Abstract
Adoptive cell transfer (ACT) of engineered T cell receptors (TCRs) for cancer immunotherapy has evolved from simple gene transfer of isolated TCRs to various affinity enhancement techniques that overcome limitations imposed by central and peripheral tolerance on TCR affinity. In the current issue of the JCI, Poncette et al. used mice with human TCRαβ and HLA gene loci to discover CD4+ TCRs of optimal affinity for cancer testis antigen (CTA) NY-ESO-1. They combined this TCR with a previously discovered NY-ESO-1–specific CD8+ TCR in an ACT fibrosarcoma tumor model to demonstrate the importance of T cell help in mediating antitumor responses.
Evolution of T cell receptor engineering
The first demonstration that T cells could be engineered with a predetermined specificity through transfer of the α and β T cell receptor (TCR) genes occurred more than 30 years ago (1). Two decades later, this technology was applied clinically for the first time in adoptive cell transfer (ACT) treatment of metastatic melanoma using MART-1 melanoma antigen-specific clones recovered from tumor-infiltrating lymphocytes (TILs) (2). While in the initial trials 2 of 15 patients (13%) had objective tumor regression after treatment, subsequent trials targeting MART-1 and gp100 melanoma antigens with high-avidity TCRs (based on IFN-γ secretions with titrating amounts of cognate antigen) yielded higher response rates (30% and 20%, respectively). However, this treatment led to widespread destruction of healthy melanocytes and significant on-target toxicities (3). Nevertheless, as the high-avidity gp100 antigen was discovered by immunizing HLA-A2–transgenic mice with the gp100154–162 epitope, this study demonstrated for the first time the clinical potential of tumor antigen–specific TCRs generated in nonhuman hosts whose T cell responses to human antigens are unencumbered by limitations posed by central or peripheral tolerance (3). More recently, a murine TCR (mTCR) gene specific to the cancer testis antigen (CTA) New York esophageal squamous cell carcinoma 1 (NY-ESO-1) was isolated from HLA-A2–transgenic mice immunized with NY-ESO-1157–165 peptide and is being used in an ongoing phase II clinical trial (NCT01967823) (4). Other groups have used alloreactive settings (5), rational design through mutations in the complementarity-determining regions (CDRs) of TCRs (6), yeast (7), phage display (8), or some combination thereof to overcome tolerance-imposed limitations on tumor-associated antigen (TAA) TCR avidity.
Engineering “optimal”-affinity T cell receptors using a nontolerant host
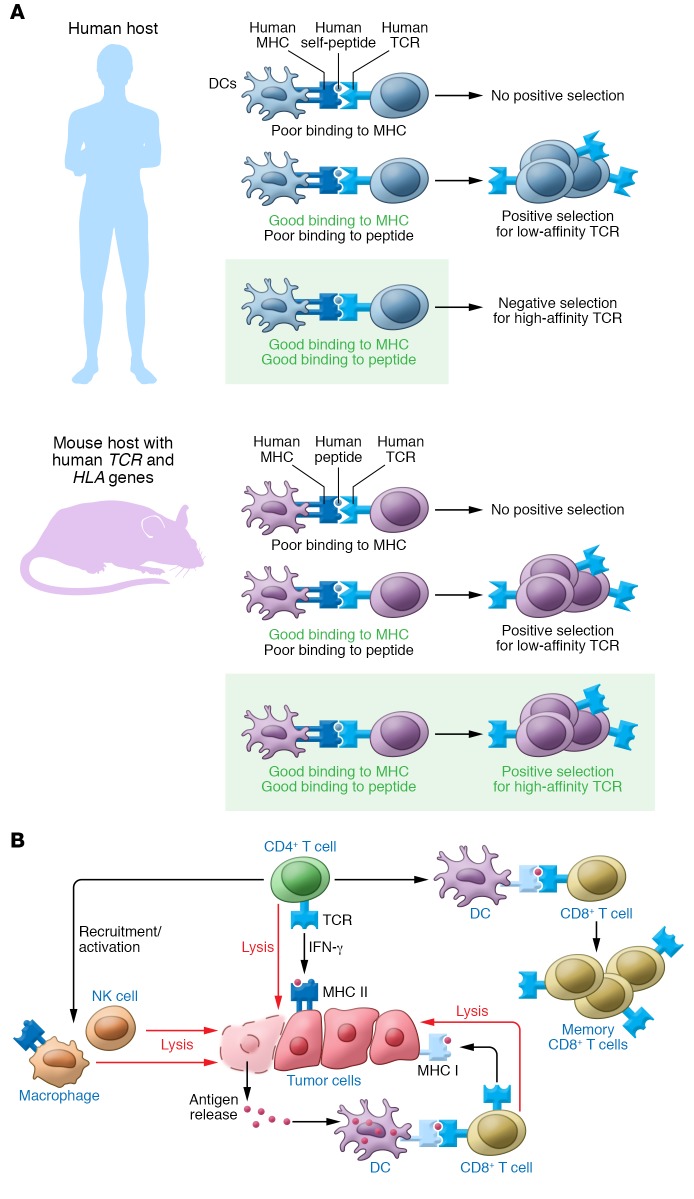
In the current issue of the JCI, Poncette and colleagues use a system that has been developed over several years by the Blankenstein laboratory to generate “optimal”-affinity TCRs that bind human HLA, but recognize self-antigens such as MART-1 and NY-ESO-1 as foreign (9). Initially, Li et al. developed ABab-transgenic mice with human, instead of murine, TCRαβ gene loci and then crossed them with HLA-A2–transgenic mice to form ABabDII mice, which express human TCRs and the human HLA-A2 MHC class I (MHC I) (10). These mice lacked tolerance to human antigens and were capable of generating human CD8+ TCRs with high affinity for TAAs (Figure 1A). Because these TCRs use human genes, they, unlike mTCRs (11), were at a lower risk of generating a humoral immune response that would hinder therapy. Moreover, they did not need to be further mutated to improve binding avidity at the risk of severe off-target toxicities (12), due in part to the fact that ABabDII mice have a broader CD8+ repertoire than HLA-A2–transgenic mice, and also because human and mouse TCRs have an intrinsically higher affinity for intra- rather than inter-species MHC (10, 13). More recently, the system has been adapted for discovery of optimal-affinity CD4+ T cells by crossing ABab mice with HLA-DR4 mice to generate ABabDR4 mice, which express human TCRs and HLA-DR4, but lack murine MHC II proteins (14).
Figure 1. Optimal-affinity CD4+ T cells for enhanced antitumor response.
(A) Mouse hosts with human TCR and HLA genes generate “optimal-affinity” TCRs. As opposed to human hosts — which display self-antigens in the thymus and delete T cells that bind self-MHC and self-peptide with high affinity in a process known as negative selection — mouse hosts that lack human peptides but have human TCRs and HLA molecules can generate TCRs that bind human MHC and human peptide with high affinity. These are so-called optimal-affinity TCRs. (B) The wide range of effector and helper roles of CD4+ T cells in the antitumor response. CD4+ T cells can directly lyse tumor cells that have constitutive or inducible MHC II expression. Additionally, they can indirectly lead to tumor cell lysis by recruiting and activating macrophages and NK cells, which can release tumor antigens that can be presented by professional antigen-presenting cells such as dendritic cells to CD8+ T cells. Finally, they can also license dendritic cells, enhancing the activation and memory formation of tumor-specific CD8+ T cells.
In this study, Poncette and colleagues used their ABabDR4 system to generate NY-ESO-1 CD4+ TCRs of optimal affinity and compared their functionality with that of NY-ESO-1 TCRs isolated from human CD4+ T cells (9) in a manner much akin to their previous work with ABabDII mice (15). This study has come at a time of mounting evidence that besides CD8+ T cells, CD4+ T cells should be targeted for ACT. Recent studies show that CD4+ T cells have important effector roles in the antitumor response, including superior recognition of tumor neoantigens in both mouse (16) and human (17) systems; MHC II–dependent tumor cell lysis (18); and MHC II–independent but INF-γ–dependent recruitment and activation of macrophages and NK cells (ref. 19 and Figure 1B). In fact, autologous transfer of NY-ESO-1–reactive CD4+ T cells alone has been shown to mediate durable remission in a patient with refractory metastatic melanoma (20). Moreover, there is significant evidence that combined CD4+ and CD8+ cell–based therapies may have synergistic effects, as CD4+ T cells play important helper roles in enhancing CD8+ activation (21), memory formation (22), and antigen spreading to nontargeted tumor epitopes (23, 24). In line with these important findings, this study also examined whether combining CD4+ and CD8+ T cells of optimal-affinity could enhance ACT efficacy.
In order to find optimal-affinity NY-ESO-1–specific CD4+ T cells, Poncette et al. immunized ABabDR4 mice with either NY-ESO-1116–135 or NY-ESO-1 full-length DNA. These TCRs were shown to recognize NY-ESO116–135–loaded, NY-ESO-1–transduced, and naturally expressing NY-ESO-1 melanoma cell lines more effectively than the ones isolated from human CD4+ T cells. Moreover, their EC50 values in a NY-ESO-1 peptide titration assay were almost a log-fold lower than 3 of 5 human-derived TCRs (10–10 vs. 10–9 M), they secreted greater maximal IFN-γ concentrations, and they had higher MFIs when stained with DR4/NY-ESO-1116–135 tetramer (9). One TCR, TCR-3598_2, was chosen for a combined CD4+ and CD8+ ACT study, as it showed no signs of alloreactivity or cross-reactivity with any other naturally processed and presented human self-peptides that contain its recognition motif (9). This TCR was combined with a previously isolated HLA-A2/NY-ESO-1157–165–reactive TCR (15) in an ACT fibrosarcoma model where CD8+ T cells could only recognize antigen on cancer cells, while the NY-ESO-1–specific CD4+ T cells could only recognize antigen cross-presented by tumor stromal cells (9). The combination of NY-ESO-1–specific CD4+ and CD8+ T cells led to tumor regression in 10 of 10 mice, although the majority of these mice did eventually develop tumors, likely due to antigen loss (9). Correspondingly, this group had the highest number of CD8+ T cells in their peripheral blood and both CD4+ and CD8+ T cells within their tumors. In contrast, the CD4+-only group showed limited therapeutic benefit, suggesting that, in this system, the primary role of CD4+ T cells is to provide T cell help, rather than directly mediate antitumor activity. That said, the lack of direct CD4+ regression and the failure of the combined-treatment group to eradicate antigen-loss variants are possibly constraints of this engineered tumor model, rather than being indicative of true biological shortcomings of the combined CD4+ and CD8+ therapy.
Concluding remarks
There are certainly many benefits to this and other TCR gene therapy approaches for designing TCRs of optimal affinity for tumor antigens. They can provide a scalable, off-the-shelf reagent for treating many patients with a wide variety of cancers that express the corresponding tumor antigen, and engineered TCRs can be prescreened to have minimal on- or off-target toxicities. However these approaches will not confer the potential advantages associated with a polyclonal T cell response of a diverse spectrum of affinities and binding orientations against a single antigen that can be achieved through artificial antigen–presenting cell–based (aAPC-based) expansions (25). Moreover, as engineered TCRs are traditionally designed based on reactivity to a single dominant epitope of a tumor antigen, TCRs with reactivity to subdominant epitopes of tumor antigens remain conspicuously unexplored. Even in the case of Poncette et al., while they confirmed natural processing of NY-ESO-1116–135 by immunizing mice with full-length NY-ESO-1 DNA, they required that all of their TCRs stain positive for DR4/NY-ESO-1116–135 tetramer (9). In the process, however, they may have screened out TCRs that target different portions of the NY-ESO-1 protein but still have potent antitumor activity. Finally, targeting of a limited number of tumor antigens can quickly lead to outgrowth of antigen-loss variants, which may ultimately limit the value of single TCR approaches. Combined CD8+ and CD4+ approaches are a good start, as they can promote antigen spreading beyond targeted tumor epitopes (23, 24). As the importance of clinically relevant ACT therapies expands, further mechanistic studies comparing the potential clinical benefit of polyclonal antitumor responses over single high-affinity TCR approaches as well as more sophisticated systems for generating diverse TCR repertoires will ultimately help us develop and optimize ACT therapies.
Acknowledgments
This work was funded in part through the National Science Foundation (to AI), NIH/NCI Cancer Center Support Grant P01-AI072677 (to JPS), R01-CA108835 (to JPS), R21-CA185819 (to JPS), R21 EB023411, TEDCO/Maryland Innovation Initiative, and the Wallace H. Coulter Foundation (to JPS).
Version 1. 12/10/2018
Electronic publication
Version 2. 01/02/2019
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2019;129(1):69–71. https://doi.org/10.1172/JCI125471.
References
- 1.Dembić Z, et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LA, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosati SF, et al. A novel murine T-cell receptor targeting NY-ESO-1. J Immunother. 2014;37(3):135–146. doi: 10.1097/CJI.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadovnikova E, Jopling LA, Soo KS, Stauss HJ. Generation of human tumor-reactive cytotoxic T cells against peptides presented by non-self HLA class I molecules. Eur J Immunol. 1998;28(1):193–200. doi: 10.1002/(SICI)1521-4141(199801)28:01<193::AID-IMMU193>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Robbins PF, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180(9):6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holler PD, Chlewicki LK, Kranz DM. TCRs with high affinity for foreign pMHC show self-reactivity. Nat Immunol. 2003;4(1):55–62. doi: 10.1038/ni863. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23(3):349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 9.Poncette L, Chen X, Lorenz FKM, Blankenstein T. Effective NY-ESO-1–specific MHC II–restricted T cell receptors from antigen-negative hosts enhance tumor regression. J Clin Invest. 2019;129(1):324–335. doi: 10.1172/JCI120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li LP, et al. Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat Med. 2010;16(9):1029–1034. doi: 10.1038/nm.2197. [DOI] [PubMed] [Google Scholar]
- 11.Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin Cancer Res. 2010;16(23):5852–5861. doi: 10.1158/1078-0432.CCR-10-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RA, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature. 2009;458(7241):1043–1046. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Poncette L, Blankenstein T. Human TCR-MHC coevolution after divergence from mice includes increased nontemplate-encoded CDR3 diversity. J Exp Med. 2017;214(11):3417–3433. doi: 10.1084/jem.20161784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obenaus M, et al. Identification of human T-cell receptors with optimal affinity to cancer antigens using antigen-negative humanized mice. Nat Biotechnol. 2015;33(4):402–407. doi: 10.1038/nbt.3147. [DOI] [PubMed] [Google Scholar]
- 16.Kreiter S, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2016;520(7549):692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin U, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 18.Quezada SA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Diez A, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109(12):5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antony PA, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174(5):2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourgeois C, Tanchot C. Mini-review CD4 T cells are required for CD8 T cell memory generation. Eur J Immunol. 2003;33(12):3225–3231. doi: 10.1002/eji.200324576. [DOI] [PubMed] [Google Scholar]
- 23.Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207(11):2469–2477. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li K, et al. Adoptive cell therapy with CD4+ T helper 1 cells and CD8+ cytotoxic T cells enhances complete rejection of an established tumour, leading to generation of endogenous memory responses to non-targeted tumour epitopes. Clin Transl Immunology. 2017;6(10):e160. doi: 10.1038/cti.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perica K, et al. Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano. 2015;9(7):6861–6871. doi: 10.1021/acsnano.5b02829. [DOI] [PMC free article] [PubMed] [Google Scholar]