ABSTRACT
Diapause is an alternative life-history strategy that allows organisms to enter developmental arrest in anticipation of unfavorable conditions. Diapause is widespread among insects and plays a key role in enhancing overwinter survival as well as defining the seasonal and geographic distributions of populations. Next-generation sequencing has greatly advanced our understanding of the transcriptional basis for this crucial adaptation but less is known about the regulation of embryonic diapause physiology at the metabolite level. Here, we characterized the lipid and metabolite profiles of embryonic diapause in the Asian tiger mosquito, Aedes albopictus. We used an untargeted approach to capture the relative abundance of 250 lipids and 241 metabolites. We observed adjustments associated with increased energy storage, including an accumulation of lipids, the formation of larger lipid droplets and increased lipogenesis, as well as metabolite shifts suggesting reduced energy utilization. We also found changes in neuroregulatory- and insulin-associated metabolites with potential roles in diapause regulation. Finally, we detected a group of unidentified, diapause-specific metabolites which have physical properties similar to those of steroids/steroid derivatives and may be associated with the ecdysteroidal regulation of embryonic diapause in A. albopictus. Together, these results deepen our understanding of the metabolic regulation of embryonic diapause and identify key targets for future investigations.
KEY WORDS: Embryonic diapause, Developmental arrest, Untargeted metabolomics, Lipidomics
Summary: Identification of diapause-associated metabolic shifts consistent with altered nutrient storage and energy utilization as well as regulatory pathways potentially contributing to developmental arrest in the Asian tiger mosquito, Aedes albopictus.
INTRODUCTION
The ability to exploit favorable seasonal conditions and survive stressful environments is a fundamental requirement for insects inhabiting temperate regions. To synchronize their life cycles with recurring periods of seasonal variation, many insect taxa have independently evolved diapause (Danks, 1987; Tauber et al., 1986). Diapause is an alternative life-history strategy defined by a hormonally controlled developmental arrest that is initiated in anticipation of habitat deterioration (Denlinger et al., 2012; Lees, 1956). The capacity to initiate diapause allows insects to regulate their energy utilization throughout the year, maximizing allocation towards growth, development and reproduction during favorable periods in the spring and summer, then minimizing such allocations during the more inclement autumn and winter (Bradshaw et al., 2004). The adaptive significance of insect diapause is well understood; diapause enhances overwinter survival via increased nutrient storage, reduced metabolic activity and elevated stress tolerance (Hahn and Denlinger, 2011; Teets and Denlinger, 2013). Additionally, the prevalence and timing of diapause shape the geographic distribution and seasonal abundance of insect populations (Danks, 1987; Tauber and Tauber, 1976). Nevertheless, despite the long-recognized ecological importance of diapause, until recently relatively little was known about the molecular mechanisms underpinning this alternative developmental strategy (Denlinger, 2002; Ragland and Keep, 2017).
Diapause is a complex, dynamic developmental program consisting of several eco-physiological stages (Koštál, 2006; Koštál et al., 2017). First, in the induction phase, a token environmental cue is perceived during a sensitive period that may occur far in advance of dormancy. The diapause-inducing signal, often a change in photoperiod, causes the organism to begin preparing for developmental arrest. In the preparation phase, the diapause-destined organism undergoes behavioral and/or physiological adjustments to accommodate prolonged dormancy. Then, in the initiation phase, the organism enters developmental arrest, thus marking the onset of diapause. This developmental arrest can occur at any life stage, although typically a species will only arrest at a single life stage (Danks, 1987). In the maintenance phase, diapause is sustained for some genetically determined duration, even if the organism is exposed to favorable conditions. Finally, in the termination phase, the organism will gradually regain sensitivity to environmental cues that permit continued development. If unfavorable conditions persist beyond the termination phase, development may remain environmentally suppressed in post-diapause quiescence, a dormant state that can be terminated immediately upon exposure to favorable conditions.
Over the past decade, transcriptomics has greatly advanced our understanding of the molecular regulation of diapause in many species (e.g. Gong et al., 2013; Huang et al., 2015; Kang et al., 2016; Poelchau et al., 2013a,b; Poupardin et al., 2015; Qi et al., 2015; Ragland et al., 2010; Yocum et al., 2015). Numerous studies have identified significant diapause-associated changes in the expression of thousands of genes including those involved in cell proliferation, nutrient storage, metabolic activity, hormonal signaling, circadian rhythms and stress resistance (see review in Ragland and Keep, 2017). Metabolomic adjustments in diapause have also garnered significant attention primarily through narrowly targeted assays for molecules expected a priori to contribute to the diapause phenotype. For example, many studies have measured changes in the abundance of molecules associated with nutrient storage, like glycogen and triacylglycerides (e.g. Goto et al., 1998; Shimizu, 1992; Wipking et al., 1995), or cryoprotective molecules such as polyols and amino acids (e.g. Goto et al., 1998; Lafage et al., 1974; Mohammadzadeh et al., 2017). Recently, emerging untargeted metabolomic technology has enabled simultaneous assessment of a wide array of small molecules including amino acids, lipids, polyols, fatty acids and metabolic intermediates. The advantage of an untargeted metabolomics approach is that it provides an unbiased snapshot of organismal physiology, potentially identifying both expected and unexpected metabolic adjustments and informing future targeted assessments (Macel et al., 2010). The disadvantage of this approach is that it lacks the specificity and complete pathway coverage of targeted analyses. Furthermore, which metabolites are detected depends on the extraction and separation methodologies utilized (Cajka and Fiehn, 2016; Patti, 2011; Yanes et al., 2011). Finally, metabolomic approaches cannot assess the regulation of enzymes such as allosteric control or covalent modification. A small number of studies have applied untargeted analyses to characterize the metabolome of diapause at the larval, pupal and adult stages (Khodayari et al., 2013; Li et al., 2015; Lu et al., 2014), but to date no study has investigated the metabolomic profile of embryonic diapause.
List of abbreviations.
- CPT
carnitine palmitoyltransferase
- DG
diacylglyceride
- dpo
days post-oviposition
- FDR
false discovery rate
- FOXO
forkhead transcription factor
- HMDB
human metabolome database
- JH3
juvenile hormone III
- LC
liquid chromatography
- LD
long day (16 h light: 8 h dark)
- m/z
mass-to-charge ratio
- MS
mass spectrometry
- PC
phosphatidylcholine
- PCA
principal component analysis
- PEA
pathway enrichment analysis
- plasmenyl-PE
plasmenyl-phosphatidylethanolamine
- QC
quality control
- QEA
quantitative enrichment analysis
- RH
relative humidity
- RSD
relative standard deviation
- RT
retention time
- SD
short day (8 h light: 16 h dark)
- TG
triacylglyceride
The Asian tiger mosquito, Aedes albopictus (Skuse), is an emerging model system for studying embryonic diapause (Denlinger and Armbruster, 2014, 2016). Temperate populations of A. albopictus undergo a facultative embryonic diapause modulated by the photoperiod experienced by female pupae and adults in the previous generation (Hawley, 1988; Pumpuni, 1989). Under long-day photoperiods (e.g. 16 h light:8 h dark), females produce eggs that complete embryonic development and are then immediately responsive to hatching stimuli. In contrast, under short-day photoperiods (e.g. 8 h light:16 h dark), females produce eggs that complete embryonic development, enter diapause as pharate larvae within the chorion of the egg, and then undergo a genetically controlled period in which they are not responsive to hatching stimuli (Denlinger and Armbruster, 2014).
Transcriptional changes throughout the eco-physiological trajectory of diapause have been extensively characterized in A. albopictus. Previous studies have identified thousands of differentially expressed genes across multiple developmental time points, including during diapause induction (Huang et al., 2015), throughout diapause preparation (Batz et al., 2017; Poelchau et al., 2011, 2013a; Reynolds et al., 2012) and in diapause maintenance (Batz et al., 2017; Poelchau et al., 2013b). These studies have established that many metabolic genes, including those involved in gluconeogenesis and lipid metabolism, are differentially expressed throughout the course of embryonic diapause relative to expression in non-diapausing embryos (Poelchau et al., 2013a,b). Additionally, multiple stress-tolerance mechanisms are enhanced at the transcriptional level in A. albopictus diapause. For example, a greater abundance of long-chain hydrocarbons is deposited on the chorion of the egg, significantly increasing desiccation resistance (Urbanski et al., 2010). The complex molecular changes observed during diapause imply that evidence from multiple regulatory levels will enhance our understanding of the physiological basis of this crucial adaptation, though only a few studies have attempted to do so in any species (e.g. Colinet et al., 2012; Zhang et al., 2012, 2013).
In this study, we investigated the metabolome of embryonic diapause in A. albopictus. We performed shotgun lipidomics and untargeted metabolomics on A. albopictus eggs in early diapause maintenance (11 days post-oviposition, dpo) and on age-matched, non-diapause eggs to characterize diapause-associated changes in the relative abundance of lipids and metabolites. We identified specific metabolic pathways and individual metabolites that were significantly altered during early diapause. We draw upon the extensive transcriptomic data available in this species (Batz et al., 2017; Huang et al., 2015; Poelchau et al., 2011, 2013a,b; Reynolds et al., 2012) to inform the interpretation of our metabolomic data and provide novel insights regarding the molecular basis of embryonic diapause.
MATERIALS AND METHODS
Mosquito rearing and tissue generation
All experiments were completed with an F3 laboratory colony of A. albopictus. This colony was originally established in August 2015 from over 200 larvae collected from 15 tires at a recycling center in Manassas, VA, USA. Prior to the start of this experiment, the laboratory colony was maintained under a long-day photoperiod (LD; 16 h light:8 h dark) at 21°C and 80% relative humidity (RH) as described previously (Armbruster and Conn, 2006; Armbruster and Hutchinson, 2002). To generate samples for this experiment, larvae were reared under a LD photoperiod at 21°C and 80% RH and fed a near-optimal diet consisting of a slurry of dog food and brine shrimp (Armbruster and Conn, 2006). Upon pupation, pupae were divided evenly into two treatments: four biological replicates were established under an unambiguous short-day photoperiod (SD; 8 h light:16 h dark) to induce production of diapause eggs, and four biological replicates were established under a LD photoperiod to induce production of direct developing (non-diapause) eggs. Each SD and LD replicate was established with at least 150 pupae.
Adult females were provided a human blood meal 7–14 days post-eclosion. The Georgetown University Institutional Review Board (IRB) has determined that mosquito blood feeding is not human research and thus does not require IRB approval; however, the blood feeding protocol has been approved by the Georgetown University Occupational Health and Safety Committee. Beginning 4 days post-blood meal, females were provided with an oviposition cup lined with an unbleached paper towel and half-filled with deionized water. Eggs were collected daily, maintained on a wet paper towel for approximately 48 h, then gently air dried and stored in a Tupperware container under a SD photoperiod at 21°C and 80% RH. A subset of eggs from each biological replicate was used to confirm diapause incidence (see below). At 11 dpo, eggs were gently brushed from the unbleached paper towel onto a weigh boat and weighed to the nearest 1 μg using a Mettler-Toledo AX5 microbalance (Mettler-Toledo, Columbus, OH, USA). The median sample mass was 5.014 mg (range: 3.329–9.009 mg). Finally, the eggs were transferred to a 1.5 ml Eppendorf tube and snap frozen in liquid nitrogen. Snap freezing occurred between 12:00 h and 13:00 h each day (Zeitgeber time 4–5 h) and all egg samples collected on a single day were snap frozen simultaneously to limit non-biological sources of variation. Snap frozen samples were immediately stored at −80°C.
Assessing diapause incidence
To ensure that adults under a SD photoperiod produced diapause eggs and adults under a LD photoperiod produced non-diapause eggs, we quantified diapause incidence with a subset of eggs collected from each biological replicate as previously described (Urbanski et al., 2012). Briefly, 11 dpo eggs from each biological replicate (four diapause, four non-diapause) were stimulated to hatch by submersion in water and larval food slurry. The mean number of eggs assessed per replicate was 444 (range: 167–1047 eggs). We recorded the number of larvae that hatched in each biological replicate, air dried the remaining eggs, and then repeated the process 1 week later. After the second attempt to stimulate hatching, the remaining unhatched eggs were bleached (Trpiš, 1970) and the number of embryonated, unhatched eggs was counted (i.e. the number of diapause eggs). Diapause incidence was calculated for each replicate according to the following formula: (number of embryonated unhatched eggs)/(number of hatched eggs+number of embryonated unhatched eggs).
Sample preparation
Lipidomic and metabolomic sample preparation and data generation were performed at the University of Michigan Metabolomics Resource Core. All samples were shipped overnight on dry ice, then stored at −80°C until extraction. All reagents were liquid chromatography (LC)-mass spectrometry (MS) grade and were purchased from Sigma-Aldrich (St Louis, MO, USA).
For the shotgun lipidomics, eight egg samples (four diapause, four non-diapause) collected from separate biological replicates on a single day were thawed on ice then extracted using a modified Bligh-Dyer method (Bligh and Dyer, 1959). The extraction was carried out at room temperature using 400 µl of water:methanol:dichloromethane (1:1:1) and 12 internal standards from a range of lipid classes were added (Table S1). Next, the organic layer was collected and dried under a stream of nitrogen gas then re-suspended in 100 µl of acetonitrile:water:isopropanol (10:5:85) with 10 mmol l−1 ammonium acetate. Additionally, a pooled quality control (QC) sample was prepared by combining aliquots of each experimental sample.
For the untargeted metabolomics, eight additional egg samples (four diapause, four non-diapause) collected from separate biological replicates on a single day were thawed on ice and combined with 400 µl of methanol:acetonitrile:acetone (1:1:1) and five internal standards (Table S1). Next, tubes were sonicated for 5 min in a water bath to settle the eggs before each sample was probe sonicated at 40% power for 10 s. Post-sonication, egg disruption was confirmed under a dissecting microscope. To complete metabolite extraction, samples were further sonicated in a water bath for 60 min then centrifuged (14,000 g for 20 min at 4°C). Finally, 400 μl of the supernatant was transferred to a clean vial and dried under a stream of nitrogen gas. The extract was reconstituted in 50 μl methanol:water (1:1) containing 1 μl zeatin, an instrument performance standard. A pooled QC sample was also prepared by combining aliquots of each experimental sample.
Lipidome data generation
To generate the lipidome data, the eight lipid extracts were analyzed in a random order using an LC-MS/MS system. LC separation was performed on a Shimadzu UFLC XR system (Shimadzu, Kyoto, Japan) with a Waters Acquity HSS T3 column (1.8 μm particle size, 50 mm×2.1 mm i.d.; Waters, Milford, MA, USA). MS/MS analysis was performed on an AB-SCIEX 5600 tripleTOF analyzer with a DuoSpray ion source (AB-SCIEX, Redwood City, CA, USA). Each of the eight experimental samples was assessed once in positive ion mode and once in negative ion mode using MS/MS with dynamic mass exclusion. The QC sample was run four times in each mode to monitor machine drift and assess the reliability of feature quantification. In both positive and negative modes, mobile phase A was acetonitrile:water (40:60) with 10 mmol l−1 ammonium acetate and mobile phase B was acetonitrile:water:isopropanol (10:5:85) with 10 mmol l−1 ammonium acetate. The column was programmed to perform a gradient elution from 40% to 100% mobile phase B over 10 min. Mobile phase B was maintained at 100% for 2 min, then decreased to 40% over 0.1 min. Prior to the next sample injection, the column was held at 40% for 3 min. Flow rate was 0.4 ml min−1 and the column temperature was maintained at 55°C.
Metabolome data generation
To generate the untargeted metabolomic data, eight metabolite extracts were analyzed in a random order on an LC-MS system. LC separation was performed on an Agilent 1290 Infinity Binary UHPLC system (Agilent, Santa Clara, CA, USA) with a Waters Acquity HSS T3 column (1.8 μm particle size, 100 mm×2.1 mm i.d.). MS analysis was performed on an Agilent 6530 quadrupole TOF system with a Jetstream ion source. Full mass spectra were acquired over the mass-to-charge ratio (m/z) range of 50–1000 Da. Each of the eight experimental samples was analyzed once in positive ion mode and once in negative ion mode. The QC sample was run three times in each ion mode to assess machine drift and reliability of feature quantification. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in methanol. The column was programmed to perform a gradient elution from 2% mobile phase B to 75% over 20 min. Next, mobile phase B was increased to 98% over 2 min, held at this level for 8 min, then reset to 2% in 0.1 min. Prior to the next sample injection, the column was re-equilibrated for 7 min. Throughout the runs, flow rate was 0.35 ml min−1 and the column was maintained at 55°C.
Feature identification and quantification
Chromatographic peaks (i.e. features) detected during lipidomics and untargeted metabolomics were identified using the LipidBlast package and Agilent's MassHunter software, respectively. Feature alignment between samples was performed allowing up to 0.5 min retention time (RT) shift and 20 ppm mass shift between sample runs; these shifts were verified using the RTs of known internal standards. Lipid features were identified by comparing MS/MS spectra with in silico spectra in the LipidBlast database (Kind et al., 2013). Metabolite features were identified by recursively searching the dataset using Agilent's ‘Find by Feature’ algorithm. Metabolite features were then annotated by matching feature m/z and RT to an in-house library maintained by the University of Michigan Metabolomics Resource Core containing known standards obtained under identical analytical conditions (Table S2). Relative quantification of lipids and metabolites was carried out using the AB-SCIEX Multiquant and Agilent MassProfiler Pro programs, respectively. Lipidome and metabolome data are available via Metabolomics Workbench, study accession ST000722.
Lipidome and metabolome data analyses
To ensure that our datasets contained only features that could be reliably quantified, we removed features with >30% relative standard deviation (RSD) calculated across replicate measurements of QC samples (Liu et al., 2014). Both datasets were then scaled by pre-extraction egg mass to correct for variation in input amount and the data were filtered by interquartile range to remove low-information features (i.e. those features with low variability across all experimental samples in both treatment groups; Gentlemen et al., 2005). Finally, data from both positive and negative ion modes were combined to produce a single lipid set and a single metabolite set.
To visualize the overall similarity of lipidomic or metabolomic profiles between treatment groups, we generated principal component analysis (PCA) plots using data from all features (including unannotated features) via the MetaboAnalyst online toolkit (Xia and Wishart, 2011a; Xia et al., 2015). These analyses were first performed with the pooled samples included to allow for visual assessment of technical variation due to machine drift and then performed again with only the experimental samples included. Next, we used MetaboAnalyst to calculate the log2 fold-change in abundance between diapause and non-diapause eggs for each lipid and metabolite (annotated and unannotated).
For further analysis of lipid and metabolite shifts during diapause, only annotated lipids and metabolites were considered. For features identified by multiple adduct ions (e.g. cystine+H+ and cystine+Na+), the adduct ion measured with the lowest RSD across technical replicates of the QC sample was retained. The annotated data were then auto-scaled, such that metabolites were given equal weight in analysis and calculations of significance were decoupled from the measured concentration (van den Berg et al., 2006). Next, the fold-change in relative abundance between diapause and non-diapause groups was calculated for each lipid and metabolite. Significant differences in either lipids or metabolites between treatments were identified via t-test followed by a Benjamini–Hochberg false discovery rate (FDR) correction to account for multiple comparisons (Benjamini and Hochberg, 1995). The top 50 lipids, as ranked by P-value, were further analyzed by generating a heat map using MetaboAnalyst. For each lipid class, we calculated the unsaturation index, which quantifies the average number of unsaturated bonds per fatty acid (Lehmann et al., 2018). Significant differences in unsaturation index by lipid class between diapause and non-diapause eggs were identified using t-tests followed by a Benjamini–Hochberg FDR correction (Benjamini and Hochberg, 1995).
To identify functionally related sets of metabolites affected by diapause state, we performed both quantitative enrichment analysis (QEA) and pathway enrichment analysis (PEA). QEA is derived from the gene set enrichment analysis originally developed for transcriptomics and identifies coordinated changes within metabolic pathways (Xia and Wishart, 2010). PEA is conceptually similar to QEA, but further integrates topological information such that metabolites occupying more central positions in a pathway are weighted more heavily in the analysis than metabolites on the pathway periphery (Xia and Wishart, 2011b). For both QEA and PEA, the complete in-house library of identifiable metabolites was used as the background reference set (Table S2). In both analyses, we performed a global test for significant enrichment followed by a Benjamini–Hochberg FDR correction (Benjamini and Hochberg, 1995); only functional groups and pathways containing at least two metabolites annotated in our study were retained in either analysis.
Characterization of diapause-exclusive features
Features that were detected in all four diapause samples but not detected in any non-diapause samples were further investigated. All features exhibiting this pattern were unannotated metabolites. To better understand the potential diapause-associated functions of these unannotated metabolites, we examined all annotated metabolites detected with similar RTs in our experiment. LC separates molecules based on physical properties such that molecules that elute with similar RTs share similar characteristics. For each diapause-exclusive feature, we identified all annotated metabolites within ±0.5 min RT. Next, we determined the super-class, class and sub-class categorizations for each annotated metabolite based on the molecular taxonomy in the Human Metabolome Database (HMDB; Wishart et al., 2007, 2018).
RESULTS
Diapause incidence
Under a diapause-inducing SD photoperiod, the mean diapause incidence across biological replicates was 99.6% (range 99.2–100%), while under a diapause-averting LD photoperiod, the mean diapause incidence was 10.2% (range 6.3–14.0%; Table S3). Thus, our experimental conditions consistently induced the desired phenotypes.
LC-MS performance and data preparation
Across four repeat measurements of our lipidomic QC sample, the median RSD was 9.6%, while across three repeat measurements of our metabolomics QC sample, the median RSD was 4.6%. Additionally, PCA plots for both lipidomic and untargeted metabolomic datasets showed tight clustering of QC measurements indicating low machine drift throughout the experimental runs (Fig. S1). Together, these results demonstrate a high level of consistency for the analytical platform used throughout our experiments.
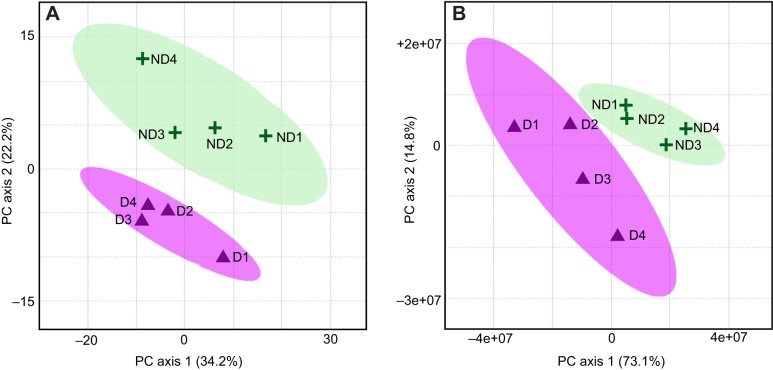
In the lipid dataset, we annotated 265 lipids in positive mode and 202 lipids in negative mode (467 total lipids). From this set we retained 400 lipids with RSD <30% across repeated QC sample measurements. We then removed duplicate adducts to produce a final dataset that contained 250 lipids. A PCA plot of these lipid features showed clear clustering of diapause and non-diapause samples (Fig. 1A) with 56.4% of the variation explained by the first two principal component axes.
Fig. 1.
Overall metabolomic and lipidomic profiles differ for diapause and non-diapause samples. Principal component analysis (PCA) plot of (A) lipidomic and (B) untargeted metabolomic datasets. Purple triangles represent biological replicates of diapause (D) eggs. Green crosses represent biological replicates of non-diapause (ND) eggs. Purple and green shaded regions surrounding the points represent the 95% confidence interval for diapause and non-diapause conditions, respectively. The percentage variance explained by each of the first two principal components is listed parenthetically.
In the untargeted metabolomics dataset, we identified 4511 features in positive mode and 1157 features in negative mode (5668 total features). From this set, we retained 5216 features with RSD <30% across QC sample measurements. We generated a PCA plot of these features and found a clear separation of diapause and non-diapause samples (Fig. 1B), with 87.9% of the variation explained by the first two principal component axes.
Degradation is a consistent concern in metabolomic studies due to thermal instability (Lerma-Ortiz et al., 2016) and/or enzymatic conversions of short half-life molecules during sample processing (Vuckovic, 2012). In our study, we did not identify ATP or ADP, but we did detect AMP and adenosine, consistent with enzymatic dephosphorylation during processing. However, we successfully annotated several molecules known to be sensitive to degradation during LC-MS sample preparation including idinosine, insosine monophosphate and arginine, suggesting acceptable metabolite recovery (Fang et al., 2015; Lerma-Ortiz et al., 2016).
Metabolite annotation is another significant challenge for untargeted metabolomics (Aksenov, et al., 2017; Dunn et al., 2013; Johnson and Gonzalez, 2012). Untargeted LC-MS datasets contain features representing various isotopes, alternative adducts, experimental artifacts and metabolites without commercially available standards for comparison (Mahieu and Patti, 2017). Thus, untargeted studies typically unambiguously annotate 2–10% of detected features (Aksenov et al., 2017). In our study, 389 features (7.5%) were annotated as specific metabolites with high confidence (Table S2). Removing duplicate adduct ions from this annotated set produced a final dataset that contained 241 metabolites.
Differentially abundant lipids and metabolites
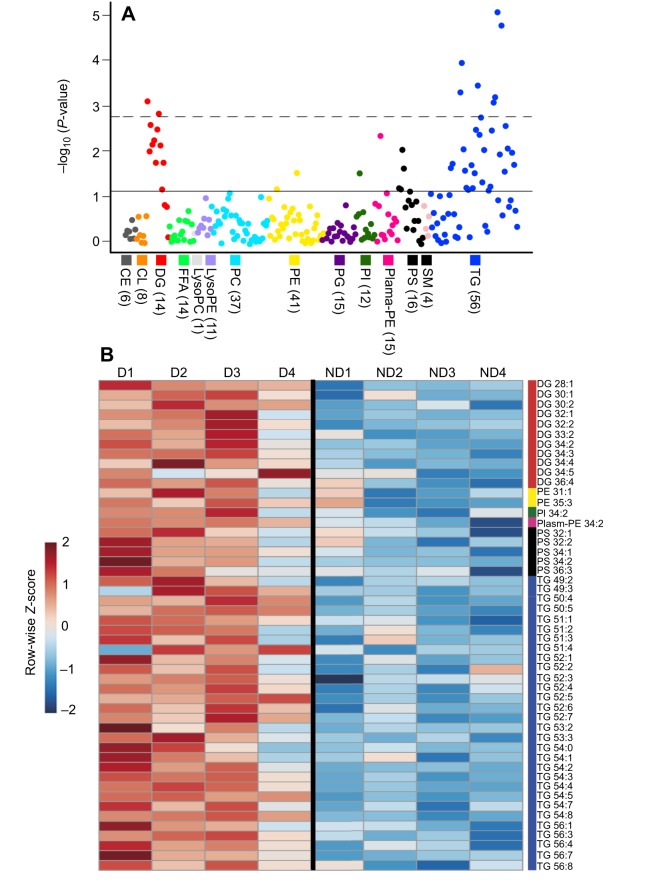
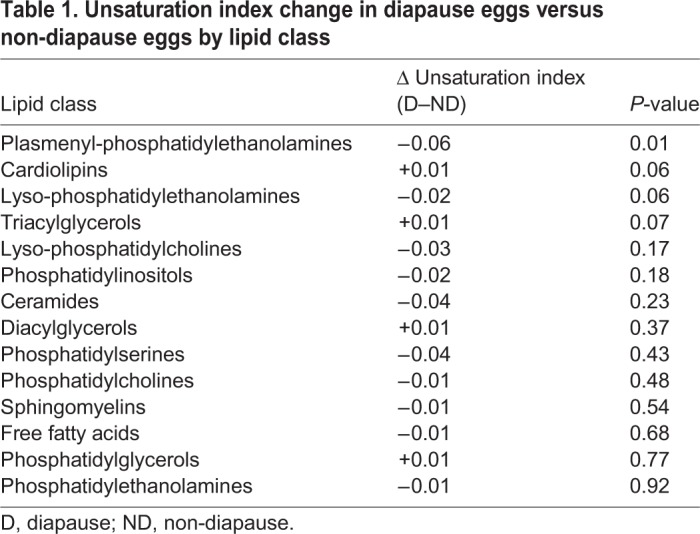
We identified nine lipids, two diacylglycerides (DGs) and seven triacylglycerides (TGs; Fig. 2A), that were significantly more abundant in diapause eggs than in non-diapause eggs. A heat map of the top 50 lipids, as ranked by P-value (Fig. 2B; Table S4), revealed that the distinct lipidome of diapause is due to lipid accumulation, primarily in the form of TGs (30 lipids, 60%) and DGs (11 lipids, 22%). There were no differences in saturation levels of these lipids, except for plasmenyl phosphatidylethanolamines (plasmenyl-PEs), which were more saturated in diapause (Table 1; t=4.13, d.f.=6, P=0.006).
Fig. 2.
Diapause eggs have increased lipid abundance, primarily in the form of diacylglycerides and triacylglycerides. (A) log10 P-value of t-test for differences in lipid abundance between biological replicates of diapause (n=4) and non-diapause (n=4) eggs. Lipids are sorted and color-coded by class; the number of measured lipids within each class is identified parenthetically. The dashed line indicates a false discovery rate (FDR)-corrected P-value of 0.05; the solid line indicates the top 50 lipids by P-value. (B) The relative abundance for each of the top 50 lipids according to P-value in A displayed as a heat map. Each row in the heat map has been normalized such that the color corresponds to the Z-score for that lipid. Red cells represent higher relative abundance (Z-score) in diapause eggs and blue cells represent lower relative abundance in diapause eggs. Colored blocks to the right of the heat map match the lipid class colors used in A. Lipid abbreviations are as follows: CE, ceramide; CL, cardiolipin; DG, diacylglyceride; FFA, free fatty acid; lysoPC, lyso-phosphatidylcholine; lysoPE, lyso-phosphatidylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; Plasm-PE plasmenyl-phosphoethanolamine; PS, phosphatidylserine; SM, sphingomyelin; TG, triacylglyceride.
Table 1.
Unsaturation index change in diapause eggs versus non-diapause eggs by lipid class
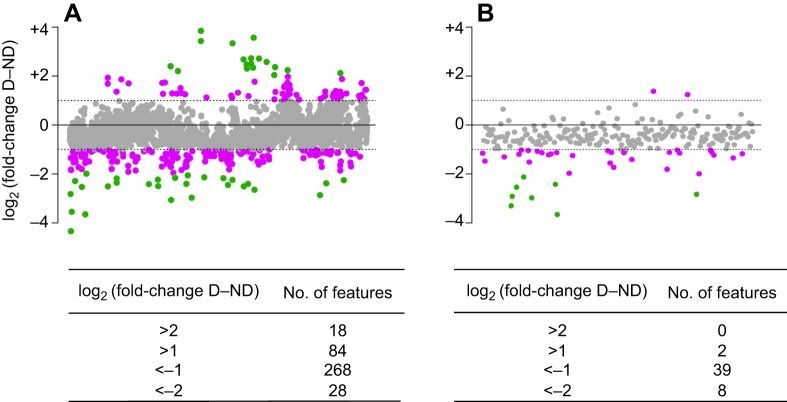
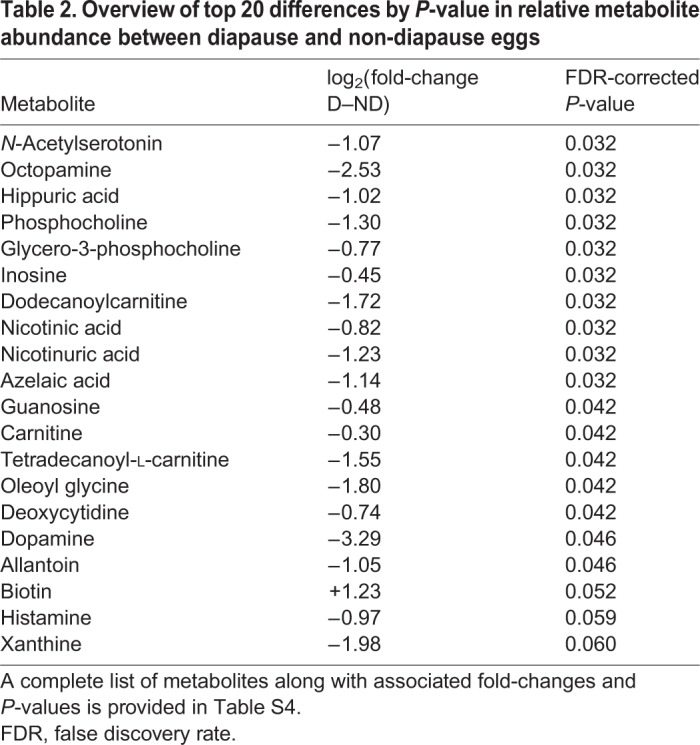
Our untargeted metabolomics dataset (Fig. 3A; Table S4) included 296 features that were less abundant (≤−1 fold-change) and 102 features that were more abundant (≥+1 fold-change) in diapause eggs relative to non-diapause eggs (Fig. 3A). Further analysis including only annotated metabolites identified 49 metabolites with fold-change values ≥|1|, 47 of which were less abundant in diapause eggs (Fig. 3B). We identified 17 annotated metabolites with significant differences in abundance (Table 2). Eleven of these metabolites had a fold-change >|1|, all of which were less abundant in diapause eggs.
Fig. 3.
Metabolites tend to exhibit lower abundance in diapause eggs. Data are log2 fold-change in relative abundance of untargeted metabolites in biological replicates of diapause eggs (n=4) relative to non-diapause eggs (n=4) for (A) all features with relative standard deviation (RSD)<30% and (B) only annotated metabolites. Dashed lines indicate a log2 fold-change of |1|. Purple dots indicate metabolites with >|1| log2 fold-change and green dots represent metabolites with >|2| log2 fold-change; counts of the number of features at each fold-change cutoff are provided in the tables below each figure. A complete list of log2 fold-change values for all metabolites in these figures is provided in Table S4.
Table 2.
Overview of top 20 differences by P-value in relative metabolite abundance between diapause and non-diapause eggs
Metabolite set enrichment analyses
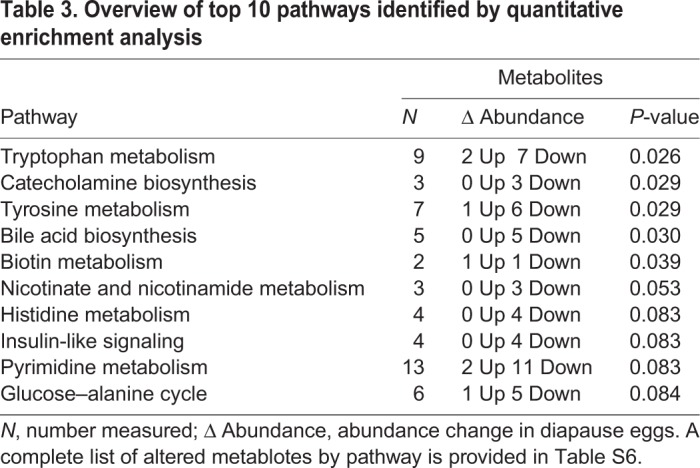
Quantitative enrichment analysis identified five metabolic pathways that were significantly enriched in diapause eggs (Table 3; Table S6). Pathway enrichment analysis identified tyrosine metabolism and tryptophan metabolism as significantly enriched in diapause eggs (Fig. S2).
Table 3.
Overview of top 10 pathways identified by quantitative enrichment analysis
Diapause-exclusive features
Among the detected features, 36 were found in all four diapause egg samples but were undetected in all four non-diapause samples. In contrast, only one feature was detected in all four non-diapause samples but undetected in all four diapause samples. None of the 36 diapause-exclusive features could be annotated but the composite spectra, masses and RTs for these unknown features are listed in Table S5. All 36 features were detected in positive ion mode and 31 of these features had RTs between 21.3 and 24.2 min. Annotated metabolites eluting within 0.5 min of the unknown, diapause-exclusive features were nearly all in the ‘lipid and lipid-like molecule’ HMDB super class (84.7% of metabolites). Further, the top three HMDB molecule classes in the co-eluting annotated dataset were ‘steroids and steroid derivatives’ (32.8%), ‘fatty acyls’ (30.0%) and ‘glycerophospholipids’ (14.3%). Within the steroid and steroid derivative class, most annotated metabolites were classified as ‘bile acids and bile acid derivatives’ (52.2%).
DISCUSSION
Diapause is a widespread adaptation that is crucial for overwinter survival as well as defining the distribution and abundance of many insect populations (Bale and Hayward, 2010; Bradshaw, 1993; Lees, 1956; Tauber et al., 1986). Despite the ecological significance of diapause, relatively little was known about the molecular regulation of this adaptation until recently (Denlinger, 2002). In the past decade, high-throughput RNA sequencing has rapidly advanced our understanding of the transcriptional basis of diapause (Koštál et al., 2017; Ragland and Keep, 2017). However, fewer studies have investigated regulatory mechanisms at the metabolite level, especially in the case of embryonic diapause. In this paper, we addressed this gap by identifying shifts in lipids, metabolites and metabolic pathways associated with embryonic diapause in A. albopictus.
Lipidomics
We identified distinct lipid profiles for eggs in early diapause maintenance (11 dpo) compared with those in age-matched, non-diapause eggs (Fig. 1A). Lipids are the primary energy storage molecule in insects and accumulation of lipids, particularly TGs, is a common hallmark of diapause (Hahn and Denlinger, 2007; Hahn and Denlinger, 2011). Blood-fed A. albopictus adult females overexpress the gene encoding fatty acid synthase when induced to produce diapause eggs compared with adult females induced to produce non-diapause eggs; this upregulation is consistent with greater maternal provisioning of lipids to diapause eggs (Huang et al., 2015). Additionally, A. albopictus diapause eggs upregulate the gene encoding lipid storage droplet protein 2 during diapause preparation (3 dpo; Poelchau et al., 2013a), differentially express 23 genes related to lipid metabolism during early diapause maintenance (11 dpo; Poelchau et al., 2013b) and have greater overall lipid abundance (Reynolds et al., 2012). Our results further reveal that the increased lipid abundance in A. albopictus diapause is primarily due to an accumulation of DGs and TGs (Fig. 2B). We identified nine lipid features (2 DGs, 7 TGs) that were significantly more abundant during diapause (Fig. 2A), although we lacked the resolution necessary to determine the specific fatty acid composition of these differentially abundant lipids.
We assessed changes in saturation across lipid classes during diapause but detected only minor changes to plasmenyl-PEs (Table 1). This result was contrary to our expectations, because changes in lipid unsaturation are frequently observed during diapause in other species (Bashan and Cakmak, 2005; Bennett et al., 1997; Izumi et al., 2009; Khani et al., 2007; Shimizu, 1992). Increased abundance of unsaturated fatty acids promotes lipid mobility and membrane fluidity at low temperatures, contributing to cold tolerance (Clark and Worland, 2008; Hazel, 1995). In A. albopictus, three genes encoding fatty acid desaturases are upregulated in blood-fed adult females under diapause-inducing conditions, consistent with increased maternal provisioning of unsaturated fatty acids (Huang et al., 2015). Furthermore, the gene encoding Δ(9)-desaturase, a rate-limiting enzyme in unsaturated fatty acid synthesis, is significantly upregulated during diapause preparation (6 dpo; Reynolds et al., 2012). In this species, diapause moderately improves cold tolerance but this effect is greatly enhanced by a period of cold acclimation (i.e. extended exposure to sub-lethal low temperatures; Hanson and Craig, 1994). Thus, it is possible that in A. albopictus, changes in lipid unsaturation may occur following exposure to low temperatures (i.e. cold acclimation), rather than diapause per se.
Metabolomics
Sample clustering in our metabolite PCA plot (Fig. 1B) indicated that the overall metabolite profile differed between diapause and non-diapause eggs. Distinct metabolome profiles for diapause and non-diapause samples have been consistently observed across insect taxa in the limited metabolomic studies to date (Dean et al., 2016; Khodayari et al., 2013; Lehmann et al., 2018; Li et al., 2015; Lu et al., 2014; Michaud and Denlinger, 2007; Purać et al., 2015; Teets et al., 2012; Vesala et al., 2012; Wang et al., 2017). In our samples, most features were less abundant (Fig. 3A) and this difference was more pronounced among our annotated metabolites (Fig. 3B). Lower metabolite abundance has also been observed during diapause in larvae of Calliphora vicina (Johnson, 2013), and adults of Tetranychus urticea (Khodayari et al., 2013), Drosophila montana and Oncopeltus fasciatus (Dean et al., 2016; Vesala et al., 2012). However, this pattern is not universal. The majority of altered metabolites are more abundant in diapausing larvae of Nasonia vitripennis (Li et al., 2015), and in diapausing pupae of Pieris napi (Lehmann et al., 2018) and Helicoverpa armigera (Lu et al., 2014).
For the remainder of the Discussion, we focus on specific metabolites and metabolic pathways that are significantly altered in diapause. We suggest potential diapause-associated roles for these metabolites and pathways. Where possible, we interpret our metabolome data with orthogonal evidence from previous transcriptional analyses in this species. We note that some pathways that were expected a priori to change under diapause conditions, such as glycolysis and the TCA cycle (Ragland et al., 2010), were not identified, likely because of limited annotation of key metabolites in these pathways.
Alterations in lipid metabolism pathways
As anticipated based on the lipidomic results (Figs 1A and 2), the abundance of several metabolites relevant to lipid metabolism was altered in diapause consistent with decreased lipid catabolism and increased lipid storage. For example, carnitine was significantly less abundant in diapause eggs (Table 2). Carnitine is bound to fatty acyl-CoA molecules via the enzymatic action of carnitine palmitoyltransferase (CPT), to produce acyl-carnitines that can be shuttled into the mitochondrial matrix where β-oxidation occurs (Bremer, 1983). The formation of acyl-carnitines is the rate-limiting step in β-oxidation and thus a critical regulatory point in the generation of energy from lipid stores (Foster, 2004). The gene encoding the CPT enzyme is significantly downregulated during diapause preparation (6 dpo) in A. albopictus, suggesting transcriptional repression of the carnitine shuttle system (Poelchau et al., 2013a). In contrast, CPT expression is not altered in early diapause (11 dpo; Reynolds et al., 2012; Poelchau et al., 2013b). However, of the 11 acyl-carnitines detected in early diapause, two were significantly less abundant (Table 2) and the remaining nine showed a downward trend (Table S4). This consistent pattern of reduced acyl-carnitine abundance supports the hypothesis that the carnitine shuttle is suppressed independent of CPT expression in early diapause, likely contributing to lipid conservation during diapause via reduced β-oxidation.
We also found that phosphocholine was less abundant in diapause eggs (Table 2). Phosphocholine is the metabolite utilized in the rate-limiting step of phosphatidylcholine (PC) synthesis via the Kennedy pathway (Pol et al., 2014; Kent, 1997). PCs are a major component of membranes and in D. melanogaster they comprise approximately 25% of the surface of lipid droplets (Pol et al., 2014). Such droplets are the primary intracellular reservoir for neutral lipids like TGs (Suzuki et al., 2011) and thus play a critical role in long-term energy storage for insects in diapause (Arrese and Soulages, 2010). Greater TG abundance activates the Kennedy pathway, which produces additional surface PCs to prevent lipid droplet coalescence (Krahmer et al., 2011). However, when the Kennedy pathway is experimentally suppressed in D. melanogaster cells, PC production is hindered, resulting in larger lipid droplets that are more resistant to lipolysis (Krahmer et al., 2011). Increased lipid accumulation and reduced lipolysis are key facets of the diapause phenotype across diverse insect taxa (Hahn and Denlinger, 2011). Aedes albopictus diapause eggs have increased neutral lipid abundance (Fig. 2) but we hypothesize that reduced phosphocholine abundance hinders PC production, thereby increasing the abundance of larger, coalescent lipid droplets. Furthermore, in A. albopictus, two genes encoding enzymes on the Kennedy pathway are downregulated during diapause preparation, consistent with reduced PC abundance and the production of larger lipid droplets (Poelchau et al., 2013b; Reynolds et al., 2012). Together, these data imply that adjustments in the Kennedy pathway may be an important component of lipolysis suppression in A. albopictus diapause.
Regulation of developmental arrest
Two catecholamines, dopamine and octopamine, were significantly less abundant in diapause (Table 2). Furthermore, the tyrosine metabolism pathway containing these molecules was significantly altered in diapause, as was catecholamine biosynthesis, a sub-pathway wholly contained within the tyrosine metabolism pathway (Table 3; Fig. S2). Dopamine and octopamine can act as neuromodulators regulating insect hormonal activity (Gilbert et al., 2000). However, their specific role can vary from promoter to inhibitor, depending on species, age and sex (e.g. Granger et al., 1996; Gruntenko et al., 2007; Kaatz et al., 1994; Pastor et al., 1991; Woodring and Hoffmann, 1994). The role of juvenile hormone III (JH3) in regulating diapause is similarly difficult to generalize. For example, repression of JH3 synthesis maintains the adult diapause of Culex pipiens (Sim and Denlinger, 2008; Spielman, 1974) but elevated JH3 abundance initiates and maintains larval diapause in Diatraea grandiosella (Yin and Chippendale, 1973). Given the variable roles played by these molecules and the lack of an identified hormonal mechanism to initiate and maintain diapause in A. albopictus (Denlinger and Armbruster, 2016), it is difficult to predict how repression of catecholamines and alterations in their associated metabolic pathway might contribute to diapause regulation in this species. However, treating A. albopictus diapause eggs with a JH3 analog terminates diapause (Suman et al., 2015) and expression of a gene encoding a JH3-degrading esterase is upregulated throughout diapause maintenance (Poelchau et al., 2013b). Together, these data suggest that low levels of JH3 may help maintain diapause in A. albopictus. Potential upstream regulation of JH3 by dopamine and octopamine merits further investigation.
Oleoyl glycine was significantly less abundant in diapause eggs (Table 2). This metabolite is an N-acyl amino acid that, in rats, increases insulin-mediated inactivation of forkhead transcription factor (FOXO) via the Akt pathway (Wang et al., 2015). Oleoyl glycine has not been studied in insects, but because many components of the insulin signaling pathway are conserved in insects and mammals (Claeys et al., 2002), a significant reduction of this metabolite in diapause A. albopictus eggs could increase FOXO activity as a result of reduced insulin-mediated phosphorylation. Such FOXO activation should result in greater fat accumulation and improved resistance to oxidative stress, as occurs in the adult diapause of C. pipiens and D. melanogaster, as well as during dauer formation in Caenorhabditis elegans, a diapause-like state in worms (Barthel et al., 2005; Sim and Denlinger, 2008). For A. albopictus, no Akt pathway genes are differentially expressed during diapause maintenance (Poelchau et al., 2013b) but the Akt gene is downregulated in diapause preparation (6 dpo; Poelchau et al., 2013a). Additionally, the insulin-suppressed microRNA bantam-5p is significantly more abundant in diapause eggs (Batz et al., 2017) consistent with reduced insulin. In our data, metabolite changes in the insulin-like signaling pathway approached significance (Table 3). Together, these data suggest that insulin-mediated regulation of FOXO via oleoyl glycine could contribute to diapause regulation in A. albopictus.
Finally, among unannotated metabolites (Table S5), 36 features were exclusively identified in all four diapause samples. Annotated metabolites co-eluting with these unknown features were primarily classified as steroids/steroid derivatives (Table S5). Ecdysteroids, a class of steroid hormones not present in our annotation database, are known to regulate larval and pupal diapause in numerous insect species (Denlinger, 2002) and high ecdysteroid abundance appears to maintain embryonic diapause in Lymantria dispar (Suzuki et al., 1993; Lee et al., 1997). In A. albopictus, both microRNAs (Batz et al., 2017) and mRNAs (Poelchau et al., 2011; Poelchau et al., 2013a,b) involved in ecdysone signaling are differentially abundant in diapause. Thus, the consistent observation of alterations related to ecdysone signaling at the transcriptional level throughout diapause, combined with this intriguing pattern of diapause-specific, steroid-like features detected in our dataset, suggest ecdysone signaling may contribute to diapause regulation in A. albopictus.
Supplementary Material
Acknowledgements
We thank the University of Michigan Metabolomic Resource Core for performing the lipidomic and untargeted metabolomic data collection. We also thank Drs Maureen Kachman and Maeva Millan for technical assistance. Finally, we thank Drs Steven Cook, Leslie Ries, Anne Rosenwald, Vladimir Koštál and Colin Brent and two anonymous reviewers for valuable feedback on earlier versions of this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Z.A.B., P.A.A.; Methodology: Z.A.B.; Formal analysis: Z.A.B.; Investigation: Z.A.B.; Writing - original draft: Z.A.B., P.A.A.; Writing - review & editing: Z.A.B., P.A.A.; Visualization: Z.A.B.; Funding acquisition: P.A.A.
Funding
This work was supported by the National Institutes of Health (grant no. R15 AI111328 to P.A.A.). Deposited in PMC for release after 12 months.
Data availability
Data from this study are available via Metabolomics Workbench, study accession ID ST000722.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.189480.supplemental
References
- Aksenov A. A., da Silva R., Knight R., Lopes N. P. and Dorrestein P. C. (2017). Global chemical analysis of biology by mass spectrometry. Nat. Rev. Chem. 1, 0054 10.1038/s41570-017-0054 [DOI] [Google Scholar]
- Armbruster P. and Conn J. E. (2006). Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 99, 1234-1243. 10.1603/0013-8746(2006)99[1234:GVOLGI]2.0.CO;2 [DOI] [Google Scholar]
- Armbruster P. and Hutchinson R. A. (2002). Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae). J. Med. Entomol. 39, 699-704. 10.1603/0022-2585-39.4.699 [DOI] [PubMed] [Google Scholar]
- Arrese E. L. and Soulages J. L. (2010). Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207-225. 10.1146/annurev-ento-112408-085356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale J. S. and Hayward S. A. L. (2010). Insect overwintering in a changing climate. J. Exp. Biol. 213, 980-994. 10.1242/jeb.037911 [DOI] [PubMed] [Google Scholar]
- Barthel A., Schmoll D. and Unterman T. G. (2005). FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 16, 183-189. 10.1016/j.tem.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Bashan M. and Cakmak O. (2005). Changes in composition of phospholipid and triacylglycerol fatty acids prepared from prediapausing and diapausing individuals of Dolycoris baccarum and Piezodorus lituratus (Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 98, 575-579. 10.1603/0013-8746(2005)098[0575:CICOPA]2.0.CO;2 [DOI] [Google Scholar]
- Batz Z. A., Goff A. C. and Armbruster P. A. (2017). MicroRNAs are differentially abundant during Aedes albopictus diapause maintenance but not diapause induction. Insect Mol. Biol. 26, 721-733. 10.1111/imb.12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. and Hochberg Y. (1995). Controlling the false discovery rate : a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289-300. [Google Scholar]
- Bennett V. A., Pruitt N. L. and Lee R. E. Jr. (1997). Seasonal changes in fatty acid composition associated with cold-hardening in third instar larvae of Eurosta solidaginis. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 167, 249-255. 10.1007/s003600050071 [DOI] [Google Scholar]
- Bligh E. G. and Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37, 911-917. 10.1139/y59-099 [DOI] [PubMed] [Google Scholar]
- Bradshaw W. E. (1993). Evolution in seasonal environments. In Seasonal Adaptation and Diapause in Insects (ed. Takeda M. and Tanaka S.), pp. 121-133. Tokyo: Bun-ichi Sôgô Shuppan. [Google Scholar]
- Bradshaw W. E., Zani P. A. and Holzapfel C. M. (2004). Adaptation to temperate climates. Evolution 58, 1748-1762. 10.1111/j.0014-3820.2004.tb00458.x [DOI] [PubMed] [Google Scholar]
- Bremer J. (1983). Carnitine – metabolism and functions. Physiol. Rev. 63, 1420-1480. 10.1152/physrev.1983.63.4.1420 [DOI] [PubMed] [Google Scholar]
- Cajka T. and Fiehn O. (2016). Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 88, 524-545. 10.1021/acs.analchem.5b04491 [DOI] [PubMed] [Google Scholar]
- Claeys I., Simonet G., Poels J., Van Loy T., Vercammen L., De Loof A. and Vanden Broeck J. (2002). Insulin-related peptides and their conserved signal transduction pathway. Peptides 23, 807-816. 10.1016/S0196-9781(01)00666-0 [DOI] [PubMed] [Google Scholar]
- Clark M. S. and Worland M. R. (2008). How insects survive the cold: molecular mechanisms - a review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 178, 917-933. 10.1007/s00360-008-0286-4 [DOI] [PubMed] [Google Scholar]
- Colinet H., Renault D., Charoy-Guével B. and Com E. (2012). Metabolic and proteomic profiling of diapause in the aphid parasitoid Praon volucre. PLoS ONE 7, e32606 10.1371/journal.pone.0032606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks H. V. (1987). Insect Dormancy: An Ecological Perspective. Ottawa: Biological Survey of Canada (Terrestrial Arthropods). [Google Scholar]
- Dean C. A. E., Teets N. M., Koštál V., Šimek P. and Denlinger D. L. (2016). Enhanced stress responses and metabolic adjustments linked to diapause and onset of migration in the large milkweed bug Oncopeltus fasciatus. Physiol. Entomol. 41, 152-161. 10.1111/phen.12140 [DOI] [Google Scholar]
- Denlinger D. L. (2002). Regulation of diapause. Annu. Rev. Entomol. 47, 93-122. 10.1146/annurev.ento.47.091201.145137 [DOI] [PubMed] [Google Scholar]
- Denlinger D. L. and Armbruster P. A. (2014). Mosquito diapause. Annu. Rev. Entomol. 59, 73-93. 10.1146/annurev-ento-011613-162023 [DOI] [PubMed] [Google Scholar]
- Denlinger D. L. and Armbruster P. A. (2016). Molecular physiology of mosquito diapause. In Advances in Insect Physiology, Vol. 51 (ed. A. S. Raikhel), pp. 329-361. Cambridge, MA: Academic Press. [Google Scholar]
- Denlinger D. L., Yocum G. D. and Rinehart J. P. (2012). Hormonal control of diapause. In Insect Endocrinology (ed. L. I. Gilbert), pp. 430-463. Cambridge, MA: Academic Press. [Google Scholar]
- Dunn W. B., Erban A., Weber R. J. M., Creek D. J., Brown M., Breitling R., Hankemeier T., Goodacre R., Neumann S., Kopka J. et al. (2013). Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics 9, S44-S66. 10.1007/s11306-012-0434-4 [DOI] [Google Scholar]
- Fang M., Ivanisevic J., Benton H. P., Johnson C. H., Patti G. J., Hoang L. T., Uritboonthai W., Kurczy M. E., Siuzdak G. (2015). Thermal degradation of small molecules: a global metabolomic investigation. Anal. Chem. 87, 10935-10941. 10.1021/acs.analchem.5b03003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. W. (2004). The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 1033, 1-16. 10.1196/annals.1320.001 [DOI] [PubMed] [Google Scholar]
- Gentlemen R., Carey V., Huber W., Irizarry R. and Dudoit S. (ed). (2005). Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York, USA: Springer-Verlag. [Google Scholar]
- Gilbert L. I., Granger N. A. and Roe R. M. (2000). The juvenile hormones: historical facts and speculations on future research directions. Insect Biochem. Mol. Biol. 30, 617-644. 10.1016/S0965-1748(00)00034-5 [DOI] [PubMed] [Google Scholar]
- Gong Z.-J., Wu Y.-Q., Miao J., Duan Y., Jiang Y.-L. and Li T. (2013). Global transcriptome analysis of orange wheat blossom midge, Sitodiplosis mosellana (Gehin) (Diptera: Cecidomyiidae) to identify candidate transcripts regulating diapause. PLoS ONE 8, e71564 10.1371/journal.pone.0071564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Fujii M., Suzuki K. and Sakai M. (1998). Factors affecting carbohydrate and free amino acid content in overwintering larvae of Enosima leucotaeniella. J. Insect Physiol. 44, 87-94. 10.1016/S0022-1910(97)00098-X [DOI] [PubMed] [Google Scholar]
- Granger N. A., Sturgis S. L., Ebersohl R., Geng C. and Sparks T. C. (1996). Dopaminergic control of corpora allata activity in the larval tobacco hornworm, Manduca sexta. Arch. Insect. Biochem. Physiol. 32, 449-466. [DOI] [PubMed] [Google Scholar]
- Gruntenko N. E., Karpova E. K., Alekseev A. A., Chentsova N. A., Bogomolova E. V., Bownes M. and Rauschenbach I. Y. (2007). Effects of octopamine on reproduction, juvenile hormone metabolism, dopamine, and 20-hydroxyecdysone contents in Drosophila. Arch. Insect Biochem. Physiol. 65, 85-94. 10.1002/arch.20187 [DOI] [PubMed] [Google Scholar]
- Hahn D. A. and Denlinger D. L. (2007). Meeting the energetic demands of insect diapause: nutrient storage and utilization. J. Insect Physiol. 53, 760-773. 10.1016/j.jinsphys.2007.03.018 [DOI] [PubMed] [Google Scholar]
- Hahn D. A. and Denlinger D. L. (2011). Energetics of insect diapause. Annu. Rev. Entomol. 56, 103-121. 10.1146/annurev-ento-112408-085436 [DOI] [PubMed] [Google Scholar]
- Hanson S. M. and Craig G. B. Jr. (1994). Cold acclimation, diapause, and geographic origin affect cold hardiness in eggs of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 31, 192-201. 10.1093/jmedent/31.2.192 [DOI] [PubMed] [Google Scholar]
- Hawley W. A. (1988). The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1, 1-39. [PubMed] [Google Scholar]
- Hazel J. R. (1995). Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57, 19-42. 10.1146/annurev.ph.57.030195.000315 [DOI] [PubMed] [Google Scholar]
- Huang X., Poelchau M. F. and Armbruster P. A. (2015). Global transcriptional dynamics of diapause induction in non-blood-fed and blood-fed Aedes albopictus. PLoS Negl. Trop. Dis. 9, e0003724 10.1371/journal.pntd.0003724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Katagiri C., Sonoda S. and Tsumuki H. (2009). Seasonal changes of phospholipids in last instar larvae of rice stem borer Chilo suppressalis Walker (Lepidoptera: Pyralidae). Entomol. Sci. 12, 376-381. 10.1111/j.1479-8298.2009.00336.x [DOI] [Google Scholar]
- Johnson B. (2013). Effect of stress and diapause in two Calliphoridae species. PhD thesis, University of Birmingham, Birmingham, UK. [Google Scholar]
- Johnson C. H. and Gonzalez F. J. (2012). Challenges and opportunities of metabolomics. J. Cell. Physiol. 227, 2975-2981. 10.1002/jcp.24002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz H., Eichmüller S. and Kreissl S. (1994). Stimulatory effect of octopamine on juvenile hormone biosynthesis in honey bees (Apis mellifera): physiological and immunocytochemical evidence. J. Insect Physiol. 40, 865-872. 10.1016/0022-1910(94)90020-5 [DOI] [Google Scholar]
- Kang D. S., Cotten M. A., Denlinger D. L. and Sim C. (2016). Comparative transcriptomics reveals key gene expression differences between diapausing and non-diapausing adults of Culex pipiens. PLoS ONE 11, e0154892 10.1371/journal.pone.0154892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C. (1997). CTP: phosphocholine cytidylyltransferase. Biochim. Biophys. Acta, Lipids Lipid Metab. 1348, 79-90. 10.1186/1471-2164-14-751 [DOI] [PubMed] [Google Scholar]
- Khani A., Moharramipour S., Barzegar M. and Naderi-Manesh H. (2007). Comparison of fatty acid composition in total lipid of diapause and non-diapause larvae of Cydia pomonella (Lepidoptera: Tortricidae). Insect Sci. 14, 125-131. 10.1111/j.1744-7917.2007.00134.x [DOI] [Google Scholar]
- Khodayari S., Moharramipour S., Larvor V., Hidalgo K. and Renault D. (2013). Deciphering the metabolic changes associated with diapause syndrome and cold acclimation in the two-spotted spider mite Tetranychus urticae. PLoS ONE 8, e54025 10.1371/journal.pone.0054025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind T., Liu K.-H., Lee D. Y., DeFelice B., Meissen J. K. and Fiehn O. (2013). LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 10, 755-758. 10.1038/nmeth.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koštál V. (2006). Eco-physiological phases of insect diapause. J. Insect Physiol. 52, 113-127. 10.1016/j.jinsphys.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Koštál V., Štětina T., Poupardin R., Korbelová J. and Bruce A. W. (2017). Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomic profiling. Proc. Natl. Acad. Sci. USA 114, 8532-8537. 10.1073/pnas.1707281114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahmer N., Guo Y., Wilfling F., Hilger M., Lingrell S., Heger K., Newman H. W., Schmidt-Supprian M., Vance D. E., Mann M. et al. (2011). Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:Phosphocholine cytidylyltransferase. Cell Metab. 14, 504-515. 10.1016/j.cmet.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafage J. P., Crowder L. A. and Watson T. F. (1974). Amino acids and total nitrogen of diapause and non-diapause larvae of the pink bollworm, Pectinophora gossypiella. Ann. Entomol. Soc. Am. 67, 472-474. 10.1093/aesa/67.3.472 [DOI] [Google Scholar]
- Lee K.-Y., Valaitis A. P. and Denlinger D. L. (1997). Further evidence that diapause in the gypsy moth, Lymantria dispar, is regulated by ecdysteroids: a comparison of diapause and nondiapause strains. J. Insect Physiol. 43, 897-903. 10.1016/S0022-1910(97)00054-1 [DOI] [PubMed] [Google Scholar]
- Lees A. D. (1956). The physiology and biochemistry of diapause. Annu. Rev. Entomol. 1, 1-16. 10.1146/annurev.en.01.010156.000245 [DOI] [Google Scholar]
- Lehmann P., Pruisscher P., Koštál V., Moos M., Šimek P., Nylin S., Agren R., Väremo L., Wiklund C., Wheat C. W. et al. (2018). Metabolome dynamics of diapause in the butterfly Pieris napi: distinguishing maintenance, termination and post-diapause phases. J. Exp. Biol. 221, jeb169508 10.1242/jeb.169508 [DOI] [PubMed] [Google Scholar]
- Lerma-Ortiz C., Jeffryes J. G., Cooper A. J. L., Niehaus T. D., Thamm A. M. K., Frelin O., Aunins T., Fiehn O., de Crécy-Lagard V., Henry C. S. et al. (2016). ‘Nothing of chemistry disappears in biology’: the top 30 damage-prone endogenous metabolites. Biochem. Soc. Trans. 44, 961-971. 10.1042/BST20160073 [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang L., Chen H., Koštál V., Simek P., Moos M. and Denlinger D. L. (2015). Shifts in metabolomic profiles of the parasitoid Nasonia vitripennis associated with elevated cold tolerance induced by the parasitoid's diapause, host diapause and host diet augmented with proline. Insect Biochem. Mol. Biol. 63, 34-46. 10.1016/j.ibmb.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Liu J. and Hekimi S. (2013). The impact of mitochondrial oxidative stress on bile acid-like molecules in C. elegans provides a new perspective on human metabolic diseases. Worm 2, e21457 10.4161/worm.21457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ser Z., Cluntun A. A., Mentch S. J. and Locasale J. W. (2014). A strategy for sensitive, large scale quantitative metabolomics. J. Vis. Exp. 27, e51358 10.3791/51358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. X., Zhang Q. and Xu W. H. (2014). Global metabolomic analyses of the hemolymph and brain during the initiation, maintenance, and termination of pupal diapause in the cotton bollworm, Helicoverpa armigera. PLoS ONE 9, e99948 10.1371/journal.pone.0099948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macel M., van Dam N. M. and Keurentjes J. J. B. (2010). Metabolomics: the chemistry between ecology and genetics. Mol. Ecol. Resour. 10, 583-593. 10.1111/j.1755-0998.2010.02854.x [DOI] [PubMed] [Google Scholar]
- Mahieu N. G. and Patti G. J. (2017). Systems-level annotation of a metabolomics data set reduces 25,000 features to fewer than 1000 unique metabolites . Anal. Chem. 89, 10397-10406. 10.1021/acs.analchem.7b02380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M. R. and Denlinger D. L. (2007). Shifts in the carbohydrate, polyol, and amino acid pools during rapid cold-hardening and diapause-associated cold-hardening in flesh flies (Sarcophaga crassipalpis): a metabolomic comparison. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 177, 753-763. 10.1007/s00360-007-0172-5 [DOI] [PubMed] [Google Scholar]
- Mohammadzadeh M., Borzoui E. and Izadi H. (2017). Physiological and biochemical differences in diapausing and nondiapausing larvae of Eurytoma plotnikovi (Hymenoptera: Eurytomidae). Environ. Entomol. 46, 1424-1431. 10.1093/ee/nvx128 [DOI] [PubMed] [Google Scholar]
- Pastor D., Piulachs M. D., Cassier P., André M. and Belles X. (1991). In vivo and in vitro study of the action of dopamine on oocyte growth and juvenile hormone production in Blattella germanica (L.) (Dictyoptera; Blattellidae). C. R. Acad. Sci. III 313, 207-212. [PubMed] [Google Scholar]
- Patti G. J. (2011). Separation strategies for untargeted metabolomics. J. Sep. Sci. 34, 3460-3469. 10.1002/jssc.201100532 [DOI] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Denlinger D. L., Elsik C. G. and Armbruster P. A. (2011). A de novo transcriptome of the Asian tiger mosquito, Aedes albopictus, to identify candidate transcripts for diapause preparation. BMC Genomics 12, 619 10.1186/1471-2164-12-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Elsik C. G., Denlinger D. L. and Armbruster P. A. (2013a). Deep sequencing reveals complex mechanisms of diapause preparation in the invasive mosquito, Aedes albopictus. Proc. R. Soc. B Biol. Sci. 280, 20130143 10.1098/rspb.2013.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M. F., Reynolds J. A., Elsik C. G., Denlinger D. L. and Armbruster P. A. (2013b). RNA-Seq reveals early distinctions and late convergence of gene expression between diapause and quiescence in the Asian tiger mosquito, Aedes albopictus. J. Exp. Biol. 216, 4082-4090. 10.1242/jeb.089508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol A., Gross S. P. and Parton R. G. (2014). Biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J. Cell Biol. 204, 635-646. 10.1083/jcb.201311051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupardin R., Schöttner K., Korbelová J., Provazník J., Doležel D., Pavlinic D., Beneš V. and Koštál V. (2015). Early transcriptional events linked to induction of diapause revealed by RNAseq in larvae of drosophilid fly, Chymomyza costata. BMC Genomics 16, 720 10.1186/s12864-015-1907-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpuni C. B. (1989). Factors influencing photoperiodic control of egg diapause in Aedes albopictus (Skuse). PhD thesis, University of Notre Dame, South Bend, IN, USA. [Google Scholar]
- Purać J., Kojić D., Popović Ž. D., Vukašinović E., Tiziani S., Günther U. L. and Grubor-Lajšić G. (2015). Metabolomic analysis of diapausing and non-diapausing larvae of the European corn borer Ostrinia nubilalis (Hbn.) (Lepidoptera: Crambidae). Acta Chim. Slov. 62, 761-767. [PubMed] [Google Scholar]
- Qi X., Zhang L., Han Y., Ren X., Huang J. and Chen H. (2015). De novo transcriptome sequencing and analysis of Coccinella septempunctata L. in non-diapause, diapause and diapause-terminated states to identify diapause-associated genes. BMC Genomics 16, 1086 10.1186/s12864-015-2309-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland G. J. and Keep E. (2017). Comparative transcriptomics support evolutionary convergence of diapause responses across Insecta. Physiol. Entomol. 42, 246-256. 10.1111/phen.12193 [DOI] [Google Scholar]
- Ragland G. J., Denlinger D. L. and Hahn D. A. (2010). Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 107, 14909-14914. 10.1073/pnas.1007075107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Poelchau M. F., Rahman Z., Armbruster P. A. and Denlinger D. L. (2012). Transcript profiling reveals mechanisms for lipid conservation during diapause in the mosquito, Aedes albopictus. J. Insect Physiol. 58, 966-973. 10.1016/j.jinsphys.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I. (1992). Comparison of fatty acid compositions in lipids of diapause and non-diapause eggs of Bombyx mori (Lepidoptera: Bombycidae). Comp. Biochem. Physiol. 102, 713-716. [Google Scholar]
- Sim C. and Denlinger D. L. (2008). Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA 105, 6777-6781. 10.1073/pnas.0802067105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A. (1974). Effect of synthetic juvenile hormone on ovarian diapause of Culex pipiens mosquitoes. J. Med. Entomol. 11, 223-225. 10.1093/jmedent/11.2.223 [DOI] [PubMed] [Google Scholar]
- Suman D. S., Wang Y. and Gaugler R. (2015). The insect growth regulator pyriproxyfen terminates egg diapause in the Asian tiger mosquito, Aedes albopictus. PLoS ONE 10, e0130499 10.1371/journal.pone.0130499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Nakamura T., Yanbe T., Kurihara M. and Kuwano E. (1993). Termination of diapause in pharate first-instar larvae of the gypsy moth Lymantria dispar japonica by an imidazole derivative KK-42. J. Insect Physiol. 39, 107-110. 10.1016/0022-1910(93)90101-V [DOI] [Google Scholar]
- Suzuki M., Shinohara Y., Ohsaki Y. and Fujimoto T. (2011). Lipid droplets: size matters. J. Electron Microsc. (Tokyo) 60, S101-S116. 10.1093/jmicro/dfr016 [DOI] [PubMed] [Google Scholar]
- Tauber M. J. and Tauber C. A. (1976). Insect seasonality: diapause maintenance, termination, and postdiapause development. Annu. Rev. Entomol. 21, 81-107. 10.1146/annurev.en.21.010176.000501 [DOI] [Google Scholar]
- Tauber M. J., Tauber C. A. and Masaki S. (1986). Seasonal Adaptations of Insects. New York, USA: Oxford University Press. [Google Scholar]
- Teets N. M. and Denlinger D. L. (2013). Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 38, 105-116. 10.1111/phen.12019 [DOI] [Google Scholar]
- Teets N. M., Peyton J. T., Ragland G. J., Colinet H., Renault D., Hahn D. A. and Denlinger D. L. (2012). Combined transcriptomic and metabolomic approach uncovers molecular mechanisms of cold tolerance in a temperate flesh fly. Physiol. Genomics 44, 764-777. 10.1152/physiolgenomics.00042.2012 [DOI] [PubMed] [Google Scholar]
- Trpiš M. (1970). A new bleaching and decalcifying method for general use in zoology. Can. J. Zool. 48, 892-893. 10.1139/z70-158 [DOI] [Google Scholar]
- Urbanski J. M., Benoit J. B., Michaud M. R., Denlinger D. L. and Armbruster P. (2010). The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proc. R. Soc. B Biol. Sci. 277, 2683-2692. 10.1098/rspb.2010.0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski J., Mogi M., O'Donnell D., DeCotiis M., Toma T. and Armbruster P. (2012). Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 179, 490-500. 10.1086/664709 [DOI] [PubMed] [Google Scholar]
- van den Berg R. A., Hoefsloot H. C. J., Westerhuis J. A., Smilde A. K. and van der Werf M. J. (2006). Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 7, 1-15. 10.1186/1471-2164-7-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesala L., Salminen T. S., Koštál V., Zahradníčková H. and Hoikkala A. (2012). Myo-inositol as a main metabolite in overwintering flies: seasonal metabolomic profiles and cold stress tolerance in a northern drosophilid fly. J. Exp. Biol. 215, 2891-2897. 10.1242/jeb.069948 [DOI] [PubMed] [Google Scholar]
- Vuckovic D. (2012). Current trends and challenges in sample preparation for global metabolomics using liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 403, 1523-1548. 10.1007/s00216-012-6039-y [DOI] [PubMed] [Google Scholar]
- Wang S., Xu Q., Shu G., Wang L., Gao P., Xi Q., Zhang Y., Jiang Q. and Zhu X. (2015). N-Oleoyl glycine, a lipoamino acid, stimulates adipogenesis associated with activation of CB1 receptor and Akt signaling pathway in 3T3-L1 adipocyte. Biochem. Bioph. Res. Co. 466, 438-443. 10.1016/j.bbrc.2015.09.046 [DOI] [PubMed] [Google Scholar]
- Wang J., Fan H., Xiong K.-C. and Liu Y.-H. (2017). Transcriptomic and metabolomic profiles of Chinese citrus fly, Bactrocera minax (Diptera: Tephritidae), along with pupal development provide insight into diapause program. PLoS ONE 12, e0181033 10.1371/journal.pone.0181033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipking W., Viebahn M. and Neumann D. (1995). Oxygen consumption, water, lipid and glycogen content of early and late diapause and non-diapause larvae of the burnet moth Zygaena trifolii. J. Insect Physiol. 41, 47-56. 10.1016/0022-1910(94)00079-V [DOI] [Google Scholar]
- Wishart D. S., Tzur D., Knox C., Eisner R., Guo A. C., Young N., Cheng D., Jewell K., Arndt D., Sawhney S. et al. (2007). HMDB: the human metabolome database. Nucleic Acids Res. 35, D521-D526. 10.1093/nar/gkl923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S., Feunang Y. D., Marcu A., Guo A. C., Liang K., Vázquez-Fresno R., Sajed T., Johnson D., Li C., Karu N. et al. (2018). HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 46, D608-D617. 10.1093/nar/gkx1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodring J. and Hoffmann K. H. (1994). The effects of octopamine, dopamine and serotonin on juvenile hormone synthesis, in vitro, in the cricket, Gryllus bimaculatus. J. Insect Physiol. 40, 797-802. 10.1016/0022-1910(94)90009-4 [DOI] [Google Scholar]
- Xia J. and Wishart D. S. (2010). MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 38, W71-W77. 10.1093/nar/gkq329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J. and Wishart D. S. (2011a). Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 6, 743-760. 10.1038/nprot.2011.319 [DOI] [PubMed] [Google Scholar]
- Xia J. and Wishart D. S. (2011b). MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 27, 2342-2344. [DOI] [PubMed] [Google Scholar]
- Xia J., Sinelnikov I. V., Han B. and Wishart D. S. (2015). MetaboAnalyst 3.0: making metabolomics more meaningful. Nucleic Acids Res. 43, W251-W257. 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes O., Tautenhahn R., Patti G. J. and Siuzdak G. (2011). Expanding coverage of the metabolome for global metabolite profiling. Anal. Chem. 83, 2152-2161. 10.1021/ac102981k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C.-M. and Chippendale G. M. (1973). Juvenile hormone regulation of the larval diapause of the southwestern corn borer, Diatraea grandiosella. J. Insect Physiol. 19, 2403-2420. 10.1016/0022-1910(73)90244-8 [DOI] [PubMed] [Google Scholar]
- Yocum G. D., Rinehart J. P., Horvath D. P., Kemp W. P., Bosch J., Alroobi R. and Salem S. (2015). Key molecular processes of the diapause to post-diapause quiescence transition in the alfalfa leafcutting bee Megachile rotundata identified by comparative transcriptome analysis. Physiol. Entomol. 40, 103-112. 10.1111/phen.12093 [DOI] [Google Scholar]
- Zhang Q., Lu Y.-X. and Xu W.-H. (2012). Integrated proteomic and metabolomic analysis of larval brain associated with diapause induction and preparation in the cotton bollworm, Helicoverpa armigera. J. Proteome Res. 11, 1042-1053. 10.1021/pr200796a [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lu Y.-X. and Xu W.-H. (2013). Proteomic and metabolomic profiles of larval hemolymph associated with diapause in the cotton bollworm, Helicoverpa armigera. BMC Genomics 14, 751 10.1186/1471-2164-14-751 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.