Abstract
Poria cocos is an edible medicinal fungus known as “Fuling” in Chinese and has been used as a Chinese traditional medicine for more than two thousand years. Pharmacological studies reveal that polysaccharide is the most abundant substance in Poria cocos and has a wide range of biological activities including antitumour, immunomodulation, anti‐inflammation, antioxidation, anti‐ageing, antihepatitis, antidiabetics and anti‐haemorrhagic fever effects. As a result, “Poria cocos polysaccharide oral solution” was developed and sold as an over‐the‐counter health supplement since 1970s. In 2015, “Polysaccharidum of Poria cocos oral solution” was approved as a drug by Chinese Food and Drug Administration for treating multiple types of cancers, hepatitis and other diseases alone or during chemo‐ or radiation therapy for patients with cancer. In this article, biochemical, preclinical and clinical studies of Poria cocos polysaccharide from 72 independent studies during the past 46 years (1970‐2016) based on PubMed, VIP (Chongqing VIP Chinese Scientific Journals Database), CNKI (China National Knowledge Infrastructure) and Wanfang database searches are summarized. The structure, pharmacological effects, clinical efficacy, immunobalancing molecular mechanism and toxicity of Poria cocos polysaccharide are deliberated to provide a general picture of Poria cocos polysaccharide as a clinically used antitumour drug.
Keywords: antitumour, clinical application, pharmacological activities, polysaccharides, Poria cocos
1. INTRODUCTION
Poria cocos (Figure 1), known as “Fuling” in Chinese, is an edible medicinal mushroom belonging to dry sclerotium of polyporaceae fungi. It has more than 2000 years of medical application history for its remarkable pharmaceutical effect.1 The bioactive components in Poria cocos include polysaccharides, triterpenoids, fatty acids, sterols and enzymes. Poria cocos polysaccharide (PCP) accounts for 84% by weight among all constituents in the dried sclerotium.2 PCP is also the main bioactive component in Poria cocos.
Figure 1.

The fruiting body of Mushroom Poria cocos. Poria cocos is an edible medicinal fungus known as “Fuling” in Chinese and has been used as a Chinese traditional medicine for more than two thousand years
1.1. The structural composition and properties of PCP
PCP is extracted from the sclerotium of Poria cocos. Different solvent extraction methods can obtain different polysaccharide fractions, such as WPS (NaOH‐HAc), PAP (1 mol/L NaOH), PCP2 (hot water), PCP1 (0.9% NaCI), PCP3‐I and PCP3‐II (0.5 mol/L NaOH), PCP4‐I and PCP4‐II (88% formic acid).3, 4 Thus, PCP is a mixture of different types of polysaccharides with the molecular weight ranged from 4.1 × 104 to 5 × 106 Da.5 Glucose, fucose, arabinose, xylose, mannose and galactose are detected in PCP. β‐Glucan is the major PCP with β‐(1→3)‐linked glucose backbone and β‐(1→6)‐linked glucose side chains as shown in Figure 2.3, 6 The β‐glucan from Poria cocos has poor water solubility but decent anticancer activity.7 Chihara et al removed the β‐(1→6) glucose in the β‐glucan of PCP by periodate oxidation and Smith degradation. The derivative is named “pachymaran,” which exhibits better anti‐S‐180 tumour activities.8 Hamuro et al further improved the water solubility issue of pachymaran by chemical carboxymethylation. The carboxymethylated pachymaran (CMP) has enhanced antitumour activity compared to that of pachymaran.9 Subsequently, different chemical modifications, such as sulfation,10 carboxymethylation plus sulfation,11 methylation, hydroxyethylation and hydroxpropylation, have been conducted and different types of modified pachymarans are reported.12 In general, these chemical modified pachymaran derivatives are water‐soluble and show improved bioactivities.
Figure 2.

A schematic diagram of β‐glucan structure in Poria cocos. β‐Glucan is the major Poria cocos polysaccharide with β‐(1→3)‐linked glucose backbone and β‐(1→6)‐linked glucose side chains. The β‐glucan from Poria cocos has poor water solubility but decent anticancer activity
1.2. PCP‐based drug in China
PCP‐based product named “compound polysaccharide oral solution” was developed in the 1970s and was very popular with consumers as health supplement products. In 2006, “Polysaccharidum of Poria cocos oral solution” was developed by Hunan Butian pharmaceutical company of China and was granted a Chinese patent (200610163425‐X). The major component (95%) in the patented product is CMP. The water solubility of CMP allows 98% of the components to be absorbed through the human digestive track. In 2015, “Polysaccharidum of Poria cocos oral solution” was approved by Chinese Food and Drug Administration with a certified drug number B20050015 for treating multiple types of cancers, hepatitis and other diseases alone or during chemo‐ or radiation therapy for patients with cancer.
1.3. The approach for the literature searching
In this article, a total of 72 publications related to different kinds of Poria cocos polysaccharides (PCPs), pachymaran, the derivatives and the biochemical/preclinical/clinical studies up to date were identified through searching PubMed, VIP (Chongqing VIP Chinese Scientific Journals Database), CNKI (China National Knowledge Infrastructure) and Wanfang database. The reported biological and pharmacological activities of PCP are classified based on these publications and shown in Figures 3 and 4. The pharmacological and other biological functions and possible molecular mechanisms shown in Figures 3 and 4 will be the major topics discussed in this article. Moreover, the data that show PCPs can overcome immunosuppression and adverse reactions associated with radiation therapy and chemotherapy13 will also be presented and discussed.
Figure 3.
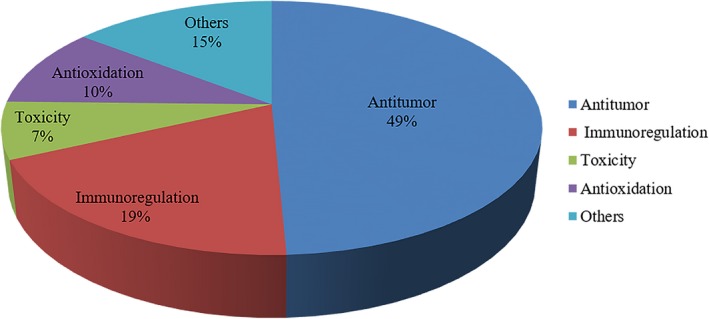
Pharmacological activities of Poria cocos polysaccharides (PCPs). Sixty‐six articles related to pharmacological activities of PCPs are summarized. Thirty‐eight per cent of studies are about antitumour activities of PCPs. Twelve per cent of studies are about antitumour mechanisms. Studies on immunoregulation, antioxidant and toxicity account for 19%, 10% and 7%, respectively. Fourteen per cent of pharmacological activity studies of PCPs are defined as “others” that are further explained in Figure 4
Figure 4.
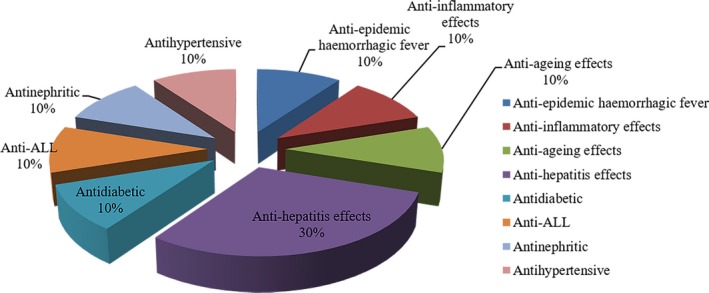
Other pharmacological activities of PCPs. Antihepatitis effects: 30%; antidiabetic effects: 10%; anti‐epidemic haemorrhagic fever: 10%; anti‐ageing effects: 10%; anti‐inflammatory effects: 10%; and anti‐acute lymphoblastic leukaemia (ALL): 10%
2. PHARMACOLOGICAL ACTIVITIES OF PCP
2.1. Antitumour
Parallel to other reported polysaccharides from fungi,14, 15, 16 PCP and its derivatives have more impressive anticancer cell proliferation activities in vivo than in vitro when the same PCP samples are tested both in cancer cell lines and in cancer cell‐injected animal models. The in vitro anticancer cell proliferation effects of PCPs are summarized in Table 1, whereas the in vivo antitumour growth effects of PCPs are summarized in Table 2 from 15 independent studies.17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30
Table 1.
Abbreviation list
| Abbreviation | Full name | Abbreviation | Full name |
|---|---|---|---|
| ALL | Acute lymphoblastic leukaemia | LCT | Lymphocytes transformation |
| ACV | Acyclovir | LLC | Lewis lung carcinoma |
| Bcl‐2 | B‐cell lymphoma‐2 | ‐L | Low dosage |
| Bcap‐37 | Breast carcinoma cells | ‐M | Medium dosage |
| Bax | Bcl‐2 Assaciated X protein | MPCP | Methylated poria cocos polysaccharide |
| BHT | Butylated hydroxytoluene | MAO | Monoamine oxidase |
| CMP | Carboxymethylated pachymaran | MDA | Malondialdehyde |
| CTX | Cyclophosphamide | NS | Normal saline |
| CA | Cortisone acetate | NK | Natural killer cell |
| CP | Hericium erinaceus polysaccharide+Lentinan+Pachymaran | OH | Hydroxyl free radical |
| CPABM | Agaricus blazei murill polysaccharide+Lentinan+CMP | PCP | Poria cocos polysaccharide |
| DAG | Dianhydrogalactitol | PCP1 | Polysaccharide extracted using 0.9% NaCI |
| DXM | Dexamethasone | PCP2 | Polysaccharide extracted using hot water |
| DPPH | 1,1‐Diphenyl‐2‐picrylhydrazyl radical 2,2‐Diphenyl‐1‐(2,4,6‐trinitrophenyl)hydrazyl | PCP3 | Polysaccharide extracted using 0.5 mol/L NaOH |
| EC50 | 50% effective concentration | PCP4 | Polysaccharide extracted using 88% formic acid |
| Ea | Active erythrocyte rosettle test | PTPP | Phosphorylation of protein tyrosine phosphatase |
| Et | Total erythrocyte rosettle test | PT | Pachymaran and triterpens |
| EAC | Ehrlich ascites carcinoma cells | PS | Pachymaran (Sulphated) |
| FP | Ferulic acid pachymaran | Pt | Cisplatin |
| HBsAg | Hepatitis B surface antigen | PAP | Acidic pachymaran |
| HBeAg | Hepatitis Be Antigen | SGC‐7901 | Gastric carcinoma cells |
| HE‐PCP | Hydroxyethylated poria cocos polysaccharide | SOD | Superoxide dismutase |
| HP‐PCP | Hydroxypropylated poria cocos polysaccharide | SGPT | Serum glutamic pyruvic transaminase |
| H22 | H22 hepatoma | TNF | Tumour necrosis factor |
| HPBL | Human peripheral blood lymphocyte | TPK | Tyrosine protein kinase |
| ‐H | High dosage | U‐14 | U‐14 ascitic fluid tumour cells |
| IC50 | Half maximal inhibitory concentration | Vc | Vitamin C |
| IFN‐γ | Interferon‐γ | WPS | Polysaccharide extracted using NaOH‐HAc |
| KSC | Kappa‐selenocarrageenan | 5‐Fu | 5‐fluoro‐2,4(1H,3H) pyrimidinedione |
| LH | Levamisole hydrochloride | ||
| Lewis | Lung carcinoma cell |
Table 2.
Inhibition rates of PCPs on different cancer cells
| Cell types | Groups | Cancer inhibition rates (%) | P‐value | References |
|---|---|---|---|---|
| SGC‐7901 (human) | Distilled water | 0 | 17 | |
| 5‐Fu‐L | 34.98 | |||
| 5‐Fu‐M | 69.92 | |||
| 5‐Fu‐H | 85.05 | |||
| PCP2‐L | 60.09 | |||
| PCP2‐M | 90.04 | |||
| PCP2‐H | 96.10 | |||
| Bcap‐37 (human) | 5‐Fu‐L | 35.41 | ||
| 5‐Fu‐M | 61.04 | |||
| 5‐Fu‐L | 88.72 | |||
| PCP2‐L | 42.48 | |||
| PCP2‐M | 85.09 | |||
| PCP2‐H | 88.54 | |||
| HepG2 (human) | PAP1 | 14.22 ± 1.06 | 4 | |
| PAP2 | 16.65 ± 3.01 | |||
| PAP3 | 6.94 ± 2.08 | |||
| PAP4 | 15.98 ± 4.16 | |||
| PAP5 | 16.71 ± 1.72 | |||
| PAP6 | 16.02 ± 2.65 | |||
| PAP7 | 59.76 ± 5.47 | P < .05 | ||
| PAP8 | 23.45 ± 1.31 | P < .05 | ||
| PAP9 | 78.67 ± 1.68 | P < .01 | ||
| PAP10 | 82.92 ± 2.8 | P < .01 | ||
| K562 (human) | NS | 0 | 30 | |
| PS‐L | 16.95 ± 5.16 | |||
| PS‐M | 27.80 ± 3.57 | |||
| PS‐H | 52.95 ± 1.2 |
PCPs possess decent anticancer cell proliferation effect in vitro, which is measured in cell cultures where different tumour cell lines derived from human or mouse tumours have been tested, such as SGC‐7901 (human), Bcap‐37 (human), HepG2 (human) and K562 (human) cells (Table 2). For example, the inhibition rate for SGC‐7901 cells is 96% compared to that of 88% for Bcap‐37 cells.17 As shown in Table 2, the inhibition rates of PCP or its derivatives on the proliferation of cancer cells are largely concentration‐dependent, which resembles the control drug 5‐fluoro‐2,4(1H,3H) pyrimidinedione or 5‐Fu.
In contrast, in cancer cell‐injected animal models (Table 3), the inhibition rates of PCP or its derivatives on tumour growth are only partially concentration‐dependent in that within a certain range, the higher the concentrations, the higher the inhibition rates are. But beyond the range, the inhibition rates will drop. For example, the experiment conducted by Cheng et al18 showed that the inhibition rates of cancer cell growth are 0, 69%, 87%, 92% and 89%, respectively, with increasing CMP concentrations.
Table 3.
Tumour inhibition rates of PCPs in animal models
| Models | Administration route | Cell types | Groups | Tumour weight (g) | P‐value | Tumour inhibition rates (%) | P‐value | References |
|---|---|---|---|---|---|---|---|---|
| ICR mice | Intragastric administration | S‐180(mouse) | Distilled water | 2.742 ± 0.378 | 0 | 20 | ||
| 5‐Fu | 1.341 ± 0.135 | P < .001 | 45.73 | |||||
| CMP‐L | 1.972 ± 0.399 | P < .05 | 2.23 | |||||
| CMP‐H | 1.675 ± 0.412 | P < .01 | 32.22 | |||||
| 5‐Fu+CMP‐L | 1.413 ± 0.394 | P < .001 | 42.83 | |||||
| 5‐Fu+CMP‐H | 1.283 ± 0.483 | P < .001 | 48.11 | |||||
| ICR/JCL mice | Intraperitoneal injection | U‐14 (mouse) | NS | 1.385 ± 0.101 | 0 | 18 | ||
| CMP (30 mg/kg) | 0.433 ± 0.105 | P < .01 | 68.7 | |||||
| CMP (120 mg/kg() | 0.182 ± 0.121 | P < .01 | 86.9 | |||||
| CMP (180 mg/kg) | 0.108 ± 0.053 | P < .01 | 92.2 | |||||
| CMP (360 mg/kg) | 0.151 ± 0.113 | P < .01 | 89.1 | |||||
| Kunming mice | Intraperitoneal injection | S‐180 (mouse) | NS | 1.23 ± 0.11 | 0 | 21 | ||
| CTX | 0.49 ± 0.07 | P < .01 | 60.21 | |||||
| WPS‐L | 0.99 ± 0.10 | 19.53 | ||||||
| WPS‐M | 0.89 ± 0.12 | P < .01 | 28.01 | |||||
| WPS‐H | 0.72 ± 0.08 | P < .01 | 43.94 | |||||
| WPS1‐L | 0.98 ± 0.13 | 2.32 | ||||||
| WPS1‐M | 0.96 ± 0.06 | P < .1 | 22.45 | |||||
| WPS1‐H | 0.70 ± 0.07 | P < .01 | 41.57 | |||||
| WPS2‐L | 1.09 ± 0.08 | 11.45 | ||||||
| WPS2‐M | 0.98 ± 0.10 | P < .1 | 2.32 | |||||
| WPS2‐H | 0.74 ± 0.04 | P < .01 | 39.81 | |||||
| Kunming mice | Intragastric administration | S‐180 (mouse) | NS | 0.753 ± 0.191 | 0 | 22 | ||
| CTX | 0.155 ± 0.091 | P < .05 | 79 | |||||
| PCP‐L | 0.437 ± 0.117 | P < .05 | 42 | |||||
| PCP‐M | 0.482 ± 0.105 | P < .05 | 36 | |||||
| PCP‐H | 0.527 ± 0.152 | P < .05 | 30 | |||||
| BALB/c mice | Intraperitoneal injection | S‐180 (mouse) | PBS | 1.61 ± 0.32 | 0 | 19 | ||
| 5‐Fu | 0.76 ± 0.16 | 52.76 | P < .01 | |||||
| PCP3‐II‐L | 1.56 ± 0.42 | 2.46 | ||||||
| PCP3‐II‐H | 1.57 ± 0.66 | 3.02 | ||||||
| PS‐L | 1.39 ± 0.27 | 13.88 | ||||||
| PS‐H | 1.21 ± 0.41 | 34.63 | P < .05 | |||||
| CMP‐L | 1.23 ± 0.48 | 23.45 | P < .05 | |||||
| CMP‐H | 1.04 ± 0.20 | 35.27 | P < .01 | |||||
| MPCP3‐II‐L | 1.34 ± 0.46 | 16.65 | ||||||
| MPCP3‐II‐H | 1.22 ± 0.38 | 24.48 | P < .05 | |||||
| HE‐PCP3‐II‐L | 1.63 ± 0.36 | |||||||
| HE‐PCP3‐II‐H | 1.29 ± 0.29 | 20.20 | ||||||
| HP‐PCP3‐II‐L | 1.45 ± 0.27 | 10.00 | ||||||
| HP‐PCP3‐II‐H | 1.37 ± 0.48 | 14.88 | ||||||
| PBS | 1.40 ± 0.32 | 0 | ||||||
| 5‐Fu | 0.76 ± 0.27 | 46.0 | P < .01 | |||||
| PS‐2‐L | 1.09 ± 0.26 | 22.32 | ||||||
| PS‐2‐H | 0.90 ± 0.36 | 35.71 | P < .05 | |||||
| PS‐4‐L | 0.89 ± 0.18 | 36.51 | P < .05 | |||||
| PS‐4‐H | 0.97 ± 0.36 | 30.95 | ||||||
| PS‐5‐L | 0.91 ± 0.34 | 34.92 | P < .05 | |||||
| PS‐5‐H | 0.86 ± 0.26 | 38.39 | P < .05 | |||||
| PS‐6‐L | 1.14 ± 0.12 | 18.25 | ||||||
| PS‐6‐H | 1.16 ± 0.39 | 17.35 | ||||||
| PS‐9‐L | 0.92 ± 0.29 | 33.93 | P < .05 | |||||
| PS‐9‐H | 0.93 ± 0.38 | 33.33 | P < .05 | |||||
| BalB/c mice | Intraperitoneal injection | S‐180 (mouse) | PBS | 1.407 ± 0.32 | 0 | 23 | ||
| 5‐Fu | 0.867 ± 0.26 | 46 | P < .01 | |||||
| PS | 0.767 ± 0.27 | 38.39 | P < .05 | |||||
| ICR/JCL mice | Intragastric administration | S‐180 (mouse) | NS | 1.989 ± 0.594 | 0 | 24 | ||
| 5‐Fu | 0.363 ± 0.286 | P < .01 | 81.7 | |||||
| CMP‐L | 1.282 ± 0.166 | P < .05 | 35.5 | |||||
| CMP‐M | 1.159 ± 0.126 | P < .05 | 41.6 | |||||
| CMP‐H | 0.963 ± 0.364 | P < .05 | 51.6 | |||||
| Intravenous injection | S‐180 (mouse) | NS | 3.425 ± 0.958 | 0 | ||||
| 5‐Fu | 0.323 ± 0.261 | P < .01 | 90.6 | |||||
| CMP‐L | 2.106 ± 1.037 | P < .05 | 38.5 | |||||
| CMP‐M | 2.294 ± 1.037 | P < .05 | 32 | |||||
| CMP‐H | 2.049 ± 0.752 | P < .05 | 40.2 | |||||
| Intragastric administration | H22 (mouse) | NS | 1.721 ± 0.571 | 0 | ||||
| 5‐Fu | 0.269 ± 0.230 | P < .01 | 84.65 | |||||
| CMP‐L | 0.760 ± 0.470 | P < .05 | 55.58 | |||||
| CMP‐M | 1.044 ± 0.438 | P < .05 | 39.03 | |||||
| CMP‐H | 0.644 ± 0.438 | P < .01 | 62.24 | |||||
| ICR/JCL mice | Intraperitoneal injection | U‐14 (mouse) | NS | 1.225 ± 0.122 | 0 | 25 | ||
| CMP (25 mg/kg) | 0.253 ± 0.11 | P < .01 | 79.4 | |||||
| CMP (50 mg/kg) | 0.26 ± 0.137 | P < .01 | 78.8 | |||||
| CMP (100 mg/kg) | 0.089 ± 0.003 | P < .01 | 92.7 | |||||
| CMP (500 mg/kg) | 0.30 ± 0.095 | P < .01 | 75.5 | |||||
| Intravenous injection | S‐180 (mouse) | NS | 3.431 ± 1.136 | 0 | ||||
| CMP‐L | 2.237 ± 0.977 | P < .05 | 34.8 | |||||
| CMP‐M | 2.141 ± 0.969 | P < .05 | 37.6 | |||||
| CMP‐H | 1.339 ± 0.683 | P < .01 | 61 | |||||
| Intravenous injection | H22 (mouse) | NS | 2.167 ± 0.812 | 0 | ||||
| CMP‐L | 1.732 ± 0.988 | 20.1 | ||||||
| CMP‐M | 1.372 ± 0.673 | P < .05 | 36.7 | |||||
| CMP‐H | 1.485 ± 0.931 | P < .05 | 31.5 | |||||
| NIH mice | Intragastric administration | S‐180 (mouse) | NS | 1.34 ± 0.32 | 0 | 26 | ||
| 5‐Fu | 0.54 ± 0.43 | P < .001 | 59.7 | P < .01 | ||||
| CMP‐L | 0.48 ± 0.34 | P < .001 | 64.18 | P < .01 | ||||
| CMP‐M | 0.70 ± 0.36 | P < .001 | 47.76 | P < .05 | ||||
| CMP‐H | 0.51 ± 0.53 | P < .001 | 61.94 | P < .01 | ||||
| S‐180 (mouse) | NS | 1.30 ± 0.22 | 0 | |||||
| 5‐Fu | 0.67 ± 0.14 | P < .001 | 48.46 | P < .05 | ||||
| CMP‐L | 0.54 ± 0.12 | P < .001 | 58.46 | P < .01 | ||||
| CMP‐M | 0.78 ± 0.14 | P < .001 | 40.00 | P < .05 | ||||
| CMP‐H | 0.74 ± 0.16 | P < .001 | 43.08 | P < .05 | ||||
| EAC(mouse) | NS | 1.06 ± 0.16 | 0 | |||||
| 5‐Fu | 0.45 ± 0.16 | P < .001 | 57.55 | P < .01 | ||||
| CMP‐L | 0.43 ± 0.18 | P < .001 | 59.43 | P < .001 | ||||
| CMP‐H | 0.54 ± 0.21 | P < .001 | 49.06 | P < .05 | ||||
| EAC(mouse) | NS | 1.02 ± 0.15 | 0 | |||||
| 5‐Fu | 0.41 ± 0.19 | P < .001 | 59.80 | P < .01 | ||||
| CMP‐L | 0.51 ± 0.28 | P < .001 | 50.00 | P < .01 | ||||
| CMP‐H | 0.62 ± 0.25 | P < .001 | 39.22 | P < .05 | ||||
| ICR/ICJ mice | Intragastric administration | S‐180 (mouse) | NS | 2.49 ± 0.42 | 0 | 28 | ||
| PCP‐L | 1.29 ± 0.28 | P < .01 | 48.1 | |||||
| PCP‐M | 1.46 ± 0.46 | P < .01 | 41.37 | |||||
| PCP‐H | 2.25 ± 0.67 | .96 | ||||||
| CFW mice | Intraperitoneal injection | S‐180 (mouse) | NS | 5.1 ± 0.9 | 0 | 29 | ||
| PCP‐H | 2.9 ± 1.0 | 43.1 | P < .01 | |||||
| PCP‐M | 3.1 ± 1.1 | 39.2 | P < .01 | |||||
| PCP‐L | 3.2 ± 1.7 | 37.3 | P < .01 | |||||
| Rats | Intraperitoneal injection | S‐180 (mouse) | NS | 10.2 ± 2.6 | 0 | 27 | ||
| PCP | 5.77 ± 2.7 | 39.9 | P < .01 | |||||
| PS | 6.10 ± 3.0 | 43.2 | P < .01 |
The inhibition of tumour growth in vivo is measured either by the reduced tumour weight or by ultrasound compared to controls in different animal models and presented as tumour inhibition rates (%). The controls include blank and positive controls where the chemotherapeutic drug, such as 5‐fluoro‐2, 4 (1H, 3H) pyrimidinedione (5‐Fu), is used. PCPs have potent antitumour activities in different animal tumour models when compared to blank or 5‐Fu controls (Table 3). When PCP is modified with different chemical groups, such as carboxymethyl, sulphate, methyl, hydroxypropyl and hydroxyethyl, the derivatives exhibit better inhibition rates. For example, Wang et al extracted six polysaccharides from the fresh sclerotium of Poria cocos using different solvents sequentially, including PCP1 (0.9% NaCl), PCP2 (hot water), PCP3‐I and PCP3‐II (0.5 mol/L NaOH), PCP4‐I and PCP4‐II (88% formic acid). They then modified PCP3‐II chemically and obtained five different PCP3‐II derivatives.3 They showed19 that all the derivatives inhibit tumour growth better than PCP3‐II with the inhibiting rates increased by 36% and 35% for sulphated and carboxymethyled derivatives, respectively, and by 24%, 15% and 20% for methylated, hydroxypropylated and hydroxyethylated derivatives, respectively. They further demonstrated that the increased degree of chemical modifications and the increased molecular weight in the derivatives correlate with better inhibiting rates in vivo.
Furthermore, the curing effects can be further enhanced when PCPs are combined with chemotherapeutic drugs in different animal models. In addition, the combined therapy also reduces the adverse effects associated with chemotherapeutic drugs (Table 4). For example, Liu et al31 Tang et al32 and Chen et al29 reported that PCPs exhibit better tumour inhibition rates when it is used with other chemotherapeutic drugs. For instance, inhibition rates were 46% when PCP is used with 5‐Fu compared with the inhibition rates of 41% when 5‐Fu is used alone.29
Table 4.
Tumour inhibition rates of PCPs ± chemotherapy on mice
| Models | Administration routs | Cell types | Groups | Tumour weight(g) | P‐value | Tumour inhibition rates (%) | P‐value | References |
|---|---|---|---|---|---|---|---|---|
| Kunming mice | Intragastric administration | S‐180 (mouse) | NS | 3.01 ± 0.38 | 0 | 31 | ||
| CTX | 0.84 ± 0.21 | P < .01 | 72.1 | |||||
| CPABM‐L | 2.10 ± 0.28 | P < .01 | 30.2 | |||||
| CPABM‐M | 1.43 ± 0.24 | P < .01 | 52.5 | |||||
| CPABM‐H | 2.69 ± 0.32 | 10.6 | ||||||
| CPABM+CTX‐L | 0.52 ± 0.16 | P < .01 | 82.7 | |||||
| CPABM+CTX‐M | 0.50 ± 0.19 | P < .01 | 83.4 | |||||
| CPABM+CTX‐H | 1.03 ± 0.31 | P < .01 | 65.8 | |||||
| Kunming and NIH mice | Intragastric administration | S‐180 (mouse) | NS | 1.89 ± 0.24 | 0 | 32 | ||
| CP‐L | 1.18 ± 0.29 | P < .05 | 37.73 ± 13.11 | |||||
| CP‐M | 1.03 ± 0.36 | P < .05 | 44.74 ± 19.33 | |||||
| CP‐H | 0.98 ± 0.26 | P < .05 | 48.34 ± 13.08 | |||||
| CFW mice | Intraperitoneal injection | S‐180 (mouse) | NS | 1.4 ± 0.6 | 0 | 29 | ||
| 5‐Fu | 0.7 ± 0.2 | 50 | P < .05 | |||||
| CTX | 1.1 ± 0.4 | 21.4 | ||||||
| DAG | 0.9 ± 0.4 | 35.7 | P < .05 | |||||
| PCP | 1.0 ± 0.4 | 28.6 | ||||||
| PCP+5‐Fu | 0.9 ± 0.3 | 35.7 | P < .05 | |||||
| PCP+CTX | 0.8 ± 0.3 | 42.9 | P < .05 | |||||
| PCP+DAG | 0.8 ± 0.3 | 42.9 | P < .05 |
2.2. Antitumour mechanisms
PCPs exert their antitumour activity via assisting the host to overcome adverse biological stresses, to increase immunity against the tumours and to promote the apoptosis of tumour cells directly. The possible mechanisms reported so far are summarized in Table 5A‐D and Figure 5, 33, 34, 35, 36, 37, 38 and discussed below.
Table 5.
(A) Antitumour mechanisms of PCP: impact on expression rates of Fas, Bcl‐2 and Bax, the ratio of lymphocyte, killing activity of NK cell, IFN‐γ, TNF, phagocytic ability and IL‐2 (B) Antitumour mechanisms of PCP: impact on IFN‐γ, TNF, phagocytic ability, IL‐6, TPK in cytoplasm and cytomembrane, PTPP in cytoplasm and cytomembrane (C) Antitumour mechanisms of PCP: impact on phagocytic ability, thymus index, spleen index and IFN‐r (D) Antitumour mechanisms of PCP: impact on haemolysin, IL‐4, IgA in serum, IgG in serum and IgM in serum
| Models | Administration routs | Cell types | Groups | Expression rates (%) | The ratio of lymphocyte (%) | Killing activity of NK cell (%) | IFN‐γ (IU/mL) | TNF (ug/mL) | Phagocytic ability (%) | IL‐2 (IU) | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fas | Bcl‐2 | Bax | |||||||||||
| (A) | |||||||||||||
| BALB/c mice | Intraperitoneal injection | S‐180 | PBS | 4.12 | 47.96 | 2.45 | 33 | ||||||
| PS | 64.99 | 17.97 | 48.05 | ||||||||||
| BALB/c mice | Intraperitoneal injection | H22 | NS | 46.52 | 45.38 | 0.08 (TNF‐α) | 34 | ||||||
| CMP‐L | 52.55 | 52.64 | 0.128 (TNF‐α) | ||||||||||
| CMP‐M | 62.39 | 61.88 | 0.134 (TNF‐α) | ||||||||||
| CMP‐H | 56.15 | 54.45 | 0.135 (TNF‐α) | ||||||||||
| NIH mice | Intragastric administration | EAC | NS | 12.01 | 8.05 × 10^5 | 26 | |||||||
| 5‐Fu | 16.01 | 12.28 × 10^5 | |||||||||||
| CMP‐L | 14.82 | 8.5 × 10^5 | |||||||||||
| CMP‐H | 15.56 | 9.69 × 10^5 | |||||||||||
| NS | 24.92 | ||||||||||||
| NS+CTX | 13.01 | ||||||||||||
| CMP‐L+CTX | 19.52 | ||||||||||||
| CMP‐H+CTX | 21.67 | ||||||||||||
| CFW mice | Intraperitoneal injection | S‐180 | NS | 9.3 | 29 | ||||||||
| PCP‐L | 21.9 | ||||||||||||
| PCP‐H | 24.5 | ||||||||||||
| Models | Administration routs | Cell types | Groups | IFN‐γ (IU/mL) | TNF (ug/mL) | Phagocytic ability (%) | IL‐6 (IU) | TPK in cytoplasm (min−1× μn−1) | TPK in cytomembrane (min−1 × μg−1) | PTPP in cytoplasm (min−1 × μg−1) | PTPP in cytomembrane (min−1 × μg−1) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (B) | ||||||||||||
| ICR/ICJ mice | Subcutaneous injection | LLC | NS | 24.44 | 36 | |||||||
| CMP | 59.26 | |||||||||||
| S‐180 | NS | 26.88 | ||||||||||
| CMP | 45.92 | |||||||||||
| Cancer cells | HPBL | NS | 5.0 | 1299.3 | 35 | |||||||
| CMP‐L | 22.2 | 1356.3 | ||||||||||
| CMP‐M | 41.9 | 1837.4 | ||||||||||
| CMP‐H | 48.3 | 1880.6 | ||||||||||
| Cancer cells | HL‐60 | Control | 105.1 | 197.8 | 1.93 | 0.97 | 37 | |||||
| PCP | 62.5 | 158.1 | 6.08 | 3.14 | ||||||||
| Cancer cells | HPBL | Control | 3400 | 315 (U/mL) | 4232 | 38 | ||||||
| CMP | 6216 | 450 (U/mL) | 6478 | |||||||||
| Models | Administration routs | Groups | Phagocytic ability (%) | Thymus index | Spleen index | IFN‐γ (pg/mL) | References |
|---|---|---|---|---|---|---|---|
| (C) | |||||||
| Kunming mice | Intragastric administration | NS | 45.5 | 2.19 | 2.10 | 42 | |
| LH | 67.83 | 2.73 | 2,92 | ||||
| PT‐L | 58.83 | 2.72 | 2.85 | ||||
| PT‐M | 70.17 | 2.74 | 2.93 | ||||
| PT‐H | 69.67 | 2.73 | 2.91 | ||||
| C57BL/6 mice | Intravenous injection | NS | 1.15 | 44 | |||
| Pt | 0.42 | ||||||
| PCP‐L | 1.1 | ||||||
| PCP‐H | 1.41 | ||||||
| Kunming mice | Intraperitoneal injection | NS | 3.09 | 45 | |||
| DXM | 1.11 | ||||||
| PS+DXM | 1.89 | ||||||
| PS | 2.84 | ||||||
| BALB/c mice | Intraperitoneal injection | NS | 77.00 | 43 | |||
| CMP | 248.33 | ||||||
| NIH mice | Intragastric administration | NS | 58.36 | 47.14 | 73.00 | 26 | |
| CTX | 37.64 | 23.17 | 42.34 | ||||
| CMP‐L | 48.36 | 27.91 | 43.55 | ||||
| CMP‐M | 51.09 | 30.28 | 54.88 | ||||
| CMP‐H | 55.18 | 36.73 | 58.69 | ||||
| ICR/ICJ mice | Subcutaneous injection | NS | 39.65 | 36 | |||
| CMP | 59.26 | ||||||
| NS | 30.39 | ||||||
| CA | 18.86 | ||||||
| CA+CMP | 34.81 | ||||||
| CMP | 50.48 | ||||||
| NS | 24.75 | 56.84 | |||||
| CTX | 22.25 | 45.7 | |||||
| CTX+CMP | 19.08 | 45.57 | |||||
| CMP | 33.82 | 69.87 | |||||
| Models | Administration routs | Groups | Haemolysin (OD450) | IL‐4 (pg/mL) | IgA in serum (μg/mL) | IgG in serum (ng/mL) | IgM in serum (ng/mL) | References |
|---|---|---|---|---|---|---|---|---|
| (D) | ||||||||
| Kunming mice | Intragastric administration | NS | 61.45 | 286.64 | 2.23 | 46 | ||
| LH | 97.32 | 378.46 | 2.81 | |||||
| PCP (60%)‐L | 103.67 | 376.19 | 2.78 | |||||
| PCP (60%)‐M | 122.38 | 570.30 | 3.53 | |||||
| PCP (60%)‐H | 139.65 | 706.79 | 4.58 | |||||
| Kunming mice | Intragastric administration | NS | 0.4081 | 67.8 | 852.1 | 655.4 | 47 | |
| CTX | 0.1891 | 43.8 | 585.5 | 302.4 | ||||
| CTX+PCP‐L | 0.2630 | 56.6 | 773.3 | 465.2 | ||||
| CTX+PCP‐H | 0.2949 | 64.1 | 822.5 | 504.1 | ||||
| PCP | 0.4967 | 84.1 | 1071.2 | 655.4 | ||||
Figure 5.
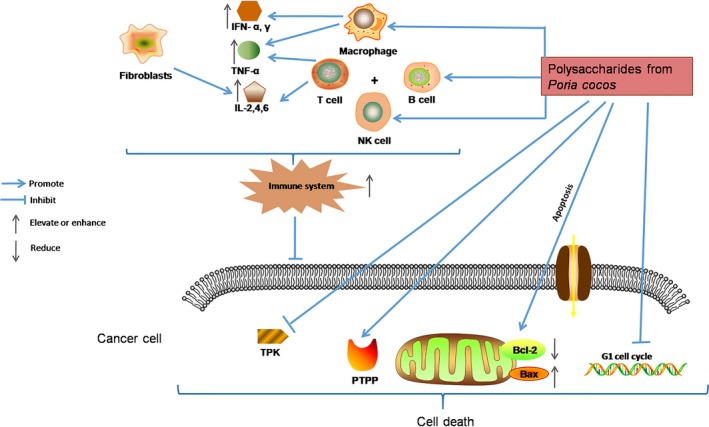
Possible antitumour mechanisms of PCPs. PCPs exert their antitumour activity via assisting the host to overcome adverse biological stresses, to assist the host to enhance the lethality of macrophages, T cells, B cells and NK cells by releasing cytokines to increase immunity, and to promote the apoptosis of tumour cells directly by up‐regulating the expression of apoptosis‐related genes
2.2.1. Enhancing the innate immunity through activating the immune cells
Polysaccharides could activate effector immune cells, such as macrophages, lymphocytes and natural killer (NK) cells to activate the innate immune system to exert antitumour activity by accelerating the host's defence mechanisms.33 It is reported that the ratio of lymphocytes could increase to 62% in the treatment group compared with control group (47%)34 and phagocytic ability of macrophages could reach 59% compared with control group of 27%.36
2.2.2. Increasing cytokine levels
TNF (tumour necrosis factor) secreted by macrophages and lymphocytes is used as a drug for tumour biotherapy. IL‐6 (interleukin‐6) secreted by T lymphocytes and fibroblasts improves the killing ability of NK cells. Miu et al and Chen et al found that carboxymethyl pachymaran could improve the levels of IFN‐γ, IL‐2, TNF and IL‐6.35, 38
2.2.3. Stimulating the expression of apoptosis‐related genes
Bcl‐2 and Bax are members of the Bcl‐2 family, which are important for the regulation of apoptosis. The Bax/Bcl‐2 ratio determines the survival of cells. Meng reported that sulphated pachymaran could enhance the expression of apoptosis‐related genes Fas and Bax and reduce the expression of Bcl‐2 gene. The increased Bax/Bcl‐2 ratio is responsible for the apoptosis of S180 tumour cells in the mouse model.33 Zhang et al showed that WPS could inhibit the growth of human breast carcinoma MCF‐7 cells by inducing G1 arrest of the cell cycle and by elevating the Bax/Bcl‐2 ratio.39
2.2.4. Regulating the activities of TPK and PTPP in cancer cells
TPK (tyrosine protein kinase) and PTPP (phosphotyrosine protein phosphatase) are two important enzymes controlling the growth, proliferation and differentiation of cells. Several studies reported that the activities of TPK and PTPP have changed significantly when the cells become cancerous, but PCP normalizes the activities of TPK and PTPP in cancer cells and slowdowns cancer cell growth.37
2.3. Immunomodulation
Studies showed that PCP could enhance host immune function and activate the immune response.26, 36, 40, 41, 42, 43, 44, 45, 46, 47 It is reported that PCPs could modulate their specific immune response via the activation of T cells.40 Pachymaran strongly enhances the generation of alloreactive cytotoxic T lymphocytes in vivo.41 Generally speaking, the strength of the immune function can be judged through three aspects: the capability of macrophage's phagocytosis, the cellular immune function by detecting the index of immune organs42, 43 and the humoural immunity function by measuring the production of antibodies and serum haemolysins. As shown in Table 5C,D, Zhang et al and Xu et al found that the capability of macrophage's phagocytosis, thymus index and spleen index have significantly improved by PCP.26, 42 Zhang et al and Peng et al reported that the levels of IgA, IgG and IgM in serum are increased and the levels of IFN‐γ and IL‐4 are also enhanced by PCPs.46, 47, 48
2.4. Antioxidation
Free radicals refer to the dissociative molecules, atoms or ions with unpaired electron that reacting rapidly with other substances. Under certain range, free radicals can help to eliminate microorganisms intruding into body or abnormal cells. However, if free radicals are excessively produced, they would attack the normal cells and tissues. Therefore, human body constantly produces and removes free radicals to maintain a dynamic balance. Studies showed that PCP has antioxidant activity by scavenging free radicals. As shown in Table 6,49, 50, 51, 52, 53 Li et al and Zhang et al found that PCP2 and FP could clear free radicals (O2 −, ·OH and DPPH·) and the clearance rate of DPPH· could reach 93%.51, 52 Wang et al also found that carboxymethylated PCP has DPPH, O2 − and ·OH radical‐scavenging activity in vitro.54, 55 Superoxide dismutase (SOD) is an important antioxidant enzyme in organisms and Chen et al showed that CMP could increase the expression of SOD and reduce the amount of MAD (malondialdehyde).53
Table 6.
Antioxidant effects of PCPs
| Groups | Deoxidization (abs) | Antioxidation (abs) | Clearance rate (%) | EC50 (g/L) | MDA in serum (nmol/mL) | MDA in hepar (nmol/mg·pro) | SOD activity in serum (U/mL) | SOD activity in hepar (U/mg·pro) | IC50 (mg/mL) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| BHT | 0.912 | 0.406 | 49 | |||||||
| PCP | 0.490 | 0.546 | ||||||||
| CMP | 2.5 (OH) | 50 | ||||||||
| Vc | 0.2 (·OH) | |||||||||
| CMP | >1.5 (O2 −) | |||||||||
| Vc | 1.5 (O2 −) | |||||||||
| PCP (8 mg/mL) | 76.7 (·OH) | 51 | ||||||||
| PCP (10 mg/mL) | 59.3 (O2 −) | |||||||||
| PCP (5 mg/mL) | 93.4 (DPPH) | |||||||||
| FP (4 mg/mL) | 58.72 (·OH) | 2.5(·OH) | 52 | |||||||
| FP (4 mg/mL) | 39.7 (DPPH·) | |||||||||
| CMP | 2.57 (O2 −) | 53 | ||||||||
| 7.66 (·OH) | ||||||||||
| 4.56 (H2O2) | ||||||||||
| NS | 12.38 | 40.54 | 9.12 | 187.56 | ||||||
| CMP‐L | 12.05 | 31.75 | 9.87 | 190.52 | ||||||
| CMP‐M | 11.52 | 28.53 | 10.25 | 204.78 | ||||||
| CMP‐H | 8.74 | 24.62 | 12.67 | 224.63 |
2.5. Other pharmacological activities
PCPs have other biological effects, such as anti‐inflammatory,56 anti‐ageing,57 antihepatitis,58, 59 antidiabetic,60 anti‐ALL (acute lymphoblastic leukaemia),61 anti‐nephritic62 and antihypertensive effects.63 These pharmacological activities are summarized in Table 7A,B. Hou et al observed that PCP reduces the size of granuloma.56 As an anti‐ageing reagent, PCPs enhance the activities of both T‐SOD and Cu‐SOD and reduce MAD and MAO (monoamine oxidase) activities.57 To understand the antihepatitis effects of CMP, it is found that CMP reduces the expression of HBsAg and HBeAg in a concentration‐dependent manner.58 Zheng et al reported that PCP could reduce blood glucose level and increase the weight of mice in a diabetic mouse model.60 Meanwhile, CMP significantly improves the survival rates of mice suffering acute lymphoblastic leukaemia or ALL.61
Table 7.
(A) Other pharmacological effects of PCPs: anti‐inflammatory, anti‐ageing and antihepatitis effects (B) Other pharmacological effects of PCPs: antidiabetic and anti‐acute lymphoblastic leukaemia effects
| Other effects | Groups | Swelling degree (mg) | Granuloma (mg/10 g) | T‐SOD | Cu‐SOD | MDA | MAO | Death time of swimming (min) | Inhibition of HBsAg (%) | Inhibition of HBeAg (%) | Inhibition of Anti‐HBc | Inhibition of SGPT | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | |||||||||||||
| Anti‐inflammatory effects | NS | 6.2 | 47 | 56 | |||||||||
| DXM | 3.2 | 13 | |||||||||||
| PCP‐L | 3.6 | 36 | |||||||||||
| PCP‐M | 7.4 | 40 | |||||||||||
| PCP‐H | 8.5 | 38 | |||||||||||
| Anti‐ageing effects | Double distilled water | 108 | 46 | 7.1 | 16 | 2.48 | 57 | ||||||
| PCP‐L | 114 | 54 | 6.7 | 19 | 3.68 | ||||||||
| PCP‐M | 125 | 60 | 6.5 | 16 | 4.93 | ||||||||
| PCP‐H | 127 | 62 | 6.3 | 13 | 6.15 | ||||||||
| Antihepatitis effects | ACV‐L | 23.5 | 18.7 | 58 | |||||||||
| ACV‐M | 50.7 | 30.5 | |||||||||||
| ACV‐H | 61.4 | 51.1 | |||||||||||
| CMP (1.5 g/L) | 37.4 | 30.4 | |||||||||||
| CMP (3.0 g/L) | 46.3 | 47.0 | |||||||||||
| CMP (6.0 g/L) | 56.2 | 58.5 | |||||||||||
| CMP (12.0 g/L) | 71.8 | 65.3 | |||||||||||
| Traditional Chinese medicine | 22.3 | 36.3 | 28.5 | 32.3 | 59 | ||||||||
| CMP‐induced IFN‐α+ Traditional Chinese medicine | 52.1 | 95.4 | 75 | 80 | |||||||||
| Other effects | Groups | The weight of mice after 30 days (g) | Blood glucose of mice after 30 days (mg %) | Life prolonging rates (%) | Expression rates of Bcl‐2 | References |
|---|---|---|---|---|---|---|
| (B) | ||||||
| Antidiabetic effects | Distilled water | 163.8 | 19.72 | 60 | ||
| Melbine | 168.64 | 11.35 | ||||
| PCP‐L | 201.32 | 15.4 | ||||
| PCP‐H | 213.58 | 15.16 | ||||
| Effects on ALL mice | NS | 0 | 18.4 | 61 | ||
| CTX | 58.84 | 40.5 | ||||
| KSC | 25.83 | 68.9 | ||||
| CMP | 35.88 | 36.3 | ||||
| KSC+CMP | 43.97 | 40.1 | ||||
| KSC+CMP+CTX | 79.68 | 26.7 | ||||
IFN‐α was obtained from human peripheral blood lymphocytes treated with CMP.
3. CLINICAL EFFICACY OF PCP
Seven clinical studies on PCPs were found through literature search.59, 64, 65, 66, 67, 68 The clinical data are summarized in Table 8. Most of studies are related to the antitumour effects of CMP where these studies use IL‐2 or IFN‐α obtained from human peripheral blood lymphocytes induced by CMP in combination with chemotherapy or radiotherapy. Sheng et al reported that the total effective rate could reach 97% when CMP‐induced IL‐2 is combined with chemotherapy during cancer treatment compared to that of 27% by chemotherapy alone.65 The effectiveness is defined as improving the symptoms of the disease by increasing appetite, elevating the levels of cAMP in blood circulation, regulating the ratio of cAMP/cGMP, protecting and restoring damaged liver and reducing the side effects of chemotherapy.66 In treating epidemic haemorrhagic fever, cure rate could reach 100% when CMP‐induced IFN‐α is combined with normal therapy compared to that of 63% with normal therapy alone.64 Chen et al also found that the total effective rate could reach 90% during hepatitis treatment.66 The changes of immune functions are measured with Et (total erythrocyte rosettle test), Ea (active erythrocyte rosettle test) and LCT (lymphocytes transformation) during PCP treatment. These values are significantly increased compared to the control groups when PCP is combined with chemotherapy67 (Table 8).
Table 8.
PCP‐related clinical studies
| Diseases types | Cases | Groups | Total effective rate (%) | NK cell activity (%) | Cure rate (%) | Et (%) | LCT (%) | Ea (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| Antitumour effects | 71 | Mitomycin/cis‐platinum/pharmorubicin/5‐Fu | 25.9 | 63 | |||||
| Mitomycin/cis‐platinum/pharmorubicin/5‐Fu + CMP‐induced IL‐2 | 86.7 | ||||||||
| 44 | Mitomycin/cis‐platinum/doxorubicin/5‐Fu + CMP‐induced IL‐2 | 86.4 | 65 | ||||||
| 37 | 60Co radiotherapy + CMP‐induced IL‐2 | 97.3 | |||||||
| 77 | 60Co radiotherapy + CMP‐induced IFN‐α | 97.4 | 59 | ||||||
| 25 | Mitomycin/cis‐platinum/pharmorubicin/5‐Fu+ CMP‐induced IFN‐α | 92 | |||||||
| Effects on epidemic haemorrhagic fever | 128 | Balanced salt solution | 60 | 68.7 | 64 | ||||
| Balanced salt solution+ IFN‐α | 63 | 100 | |||||||
| Antihepatitis effects | 35 | CMP | 88.57 | 54.28 | 66 | ||||
| 30 | CMP | 90 | 36.67 | ||||||
| 30 | CMP | 90 | 30 | ||||||
| 60 | Mitomycin/cis‐platinum/pharmorubicin/5‐Fu | 30.7 | 38.7 | 20.5 | 67 | ||||
| Mitomycin/cis‐platinum/pharmorubicin/5‐Fu+ PCP | 38.5 | 50.4 | 27.5 | ||||||
| 50 | Control | 47.4 | 46.2 | 23.3 | 68 | ||||
| PCP | 49.2 | 55.4 | 33.3 |
IL‐2 and IFN‐α were obtained from human peripheral blood lymphocytes treated with CMP.
4. TOXICITY
CMP has very low toxicity. It is reported by Chen et al69 that the mice moves freely, no abnormal reaction is observed when CMP is used during standard acute toxicity test. No teratogenic effects are detected on rats when CMP is used in the teratogenic tests. No toxic reaction is witnessed when the dogs are continuously injected by intravenous injection reported by Ye et al70 They further demonstrated that blood pressure, heart rate, electrocardiogram and breathy of dogs are not affected after intravenously injecting either 400 mg/kg or 800 mg/kg CMP. In consistent, Chai et al71 reported that CMP has no adverse effects on mice as well. Wang et al showed that CMP could enhance the tumour inhibition rate of 5‐FU and decrease the liver injuries simultaneously caused by 5‐FU in CT26 tumour‐bearing mice.72
5. FUTURE PERSPECTIVES
In this article, the structure, pharmacological effects, clinical efficacy, immunobalancing molecular mechanism and toxicity of PCPs are summarized in Figures 1, 2, 3, 4, 5 and Tables 1, 2, 3, 4, 5, 6, 7, 8. The broad spectrum of therapeutic properties, relatively low toxicity and low costs make PCPs attractive immune therapeutics for treating not only different types of cancers but also hepatitis B and other diseases.
Both advantages and disadvantages of PCPs as drugs rely on their complicated polysaccharide structure‐dependent immune regulatory functions. Thus, there is a great need for clarifying the active ingredients in PCPs besides β‐glucan/pachymaran and their molecular targets responsible for their drug effects. In addition, how to standardize the quality of PCPs, especially the degree of chemical modifications of pachymaran derivatives and how to perform reliable pharmacokinetic studies of PCPs are some of important issues to be solved in near future to make use of PCPs for treating patients with cancer worldwide.
CONFLICT OF INTEREST
The authors declare no conflict of interests. All grants and funding agencies play no role in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
ACKNOWLEDGEMENTS
This research was supported by Natural Science Foundation of China (Grant No. 81672585); Key Technology Fund of Shandong Province (Grant 2016ZDJS07A07); and the “Double First‐Class” fund of Shandong Province to LZ. All authors have approved the final article.
Li X, He Y, Zeng P, et al. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J Cell Mol Med. 2019;23:4–20. 10.1111/jcmm.13564
REFERENCES
- 1. Wang YZ, Zhang J, Zhao YL, et al. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: a review. J Ethnopharmacol. 2013;147:265‐276. [DOI] [PubMed] [Google Scholar]
- 2. Wen YQ, Jia B, Peng T. Studied on pharmacology of Poria cocos polysaccharides and their modifications. Lishizhen Med Mater Med Res. 2014;25:432‐434. [Google Scholar]
- 3. Wang YF, Zhang M, Ruan D, et al. Chemical components and molecular mass of six polysaccharides isolated from the sclerotium of Poria cocos . Carbohyd Res. 2004;339:327‐334. [DOI] [PubMed] [Google Scholar]
- 4. Lu HJ, Lu Y, Liu YW. Studies on the fractionation, structure analysis and bioactivities of Poria cocos acidic polysaccharides. Hubei: Hubei University of Chinese Medicine; 2014. [Google Scholar]
- 5. Jiao QC, Wang AY. Sulfation of pachyman with chlorosulfonic acid using the improved Wolfrom method. Chinese J Polym Sci. 2006;24:637‐646. [Google Scholar]
- 6. Jin Y, Zhang LN, Chen L, et al. Effect of culture media on the chemical and physical characteristics of polysaccharides isolated from Poria cocos mycelia. Carbohyd Res. 2003;338:1507‐1515. [DOI] [PubMed] [Google Scholar]
- 7. Jia XJ, Ma LS, Li P, et al. Prospects of Poria cocos polysaccharides: isolation process, structural features and bioactivities. Trend Food Sci Tech. 2016;54:52‐62. [Google Scholar]
- 8. Chihara G, Hamuro J, Maeda Y, et al. Antitumour polysaccharide derived chemically from natural glucan (pachyman). Nature. 1970;225:943‐944. [DOI] [PubMed] [Google Scholar]
- 9. Hamuro J, Yamashita Y, Ohsaka Y, et al. Carboxymethylpachymaran, a new water soluble polysaccharide with marked antitumour activity. Nature. 1971;233:486‐488. [DOI] [PubMed] [Google Scholar]
- 10. Huang QL, Zhang LN. Solution properties of (1→3)‐α‐D‐glucan and its sulfated derivative from Poria cocos mycelia via fermentation tank. Biopolymers. 2005;79:28‐38. [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Zhang L, Cheung PCK. Immunopotentiation and anti‐tumor activity of carboxymethylated‐sulfated β‐(1→3)‐d‐glucan from Poria cocos . Int Immunopharmacol. 2010;10:398‐405. [DOI] [PubMed] [Google Scholar]
- 12. Wang YF, Zhang LN, Li YQ, et al. Correlation of structure to antitumor activities of five derivatives of a β‐glucan from Poria cocos sclerotium. Carbohyd Res. 2004;339:2567‐2574. [DOI] [PubMed] [Google Scholar]
- 13. Gao XJ, Sun YR, Li YB. Antitumor effects and pharmacological research of pachymaran. Acta Chin Med Pharm. 1996;1:45. [Google Scholar]
- 14. Zhou ZJ, Han ZR, Zeng YY, et al. Chinese FDA approved fungal glycan‐based drugs: an overview of structures, mechanisms and clinical related studies. Transl Med. 2014;4:141. [Google Scholar]
- 15. Chang YJ, Zhang M, Liu Y, et al. Preclinical and clinical studies of Coriolus Versicolor polysaccharopeptide as an immunotherapeutic in China. Discov Med. 2017;23:207‐219. [PubMed] [Google Scholar]
- 16. Jiang YF, Chang YJ, Liu Y, et al. Overview of Ganoderma Sinense polysaccharide–an adjunctive drug used during concurrent chemo/radiation therapy for cancer treatment in China. Biomed Pharmacother. 2017;96:865‐870. [DOI] [PubMed] [Google Scholar]
- 17. Wang XF, Liu CY, Dou DQ. Studies on the effective components of Poria cocos in antitumor activity. Liaoning J Tradit Chin Med. 2014;41:1240‐1243. [Google Scholar]
- 18. Chen JS, Zhao J, Huang H. The preparation and study on pharmacodynamics of antitumor of carboxymethy pachymaran. Youjiang Med J. 2008;36:386‐388. [Google Scholar]
- 19. Wang YF. The structure and biological activity of Poria cocos sclerotium polysaccharide and its derivatives. Wuhan: Wuhan University; 2004. [Google Scholar]
- 20. Wang AY, Chen Q, Li CF, et al. Antitumor function and mechanism study of modified pachymaran. Chin Herbal Med. 2009;40:268‐271. [Google Scholar]
- 21. Bian C. The preparation of water‐soluble pachymaran and the study of its antitumor activities. Huazhong Agricultural University. 2006: 65.
- 22. Luo LQ. Studies on antitumor effects and mechanisms of Chinese medicine polysaccharide in signal transduction pathway. Hubei: Hubei University of Chinese Medicine; 2005. [Google Scholar]
- 23. Tan XT, Wang YF, Zhang LN, et al. Histological observation on antitumor effect of Poria cocos polysaccharide modified by chemical technology. J Wuhan Univ. 2004;25:652‐654. [Google Scholar]
- 24. Chen CX, Zhao DM, Zhang XJ, et al. Antitumor experiments of carboxymethyl pachymaran. Fujian J Tradit Chin Med. 2002;33:38‐40. [Google Scholar]
- 25. Chen CX. The antitumor activities and immune effects of carboxymethyl pachymaran. Acta Edulis Fungi. 2001;8:39‐44. [Google Scholar]
- 26. Xu LB, Xiao MY, Fan XH. Studies on immune function and antitumor effect of carboxymethyl pachymaran oral liquid. Chin Tradit Pat Med. 2000;22:44‐46. [Google Scholar]
- 27. Zhao JF, Me YJ, Chen YJ, et al. The preparation and anti‐tumor effect of sulfonyl pachymaran. J Shenyang Pharm Univ. 1996;13:125‐128. [Google Scholar]
- 28. Lv SC, Cao QL, Zhang L, et al. The effects of pachymaran on immune function in normal and tumor‐burdened mice. J First Military Med Univ. 1990;10:267‐268. [Google Scholar]
- 29. Chen DN, Fan YJ, Zhou J, et al. The antitumor and related pharmacological effects of pachymaran. Chin J Chin Mater Med. 1987;12:43‐45. [Google Scholar]
- 30. Tong L, Huang TY, Li JL, et al. The effects of botanical polysaccharides on the proliferation of S180 and K562 cells and the levels of sialic acid, phospholipid and cholesterol. Chin J Integr Med. 1994;14:482‐484. [PubMed] [Google Scholar]
- 31. Liu JC, Su FQ, Zhao XM. The antitumor effects of pachymaran after chemotherapy. Pharmacol Clin Chin Mater Med. 2006;22:73‐76. [Google Scholar]
- 32. Tang SS, Zhu XQ. The antitumor and immune effects of compound fungal polysaccharides. Basic Clin Med. 2004;24:599‐600. [Google Scholar]
- 33. Meng YL, Cai LH, Wu HF, et al. Histological observation on antitumor effect of Poria cocos polysaccharide modified by chemical technology. J Wuhan Univ. 2007;28:67‐69. [Google Scholar]
- 34. Ji F, Li PF, Xu SY, et al. The preparation and antitumor effect of carboxymethy pachymaran in vivo. Chin J Microecol. 2003;15:22‐23. [Google Scholar]
- 35. Miu JC, Yang JC, Sheng WH. The effects of carboxymethyl pachymaran on increasing lymphocyte induce cytokines. Chin Tradit Pat Med. 1999;21:25‐27. [Google Scholar]
- 36. Chai BL, Lin ZB, Cao SL. The effects of carboxymethyl pachymaran on phagocytizing function of macrophages. Beijing Med J. 1983;15:9‐11. [Google Scholar]
- 37. Yang JS, Qin XP, Zhang N, et al. Effects of two fungal polysaccharides on protein tyrosine phosphorylation of HL‐60 cells. Chin J Pharm. 2000;35:17‐19. [Google Scholar]
- 38. Chen CX. The application of carboxymethylpachymaran‐inducers. Acta Edulis Fungi. 1998;3:41‐42. [Google Scholar]
- 39. Zhang M, Chiu LCM, Cheung PCK, et al. Growth‐inhibitory effects of a β‐glucan from the mycelium of Poria cocos on human breast carcinoma MCF‐7 cells: cell‐cycle arrest and apoptosis induction. Oncol Rep. 2006;15:637‐643. [PubMed] [Google Scholar]
- 40. Ma CY, Chang WC, Chang HM, et al. Immunomodulatory effect of the polysaccharide‐rich fraction from sclerotium of medicinal mushroom Poria cocos F.A. Wolf (Aphyllophoromycetideae) on Balb/c Mice. Int J Med Mushrooms. 2010;12:111‐121. [Google Scholar]
- 41. Hamuro J. β (1→3) Glucan‐mediated augmentation of alloreactive murine cytotoxic T‐lymphocytes in vivo. Cancer Res. 1978;38:3060‐3065. [PubMed] [Google Scholar]
- 42. Zhang WY, Yang Y, Chen CL, et al. The effects of Poria cocos on immune function of immune deficit mice. Chin J Vet Sci. 2014;34:283‐287. [Google Scholar]
- 43. Liu YY, Chen YX, Hou AJ. The effects of carboxymethyl pachymaran on lymphocyte secreting cytokines in mice. Pharmacol Clin Chin Mater Med. 2006;22:71‐73. [Google Scholar]
- 44. Zhang MX, Li YW, Zhang DS, et al. Inhibition of spontaneous lung metastasis of pachymaran on Lewis lung cancer in mice and its mechanism. Drug Clinic. 2013;28:842‐846. [Google Scholar]
- 45. Wei XJ, Hu TJ, Gao JF, et al. The preliminary research on the pharmacological effects of sulfation pachymaran. Vet Pharm Feed Additives. 2009;14:1‐2. [Google Scholar]
- 46. Zhang ZJ, Feng X, Jiang J, et al. The effects of pachymaran on the biosynthesis of IgA, IgG and IgM in serum in mice. Chin J Immunol. 2013;29:1213‐1215. [Google Scholar]
- 47. Peng XB, Qiu XH, Yu CL, et al. The effects of pachymaran on humoral immunity function in immunosuppressive mice caused of cyclophosphamide. Pharmacol Clin Chin Mater Med. 2013;29:69‐72. [Google Scholar]
- 48. Chu M, Wang D, Zhang Y, et al. Pachyman treatment improves CD4 + CD25 + Treg counts and serum interleukin 4 and interferon γ levels in a mouse model of Kawasaki disease. Mol Med Rep. 2012;12:1237. [DOI] [PubMed] [Google Scholar]
- 49. Zheng LL, Jiang JP, Xu HS, et al. The response surface method to optimize pachymaran extraction technology and research on its antioxidant activity. Chin J Tradit Chin Med Pharm. 2014;29:918‐922. [Google Scholar]
- 50. Cao Y, Gao WY, Zhang LM, et al. Studies on the antioxidative activities of pachymaran. Food R&D. 2009;30:148‐151. [Google Scholar]
- 51. Li YL, Zhang ZX, Hu L. Studies on the antioxidative activities of pachymaran. Nat Prod Res Develop. 2012;24:1126‐1128. [Google Scholar]
- 52. Zhang Q, Zhang LM. Studies on the antioxidative activities of ferulic acid pachymaran. Mod Food Sci Tech. 2011;27:1077‐1080. [Google Scholar]
- 53. Cheng SM, Liu Y, Mei GM, et al. Studies on the antioxidative activities of carboxymethyl pachymaran. Food R&D. 2013;34:1‐5. [Google Scholar]
- 54. Wang Y, Yu Y, Mao J. Carboxymethylated β‐Glucan derived from Poria cocos with biological activities. J Agr Food Chem. 2009;57:10913‐10915. [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Liu S, Yang Z, et al. Oxidation of β‐glucan extracted from Poria Cocos and its physiological activities. Carbohyd Polym. 2011;85:798‐802. [Google Scholar]
- 56. Hou AJ, Peng SP, Xiang R. Studies on the anti‐inflammatory effects of pachymaran. Pharmacol Clin Chin Mater Med. 2003;19:15‐16. [Google Scholar]
- 57. Hou AJ, Chen TY, Peng SP, et al. Studies on the anti‐aging effects of pachymaran. Pharmacol Clin Chin Mater Med. 2004;20:10‐11. [Google Scholar]
- 58. Duan HP, Hou AJ, Lu FE, et al. Studies on carboxymethyl pachymaran on the impact of HBV transfection cells expressing function. Chin J Exp Clin Virol. 2005;19:290‐292. [Google Scholar]
- 59. Yang JC, Liu JS, Sheng WH, et al. The application of Chinese medicine effective component in interferon biotechnology experiment and clinical research. Prog Biotechnol. 1993;3:30‐34. [Google Scholar]
- 60. Zheng CY. Studies on the anti‐diabetic effects of pachymaran. Chin Healthc Innov. 2010;5:12‐13. [Google Scholar]
- 61. Yang HX, Bo XZ, Yang Y, et al. Studies on the anti‐leukemia effects of pachymaran combined with selenium in mice. J Leukemia. 2006;15:94‐95. [Google Scholar]
- 62. Hattori T, Hayashi K, Nagao T, et al. Studies on antinephritic effects of plant components (3): effect of pachyman, a main component of Poria Cocos Wolf on original‐type anti‐GBM nephritis in rats and its mechanisms. Jpn J Pharmacol. 1992;59:89‐96. [DOI] [PubMed] [Google Scholar]
- 63. Wang CY, Chen Q, Shen B, et al. Synthesis and antihypertensive activity of pachyman sulfate. Curr Top Nutraceut Res. 2016;14:215‐218. [Google Scholar]
- 64. Chen CX. The clinical trials of carboxymethyl pachymaran inducers. J Fujian Univ Tradit Chin Med. 2003;13:20‐22. [Google Scholar]
- 65. Sheng WH, Yang JC, Gao YM, et al. Experimental and clinical studies on carboxymethyl pachymaran on promoting the induction of IL‐2. Chin J Cancer Biother. 1996;3:51‐54. [Google Scholar]
- 66. Chen CX. The medical function of carboxymethyl pachymaran. Acta Edulis Fungi. 1994;3:38. [Google Scholar]
- 67. Lv SC, Cao QL. The observations of pachymaran on the cell immune function of patients with lung cancer and clinical curative effects. Shanghai J Immunol. 1994;14:109. [Google Scholar]
- 68. Lv SC, Kong W, Cao QL, et al. The effects of pachymaran on immune function of tumor‐burdened mice, the elderly and patients with tumor. J Curr Phys. 1996;1:5‐7. [Google Scholar]
- 69. Chen C, Ye J, Lin J. Carboxymethyl tuckahoe polysaccharide the safety of the experiment. Fujian J Tradit Chin Med. 2003;34:27‐30. [Google Scholar]
- 70. Ye JR, Lin JF, Lin DJ, et al. Toxicity studies on sodium carboxymethyl pachymaran. Fujian Med J. 1988;10:29‐32. [Google Scholar]
- 71. Chai BL, Lin ZB, Cao SL, et al. Pharmacological effects and toxicity about carboxymethyl pachymaran. Beijing Med J. 1984;16:47‐48. [Google Scholar]
- 72. Wang C, Huo X, Gao L, et al. Hepatoprotective effect of carboxymethyl pachyman in fluorouracil‐treated CT26‐bearing mice. Molecules. 2017;22:756. [DOI] [PMC free article] [PubMed] [Google Scholar]


