Abstract
The development of acute kidney injury (AKI) in patients with sepsis causes significant morbidity and mortality. The pathogenesis of AKI in sepsis is incompletely understood. In this issue of the JCI, Hato et al. investigate the renal translatome during bacterial sepsis and identify the global shutdown of renal protein translation mediated by the eukaryotic translation initiation factor 2-α kinase 2/eukaryotic translation initiation factor 2α (EIF2AK2/eIF2α) axis as a major pathway in mediating septic AKI. The results of this study suggest that inhibiting this pathway could be a potential therapeutic strategy for preventing septic AKI.
Acute kidney injury in sepsis
The development of acute kidney injury (AKI) remains a major cause of increased mortality among critically ill patients with bacterial sepsis (1). Despite the availability of dialytic renal replacement therapy, the benefit of increasing the delivered dose or earlier initiation of dialysis in septic patients with AKI remains controversial (2–4). Unfortunately, no therapeutic option is available for preventing or reversing sepsis-associated AKI. The lack of therapeutic options, in part, stems from an incomplete understanding of the drivers of kidney injury during sepsis.
Host defense: disease resistance and tolerance
Host defense against pathogens requires both disease resistance and disease tolerance (5). Disease resistance against an infection encompasses the ability of the host to detect and eliminate invading pathogens. Disease tolerance involves activation of adaptive mechanisms to limit tissue damage caused by the inflammatory response. Adaptive pathways in disease tolerance include metabolic and physiologic responses that occur at cellular, tissue, and systemic levels. The most recent consensus definition of sepsis states that it is a condition with life-threatening organ dysfunction due to dysregulated host defense against infection (6). Yet our understanding of which aspects of the host defense are dysregulated, leading to pathology, and which manifestations are adaptive, promoting survival and tissue protection, is incomplete. Our understanding of septic kidney disease is also incomplete. There is a growing appreciation of the need to better understand and identify disease tolerance pathways in the context of sepsis-induced AKI (7).
Stress-response adaptation pathways, including the integrated stress response (ISR) and unfolded protein response (UPR) in response to ER stress, are central to both the resistance and tolerance arms of host defense. ISR and UPR are both highly conserved pathways that respond to cellular stressors and activate adaptive pathways to recover cellular homeostasis. In the case of disease resistance, many antimicrobial pathways are activated by both ISR and UPR pathways (8, 9). A shared effect of the ISR and UPR pathways is regulation of protein translation, whereby there is a marked decrease in global protein translation along with increased expression and translation of a select group of genes that are activated to support cellular recovery. In both pathways, if the stress becomes too excessive or prolonged, pathways of cellular apoptosis are engaged. This apoptotic arm of these stress-response pathways is an attractive therapeutic target in states of excessive and chronic injury and/or inflammation.
The ISR is initiated by eukaryotic translation initiation factor 2α (eIF2α) phosphorylation. The four eIF2α kinases that make up the ISR include dsRNA-dependent protein kinase (PKR, also known as eukaryotic translation initiation factor 2-α kinase 2 [EIF2AK2]), PKR-like ER kinase (PERK), heme-regulated eIF2α kinase (HRI), and general control nonderepressible 2 (GCN2) (10). Although each kinase was initially described as responsive to distinct stressors (dsRNA for PKR, UPR for PERK, heme for HRI, and amino acid deficiency for GCN2), many are activated by multiple stimuli. For example, in addition to responding to dsRNA and promoting antiviral responses, PKR is also activated by other stimuli, including bacteria, fatty acids, ER stress, cytokines, and DNA damage (11).
ISR and protein translation in septic AKI
In this issue, Hato et al. provide evidence for PKR-mediated global protein translational shutdown as a potential mechanism of renal dysfunction in the context of sepsis and as a potential therapeutic target to minimize septic AKI (12). In a careful time-course analysis of the kidney after LPS challenge, a model of bacterial sepsis, Hato et al. have provided an impressive database of the dynamics of the translatome of the septic mouse kidney. These data were generated using nascent proteomics, in which only newly synthesized proteins are labeled and identified by mass spectrometry, Ribo-Seq, which captures active mRNA translation, and RNA-Seq, which captures the transcriptome. Protein translation within the kidney initially increased during the first few hours after LPS challenge, consistent with the inflammatory response; however, there was a dramatic decrease in translation of most proteins at later time points. A subset of proteins was not affected by the global shutdown of translation, and these included antiviral proteins, which corresponded to increased expression of EIF2AK2, but not the other ISR kinases. Without affecting the overall inflammatory response to LPS, systemic treatment with ISRIB, an ISR inhibitor, protected mice against the late suppression of protein translation as well as AKI development when given within the first hour after LPS challenge. These findings are compelling and raise the possibility that ISR activation, via the EIF2AK2/eIF2α pathway, and subsequent suppression of protein translation in the kidney is a maladaptive, rather than an adaptive, response to sepsis and is central to the development of sepsis-induced AKI (Figure 1). In addition to identifying a new, potential therapeutic target for preventing or treating septic AKI, Hato et al. have also provided a vast and impressive data set that captures the proteome, translatome, and transcriptome of the kidney across multiple time points during sepsis (12). This database allows for a dynamic and comprehensive picture of gene expression from transcription to protein translation across the timeline of sepsis. These data, available to the scientific community, can be used to further understand the effect of sepsis on the kidney and identify other targetable pathways.
Figure 1. Targeting the ISR in bacterial sepsis to limit kidney injury.
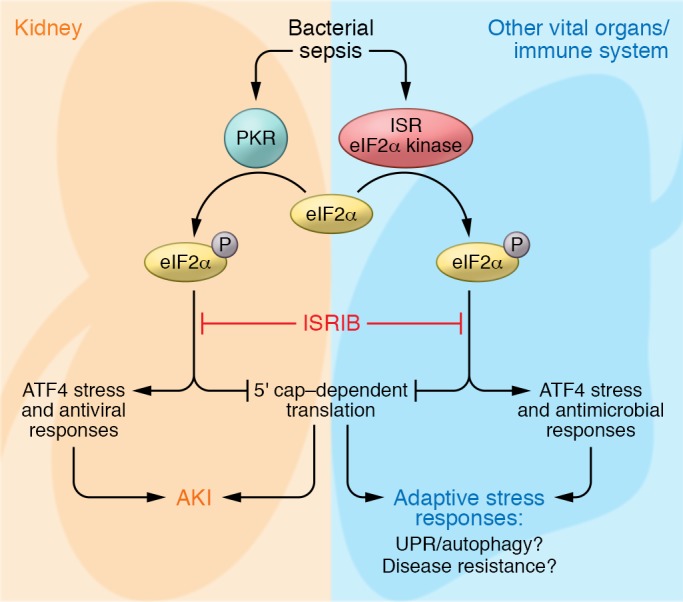
Inhibition of the PKR/eIF2α axis limits global renal protein translation shutdown and the development of AKI in mouse models of bacterial sepsis. Further research will be needed to determine whether systemic inhibition of ISR will deter adaptive stress responses in other organs or alter pathogen control by the immune system.
Potential limitations in inhibiting stress-adaptation pathways
Before attempting clinical translation of ISR inhibition in septic patients to prevent AKI, there are several aspects of this approach, especially in the context of bacterial sepsis, that will require further investigation (Figure 1). First, there is a significant dynamic and time-specific aspect to the control of protein translation, indicating that appropriate timing of the proposed therapeutic intervention to prevent global protein translational shutdown will be important. Could ISR inhibition disrupt proteostasis and cause the development of UPR and ER stress? Furthermore, treatment with ISRIB was only protective when given during the early time period after LPS administration. In animal models, the time of initiation of sepsis is controlled. Further research will be needed before we can identify with confidence where a patient presents in the timeline of sepsis. Second, disease resistance could also be compromised, as ISR pathways are important in pathogen clearance (9). Third, there is also growing evidence that autophagy is a critical protective mechanism against sepsis pathology (13), adding concern for inadvertent disruption of autophagy with ISR inhibition due to the integral link between autophagy and the ISR (14). Hato et al. used a sublethal dose of LPS in their investigation of the renal translatome, and only kidney function, as estimated by serum creatinine, and kidney injury biomarkers were assessed. As sepsis is a systemic disease that affects multiple organs, could inhibition of ISR in the kidney be protective, but cause cellular stress in other organs that are dependent on ISR for tissue tolerance and survival? Although the development of AKI dramatically increases mortality in sepsis, it remains unclear whether the kidneys are the limiting organs in all cases of mortality with septic AKI. A recent clinical trial of early and late initiation of dialysis in septic patients who develop AKI noted that approximately a quarter of patients with spontaneous recovery of renal function in the late dialysis group subsequently died, suggesting that the kidneys were not likely the limiting organs for the survival of those patients (4). Finally, there is emerging evidence that immunosuppression occurs during the late stages of sepsis (15). This immunosuppression associates with secondary opportunistic infections, including latent viral reactivation, and increased mortality (16, 17). Although PKR activation and induction of antiviral programs appear to be pathologic and inappropriate in the context of a bacterial stimulus, activation of antiviral responses, either locally in the kidney or systemically, may be an adaptive program designed to limit opportunistic infections during the immunosuppressive phase of sepsis. Thus, the effects of preventing adaptive ISR protein translation shutdown in the kidney as well as in other vital organs and the immune system during sepsis will need to be further investigated.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K08DK110424) and an American Society of Nephrology Carl W. Gottschalk Research Scholar Grant.
Version 1. 12/03/2018
Electronic publication
Version 2. 01/02/2019
Print issue publication
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Reference information: J Clin Invest. 2019;129(1):60–62. https://doi.org/10.1172/JCI125432.
See the related article at Bacterial sepsis triggers an antiviral response that causes translation shutdown.
References
- 1.Uchino S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Palevsky PM, O’Connor T, Zhang JH, Star RA, Smith MW. Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. Clin Trials. 2005;2(5):423–435. doi: 10.1191/1740774505cn116oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RENAL Replacement Therapy Study Investigators. et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 4.Barbar SD, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335(6071):936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol. 2017;13(3):143–151. doi: 10.1038/nrneph.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssens S, Pulendran B, Lambrecht BN. Emerging functions of the unfolded protein response in immunity. Nat Immunol. 2014;15(10):910–919. doi: 10.1038/ni.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues LOCP, Graça RSF, Carneiro LAM. Integrated stress responses to bacterial pathogenesis patterns. Front Immunol. 2018;9:1306. doi: 10.3389/fimmu.2018.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17(10):1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang R, Tang D. PKR-dependent inflammatory signals. Sci Signal. 2012;5(247):pe47. doi: 10.1126/scisignal.2003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hato T, et al. Bacterial sepsis triggers an antiviral response that causes translation shutdow. J Clin Invest. 2019;129(1):296–309. doi: 10.1172/JCI123284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Escobar DA, Botero AM, Gomez H, Zuckerbraun BS. Autophagy: Chapter 17. Sepsis-induced autophagy is a protective mechanism against cell death. In: Hayat MA, ed. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Vol. 2. San Diego, California, USA: Academic Press; 2014. [Google Scholar]
- 14.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boomer JS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walton AH, et al. Reactivation of multiple viruses in patients with sepsis. PLoS One. 2014;9(2):e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]


