Abstract
Berberine, a natural isoquinoline alkaloid, is used in herbal medicine and has recently been shown to have efficacy in the treatment of mood disorders. Furthermore, berberine modulates neurotransmitters and their receptor systems within the central nervous system. However, the detailed mechanisms of its action remain unclear. This review summarizes the pharmacological effects of berberine on mood disorders. Therefore, it may be helpful for potential application in the treatment of mood disorders.
Keywords: anxiety, berberine, bipolar disorder, depression, mechanism, pharmacology
1. INTRODUCTION
Mood disorders are common, chronic, recurrent mental illnesses that affect millions of individuals worldwide.1, 2 The primary mood disorders are major depressive disorder and bipolar disorder. Most patients with mood disorders receive some benefit from available treatments.3, 4 However, full remission of clinical symptoms is rarely achieved owing to complex pathophysiology. Moreover, many classes of antidepressant have serious side‐effects such as drowsiness, dryness of mouth, headache, nausea, and sexual dysfunction.5, 6 There is, therefore, an urgent need to develop alternative drugs. Berberine is an herbal drug used in traditional Chinese medicine that has recently been shown to alleviate mood disorders in a number of ways.7, 8 Berberine, therefore, has the potential to become a mainstream drug for treating mood disorders. This article reviews the literature on the pharmacological effects of berberine in the treatment of various mood disorders. We focus on underlying mechanisms and pathways that mediate the multiple pharmacological actions of berberine. The applicability of berberine to mood disorders is also discussed in this review.
2. BERBERINE
Berberine, a natural isoquinoline alkaloid, is widely used in traditional Chinese medicine.9 It is isolated from several herbal species, including Berberis Hydrastis canadensis (goldenseal), Xanthorhiza simplicissima (yellow root), Phellodendron anureses (Amur cork tree), Coptis chinensis (Chinese goldthread), Tinospora cordifolia, Argemone mexicana (prickly poppy), and Eschscholzia californica (Californian poppy).10 However, it has poor bioavailability, which seriously limits its application and development.11 It is an intense yellow powder, odourless with a characteristic alkaloidal bitter taste. It is very slightly soluble in water and ethanol and is sparingly soluble in methanol. The chloride or sulphate salts of berberine are relatively more soluble and are used clinically.12 Berberine has multiple therapeutic actions, including antioxidant, anti‐inflammatory, antitumour, antimicrobial, hepatoprotective, hypolipidemic, and hypoglycemic actions.13 The pharmacology of berberine is well documented; however, there is renewed interest in berberine because of its benefits in various neurodegenerative and neuropsychiatric disorders.7 Wang et al reported that berberine could easily cross the blood‐brain barrier on systemic administration14 and recent studies show that berberine has a protective effect on central nervous system disorders, such as Alzheimer's, cerebral ischaemia, mental depression, schizophrenia, and anxiety.7, 12, 15 Here, we review the pharmacology of berberine in detail and highlight its efficacy in the treatment of mood disorders.
3. PHARMACOLOGICAL EFFECT OF BERBERINE ON DEPRESSION
3.1. The effect of berberine on neurotransmitters in depression
The original monoamine hypothesis postulates that the dysregulation of serotonin, norepinephrine, and dopamine has been theorized to be a core pathogenic factor in depression. Dysregulation of neurotransmitters can cause depression.16 Berberine can regulate brain neurotransmitters, especially biogenic amines.12, 17 Norepinephrine (NE), serotonin (5‐HT) and dopamine (DA) are neurotransmitters released from neurons during synaptic transmission in the nervous system.12 The studies demonstrated that berberine (10, 20 mg/kg, p.o.), dramatically reduced the immobility time during the forced swim test and the tail suspension test.18 Either acute or chronic administration of berberine at low doses results in increased levels of NE, 5‐HT, and DA in whole‐brain samples. Kulkarni et al showed that acute administration of berberine (5 mg/kg, i.p.) in mice caused increased levels of norepinephrine (31%), serotonin (47%), and dopamine (31%). Chronic administration of berberine (5 mg/kg, ip) for 15 days significantly increased the levels of norepinephrine (29%), serotonin (19%) as well as dopamine (52%).12 These increases in biogenic amine levels are attributed to the inhibition of monoamine oxidase by berberine.19, 20 Berberine can also inhibit the release of NE via activation of adrenergic α2 autoreceptors21 and can affect DA in a manner that antagonizes D2 and agonizes D1 receptors.22, 23 Substance P shows a strong negative correlation with serum concentrations of the primary 5‐HT metabolite, 5‐hydroxyindoleacetic acid.24 Berberine reverses the increase in substance P levels induced by reserpine in the cerebral cortex and hippocampus.25
Sigma receptors play an important role in the modulation of various neurotransmitters. Sigma ligands can modulate the activity of the neurotransmitter systems, such as noradrenergic, serotonergic, dopaminergic, and glutamatergic ones.26 Meurs et al reported that sigma 1 receptor‐mediated increase in hippocampal extracellular dopamine.27 Moreover, some sigma agonists are found to have antidepressant‐like activity perhaps with fewer side‐effects.28 These receptors are a promising therapeutic target for neuropsychiatric disorders, in particular, for depression. Recent studies have provided further evidence for the involvement of sigma receptors in the pathophysiology of major depression psychiatric disturbances.28, 29, 30, 31 Berberine affects sigma receptor 1 similarly to many antidepressant drugs32 indicating its potential for the treatment of major depression.
3.2. The effect of berberine on antioxidation in depression
Clinical studies of patients with depression have shown disturbances to oxidation, such as elevated lipid peroxidation products and reduced levels of superoxide dismutase.33 Patients with depression also accompanied changes in brain volume. Increases in reactive oxygen species (ROS) and decreased antioxidant defences may cause oxidative modifications of proteins and DNA. The damage of stability of proteins and DNA may result in apoptois, and in part explain the brain volumetric changes evident in depression.34 Arora et al found that treatment with berberine (10 and 20 mg/kg) produced a significant reduction in lipid peroxide levels in the cerebral cortex of reserpine administered rats.25 Berberine inhibits the generation of ROS by suppressing overexpression of the nicotinamide adenine dinucleotide phosphate oxidase (NOX) enzyme complex.35, 36 Meanwhile, berberine treatment restored the levels of nonprotein thiols, superoxide dismutase and catalase, which were significantly decreased by reserpine in the cerebral cortex and hippocampus.25, 37
Lukic et al provided evidence that depression is characterized by up‐regulation of nuclear transcription factor‐κB (NF‐κB). Major depressive disorder subjects exhibited higher levels of NF‐κB compared to controls.38 NF‐κB activity is regulated at least in part by the intensity of intracellular oxidative stress.39, 40 Li et al reported that NF‐κB can be activated by oxidative stress (such as by exposure to H2O2).41 Berberine interacts directly with nucleic acids, and with several proteins, including p53, NF‐κB, and oestrogen receptors.42, 43, 44 Arora et al also observed a significant increase in levels of NF‐κB and caspase‐3 in the cerebral cortex and hippocampus of reserpine‐treated rats and treatment with berberine down‐regulated the elevated levels of NF‐κB and caspase‐3.25 Chronic berberine treatment inhibited NF‐κB signalling pathway in the hippocampus and prevented the depressive deficits both in sucrose preference test and novelty‐suppressed feeding test in mice induced by chronic unpredictable mild stress.45 These studies indicate that berberine may be of use as an antidepressant through the NF‐κB signalling pathway, which may be activated by oxidative stress.
3.3. The effect of berberine on nitric oxide synthesis in depression
Systemic inhibition of nitric oxide synthase induces antidepressant‐like effects in the rat hippocampus. The neuronal nitric oxide synthase inhibitor significantly decreased immobility time.46 Pharmacological manipulation of nitric oxide pathway by berberine can be observed in a reserpine‐induced model of depression. Berberine (5 and 10 mg/kg, ip) reversed the increased of immobility period induced by reserpine.12 Evidence has suggested that neuronal nitric oxide synthase inhibition increases serotonin signalling and activaties prosencephalic 5HT1A receptors.47 There is also a close connection between adenosine monophosphate‐activated protein kinase (AMPK) pathway and nitric‐oxide synthesis. AMPK plays an important role in regulating NO synthesis in endothelial cells.48 The activity of AMPK pathway is regulated by berberine.49 AMPK is an upstream kinase of endothelial nitric oxide synthase (eNOS) that promotes eNOS phosphorylation, complex formation between eNOS and heat shock 90 kDa protein, and nitric oxide (NO) production.50, 51 Recent evidence has shown that reduced nitric oxide levels can induce antidepressant‐like effects.50 Moreover, the L‐arginine‐NO‐cyclic guanosine monophosphate signalling pathway is important in the antidepressant action of berberine chloride.46 Excessive levels of cyclic guanosine monophosphate can produce a depression‐like state, while reduced levels can produce antidepressant‐like actions.52
3.4. The effect of berberine on neuroinflammation in depression
Neuroinflammation may have a role in the pathogenesis of depression.37, 53 Inflammation‐associated disorder of serotonergic and glutamatergic neurotransmission ultimately induces depression‐like behaviour.54 Mice induced by chronic unpredictable mild stress display enhanced levels of pro‐inflammatory cytokines, including interleukin‐6, interleukin‐1‐beta, and tumour necrosis factor β in hippocampus. Then the increased pro‐inflammatory cytokines were decreased by orally administration berberine.45 In addition, treatment with berberine attenuated the increased levels of the interleukin‐1‐beta in reserpine‐treated rats.25 It has been suggested that pro‐inflammatory cytokines may affect the catabolism and disposition of various neurotransmitters through activation of indoleamine 2,3‐dioxygenase (IDO).55, 56 Increased IDO activity may also decrease tryptophan availability, impacting serotonergic neurotransmission.57 Berberine, a newly identified IDO inhibitor, significantly decreased the production of kynurenine in A549 cells.58 Increased kynurenine may metabolize to neurotoxic metabolites, such as quinolinic acid, thereby, influencing glutamatergic neurotransmission.57
3.5. The effect of berberine on neurotrophic factors in depression
Nerve growth factor plays a role in the modulation of synaptic function and plasticity in the CNS.58 Berberine potentiates nerve growth factor (NGF) activity, which can increase NGF‐induced neurite outgrowth in a dose‐dependent manner.59 In some depression models, berberine decreased ROS levels,36 and increased NGF‐mediated neurite outgrowth via the phosphoinositide 3‐kinase/protein kinase B/nuclear factor‐E2‐related factor 2‐dependent pathway.36 In addition, berberine has a neuroprotective effect in a dose‐dependent manner, low‐dose berberine significantly increased cell viability, while high‐dose berberine inhibited cell viability.60
An antidepressant effect of berberine results from elevation of brain‐derived neurotrophic factor (BDNF) levels.17, 61 Bombi et al reported that berberine restored the decreased level of BDNF mRNA in the rat hippocampus following withdrawal from repeated morphine injection.17 Our recent study indicated that berberine exerts antidepressant‐like effects in ovariectomized mice, partly through the effects of berberine on BDNF‐ cyclic adenosine monophosphate (cAMP) response element‐binding protein and eukaryotic elongation factor 2 (eEF2) pathways.61 The BDNF‐ cAMP‐response element binding protein (CREB) pathway is a well‐established antidepressant pathway which is critical for antidepressant action. Furthermore, eEF2 is involved in the actions of rapid‐onset antidepressants.62, 63 The reductions of hippocampal BDNF and phosphorylated eEF2 levels in ovariectomized mice are reversed by berberine treatment.61 These studies suggest that berberine may rapidly produce antidepressant‐like behaviour.
3.6. The effect of berberine on hormonal regulation in depression
Hormonal imbalance can cause a variety of neurological disorders. One of the most common examples is the link between diabetes and major depressive disorder.64, 65 A growing number of studies have demonstrated that berberine can affect mood by regulating plasma corticosterone levels. Palmatine, a quaternary protoberberine alkaloid, produced antidepressant‐like activity by decreasing plasma corticosterone levels.66 Phellodendron, which is rich in berberine, reduced the effects of cortisol exposure and perceived daily stress.67 Treatment with berberine attenuated the depressive‐like behaviour induced by repeated corticosterone injection.68 Fluctuations in gonadal hormone levels are believed to contribute to these depressive conditions.69 Although, some studies show an effect of berberine on gonadal hormone levels, it is not known whether the antidepressant effects of berberine involve gonadal hormonal regulation.
3.7. New perspectives of berberine action in depression
Studies in humans have shown an association between irritable bowel syndrome and depression.70 Zhu et al investigated the mechanism of berberine by examining alterations to gastrointestinal tract histopathology and the gut flora profile in a chronic mild stress rat model. Berberine reversed the physical damage brought about by stress within the gastric mucosa and intestinal microvilli of the stomach, ileum, caecum, and colon.71 This study showed that high concentrations of berberine can protect rats from various symptoms of chronic stress and depression, indicating its potential clinical use.
3.8. The effect of berberine on bipolar affective disorder
In recent years, prolyl oligopeptides (POPs) have gained prominence as targets for the treatment of bipolar affective disorder.72 POP has been reported to participate in the processing of neuropeptide precursors.73 Moreover, neuroprotective effects of POPs inhibitors have been reported in experimental animals.74, 75 Berberine inhibits POPs in a dose‐dependent manner.72 However, few studies have reported the effects of berberine in bipolar disorder. As noted below, some neurotransmitters such as dopamine, glutamate, and γ‐aminobutyric acid (GABA) are responsible for mood cycling, while, dopamine and glutamate increase transmission during the manic phase.76, 77 More evidence is required to substantiate a relationship between bipolar affective disorder and berberine.
4. PHARMACOLOGICAL EFFECT OF BERBERINE ON ANXIETY
Anxiety is an aversive emotional state that affects approximately one‐eighth of the worldwide population.78 A significant anxiolytic effect of berberine can be observed in the elevated plus‐maze test. Berberine increased the time spent in and the exploration of the open arms, and decreased the entries to and time spent in the closed arms.17, 79
It is worth noting that berberine regulates biogenic amines in a concentration‐dependent manner. At a low dose, berberine (10 and 20 mg/kg) is effective in depression by increasing levels of NE, 5‐HT and DA.18 In contrast, high doses of berberine (100, 500 mg/kg) decreased concentrations of biogenic amines.18, 79 Furthermore, berberine increased the concentrations of their metabolites in the brain stem. The anxiolytic mechanism of berberine might be related to the increased turnover rates of monoamines in the brain stem and decreased serotonergic system activity. Moreover, berberine decreased serotonergic system activity via activation of somatodendritic 5‐HT1A autoreceptors and inhibition of post‐synaptic 5‐HT1A and 5‐HT2 receptors.79 Berberine also has an inhibitory effect on glutamate receptors and can reduce glutamate, 5‐HT and NE levels.80, 81
Extensive comorbidity among depressive disorders and anxiety disorders indicates related disease etiologies.82, 83 Dysregulation of GABA in anxiety has been reported in a number of studies.84 Benzodiazepines, GABAA receptor agonists, are commonly used in the clinical treatment of anxiety. Berberine alkaloids bind with the high‐affinity benzodiazepine site on the GABAA receptor.84, 85 Anxiety research has predominantly focused on the neurotransmitter systems, including GABAergic and serotoninergic systems. However, Kuloglu et al recently established a link between oxidative stress and certain anxiety disorders, demonstrating that other systems, such as oxidative metabolism, can affect the regulation of anxiety.86 Although the antioxidant effect of berberine has been confirmed,87 it has not been reported in animal models of anxiety. Further studies are warranted to validate this link and to understand the pathogenic mechanisms involved.
5. TOLERABILITY AND SAFETY
Berberine has been used in the clinic for several decades.88, 89 It displays various pharmacological effects, including efficacy against gastroenteritis, abdominal pain, and diarrhoea. It also has anti‐microbial, anti‐diabetic, and anti‐inflammatory properties.88, 90 Chronic administration of berberine (1200‐2000 mg/d) for at least 2 months significantly decreased total cholesterol levels and low‐density lipoprotein cholesterol without major adverse effects.91 Yin et al and Dong et al studied the anti‐diabetic properties of berberine (500‐1500 mg daily for 3 months) 92 and they hypothesized that berberine induces a significant reduction in postprandial glucose levels. In addition, in patients randomized to receive 800 mg of berberine hydrochloride daily for 2 months, a trend of improvement was observed for IBS symptom scores compared with placebo.74
Berberine does not display any genotoxic, cytotoxic, or mutagenic activity.7 However, berberine has remarkable cytotoxicity on a wide range of cancer cell lines because of its protoberberine skeleton.73 Standard doses of berberine are usually well‐tolerated and adverse reactions are rare. In contrast, high doses have been associated with arterial hypotension, dyspnoea, flu‐like symptoms, gastrointestinal discomfort, constipation, and cardiac damage.93 Yin et al reported that approximately 34.5% of patients treated with berberine (500 mg three times daily) experienced transient adverse gastrointestinal effects.92 Moreover, berberine can lower blood sugar levels and blood pressure and should, therefore, be used with caution in people with diabetes or low blood pressure.94, 95 Berberine is a potent displacer of bilirubin when tested in vitro using plasma from jaundiced new‐born babies and, therefore, poses a risk of kernicterus.96 Its use should, therefore, be avoided in jaundiced infants and pregnant woman, even in small doses. Although berberine has not been administered to patients with depression, as a traditional Chinese herbal medicine, berberine has been used in the East for hundreds of years. However, berberine targets are involved in a wide range of molecular activities and can alter many pathological states. Further research is required to test whether berberine is a promising candidate for the treatment of mood disorders.
6. CONCLUSION
Based on the published findings, we conclude that berberine may be a potential treatment for mood disorders. Berberine in various mood disorders and its mechanism of action are summarized in Table 1. Schematic representation of the mechanism of berberine involved with depression is shown in Figure 1. Further research into the safety profile of berberine is required before its clinical application.
Table 1.
Berberine in various mood disorders and its mechanism of action
| Mood disorders | Mechanism | References |
|---|---|---|
| Depression | Inhibition of monoamine oxidase activity | 19, 20 |
| Increase of NE, 5‐HT and DA levels | 12 | |
| Inhibition of α2 auto receptors | 21 | |
| Antagonism of D2 receptors and agonism of D1 receptors | 22, 23 | |
| Involvement with substance P | 25 | |
| Involvement with sigma receptor | 32 | |
| Inhibition of NOX and ROS | 35, 36 | |
| Reduction of lipid peroxide and superoxide dismutase levels | 33 | |
| Involvement of the l‐arginine‐NO‐cGMP pathway | 46 | |
| Involvement with tumour necrosis factor β, interleukin‐6, interleukin‐1‐beta, IDO, kynurenine levels | 45, 55, 56 | |
| Induction of NGF secretion | 59 | |
| Activation of phosphoinositide 3‐kinase/protein kinase/nuclear factor‐E2‐related factor 2‐mediated regulation | 36 | |
| Activation of BDNF‐cAMP response element‐binding protein and eEF2 pathways | 61 | |
| Protect gastrointestinal tract | 71 | |
| Decrease of plasma corticosterone levels | 66 | |
| Fluctuations in gonadal hormone levels | 69 | |
| Bipolar disorder | Activation of POP | 72 |
| Anxiety | Decrease of 5‐HT, NE, and DA levels | 18 |
| Binding with GABAA receptor | 84, 85 | |
| Inhibition of glutamate receptors | 80, 81 | |
| Increase in turnover rates of monoamines | 79 | |
| Activation of 5‐HT1A and inhibition of 5‐HT1A and 5‐HT2 receptors | 79 |
Figure 1.
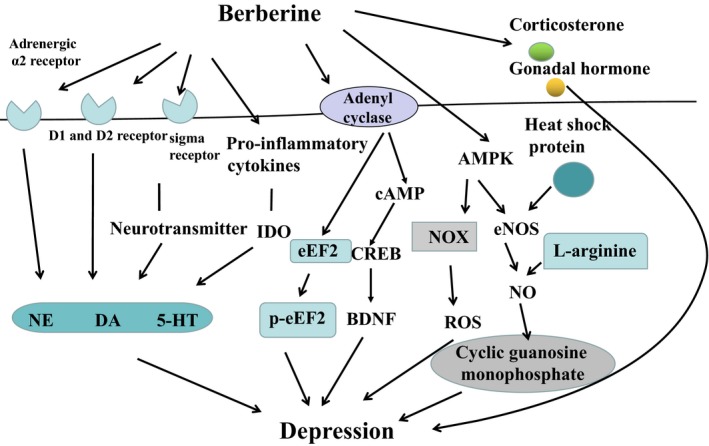
Schematic representation of molecular mechanism of berberine involved with depression. The schematic drawing showed possible regulatory network of berberine in depression. Black arrows indicate stimulation. Some mechanisms are indicated by abbreviations. D1: dopamine 1 receptor; D2: dopamine 2 receptor; NE: norepinephrine; 5‐HT: serotonin; DA: dopamine; ROS: reactive oxygen species; NOX: nicotinamide adenine dinucleotide phosphate oxidase; AMPK: adenosine monophosphate‐activated protein kinase; eNOS: endothelial nitric oxide synthase; NO: nitric oxide; IDO: indoleamine‐ 2,3‐dioxygenase; BDNF: brain‐derived neurotrophic factor; eEF2: eukaryotic elongation factor 2; cAMP: cyclic adenosine monophosphate; CREB: cAMP‐response element binding protein
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by grants from the Natural Science Foundation of China (NSFC) (31571126 and 31300850).
Fan J, Zhang K, Jin Y, et al. Pharmacological effects of berberine on mood disorders. J Cell Mol Med. 2019;23:21–28. 10.1111/jcmm.13930
Funding information
This work was supported by grants from the Natural Science Foundation of China (NSFC) (81871070, 31571126, 31471120) and Jilin Science and Technology Agency funding (Grant Nos. 20160307025YY, 20180519003JH, 20170414034GH and 20180414050GH)
Contributor Information
Jiaming Zhu, Email: zhujiaming75@sina.com.
Ranji Cui, Email: cuiranji@jlu.edu.cn.
REFERENCES
- 1. Sanacora G, Zarate CA, Krystal JH, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34:244‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905‐1917. [DOI] [PubMed] [Google Scholar]
- 4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28‐40. [DOI] [PubMed] [Google Scholar]
- 5. Mochizuki D, Tsujita R, Yamada S, et al. Neurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in rats. Psychopharmacology. 2002;162:323‐332. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Z, Zhang HT, Bootzin E, et al. Association of changes in norepinephrine and serotonin transporter expression with the long‐term behavioral effects of antidepressant drugs. Neuropsychopharmacology. 2009;34:1467‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kulkarni SK, Dhir A. Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother Res. 2010;24:317‐324. [DOI] [PubMed] [Google Scholar]
- 8. Kumar A, Ekavali Chopra K, et al. Current knowledge and pharmacological profile of berberine: an update. Eur J Pharmacol. 2015;761:288‐297. [DOI] [PubMed] [Google Scholar]
- 9. Karnam KC, Ellutla M, Bodduluru LN, et al. Preventive effect of berberine against DMBA‐induced breast cancer in female Sprague Dawley rats. Biomed Pharmacother. 2017;92:207‐214. [DOI] [PubMed] [Google Scholar]
- 10. Cicero AF, Baggioni A. Berberine and its role in chronic disease. Adv Exp Med Biol. 2016;928:27‐45. [DOI] [PubMed] [Google Scholar]
- 11. Xiao D, Liu Z, Zhang S, et al. Berberine derivatives with different pharmacological activities via structural modifications. Mini Rev Med Chem. 2017; 10.2174/1389557517666170321103139 [DOI] [PubMed] [Google Scholar]
- 12. Kulkarni SK, Dhir A. On the mechanism of antidepressant‐like action of berberine chloride. Eur J Pharmacol. 2008;589:163‐172. [DOI] [PubMed] [Google Scholar]
- 13. Wang K, Feng X, Chai L, et al. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab Rev. 2017;49:1‐19. [DOI] [PubMed] [Google Scholar]
- 14. Wang X, Wang R, Xing D, et al. Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of Coptidis rhizoma extract. Life Sci. 2005;77:3058‐3067. [DOI] [PubMed] [Google Scholar]
- 15. Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin‐1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 2006;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobsen JP, Medvedev IO, Caron MG. The 5‐HT deficiency theory of depression: perspectives from a naturalistic 5‐HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;367:2444‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee B, Sur B, Yeom M, et al. Effect of berberine on depression‐ and anxiety‐like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J Physiol Pharmacol. 2012;16:379‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng WH, Lo KL, Lee YH, et al. Berberine produces antidepressant‐like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007;81:933‐938. [DOI] [PubMed] [Google Scholar]
- 19. Kong LD, Cheng CH, Tan RX. Monoamine oxidase inhibitors from rhizoma of Coptis chinensis. Planta Med. 2001;67:74‐76. [DOI] [PubMed] [Google Scholar]
- 20. Kuznetsova LP, Sochilina EE, Faddeeva MD, et al. Effect of some isoquinoline alkaloids on enzymatic activity of acetylcholinesterase and monoamine oxidase. Ukr Biokhim Zh. 1999;2005(77):147‐153. [PubMed] [Google Scholar]
- 21. Yao WX, Ling BD, Chen B, et al. [Blocking action of berberine on alpha 2 and alpha 1 adrenoceptors in the rat vas deferens and anococcygeus muscle]. Zhongguo Yao Li Xue Bao. 1986;7:511‐515. [PubMed] [Google Scholar]
- 22. Chu H, Jin G, Friedman E, et al. Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol Neurobiol. 2008;28:491‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kulkarni SK, Dhir A. An overview of curcumin in neurological disorders. Indian J Pharm Sci. 2010;72:149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwarz MJ, Späth M, Müller‐Bardorff H, et al. Relationship of substance P, 5‐hydroxyindole acetic acid and tryptophan in serum of fibromyalgia patients. Neurosci Lett. 1999;259:196‐198. [DOI] [PubMed] [Google Scholar]
- 25. Arora V, Chopra K. Possible involvement of oxido‐nitrosative stress induced neuro‐inflammatory cascade and monoaminergic pathway: underpinning the correlation between nociceptive and depressive behaviour in a rodent model. J Affect Disord. 2013;151:1041‐1052. [DOI] [PubMed] [Google Scholar]
- 26. Skuza G. Potential antidepressant activity of sigma ligands. Pol J Pharmacol. 2003;55:923‐934. [PubMed] [Google Scholar]
- 27. Meurs A, Clinckers R, Ebinger G, et al. Sigma 1 receptor‐mediated increase in hippocampal extracellular dopamine contributes to the mechanism of the anticonvulsant action of neuropeptide Y. Eur J Neurosci. 2007;26:3079‐3092. [DOI] [PubMed] [Google Scholar]
- 28. Takebayashi M, Hayashi T, Su TP. A perspective on the new mechanism of antidepressants: neuritogenesis through sigma‐1 receptors. Pharmacopsychiatry. 2004;37(Suppl 3):S208‐S213. [DOI] [PubMed] [Google Scholar]
- 29. Takebayashi M, Hayashi T, Su TP. Nerve growth factor‐induced neurite sprouting in PC12 cells involves sigma‐1 receptors: implications for antidepressants. J Pharmacol Exp Ther. 2002;303:1227‐1237. [DOI] [PubMed] [Google Scholar]
- 30. Su TP, Hayashi T. Understanding the molecular mechanism of sigma‐1 receptors: towards a hypothesis that sigma‐1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073‐2080. [DOI] [PubMed] [Google Scholar]
- 31. Peeters M, Romieu P, Maurice T, et al. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur J Neurosci. 2004;19:2212‐2220. [DOI] [PubMed] [Google Scholar]
- 32. Kulkarni SK, Dhir A. sigma‐1 receptors in major depression and anxiety. Expert Rev Neurother. 2009;9:1021‐1034. [DOI] [PubMed] [Google Scholar]
- 33. Sarandol A, Sarandol E, Eker SS, et al. Major depressive disorder is accompanied with oxidative stress: short‐term antidepressant treatment does not alter oxidative‐antioxidative systems. Hum Psychopharmacol. 2007;22:67‐73. [DOI] [PubMed] [Google Scholar]
- 34. Maes M, Galecki P, Chang YS, et al. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:676‐692. [DOI] [PubMed] [Google Scholar]
- 35. Zhou XQ, Zeng XN, Kong H, et al. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci Lett. 2008;447:31‐36. [DOI] [PubMed] [Google Scholar]
- 36. Cui HS, Matsumoto K, Murakami Y, et al. Berberine exerts neuroprotective actions against in vitro ischemia‐induced neuronal cell damage in organotypic hippocampal slice cultures: involvement of B‐cell lymphoma 2 phosphorylation suppression. Biol Pharm Bull. 2009;32:79‐85. [DOI] [PubMed] [Google Scholar]
- 37. Arora V, Kuhad A, Tiwari V, et al. Curcumin ameliorates reserpine‐induced pain‐depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology. 2011;36:1570‐1581. [DOI] [PubMed] [Google Scholar]
- 38. Lukic I, Mitic M, Djordjevic J, et al. Lymphocyte levels of redox‐sensitive transcription factors and antioxidative enzymes as indicators of pro‐oxidative state in depressive patients. Neuropsychobiology. 2014;70:1‐9. [DOI] [PubMed] [Google Scholar]
- 39. Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709‐720. [DOI] [PubMed] [Google Scholar]
- 40. Shi X, Li X, Li D, et al. β‐Hydroxybutyrate activates the NF‐κB signaling pathway to promote the expression of pro‐inflammatory factors in calf hepatocytes. Cell Physiol Biochem. 2014;33:920‐932. [DOI] [PubMed] [Google Scholar]
- 41. Li N, Karin M. Is NF‐kappaB the sensor of oxidative stress. FASEB J. 1999;13:1137‐1143. [PubMed] [Google Scholar]
- 42. Giri P, Kumar GS. Isoquinoline alkaloids and their binding with polyadenylic acid: potential basis of therapeutic action. Mini Rev Med Chem. 2010;10:568‐577. [DOI] [PubMed] [Google Scholar]
- 43. Maiti M, Kumar GS. Polymorphic nucleic Acid binding of bioactive isoquinoline alkaloids and their role in cancer. J Nucleic Acids. 2010;2010 https://www.hindawi.com/journals/jna/2010/593408/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu N, Yang H, Cui M, et al. Evaluation of alkaloids binding to the parallel quadruplex structure [d(TGGGGT)]4 by electrospray ionization mass spectrometry. J Mass Spectrom. 2012;47:694‐700. [DOI] [PubMed] [Google Scholar]
- 45. Liu YM, Niu L, Wang LL, et al. Berberine attenuates depressive‐like behaviors by suppressing neuro‐inflammation in stressed mice. Brain Res Bull. 2017;134:220‐227. [DOI] [PubMed] [Google Scholar]
- 46. Joca SR, Guimarães FS. Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant‐like effects. Psychopharmacology. 2006;185:298‐305. [DOI] [PubMed] [Google Scholar]
- 47. Hiroaki‐Sato VA, Sales AJ, Biojone C, et al. Hippocampal nNOS inhibition induces an antidepressant‐like effect: involvement of 5HT1A receptors. Behav Pharmacol. 2014;25:187‐196. [DOI] [PubMed] [Google Scholar]
- 48. Caliceti C, Rizzo P, Cicero AF. Potential benefits of berberine in the management of perimenopausal syndrome. Oxid Med Cell Longev. 2015;2015:723093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee KH, Lo HL, Tang WC, et al. A gene expression signature‐based approach reveals the mechanisms of action of the Chinese herbal medicine berberine. Sci Rep. 2014;4:6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morrow VA, Foufelle F, Connell JM, et al. Direct activation of AMP‐activated protein kinase stimulates nitric‐oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629‐31639. [DOI] [PubMed] [Google Scholar]
- 51. Barbosa VA, Luciano TF, Marques SO, et al. Acute exercise induce endothelial nitric oxide synthase phosphorylation via Akt and AMP‐activated protein kinase in aorta of rats: role of reactive oxygen species. Int J Cardiol. 2013;167:2983‐2988. [DOI] [PubMed] [Google Scholar]
- 52. Heiberg IL, Wegener G, Rosenberg R. Reduction of cGMP and nitric oxide has antidepressant‐like effects in the forced swimming test in rats. Behav Brain Res. 2002;134:479‐484. [DOI] [PubMed] [Google Scholar]
- 53. Herken H, Gurel A, Selek S, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247‐252. [DOI] [PubMed] [Google Scholar]
- 54. Neurauter G, Schröcksnadel K, Scholl‐Bürgi S, et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008;9:622‐627. [DOI] [PubMed] [Google Scholar]
- 55. O'Connor JC, Lawson MA, André C, et al. Lipopolysaccharide‐induced depressive‐like behavior is mediated by indoleamine 2,3‐dioxygenase activation in mice. Mol Psychiatry. 2009;14:511‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y, Lawson MA, Dantzer R, et al. LPS‐induced indoleamine 2,3‐dioxygenase is regulated in an interferon‐gamma‐independent manner by a JNK signaling pathway in primary murine microglia. Brain Behav Immun. 2010;24:201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kang A, Hao H, Zheng X, et al. Peripheral anti‐inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti‐depression efficacy. J Neuroinflammation. 2011;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vaynman S, Ying Z, Gomez‐Pinilla F. Interplay between brain‐derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic‐plasticity. Neuroscience. 2003;122:647‐657. [DOI] [PubMed] [Google Scholar]
- 59. Yu CJ, Zheng MF, Kuang CX, et al. Oren‐gedoku‐to and its constituents with therapeutic potential in Alzheimer's disease inhibit indoleamine 2, 3‐dioxygenase activity in vitro. J Alzheimers Dis. 2010;22:257‐266. [DOI] [PubMed] [Google Scholar]
- 60. Shigeta K, Ootaki K, Tatemoto H, et al. Potentiation of nerve growth factor‐induced neurite outgrowth in PC12 cells by a Coptidis Rhizoma extract and protoberberine alkaloids. Biosci Biotechnol Biochem. 2002;66:2491‐2494. [DOI] [PubMed] [Google Scholar]
- 61. Fan J, Li B, Ge T, et al. Berberine produces antidepressant‐like effects in ovariectomized mice. Sci Rep. 2017;7:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xue W, Zhou X, Yi N, et al. Yueju pill rapidly induces antidepressant‐like effects and acutely enhances BDNF expression in mouse brain. Evid Based Complement Alternat Med. 2013;2013:184367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Réus GZ, MAB DS, Strassi AP, et al. Pathophysiological mechanisms involved in the relationship between diabetes and major depressive disorder. Life Sci. 2017;183:78‐82. [DOI] [PubMed] [Google Scholar]
- 65. Demakakos P, Muniz‐Terrera G, Nouwen A. Type 2 diabetes, depressive symptoms and trajectories of cognitive decline in a national sample of community‐dwellers: a prospective cohort study. PLoS ONE. 2017;12:e0175827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dhingra D, Bhankher A. Behavioral and biochemical evidences for antidepressant‐like activity of palmatine in mice subjected to chronic unpredictable mild stress. Pharmacol Rep. 2014;66:1‐9. [DOI] [PubMed] [Google Scholar]
- 67. Talbott SM, Talbott JA, Pugh M. Effect of Magnolia officinalis and Phellodendron amurense (Relora®) on cortisol and psychological mood state in moderately stressed subjects. J Int Soc Sports Nutr. 2013;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shen JD, Ma LG, Hu CY, et al. Berberine up‐regulates the BDNF expression in hippocampus and attenuates corticosterone‐induced depressive‐like behavior in mice. Neurosci Lett. 2016;614:77‐82. [DOI] [PubMed] [Google Scholar]
- 69. Wang P, Liu C, Liu L, et al. The Antidepressant‐like Effects of Estrogen‐mediated Ghrelin. Curr Neuropharmacol. 2015;13:524‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marshall JK, Thabane M, Garg AX, et al. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut. 2010;59:605‐611. [DOI] [PubMed] [Google Scholar]
- 71. Zhu X, Sun Y, Zhang C, et al. Effects of berberine on a rat model of chronic stress and depression via gastrointestinal tract pathology and gastrointestinal flora profile assays. Mol Med Rep. 2017;15:3161‐3171. [DOI] [PubMed] [Google Scholar]
- 72. Tarrago T, Kichik N, Seguí J, et al. The natural product berberine is a human prolyl oligopeptidase inhibitor. ChemMedChem. 2007;2:354‐359. [DOI] [PubMed] [Google Scholar]
- 73. Cahlíková L, Hulová L, Hrabinová M, et al. Isoquinoline alkaloids as prolyl oligopeptidase inhibitors. Fitoterapia. 2015;103:192‐196. [DOI] [PubMed] [Google Scholar]
- 74. Toide K, Iwamoto Y, Fujiwara T, et al. JTP‐4819: a novel prolyl endopeptidase inhibitor with potential as a cognitive enhancer. J Pharmacol Exp Ther. 1995;274:1370‐1378. [PubMed] [Google Scholar]
- 75. Shishido Y, Furushiro M, Tanabe S, et al. Effects of prolyl endopeptidase inhibitors and neuropeptides on delayed neuronal death in rats. Eur J Pharmacol. 1999;372:135‐142. [DOI] [PubMed] [Google Scholar]
- 76. Lahera G, Freund N, Sáiz‐Ruiz J. Salience and dysregulation of the dopaminergic system. Rev Psiquiatr Salud Ment. 2013;6:45‐51. [DOI] [PubMed] [Google Scholar]
- 77. Michael N, Erfurth A, Ohrmann P, et al. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology. 2003;168:344‐346. [DOI] [PubMed] [Google Scholar]
- 78. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990‐1997: results of a follow‐up national survey. JAMA. 1998;280:1569‐1575. [DOI] [PubMed] [Google Scholar]
- 79. Peng WH, Wu CR, Chen CS, et al. Anxiolytic effect of berberine on exploratory activity of the mouse in two experimental anxiety models: interaction with drugs acting at 5‐HT receptors. Life Sci. 2004;75:2451‐2462. [DOI] [PubMed] [Google Scholar]
- 80. Ye M, Fu S, Pi R, et al. Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. J Pharm Pharmacol. 2009;61:831‐837. [DOI] [PubMed] [Google Scholar]
- 81. Vaziri Z, Abbassian H, Sheibani V, et al. The therapeutic potential of Berberine chloride hydrate against harmaline‐induced motor impairments in a rat model of tremor. Neurosci Lett. 2015;590:84‐90. [DOI] [PubMed] [Google Scholar]
- 82. Murphy JM, Horton NJ, Laird NM, et al. Anxiety and depression: a 40‐year perspective on relationships regarding prevalence, distribution, and comorbidity. Acta Psychiatr Scand. 2004;109:355‐375. [DOI] [PubMed] [Google Scholar]
- 83. Gamez W, Watson D, Doebbeling BN. Abnormal personality and the mood and anxiety disorders: implications for structural models of anxiety and depression. J Anxiety Disord. 2007;21:526‐539. [DOI] [PubMed] [Google Scholar]
- 84. Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Adeyemi OO, Yemitan OK, Taiwo AE. Neurosedative and muscle‐relaxant activities of ethyl acetate extract of Baphia nitida AFZEL. J Ethnopharmacol. 2006;106:312‐316. [DOI] [PubMed] [Google Scholar]
- 86. Kuloglu M, Atmaca M, Tezcan E, et al. Antioxidant enzyme and malondialdehyde levels in patients with panic disorder. Neuropsychobiology. 2002;46:186‐189. [DOI] [PubMed] [Google Scholar]
- 87. Racková L, Májeková M, Kost’álová D, et al. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg Med Chem. 2004;12:4709‐4715. [DOI] [PubMed] [Google Scholar]
- 88. Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999‐1012. [DOI] [PubMed] [Google Scholar]
- 89. Li B, Zhu WL, Chen KX. [Advances in the study of berberine and its derivatives]. Yao Xue Xue Bao. 2008;43:773‐787. [PubMed] [Google Scholar]
- 90. Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin Investig Drugs. 2010;19:1297‐1307. [DOI] [PubMed] [Google Scholar]
- 91. Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid‐ and glucose‐lowering properties: from in vitro evidence to clinical studies. Atherosclerosis. 2015;243:449‐461. [DOI] [PubMed] [Google Scholar]
- 92. Cicero AF, Ferroni A, Ertek S. Tolerability and safety of commonly used dietary supplements and nutraceuticals with lipid‐lowering effects. Expert Opin Drug Saf. 2012;11:753‐766. [DOI] [PubMed] [Google Scholar]
- 93. Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285‐292. [DOI] [PubMed] [Google Scholar]
- 95. Trimarco V, Cimmino CS, Santoro M, et al. Nutraceuticals for blood pressure control in patients with high‐normal or grade 1 hypertension. High Blood Press Cardiovasc Prev. 2012;19:117‐122. [DOI] [PubMed] [Google Scholar]
- 96. Chan E. Displacement of bilirubin from albumin by berberine. Biol Neonate. 1993;63:201‐208. [DOI] [PubMed] [Google Scholar]


