Abstract
Bacterial interspecies interactions in the oral cavity influence the structural development of cariogenic biofilms and dental caries. Visualization of the biofilm architecture and bacterial localization within biofilms is essential for understanding bacterial interactions. We herein demonstrated that the spatial localization of Streptococcus mutans within dual-species biofilms was altered in a manner that depended on the partner. Furthermore, we found that these biofilms influenced the survival of S. mutans against disinfectants. The present results provide information on how S. mutans interact with other bacteria in multi-species cariogenic biofilms.
Keywords: Streptococcus mutans, cariogenic biofilm, interspecies interaction, chlorhexidine tolerance, spatial distribution
In natural environments, most microbes live in microbial communities called biofilms (17) in which cells are enclosed in self-produced extracellular polymeric substances (EPS), which comprise various substances, including DNA, protein, and polysaccharides (7). Biofilms exhibit increased tolerance to disinfectant chemicals, antibiotics, and host immune systems and are widely recognized as causative agents of infectious diseases and environmental contaminants (11). Based on previous studies on model mono-species biofilms, our current understanding of biofilm physiology is increasing; however, multi-species biofilms are dominant in the natural environment, such as the oral cavity, and interspecies communication shapes the behavior and phenotype of multi-species biofilms (8, 22). Interspecies communication often consequently influences the-architecture of the biofilm community, thereby affecting bacterial spatial distribution (4, 5, 12). Imaging of the spatial distribution of cells in multi-species biofilms is important for fully understanding the role of interspecies interactions. Streptococcus mutans is one of the main bacteria causing dental caries, one of the most prevalent oral infectious diseases caused by oral biofilms (9). The human oral cavity contains more than 700 different bacterial species, and each of these bacterial species interacts with at least one other species in the oral cavity (1, 15). Interspecies interactions within cariogenic biofilms containing multiple bacterial species are considered to influence biofilm architecture and properties. A recent study reported that a co-culture with oral bacteria affects gene expression in S. mutans (20, 21, 25). However, the effects of these interspecies interactions on the structure of multi-species biofilms and/or spatial distribution of S. mutans in cariogenic biofilms remain unclear.
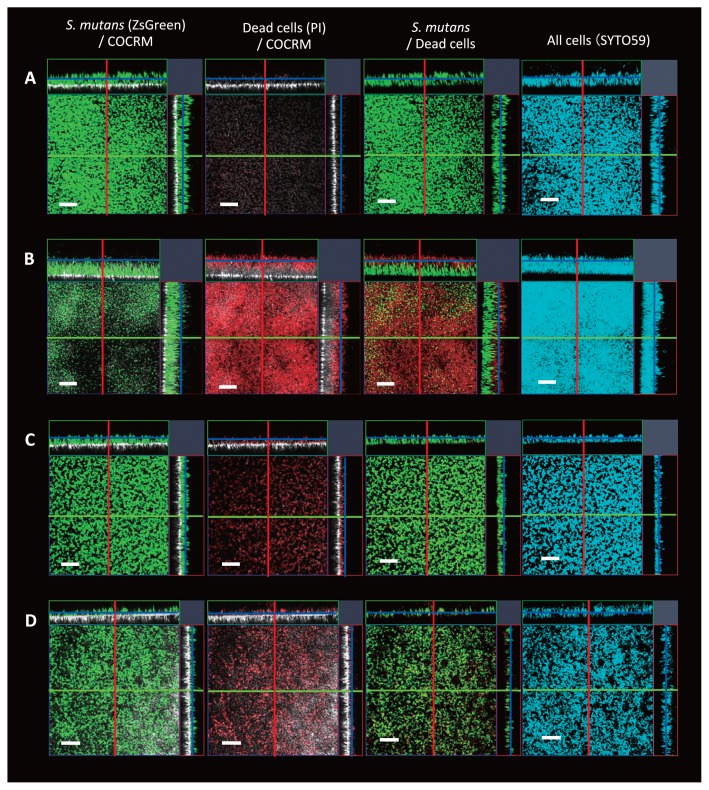
We previously applied a non-invasive real-time imaging system called continuous-optimizing confocal reflection microscopy (COCRM), which allows the visualization of bacteria and attached surfaces using light reflected from a laser (13, 14, 26). We used confocal laser scanning microscopy (CLSM) and COCRM to simultaneously visualize specific oral bacteria and tooth surfaces. S. mutans strains that constitutively express fluorescent proteins were constructed for this purpose. We constructed a strain in which ZsGreen was expressed under the control of a ldh promoter, and was inserted into the SMU_1405c locus of the S. mutans genome; this enabled the maintenance of constitutive expression of the fluorescent protein without the use of antibiotics as well as the successful distinction of S. mutans from other bacteria, even in thick biofilms on hydroxyapatite (HA) discs (Table S1 and S2). The CLSM image of ZsGreen-tagged S. mutans stained with nucleic acid-staining dye SYTO 59 completely merged with the image showing the green fluorescence of cells, indicating that the entire population of S. mutans expressed the desired fluorescent protein at the bottom cells in thick biofilms on HA discs (Fig. 1A).
Fig. 1.
Images showing biofilms and localization of S. mutans on hydroxyapatite (HA) discs. (A) Cross-sectional images of a S. mutans mono-species biofilm. Blue cells (stained with SYTO59) and green cells (S. mutans) are completely merged. (B–D) Dual-species biofilms containing S. mutans and (B) S. mitis, (C) Aa, or (D) L. casei. The (A) and (C) layer is 20 μm, the (B) layer is 50 μm, and the (D) layer is 5 μm from the HA disc surface in the vertical lay. Green: S. mutans labeled with ZsGreen; Red: dead cells stained with PI; White: HA disc surface; Blue: whole cells with SYTO59. Scale bars indicate 50 μm. The purple lines indicate the positions at which the cross-sections for the X–Y image were taken. Red and green lines indicate the positions at which the X–Z or Y–Z image were taken. Representative images of at least three independent experiments are shown.
To examine multi-species biofilms, S. mitis TU003, Aggregatibacter actinomycetemcomitans (Aa) ATCC 43718, and Lactobacillus casei subsp. casei JCM 8129 were selected for the co-inoculation with S. mutans. S. mitis is a pioneer colonizer of tooth surfaces in the human oral cavity, and its colonization is important for other oral bacteria to adhere to the tooth surface and build dental plaque biofilms (6). Aa causes localized aggressive periodontitis (2), and Aggregatibacter spp. co-aggregate with Streptococcus spp. in human plaques (19). Furthermore, S. mutans quorum sensing is activated by Aa, suggesting that these bacteria interact with each other in oral biofilms in vivo (21). L. casei is known to enhance the demineralization of tooth enamel when co-cultured with S. mutans (23).
Each overnight culture was diluted OD600 of 0.01 in fresh medium and then incubated anaerobically at 37°C for 24 h in polystyrene microtiter plates containing brain heart infusion (BHI) medium (Becton Dickinson, Sparks, MD, USA) on human saliva-coated HA discs (diameter, 7 mm; Clarkson Chromatography Products, South Williamsport, PA, USA) in a 24-well plate (IWAKI non-treated microplate flat bottom, polystyrene) (3). After the incubation, HA discs with biofilms were washed with phosphate-buffered saline (PBS). Biofilms were stained with 0.75 μM propidium iodide (PI; Thermo Fisher Scientific, Waltham, MA, USA) for 30 min. Confocal microscopy images were acquired using the upright confocal laser scanning microscope LSM880 (Carl Zeiss, Oberkochen, Germany) equipped with the W N-Achroplan 40×water-dipping objective lens with a 0.80 numerical aperture (Carl Zeiss). ZsGreen was excited by a two-photon laser (880 nm) and detected with a 499–545 nm band-pass filter, whereas PI was excited by an argon laser (514 nm) and detected with a 590–655 nm band-pass filter. HA disc surfaces were illuminated with an HeNe633 laser (633 nm), and reflected light was collected through a 627–639 nm band-pass filter. A mono-culture of S. mutans showed thin biofilm formation under the present experimental conditions, and a few dead cells were observed in biofilm aggregates (Fig. 1A). When S. mutans was co-cultured with S. mitis, the dual-species biofilm was flat with increased thickness (Fig. 1B). S. mutans occupied the bottom layer of the biofilm. PI-stained cells were observed in the upper layer of the biofilm (Fig. 1B). This result indicated that cells in the upper layer of the biofilm were dead cells. Biofilms on HA discs were also subjected to a CFU assay. Biofilms grown in BHI were disrupted by thorough pipetting and incubated anaerobically on BHI agar plates. Since an erythromycin-resistant gene was inserted in the S. mutans genome together with ZsGreen, erythromycin (10 μg mL−1) was added to distinguish S. mutans from S. mitis and this was followed by a comparison with BHI plates without erythromycin. The plates were incubated at 37°C for 48 h. This assay confirmed that most of the living cells in the biofilm were S. mutans (Fig. S1). Thus, the S. mutans and S. mitis dual-species biofilm showed a layered structure, and live S. mutans cells were completely covered by dead cells. When S. mutans was co-cultured with Aa, negligible effects on biofilm thickness were observed (Fig. 1C). The population of dead cells was slightly greater than that in the S. mutans mono-biofilm. Although the co-culture with S. mutans showed markedly (10−5) lower CFU of Aa cells than the monoculture of Aa, few Aa survived in this dual-species biofilm, suggesting that S. mutans co-exists with Aa in the dual species biofilm (Fig. S1 and S2). The co-culture of S. mutans with L. casei showed lower cell density in biofilm aggregates than that of the mono-species biofilm (Fig. 1D). The present results demonstrated that S. mutans is the most viable cell in all combinations of dual-species biofilms (Fig. S1), and its localization within biofilms is altered depending on the combination.
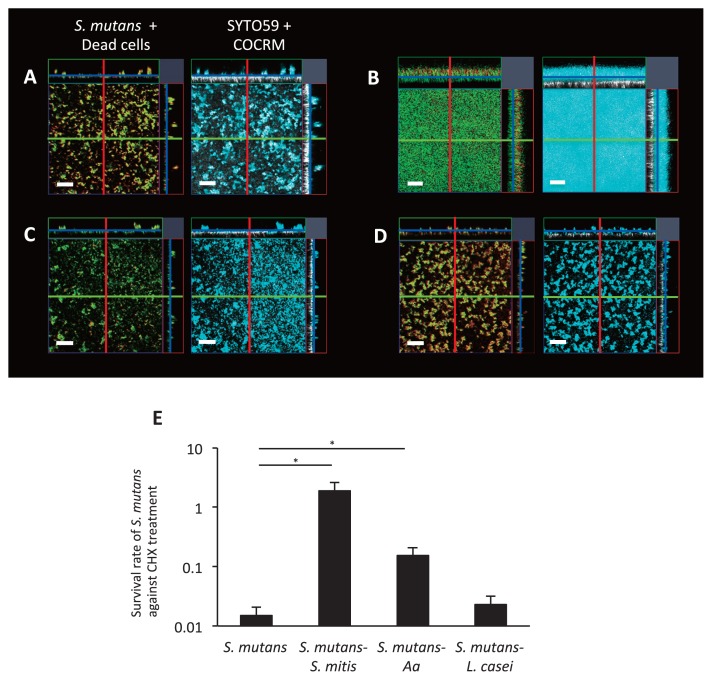
We then investigated whether a co-culture of oral bacteria affected the phenotypic properties of S. mutans, such as chemical tolerance. We tested the tolerance of mono- and dual-species biofilms to chlorhexidine (CHX), which is frequently used for oral disinfection and is approved by the Food and Drug Administration (24). Briefly, biofilms grown on HA discs were incubated with 0.05% CHX for 5 min, washed with PBS twice, and imaged using CLSM. Confocal images of biofilms and CFU assays showed that most of the cells in S. mutans mono-biofilms were dead after the CHX treatment (Fig. 2A, E, and S3). In contrast, cells in the bottom layer of the S. mutans-S. mitis dual-species biofilm remained alive after the CHX treatment (Fig. 2B). Notably, the co-culture with S. mitis significantly increased S. mutans viability after the CHX treatment (Fig. 2E and S3). Similarly, significantly increased CHX tolerance was observed in the S. mutans-Aa dual-species biofilm (Fig. 2C, E, and S3). However, the co-culture with L. casei did not affect the CHX tolerance of S. mutans (Fig. 2D, E, and S3). S. mitis and L. casei mono-species biofilms were highly tolerant to CHX, which was consistent with previous findings (10), suggesting that these biofilms are less permeable to CHX (Fig. S4).
Fig. 2.
Impact of interspecies interactions in biofilms on the chemical tolerance of S. mutans. (A–D) Cross-sectional images of biofilms after the chlorhexidine (CHX) treatment. Representative images of (A) S. mutans mono-species biofilms and dual-species biofilms of S. mutans with (B) S. mitis, (C) Aa, or (D) L. casei. Green shows S. mutans labeled with ZsGreen. Red shows dead cells stained with PI. White shows the HA disc surface taken with COCRM. Blue shows whole cells stained with SYTO59. (E) Survival ratio of S. mutans cells on HA disc after the CHX treatment. PBS was used as a control for the CHX treatment and CFU were counted to measure viable cells. The average values with standard errors from at least three independent experiments are shown. The significance of differences (*, P<0.05) was evaluated using the Student’s t-test.
A possible explanation for the increased tolerance of S. mutans co-cultured with S. mitis is the layered architecture of the dual-species biofilm; dead cells in the upper layer of the biofilm protect S. mutans in the bottom layer from chemical disinfectants.
This is consistent with a previous study that demonstrated that peripheral cells in a biofilm protected interior cells from external stresses (18). The dead cells on the upper layer may have been due to bacteriocins and/or H2O2, which are known to be produced by the S. mutans and S. mitis groups (e.g. S. mitis and S. sanguinis), respectively (16). In addition, other mechanisms that increase the CHX tolerance of S. mutans in dual-species biofilms may exist because the S. mutans-Aa biofilm did not show a bilayer structure.
The present results suggest that bacterial spatial distribution within multi-species biofilms differs depending on the combination of bacteria. Moreover, multi-species biofilms exert a strong impact on the tolerance of S. mutans to disinfectants. We consider the present study to be useful for advancing research on cariogenic biofilm formation, including that for developing efficient tooth preservation and treatment approaches.
Supplemental Material
Acknowledgements
This work was supported by JST ERATO Grant Number JPMJER1502, Japan.
References
- 1.Aas J.A., Paster B.J., Stokes L.N., Olsen I., Dewhirst F.E. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage G.C. Comparison of the microbiological features of chronic and aggressive periodontitis. Periodontol. 2010;53:70–88. doi: 10.1111/j.1600-0757.2010.00357.x. 2000. [DOI] [PubMed] [Google Scholar]
- 3.Cai J.N., Jung J.E., Dang M.H., Kim M.A., Yi H.K., Jeon J.G. Functional relationship between sucrose and a cariogenic biofilm formation. PLoS One. 2016;11:e0157184. doi: 10.1371/journal.pone.0157184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalcanti I.M.G., Del Bel Cury A.A., Jenkinson H.F., Nobbs A.H. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol Oral Microbiol. 2016;32:60–73. doi: 10.1111/omi.12154. [DOI] [PubMed] [Google Scholar]
- 5.Christensen B.B., Haagensen J.A.J., Heydorn A., Molin S. Metabolic commensalism and competition in a two-species microbial consortium. Appl Environ Microbiol. 2002;68:2495–2502. doi: 10.1128/AEM.68.5.2495-2502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzsimmons S., Evans M., Pearce C., Sheridan M.J., Wientzen R., Bowden G., Cole M.F. Clonal diversity of Streptococcus mitis biovar 1 isolates from the oral cavity of human neonates. Clin Diagn Lab Immunol. 1996;3:517–522. doi: 10.1128/cdli.3.5.517-522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flemming H.C., Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 8.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 9.Hamada S., Slade H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera D., Roldán S., Santacruz I., Santos S., Masdevall M., Sanz M. Differences in antimicrobial activity of four commercial 0.12% chlorhexidine mouthrinse formulations: an in vitro contact test and salivary bacterial counts study. J Clin Periodontol. 2003;30:307–314. doi: 10.1034/j.1600-051x.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 11.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Inaba T., Ichihara T., Yawata Y., Toyofuku M., Uchiyama H., Nomura N. Three-dimensional visualization of mixed species biofilm formation together with its substratum. Microbiol Immunol. 2013;57:589–593. doi: 10.1111/1348-0421.12064. [DOI] [PubMed] [Google Scholar]
- 13.Inaba T., Oura H., Morinaga K., Toyofuku M., Nomura N. The Pseudomonas quinolone signal inhibits biofilm development of Streptococcus mutans. Microbes Environ. 2015;30:189–191. doi: 10.1264/jsme2.ME14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyokawa T., Usuba R., Obana N., Yokokawa M., Toyofuku M., Suzuki H., Nomura N. A versatile and rapidly deployable device to enable spatiotemporal observations of the sessile microbes and environmental surfaces. Microbes Environ. 2017;32:88–91. doi: 10.1264/jsme2.ME16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolenbrander P.E., Palmer R.J., Periasamy S., Jakubovics N.S. Oral multispecies biofilm development and the key role of cellcell distance. Nat Rev Microbiol. 2010;8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- 16.Kreth J., Merritt J., Shi W., Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerchner J., Wolf A., Buchholz F., Mertens F., Neu T.R., Harms H., Maskow T. Miniaturized calorimetry—A new method for real-time biofilm activity analysis. J Microbiol Methods. 2008;74:74–81. doi: 10.1016/j.mimet.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Liu J., Prindle A., Humphries J., Gabalda-Sagarra M., Asally M., Lee D.D., Ly S., Garcia-Ojalvo J., Süel G.M. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature. 2015;523:550–554. doi: 10.1038/nature14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark Welch J.L., Rossetti B.J., Rieken C.W., Dewhirst F.E., Borisy G.G. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci USA. 2016;113:E791–E800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redanz S., Standar K., Podbielski A., Kreikemeyer B. A five-species transcriptome array for oral mixed-biofilm studies. PLoS One. 2011;6:e27827. doi: 10.1371/journal.pone.0027827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szafrański S.P., Deng Z-L., Tomasch J., Jarek M., Bhuju S., Rohde M., Sztajer H., Wagner-Döbler I. Quorum sensing of Streptococcus mutans is activated by Aggregatibacter actinomycetemcomitans and by the periodontal microbiome. BMC Genomics. 2017;18:238. doi: 10.1186/s12864-017-3618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tashiro Y., Yawata Y., Toyofuku M., Uchiyama H., Nomura N. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ. 2013;28:13–24. doi: 10.1264/jsme2.ME12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdez R.M.A., dos Santos V.R., Caiaffa K.S., Danelon M., Arthur R.A., Negrini T.C., Delbem A.C.B., Duque C. Comparative in vitro investigation of the cariogenic potential of bifidobacteria. Arch Oral Biol. 2016;71:97–103. doi: 10.1016/j.archoralbio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Varoni E., Tarce M., Lodi G., Carrassi A. Chlorhexidine (CHX) in dentistry: state of the art. Minerva Stomatol. 2012;61:399–419. [PubMed] [Google Scholar]
- 25.Wen Z.T., Yates D., Ahn S., Burne R.A. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yawata Y., Toda K., Setoyama E., Fukuda J., Suzuki H., Uchiyama H., Nomura N. Monitoring biofilm development in a microfluidic device using modified confocal reflection microscopy. J Biosci Bioeng. 2010;110:377–380. doi: 10.1016/j.jbiosc.2010.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.