectomycorrhizal fungi with an emphasis on small-secreted proteins. Front Microbiol. 2015; 6: 1278 10.3389/fmicb.2015.01278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krijger J-J, Thon MR, Deising HB, Wirsel SGR. Compositions of fungal secretomes indicate a greater impact of phylogenetic history than lifestyle adaptation. BMC Genomics. 2014; 15: 722 10.1186/1471-2164-15-722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Wit, Pierre J G M, Mehrabi R, Van den Burg, Harrold A, Stergiopoulos I. Fungal effector proteins: past, present and future. Mol Plant Pathol. 2009; 10: 735–747. 10.1111/j.1364-3703.2009.00591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Fernandez R, Jorrin-Novo JV. Contribution of proteomics to the study of plant pathogenic fungi. J Proteome Res. 2012; 11: 3–16. 10.1021/pr200873p [DOI] [PubMed] [Google Scholar]
- 10.Win J, Chaparro-Garcia A, Belhaj K, Saunders DGO, Yoshida K, Dong S, et al. Effector biology of plant-associated organisms: concepts and perspectives. Cold Spring Harb Symp Quant Biol. 2012; 77: 235–247. 10.1101/sqb.2012.77.015933 [DOI] [PubMed] [Google Scholar]
- 11.Kaffarnik, Florian A R, Jones, Alexandra M E, Rathjen JP, Peck SC. Effector proteins of the bacterial pathogen Pseudomonas syringae alter the extracellular proteome of the host plant, Arabidopsis thaliana. Mol. Cell Proteomics. 2009; 8: 145–156. 10.1074/mcp.M800043-MCP200 [DOI] [PubMed] [Google Scholar]
- 12.Strehmel N, Mönchgesang S, Herklotz S, Krüger S, Ziegler J, Scheel D. Piriformospora indica stimulates root metabolism of Arabidopsis thaliana. Int J Mol Sci. 2016; 17 10.3390/ijms17071091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippmann R, Kaspar S, Rutten T, Melzer M, Kumlehn J, Matros A, et al. Protein and metabolite analysis reveals permanent induction of stress defense and cell regeneration processes in a tobacco cell suspension culture. Int J Mol Sci. 2009; 10: 3012–3032. 10.3390/ijms10073012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogueira-Lopez G, Greenwood DR, Middleditch M, Winefield C, Eaton C, Steyaert JM, et al. The apoplastic secretome of Trichoderma virens during interaction with maize roots shows an inhibition of plant defence and scavenging oxidative stress secreted proteins. Front Plant Sci. 2018; 9: 409 10.3389/fpls.2018.00409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiapello M, Perotto S, Balestrini R. Symbiotic Proteomics—State of the art in plant–mycorrhizal fungi interactions. In: Magdeldin S, editor. Recent Advances in Proteomics Research. 2015. 10.5772/61331 [DOI] [Google Scholar]
- 16.Kloppholz S, Kuhn H, Requena N. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr. Biol. 2011; 21: 1204–1209. 10.1016/j.cub.2011.06.044 [DOI] [PubMed] [Google Scholar]
- 17.Plett JM, Kemppainen M, Kale SD, Kohler A, Legué V, Brun A, et al. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 2011; 21: 1197–1203. 10.1016/j.cub.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 18.Plett JM, Daguerre Y, Wittulsky S, Vayssières A, Deveau A, Melton SJ, et al. Effector MISSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proc. Natl. Acad. Sci. U.S.A. 2014; 111: 8299–8304. 10.1073/pnas.1322671111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lum G, VanBuren R, Ming R, Min XJ. Secretome prediction and analysis in Sacred Lotus (Nelumbo nucifera Gaertn.). Tropical Plant Biol. 2013; 6: 131–137. 10.1007/s12042-013-9121-5 [DOI] [Google Scholar]
- 20.Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000; 408: 796–815. 10.1038/35048692 [DOI] [PubMed] [Google Scholar]
- 21.Krause C, Richter S, Knöll C, Jürgens G. Plant secretome—from cellular process to biological activity. Biochim. Biophys. Acta. 2013; 1834: 2429–2441. 10.1016/j.bbapap.2013.03.024 [DOI] [PubMed] [Google Scholar]
- 22.Agrawal GK, Jwa N-S, Lebrun M-H, Job D, Rakwal R. Plant secretome: unlocking secrets of the secreted proteins. Proteomics. 2010; 10: 799–827. 10.1002/pmic.200900514 [DOI] [PubMed] [Google Scholar]
- 23.Patnaik D, Khurana P. Germins and germin like proteins: an overview. Indian J Exp Biol. 2001; 39: 191–200. [PubMed] [Google Scholar]
- 24.El-Sharkawy I, Mila I, Bouzayen M, Jayasankar S. Regulation of two germin-like protein genes during plum fruit development. J. Exp. Bot. 2010; 61: 1761–1770. 10.1093/jxb/erq043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyawaki K, Tabata R, Sawa S. Evolutionarily conserved Cle peptide signaling in plant development, symbiosis, and parasitism. Curr. Opin. Plant Biol. 2013; 16: 598–606. 10.1016/j.pbi.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 26.Mortier V, Den Herder G, Whitford R, van de Velde W, Rombauts S, D'Haeseleer K, et al. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 2010; 153: 222–237. 10.1104/pp.110.153718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assis RdAB, Polloni LC, Patané JSL, Thakur S, Felestrino ÉB, Diaz-Caballero J, et al. Identification and analysis of seven effector protein families with different adaptive and evolutionary histories in plant-associated members of the Xanthomonadaceae. Sci. Rep. 2017; 7: 16133 10.1038/s41598-017-16325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tawk C, Sharan M, Eulalio A, Vogel J. A systematic analysis of the RNA-targeting potential of secreted bacterial effector proteins. Sci. Rep. 2017; 7: 9328 10.1038/s41598-017-09527-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG, et al. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004; 16: 672–693. 10.1105/tpc.019703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Ding Y, Wang J, Hillmer S, Miao Y, Lo SW, et al. Expo, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell. 2010; 22: 4009–4030. 10.1105/tpc.110.080697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Floerl S, Majcherczyk A, Possienke M, Feussner K, Tappe H, Gatz C, et al. Verticillium longisporum infection affects the leaf apoplastic proteome, metabolome, and cell wall properties in Arabidopsis thaliana. PLoS ONE. 2012; 7: e31435 10.1371/journal.pone.0031435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng F-y, Blackburn K, Lin Y-m, Goshe MB, Williamson JD. Absolute protein quantification by LC/MS(E) for global analysis of salicylic acid-induced plant protein secretion responses. J Proteome Res. 2009; 8: 82–93. 10.1021/pr800649s [DOI] [PubMed] [Google Scholar]
- 33.Verma S, Varma A, Rexer K-H, Hassel A, Kost G, Sarbhoy A, et al. Piriformospora indica, gen. et sp. nov., a new root-colonizing Fungus. Mycologia. 1998; 90: 896 10.2307/3761331 [DOI] [Google Scholar]
- 34.Sun C, Shao Y, Vahabi K, Lu J, Bhattacharya S, Dong S, et al. The beneficial fungus Piriformospora indica protects Arabidopsis from Verticillium dahliae infection by downregulation plant defense responses. BMC plant biology. 2014; 14: 268 10.1186/s12870-014-0268-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdelaziz ME, Kim D, Ali S, Fedoroff NV, Al-Babili S. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na(+)/K(+) homeostasis under salt stress conditions. Plant Sci. 2017; 263: 107–115. 10.1016/j.plantsci.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 36.Bakshi M, Sherameti I, Meichsner D, Thürich J, Varma A, Johri AK, et al. Piriformospora indica reprograms gene expression in Arabidopsis phosphate metabolism mutants but does not compensate for phosphate limitation. Front Microbiol. 2017; 8: 1262 10.3389/fmicb.2017.01262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahrmann U, Strehmel N, Langen G, Frerigmann H, Leson L, Ding Y, et al. Mutualistic root endophytism is not associated with the reduction of saprotrophic traits and requires a noncompromised plant innate immunity. New Phytol. 2015. 10.1111/nph.13411 [DOI] [PubMed] [Google Scholar]
- 38.Lahrmann U, Zuccaro A. Opprimo ergo sum—evasion and suppression in the root endophytic fungus Piriformospora indica. Molecular plant-microbe interactions: MPMI. 2012; 25: 727–737. 10.1094/MPMI-11-11-0291 [DOI] [PubMed] [Google Scholar]
- 39.Nakano RT, Yamada K, Bednarek P, Nishimura M, Hara-Nishimura I. ER bodies in plants of the Brassicales order: biogenesis and association with innate immunity. Front Plant Sci. 2014; 5: 73 10.3389/fpls.2014.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunning BES. The identity of mystery organelles in Arabidopsis plants expressing GFP. Trends Plant Sci. 1998; 3: 417 10.1016/S1360-1385(98)01336-3 [DOI] [PubMed] [Google Scholar]
- 41.Hayashi Y. A Proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant and Cell Physiology. 2001; 42: 894–899. 10.1093/pcp/pce144 [DOI] [PubMed] [Google Scholar]
- 42.Nagano AJ, Matsushima R, Hara-Nishimura I. Activation of an ER-body-localized beta-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol. 2005; 46: 1140–1148. 10.1093/pcp/pci126 [DOI] [PubMed] [Google Scholar]
- 43.Sherameti I, Venus Y, Drzewiecki C, Tripathi S, Dan VM, Nitz I, et al. PYK10, a beta-glucosidase located in the endoplasmatic reticulum, is crucial for the beneficial interaction between Arabidopsis thaliana and the endophytic fungus Piriformospora indica. The Plant journal: for cell and molecular biology. 2008; 54: 428–439. 10.1111/j.1365-313X.2008.03424.x [DOI] [PubMed] [Google Scholar]
- 44.Nakano RT, Piślewska-Bednarek M, Yamada K, Edger PP, Miyahara M, Kondo M, et al. PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana. Plant J. 2017; 89: 204–220. 10.1111/tpj.13377 [DOI] [PubMed] [Google Scholar]
- 45.Ahn YO, Shimizu B-i, Sakata K, Gantulga D, Zhou C, Zhou Z, et al. Scopolin-hydrolyzing beta-glucosidases in roots of Arabidopsis. Plant Cell Physiol. 2010; 51: 132–143. 10.1093/pcp/pcp174 [DOI] [PubMed] [Google Scholar]
- 46.Kai K, Mizutani M, Kawamura N, Yamamoto R, Tamai M, Yamaguchi H, et al. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl COA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. 2008; 55: 989–999. 10.1111/j.1365-313X.2008.03568.x [DOI] [PubMed] [Google Scholar]
- 47.Filipský T, Říha M, Macáková K, Anzenbacherová E, Karlíčková J, Mladěnka P. Antioxidant effects of coumarins include direct radical scavenging, metal chelation and inhibition of ROS-producing enzymes. Curr Top Med Chem. 2015; 15: 415–431. [DOI] [PubMed] [Google Scholar]
- 48.Maier W, Schmidt J, Nimtz M, Wray V, Strack D. Secondary products in mycorrhizal roots of tobacco and tomato. Phytochemistry. 2000; 54: 473–479. 10.1016/S0031-9422(00)00047-9 [DOI] [PubMed] [Google Scholar]
- 49.Sun H, Wang L, Zhang B, Ma J, Hettenhausen C, Cao G, et al. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot. 2014; 65: 4305–4315. 10.1093/jxb/eru203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyun TK, Albacete A, van der Graaff E, Eom SH, Grosskinsky DK, Bohm H, et al. The Arabidopsis PLAT domain protein1 promotes abiotic stress tolerance and growth in tobacco. Transgenic Res. 2015; 24: 651–663. 10.1007/s11248-015-9868-6 [DOI] [PubMed] [Google Scholar]
- 51.Hyun TK, van der Graaff E, Albacete A, Eom SH, Grosskinsky DK, Bohm H, et al. The Arabidopsis PLAT domain protein1 is critically involved in abiotic stress tolerance. PLoS ONE. 2014; 9: e112946 10.1371/journal.pone.0112946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bateman A, Sandford R. The PLAT domain. A new piece in the PKD1 puzzle. Current Biology. 1999; 9: R588–S2. 10.1016/S0960-9822(99)80380-7 [DOI] [PubMed] [Google Scholar]
- 53.Allard JB, Brock TG. Structural organization of the regulatory domain of human 5-lipoxygenase. Curr Protein Pept Sci. 2005; 6: 125–131. [DOI] [PubMed] [Google Scholar]
- 54.UniProt. The universal protein knowledgebase. Nucleic Acids Res. 2017; 45: D158–D169. 10.1093/nar/gkw1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009; 25: 288–289. 10.1093/bioinformatics/btn615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000; 25: 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gene Ontology Consortium. Going forward. Nucleic Acids Res. 2015; 43: D1049–56. 10.1093/nar/gku1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis npr1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997; 88: 57–63. 10.1016/S0092-8674(00)81858-9 [DOI] [PubMed] [Google Scholar]
- 59.Sarry J-E, Kuhn L, Ducruix C, Lafaye A, Junot C, Hugouvieux V, et al. The early responses of Arabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics. 2006; 6: 2180–2198. 10.1002/pmic.200500543 [DOI] [PubMed] [Google Scholar]
- 60.Huang D, Wu W, Abrams SR, Cutler AJ. The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. J. Exp. Bot. 2008; 59: 2991–3007. 10.1093/jxb/ern155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad TK, Stewart CR. cDNA clones encoding Arabidopsis thaliana and Zea mays mitochondrial chaperonin HSP60 and gene expression during seed germination and heat shock. Plant Mol Biol. 1992; 18: 873–885. 10.1007/BF00019202 [DOI] [PubMed] [Google Scholar]
- 62.Virk N, Li D, Tian L, Huang L, Hong Y, Li X, et al. Arabidopsis Raf-like mitogen-activated protein kinase kinase kinase gene RAF43 is required for tolerance to multiple abiotic stresses. PLoS ONE. 2015; 10: e0133975 10.1371/journal.pone.0133975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo W-Y, Huang C-H, Jinn T-L. Chaperonin 20 might be an iron chaperone for superoxide dismutase in activating iron superoxide dismutase (FESOD). Plant Signal Behav. 2013; 8: e23074 10.4161/psb.23074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X-F, Jiang T, Wu Z, Du S-Y, Yu Y-T, Jiang S-C, et al. Cochaperonin CPN20 negatively regulates abscisic acid signaling in Arabidopsis. Plant Mol Biol. 2013; 83: 205–218. 10.1007/s11103-013-0082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boyce JM, Knight H, Deyholos M, Openshaw MR, Galbraith DW, Warren G, et al. The sfr6 mutant of Arabidopsis is defective in transcriptional activation via CBF/DREB1 and DREB2 and shows sensitivity to osmotic stress. Plant J. 2003; 34: 395–406. 10.1046/j.1365-313X.2003.01734.x [DOI] [PubMed] [Google Scholar]
- 66.Xu J, Tran T, Padilla Marcia CS, Braun DM, Goggin FL. Superoxide-responsive gene expression in Arabidopsis thaliana and Zea mays. Plant Physiol. Biochem. 2017; 117: 51–60. 10.1016/j.plaphy.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 67.Kovacs D, Kalmar E, Torok Z, Tompa P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008; 147: 381–390. 10.1104/pp.108.118208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorsuch PA, Sargeant AW, Penfield SD, Quick WP, Atkin OK. Systemic low temperature signaling in Arabidopsis. Plant Cell Physiol. 2010; 51: 1488–1498. 10.1093/pcp/pcq112 [DOI] [PubMed] [Google Scholar]
- 69.Kim BH, Kim SY, Nam KH. Genes encoding plant-specific class III peroxidases are responsible for increased cold tolerance of the brassinosteroid-insensitive 1 mutant. Molecules and Cells. 2012; 34: 539–548. 10.1007/s10059-012-0230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marmagne A, Rouet M-A, Ferro M, Rolland N, Alcon C, Joyard J, et al. Identification of new intrinsic proteins in Arabidopsis plasma membrane proteome. Mol. Cell Proteomics. 2004; 3: 675–691. 10.1074/mcp.M400001-MCP200 [DOI] [PubMed] [Google Scholar]
- 71.Marín M, Thallmair V, Ott T. The intrinsically disordered N-terminal region of AtREM1.3 remorin protein mediates protein-protein interactions. J Biol Chem. 2012; 287: 39982–39991. 10.1074/jbc.M112.414292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jarsch IK, Ott T. Perspectives on remorin proteins, membrane rafts, and their role during plant-microbe interactions. Mol Plant Microbe Interact. 2011; 24: 7–12. 10.1094/MPMI-07-10-0166 [DOI] [PubMed] [Google Scholar]
- 73.Tsai AY-L, Kunieda T, Rogalski J, Foster LJ, Ellis BE, Haughn GW. Identification and characterization of Arabidopsis seed coat mucilage proteins. Plant Physiol. 2017; 173: 1059–1074. 10.1104/pp.16.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma W, Muthreich N, Liao C, Franz-Wachtel M, Schütz W, Zhang F, et al. The mucilage proteome of maize (Zea mays L.) primary roots. J Proteome Res. 2010; 9: 2968–2976. 10.1021/pr901168v [DOI] [PubMed] [Google Scholar]
- 75.Foster J, Luo B, Nakata PA. An Oxalyl-Coa dependent pathway of oxalate catabolism plays a role in regulating calcium oxalate crystal accumulation and defending against oxalate-secreting phytopathogens in Medicago truncatula. PLoS ONE. 2016; 11: e0149850 10.1371/journal.pone.0149850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emanuelsson O, Brunak S, Heijne G von, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protocols. 2007; 2: 953–971. 10.1038/nprot.2007.131 [DOI] [PubMed] [Google Scholar]
- 77.Small I, Peeters N, Legeai F, Lurin C. Predotar. A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics. 2004; 4: 1581–1590. 10.1002/pmic.200300776 [DOI] [PubMed] [Google Scholar]
- 78.Keymer A, Pimprikar P, Wewer V, Huber C, Brands M, Bucerius SL, et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife. 2017; 6 10.7554/eLife.29107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sirrenberg A, Gobel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, et al. Piriformospora indica affects plant growth by auxin production. Physiologia plantarum. 2007; 131: 581–589. 10.1111/j.1399-3054.2007.00983.x [DOI] [PubMed] [Google Scholar]
- 80.Kai K, Shimizu B-i, Mizutani M, Watanabe K, Sakata K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry. 2006; 67: 379–386. 10.1016/j.phytochem.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 81.Vahabi K, Sherameti I, Bakshi M, Mrozinska A, Ludwig A, Reichelt M, et al. The interaction of Arabidopsis with Piriformospora indica shifts from initial transient stress induced by fungus-released chemical mediators to a mutualistic interaction after physical contact of the two symbionts. BMC Plant Biol. 2015; 15: 58 10.1186/s12870-015-0419-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nongbri PL, Vahabi K, Mrozinska A, Seebald E, Sun C, Sherameti I, et al. Balancing defense and growth—Analyses of the beneficial symbiosis between Piriformospora indica and Arabidopsis thaliana. Symbiosis. 2012; 58: 17–28. 10.1007/s13199-012-0209-8 [DOI] [Google Scholar]
- 83.Hui F, Liu J, Gao Q, Lou B. Piriformospora indica confers cadmium tolerance in Nicotiana tabacum. J Environ Sci (China). 2015; 37: 184–191. 10.1016/j.jes.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 84.Peskan-Berghöfer T, Vilches-Barro A, Müller TM, Glawischnig E, Reichelt M, Gershenzon J, et al. Sustained exposure to abscisic acid enhances the colonization potential of the mutualist fungus Piriformospora indica on Arabidopsis thaliana roots. New Phytol. 2015. 10.1111/nph.13504 [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Kars I, Essenstam B, Liebrand TWH, Wagemakers L, Elberse J, et al. Fungal endopolygalacturonases are recognized as microbe-associated molecular patterns by the arabidopsis receptor-like protein Responsiveness to Botrytis polygalacturonases1. Plant Physiol. 2014; 164: 352–364. 10.1104/pp.113.230698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong S, Tian Z, Chen PJ, Senthil Kumar R, Shen CH, Cai D, et al. The maturation zone is an important target of Piriformospora indica in Chinese cabbage roots. J. Exp. Bot. 2013; 64: 4529–4540. 10.1093/jxb/ert265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H, Kim M-S, Krishnamachari V, Payton P, Sun Y, Grimson M, et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta. 2007; 226: 839–851. 10.1007/s00425-007-0530-2 [DOI] [PubMed] [Google Scholar]
- 88.Shrivastava N, Jiang L, Li P, Sharma AK, Luo X, Wu S, et al. Proteomic approach to understand the molecular physiology of symbiotic interaction between Piriformospora indica and Brassica napus. Sci Rep. 2018; 8: 5773 10.1038/s41598-018-23994-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christensen AB, Thordal-Christensen H, Zimmermann G, Gjetting T, Lyngkjaer MF, Dudler R, et al. The germinlike protein GLP4 exhibits superoxide dismutase activity and is an important component of quantitative resistance in wheat and barley. Mol Plant Microbe Interact. 2004; 17: 109–117. 10.1094/MPMI.2004.17.1.109 [DOI] [PubMed] [Google Scholar]
- 90.Yin K, Han X, Xu Z, Xue H. Arabidopsis GLP4 is localized to the Golgi and binds auxin in vitro. Acta Biochim Biophys Sin (Shanghai). 2009; 41: 478–487. 10.1093/abbs/gmp036 [DOI] [PubMed] [Google Scholar]
- 91.Ham B-K, Li G, Kang B-H, Zeng F, Lucas WJ. Overexpression of Arabidopsis plasmodesmata germin-like proteins disrupts root growth and development. Plant Cell. 2012; 24: 3630–3648. 10.1105/tpc.112.101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson JM, Thürich J, Petutschnig EK, Altschmied L, Meichsner D, Sherameti I, et al. A poly(A) ribonuclease controls the cellotriose-based interaction between Piriformospora indica and its host Arabidopsis. Plant physiology. 2018. 10.1104/pp.17.01423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carminati A, Vetterlein D. Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources. Ann Bot. 2013; 112: 277–290. 10.1093/aob/mcs262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaiser C, Kilburn MR, Clode PL, Fuchslueger L, Koranda M, Cliff JB, et al. Exploring the transfer of recent plant photosynthates to soil microbes: mycorrhizal pathway vs direct root exudation. New Phytol. 2015; 205: 1537–1551. 10.1111/nph.13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Francoz E, Ranocha P, Burlat V, Dunand C. Arabidopsis seed mucilage secretory cells: regulation and dynamics. Trends Plant Sci. 2015; 20: 515–524. 10.1016/j.tplants.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 96.Nagahashi G, Douds DD. Isolated root caps, border cells, and mucilage from host roots stimulate hyphal branching of the arbuscular mycorrhizal fungus, Gigaspora gigantea. Mycological Research. 2004; 108: 1079–1088. [DOI] [PubMed] [Google Scholar]
- 97.Hilbert M, Voll LM, Ding Y, Hofmann J, Sharma M, Zuccaro A. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol. 2012; 196: 520–534. 10.1111/j.1469-8137.2012.04275.x [DOI] [PubMed] [Google Scholar]
- 98.Burget EG, Verma R, Mølhøj M, Reiter W-D. The biosynthesis of l-Arabinose in plants: molecular cloning and characterization of a Golgi-localized UDP-d-Xylose 4-epimerase encoded by the mur4 gene of Arabidopsis. Plant Cell. 2003; 15: 523–531. 10.1105/tpc.008425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McClerklin SA, Lee SG, Nwumeh R, Jez JM, Kunkel BN. Indole-3-Acetaldehyde dehydrogenase-dependent Auxin synthesis contributes to virulence of Pseudomonas syringae Strain DC3000; 2017. [DOI] [PMC free article] [PubMed]
- 100.Hong Y, Wang TW, Hudak KA, Schade F, Froese CD, Thompson JE. An ethylene-induced cDNA encoding a lipase expressed at the onset of senescence. Proc. Natl. Acad. Sci. U.S.A. 2000; 97: 8717–8722. 10.1073/pnas.140213697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Minor W, Steczko J, Stec B, Otwinowski Z, Bolin JT, Walter R, et al. Crystal structure of soybean lipoxygenase L-1 at 1.4 A resolution. Biochemistry. 1996; 35: 10687–10701. 10.1021/bi960576u [DOI] [PubMed] [Google Scholar]
- 102.Shin R, An J-M, Park C-J, Kim YJ, Joo S, Kim WT, et al. Capsicum annuum tobacco mosaic virus-induced clone 1 expression perturbation alters the plant's response to ethylene and interferes with the redox homeostasis. Plant Physiol. 2004; 135: 561–573. 10.1104/pp.103.035436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tomchick DR, Phan P, Cymborowski M, Minor W, Holman TR. Structural and functional characterization of second-coordination sphere mutants of soybean lipoxygenase-1. Biochemistry. 2001; 40: 7509–7517. . [DOI] [PubMed] [Google Scholar]
- 104.Camehl I, Sherameti I, Venus Y, Bethke G, Varma A, Lee J, et al. Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. The New phytologist. 2010; 185: 1062–1073. 10.1111/j.1469-8137.2009.03149.x [DOI] [PubMed] [Google Scholar]
- 105.Johnson JM, Sherameti I, Ludwig A, Nongbri PL, Sun C, Lou B, et al. Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation–A model system to study plant beneficial traits. Journal of Endocytobiosis and Cell Research. 2011: 101–113. [Google Scholar]
- 106.Scholl RL, May ST, Ware DH. Seed and molecular resources for Arabidopsis. Plant Physiol. 2000; 124: 1477–1480. 10.1104/pp.124.4.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962; 15: 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 108.Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984; 138: 141–143. 10.1016/0003-2697(84)90782-6 [DOI] [PubMed] [Google Scholar]
- 109.Vizcaíno JA, Csordas A, del-Toro N, Dianes JA, Griss J, Lavidas I, et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016; 44: D447–56. 10.1093/nar/gkv1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009; 37: e45 10.1093/nar/gkp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001; 29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Döll K. Analysis of toxigenic fungi and their mycotoxins in biotic interactions; 2014. (dissertation) Georg-August-Universität Göttingen; 2013. Available from: http://hdl.handle.net/11858/00-1735-0000-0022-5E25-E
- 113.Peterson JK, Harrison HF, Jackson DM, Snook ME. Biological activities and contents of scopolin and scopoletin in Sweetpotato clones. HortScience. 2003; 38: 1129–1133. [Google Scholar]
- 114.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008; 36: W5–9. 10.1093/nar/gkn201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selected proteins were identified by at least two unique peptides and a false discovery rate of <1%. The frequency of occurrence is given as Low (1 out of 3), Medium (2 out of 3) or High (3 out of 3 replicates) based on the frequency of detection in all replicates.
(XLSX)
Identified proteins were considered differentially enriched, when they were found in at least two replicates in one treatment and not in the other treatment. Furthermore, proteins that were found with a high frequency in one treatment and with a low frequency in the other treatment, were considered differentially secreted as well. Information about the function was obtained using the BLAST algorithm for homology searches against the UniProt database [54, 114]. The location was predicted using TargetP, SignalP and Predotar algorithms.
(XLSX)
(XLSX)
Arabidopsis roots in liquid (A) and solid media (B) were imaged using fluorescence microscopy. In both cultures, only little fungus was found in and around the root. The first pictures show the autofluorescence of the root and GFP fluorescence of P. indica. The second pictures show the bright field image. The last pictures show the overlay of all images for each row. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method.
(TIF)
(A) Venn diagram of A. thaliana proteins found in three replicates in the co-culture (generated with http://bioinformatics.psb.ugent.be/webtools/Venn/). (B) Profile for plat2 expression in WT roots and leaves. (C) Profile for plat3 expression in WT roots and leaves. For plat3, CT values were extremely low around 35–40 whereas for all other genes CT values were between 25 and 35. (D) Primer used in this study for RT-qPCR.
Dashed line represents the expression in the untreated WT plants. Error bars represent standard deviation calculated from four biological replicates. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction).
(TIF)
Expression of PYK10 and other key genes of the scopolin pathway in roots of WT and F6’H1 at 7 dpi (A) and 14 dpi (B). Error bars represent standard deviation calculated from four biological replicates. The asterisks shows the significant difference, as described in Fig 3A.
(TIF)
Fluorescence microscopy of ER-bodies in roots without and with P. indica. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method. Mock treatment in liquid culture (A) and in solid culture (B) and colonised roots at 3 dpi in liquid culture (C) and in solid culture (D) are shown. The first pictures show the autofluorescence of the root ER-Bodies. The second pictures show the bright field image. The last pictures show the overlay of all images for each row. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method.
(TIF)
Comparison of the ER-body length in Arabidopsis root cells without and with P. indica at 3 dpi. Liquid cultivation method from the secretome measurement was compared with the standard solid cultivation method. Error bars represents standard deviation. Different letters represent significant differences between treatments at p<0.05 (Two-Way ANOVA, followed by a Bonferroni correction). Per time point and treatment four roots were used and the size of 20–100 ER-Bodies per root was measured.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files with the exception of the mass spectrometry proteomics data. They have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD009563 (Reviewer account details: Username: reviewer95832@ebi.ac.uk; Password: zHk3U002).