Abstract
Objective
Hypothalamic Pro-opiomelanocortin (POMC) and Neuropeptide Y/Agouti-Related Peptide (NPY/AgRP) neurons are critical nodes of a circuit within the brain that sense key metabolic cues as well as regulate metabolism. Importantly, these neurons retain an innate ability to rapidly reorganize synaptic inputs and electrophysiological properties in response to metabolic state. While the cellular properties of these neurons have been investigated in the context of obesity, much less is known about the effects of exercise training.
Methods
In order to further investigate this issue, we utilized neuron-specific transgenic mouse models to identify POMC and NPY/AgRP neurons for patch-clamp electrophysiology experiments.
Results
Using whole-cell patch-clamp electrophysiology, we found exercise depolarized and increased firing rate of arcuate POMC neurons. The increased excitability of POMC neurons was concomitant with increased excitatory inputs to these neurons. In agreement with recent work suggesting leptin plays an important role in the synaptic (re)organization of POMC neurons, POMC neurons which express leptin receptors were more sensitive to exercise-induced changes in biophysical properties. Opposite to effects observed in POMC neurons, NPY neurons were shunted toward inhibition following exercise.
Conclusions
Together, these data support a rapid reorganization of synaptic inputs and biophysical properties in response to exercise, which may facilitate adaptations to altered energy balance and glucose metabolism.
Keywords: Melanocortin, Energy balance, Leptin receptor, Exercise, Patch-clamp, Electrophysiology
Highlights
-
•
Exercise inhibited arcuate NPY neurons.
-
•
Exercise activated arcuate POMC neurons.
-
•
Leptin receptor expressing POMC neurons are much more sensitive to response to exercise.
-
•
Exercise effects are transient, lasting only hours for NPY, while sustainable for days in POMC neurons.
1. Introduction
Exercise (single bout and/or chronic training) increases insulin sensitivity leading to improved insulin stimulated glucose uptake in muscle and reduced basal hepatic glucose production [7], [8], [10], [11], [15], [17], [20], [22], [45], [60]. Within the arcuate nucleus, the melanocortin system is an interface between signals of metabolic state and neural pathways governing energy balance and glucose metabolism [16], [54], [62]. In particular, the orexigenic neuropeptide Y/Agouti-related peptide (NPY/AgRP) neurons are activated in response to food deprivation, while the anorexigenic proopiomelanocortin (POMC)-expressing cells are inhibited [2], [3], [5], [32], [33], [66]. In addition to contributing to energy balance, the activity of arcuate NPY/AgRP and POMC neurons also have profound effects on glucose metabolism [18], [31], [50], [56]. These changes in cellular activity have been attributed to both native channel properties as well as (re)organization of synaptic connectivity [18], [24], [25]. One fascinating characteristic of this circuit is the ratio of excitatory and inhibitory synaptic inputs to arcuate NPY/AgRP and POMC neurons has been shown to change in response to metabolic hormones, such as leptin and ghrelin [13], [14], [49], [59], [63], [65], as well as alter body weight in response to energy intake or monogenic obesity models [23], [25], [46], [48], [65]. These observations support a rapid and constitutive plasticity of melanocortin synaptic organization which responds and contributes to metabolic homeostasis [23]. The plasticity of hypothalamic signaling has been studied in the context of obesity, changing energy demands/availability and humoral cues however the role of melanocortin-specific synaptic plasticity in response to exercise remains undefined. In current study, the hypothesis that exercise (single bout and chronic training) modulates the cellular activity of arcuate POMC and NPY/AgRP neurons was tested using whole-cell patch-clamp recordings in hypothalamic slices from transgenic mice. Exercise-induced effects were assessed on intrinsic membrane properties and synaptic responses, including subsets of POMC and NPY/AgRP neurons which are identified as leptin receptor expressing or non-leptin receptor expressing.
2. Material and methods
2.1. Animal care
Age (12–18 weeks) and bodyweight (25–30 g) matched male POMC-hrGFP::LepR-cre::tdtomato (PLT) and male NPY-hrGFP::LepR-cre::tdtomato (NLT) mice were used for all whole cell patch-clamp recordings. These mice allow us to identify POMC or NPY neurons that either do or do not express LepR. C57/B6 mice were used for food intake studies. All mice were housed under standard laboratory conditions (12 h on/off; lights on at 7:00 a.m.) and temperature-controlled environment (22–24 °C). All mice were provided a Harlan Teklad 2016 chow diet and water ad libitum unless otherwise noted. All experiments were performed in accordance with the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Texas Institutional Animal Care and Use Committee.
2.2. Exercise protocols
Age and bodyweight matched male mice were divided into 2 groups: sedentary and exercise. Motorized treadmills (Exer-6; Columbus Instruments, Columbus, OH) were used for exercise experiments. Food was removed from all the mice before they were placed on the treadmills. The mice were coaxed to stay on the treadmill (sedentary group) or continue running on the treadmill by means of an electric stimulus (0.25 mA × 163 V and 1 Hz) generated by a shock grid present at the treadmill base and by manually tapping their tails using a soft nylon bottle brush, as needed to enable all the mice to complete the exercise bout.
2.2.1. Exercise groups
All mice were familiarized to the treadmills for 2 days prior to the exercise bout [Prior day 1: 5 min rest on the treadmill followed by 5 min at the speed of 8 m/min and then for 5 min at the speed of 10 m/min; Prior day 2: 5 min rest on the treadmill followed by 5 min at the speed of 10 m/min and then for 5 min at the speed of 12 m/min [41].
2.2.1.1. 1 day exercise protocol
On Day 1, mice were subjected to a high intensity interval exercise (HIIE) bout [21], [41] to assess exercise-induced changes in electrophysiological characteristics of NPY/AgRP and POMC neurons. Mice were rested on the treadmill for 5 min prior to performing the 1 h of exercise consisting of 3 × 20 min intervals (5 min at the speed of 12 m/min, followed by 10 min at the speed of 17 m/min, and then 5 min at the speed of 22 m/min), without rest between intervals. This protocol was chosen because all of the mice were able to complete the entire exercise protocol, and this exercise protocol can reduce food intake in wild type mice [41].
2.2.1.2. 5 day exercise protocol
On Day 1-Day 4, mice were rested on the treadmill for 5 min prior to performing the 1 h of continuous exercise (60 min at the speed of 15 m/min). On Day 5, mice were subjected to a high intensity interval exercise (HIIE) bout.
2.2.1.3. 10 day exercise protocol
On Day 1-Day 5, mice were rested on the treadmill for 5 min prior to performing the 1 h of continuous exercise (60 min at the speed of 15 m/min). Mice were then rested in their home cages on Day 6 and Day 7. On Day 8-Day 11, mice performed 1 h continuous exercise. On Day 12, mice were subjected to a high intensity interval exercise (HIIE) bout.
2.2.2. Sedentary group
All mice were placed on the treadmills with the same time of exercise group, while the speed was 0 m/min.
Following the final exercise day (1, 5, and 10 days), mice were immediately sacrificed, and the arcuate nucleus of the hypothalamus was harvested for electrophysiology recording (See Figure S1).
2.3. Body-weight measurement
Body weights of all mice were measured on the first day before exercise and the last day after exercise.
2.4. Electrophysiology studies
2.4.1. Slice preparation
Brain slices were prepared from male mice (12–18 weeks-old) as previously described. Briefly, male mice were deeply anesthetized with i.p. injection of 7% chloral hydrate and transcardially perfused with a modified ice-cold artificial CSF (ACSF) (described below). The mice were then decapitated, and the entire brain was removed, and immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) ACSF (126 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 5 mM glucose). Coronal sections (250 μm) were cut with a Leica VT1000S Vibratome and then incubated in oxygenated ACSF at room temperature for at least 1 h before recording. The slices were bathed in oxygenated ACSF (32 °C–34 °C) at a flow rate of ∼2 ml/min. All electrophysiology recordings were performed at room temperature.
2.4.2. Whole-cell recording
The pipette solution for whole-cell recording was modified to include an intracellular dye (Alexa Fluor 350 hydrazide dye) for whole-cell recording: 120 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, and 2 mM MgATP, 0.03 mM Alexa Fluor 350 hydrazide dye (pH 7.3). Epifluorescence was briefly used to target fluorescent cells, at which time the light source was switched to infrared differential interference contrast imaging to obtain the whole-cell recording (Zeiss Axioskop FS2 Plus equipped with a fixed stage and a QuantEM:512SC electron-multiplying charge-coupled device camera). Electrophysiological signals were recorded using an Axopatch 700B amplifier (Molecular Devices); low-pass filtered at 2–5 kHz, and analyzed offline on a PC with pCLAMP programs (Molecular Devices). Membrane potential and firing rate were measured by whole-cell current clamp recordings from POMC and NPY neurons in brain slices. Recording electrodes had resistances of 2.5–5 MΩ when filled with the K-gluconate internal solution. Input resistance was assessed by measuring voltage deflection at the end of the response to a hyperpolarizing rectangular current pulse steps (500 ms of −10 to −50 pA).
Neurons were voltage-clamped at −70 mV (for excitatory postsynaptic currents) and −15 mV (for inhibitory postsynaptic currents). Frequency and peak amplitude were measured by using the Mini Analysis program (Synaptosoft, Inc.).
2.5. Blood collection and assessment of plasma leptin levels
Blood samples were collected via tail using heparinized capillary tubes immediately after HIIE for both the exercised and the time-matched sedentary controls. The blood samples were immediately centrifuged at 4 °C and 1500 g × 15 min, and plasma was stored at −80 °C until analysis.
Plasma leptin concentrations were estimated using ELISA kits – Catalog #22-LEPMS-E01 (ALPCO).
2.6. Food intake measurement
2.6.1. Food-deprivation/exercise protocol
Mice were subjected to 1 day (single bout HIIE), 5 days, and 10 days of exercise training. On the final day of exercise training, food intake was assessed after a 6 h food restriction [41]. Specifically, food was removed from all the mice at the start of the light cycle (7 AM) for a duration of 6 h. On the final exercise day, a 1 h exercise bout was performed in the 2 nd h of food restriction (“2ndh EX”). 24 h food intake was then measured at the end of the 6 h food restriction.
2.6.2. No food-deprivation/exercise protocol
Ad-libitum fed mice were subjected to 1 day (single bout HIIE). Immediately following exercise, food intake was measured over a 24 h period.
2.7. Fiber photometry
2.7.1. Experimental model
Agrp-IRES-Cre (Jackson Labs 012899, Agrptm1(cre)Lowl/J) and Pomc1-Cre (Jackson Labs 005965, POMCtg(Pomc1−Cre)16Lowl/J) were used for experimentation. All mice were habituated to handling and experimental conditions prior to experiments. All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
2.7.2. Viral injections and fiber optic implantation
Mice were anesthetized with isoflurane (1.5–3%, Clipper, 0010250) and pretreated with subcutaneous injections of ketoprofen (5 mg/kg, Santa Cruz Animal Health, sc-363115Rx) and bupivacaine (2 mg/kg, Moore Medical, 52683). Mice were placed into a stereotaxic apparatus (Stoelting, 51725D) and viral injections were performed as previously described [1], [58]. Unilateral injections of the genetically encoded calcium indicator GCaMP6s [AAV1.Syn.Flex.GCaMP6s.WPRE.SV40 (titer: 4.216e13 GC/ml, Penn Vector Core) were performed in the arcuate hypothalamic nucleus (ARC, 400 μl) according to the following coordinates: 1.35 mm posterior to bregma, 0.25 mm lateral to midline, and 6.15–6.3 mm ventral to skull. A ferrule-capped optical fiber (400 μm core, NA 0.48, Doric, MF2.5, 400/430–0.48) was implanted 0.2 mm above the injection site and secured to the skull with Metabond cement (Parkell, S380) and dental cement (Lang Dental Manufacturing, Ortho-jet BCA Liquid, B1306 and Jet Tooth Shade Powder, 143069). All mice were given at least 2 weeks for recovery and viral expression before experiments were performed.
2.7.3. Dual-wavelength fiber photometry
Dual-wavelength fiber photometry (FP) was performed as we and others have previously described [35], [58]. Two excitation wavelengths were used: 490 and 405 nm. 490 nm excites calcium-dependent fluorescence from GCaMP6s protein, providing a measure of AgRP or POMC neuron activity. 405 nm excites calcium-independent fluorescence from GCaMP6s protein and serves as a control for movement and bleaching artifacts. Excitation light intensities were modulated at different frequencies (211 and 566 Hz for 490 and 405 nm, respectively) to avoid contamination from overhead lights (120 Hz and harmonics) and cross-talk between excitation lights. Excitation lights were generated through fiber-coupled LEDs (Thorlabs, M470F3 for 490 nm and M405F1 for 405 nm) and modulated by a real-time amplifier (Tucker–Davis Technology, RZ5P). GCaMP6s emission fluorescence signals were collected through a patch cord, collimated, passed through a GFP emission filter (Thorlabs, MF525-39), and focused onto a femtowatt photoreceiver (Newport, Model 2151, gain set to AC LOW). The emission lights were converted to electrical signals and demodulated by the RZ5P real-time processor. The FP experiments were controlled by Synapse software (Tucker–Davis Technology).
2.7.4. Fiber photometry HIIE experiment
Ad libitum fed mice underwent the HIIE protocol as described above. On the second session, mice were tethered to a patch fiber to habituate to fiber photometry procedures. On test day, AgRP/POMC neuron activity was recorded during and after HIIE. All mice completed at least one running interval, and 7/13 mice completed all 3 intervals. AgRP/POMC neuron activity was analyzed during the last 10 min of running and for 10 min-post running.
2.8. Analysis and statistics
Results are reported as the mean ± SEM unless indicated otherwise, as indicated in each figure legend. Membrane potential values were not compensated to account for junction potential (−8 mV). Statistical analysis was carried out using Graph Pad Prism 7.0 software. Degrees of freedom (DF) for t statistics are marked as t(DF). All data were evaluated using a two-tailed Student's t test or ANOVA where appropriate, with *p < 0.05 being considered significant. The number of cells or mice studied for each group is shown in parentheses.
Fiber photometry data were exported from Synapse to MATLAB (MathWorks) using a script provided by Tucker–Davis Technology. The 490 and 405 nm signals were independently processed and normalized to baseline signals to determine ΔF/F, where ΔF/F = (F−Fpre-stimulus)/Fpre-stimulus and Fpre-stimulus is the median of pre-stimulus signal (end of running). Data were down-sampled to 1 Hz in MATLAB, and mean ΔF/F was calculated by integrating ΔF/F over a period of time and then dividing by the integration time.
3. Results
3.1. Exercise effects on the membrane and synaptic properties of arcuate NPY neurons
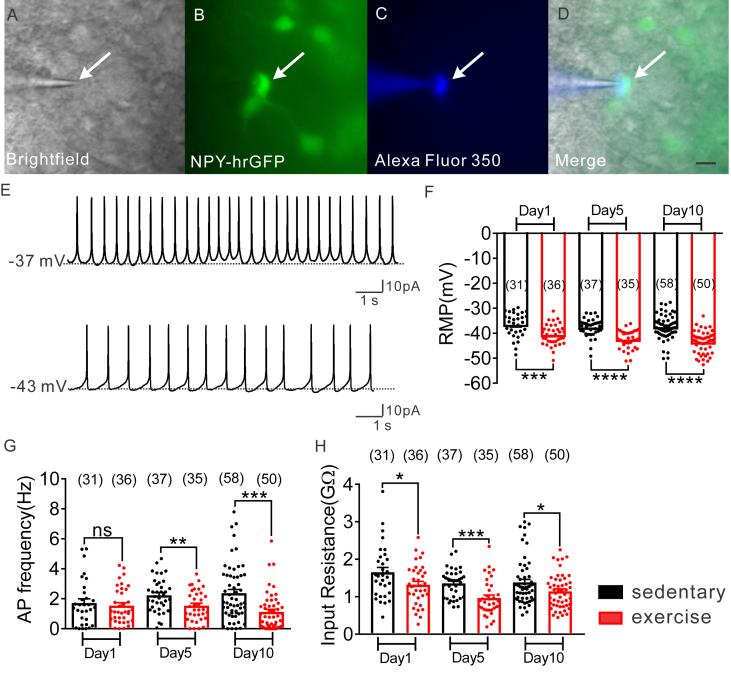
Whole-cell patch-clamp recordings were made in 247 NPY-hrGFP neurons throughout the rostro–caudal axis of the arcuate nucleus from NPY-hrGFP:LepR-cre:tdtomato mice (Figure 1A–D; Figure S2). All arcuate NPY neurons (regardless of leptin receptor expression) from sedentary mice had an average resting membrane potential of −37.4 ± 0.4 mV, a mean input resistance of 1.4 ± 0.1 GΩ, and overshooting action potentials (n = 126; Figure 1E–H). When compared to values obtained from NPY neurons of sedentary mice (1 day = −36.6 ± 0.9 mV, n = 31; 5 day = −37.7 ± 0.6 mV, n = 37; 10 day = −37.6 ± 0.6 mV, n = 58; Figure 1F), NPY neurons from exercised mice exhibited a hyperpolarized resting membrane potential (1 day = −40.7 ± 0.7 mV, t(65) = 3.654, p < 0.05, n = 36; 5 day = −42.6 ± 0.6 mV, t(70) = 5.55, p < 0.05, n = 35; 10 day = −43.7 ± 0.6 mV, t(106) = 6.866, p < 0.05,n = 50; Figure 1F). The hyperpolarized membrane potential observed in NPY neurons from exercised mice was concomitant with decreased action potential frequency and decreased input resistance (Figure 1G–H).
Figure 1.
Exercise inhibits arcuate NPY neurons. (A–D) Brightfield illumination (A) of NPY-hrGFP neuron from NPY-hrGFP::LepR-cre::tdtomato mice. (B) and (C) show the same neuron under FITC (hrGFP) and Alexa Fluor 350 illumination. Merged image of targeted NPY neuron is shown in (D). Arrow indicates the targeted cell. Scale bar = 50 μm. (E) Current-clamp recording of NPY-GFP neurons show the resting membrane potential from sedentary and exercised mice. (F–H) Histogram demonstrating the average resting membrane potential of NPY-hrGFP neurons (F), decreased action potential frequency (G), decreased input resistance (H) after 1, 5, and 10 days exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, ***p < 0.001, unpaired t test compared to controls. The number of GFP-positive neurons studied for each group is shown in parentheses.
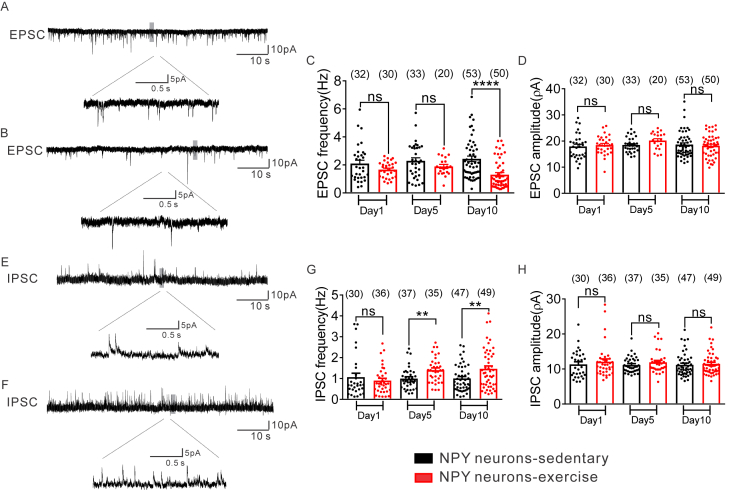
The frequency of spontaneous excitatory synaptic currents (sEPSCs) in all NPY neurons (regardless of leptin receptor expression) from sedentary mice was: 1 day = 2.1 ± 0.2 Hz, n = 32; 5 day = 2.3 ± 0.2 Hz, n = 33; 10 day = 2.4 ± 0.2 Hz, n = 53 (Figure 2A–C). When compared to values obtained from NPY neurons of sedentary mice, exercise decreased the frequency of sEPSCs to NPY neurons (1 day = 1.97 ± 0.2 Hz, t (60) = 1.657, p > 0.05, n = 30; 5 day = 1.9 ± 0.1 Hz, t (51) = 1.285, p > 0.05, n = 20; 10 day = 1.3 ± 0.1 Hz, t (101) = 4.548, p < 0.05, n = 50; Figure 2A–C), while the amplitude of sEPSCs remained unchanged (Figure 2D).
Figure 2.
Exercise suppresses excitatory synaptic inputs and enhances inhibitory synaptic inputs to arcuate NPY neurons. (A–B) Voltage clamp recording of spontaneous excitatory postsynaptic currents (sEPSCs) observed in NPY-hrGFP neuron from NPY-hrGFP::LepR-cre::tdtomato mice after 1, 5, and 10 days of sedentary (upper) or exercise activity (lower). (Vm = −70 mV). (C–D) Plots indicating the decreased sEPSC frequency (Hz) but not amplitude (pA) in arcuate NPY neurons after exercise compared with sedentary groups (black bar: sedentary; red bar: exercise). (E–F) Voltage clamp recording of spontaneous inhibitory postsynaptic currents (sIPSCs) observed in NPY-hrGFP neuron from NPY-hrGFP::LepR-cre::tdtomato mice after 1, 5, and 10 days of sedentary (upper) and exercise activity (lower). (Vm = −15 mV). (G–H) Plots indicating the increased sIPSC frequency (Hz) but not amplitude (pA) in arcuate NPY neurons after exercise compared with sedentary groups (black bar: sedentary; red bar: exercise). Data are from male mice and are expressed as mean ± SEM. *p < 0.05, ***p < 0.001, unpaired t test compared to controls. The number of GFP-positive neurons studied for each group is shown in parentheses.
The frequency of spontaneous inhibitory synaptic currents (sIPSCs) in all NPY neurons (regardless of leptin receptor expression) from sedentary mice was: 1 day = 1.1 ± 0.2 Hz, n = 30; 5 day = 1.0 ± 0.1 Hz, n = 37; 10 day = 1.0 ± 0.1 Hz, n = 47 (Figure 2E–G). When compared to values obtained from NPY neurons of sedentary mice, exercise increased frequency of sIPSCs to NPY neurons (1 day = 0.9 ± 0.1 Hz, t (64) = 0.7795, p > 0.05, n = 36; 5 day = 1.4 ± 0.1 Hz, t (70) = 3.033, p < 0.05, n = 35; 10 day = 1.5 ± 0.1 Hz, t (94) = 2.746, p < 0.05, n = 49; Figure 2E–G), while the amplitude of sIPSCs remained unchanged (Figure 2H).
Body weights were similar between sedentary and exercised NPY-hrGFP:LepR-cre::tdtomato mice from all exercise time points (Figure S3A).
3.2. Exercise effects on the membrane and synaptic properties of arcuate POMC neurons
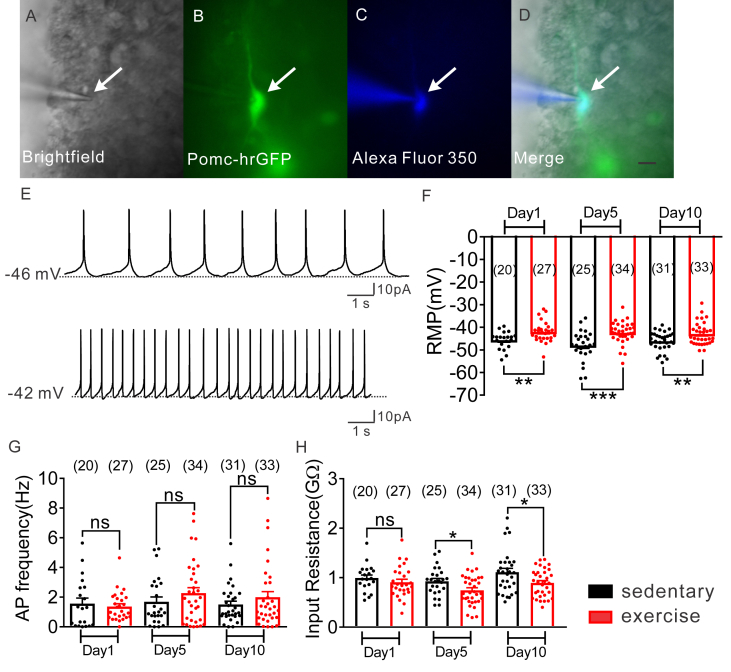
Whole-cell patch-clamp recordings were made in 170 POMC neurons throughout the rostro–caudal axis of the arcuate nucleus from POMC-hrGFP::LepR-cre::tdtomato mice (Figure 3A–D; Figure S4). All arcuate POMC neurons (regardless of leptin receptor expression) from sedentary mice had an average resting membrane potential of −46.7 ± 0.6 mV, a mean input resistance of 1.0 ± 0.04GΩ, and overshooting action potentials (n = 76; Figure 3E–H). When compared to values obtained from POMC neurons of sedentary mice (1 day = −45.7 ± 0.9 mV, n = 20; 5 day = −48.0 ± 1.3 mV, n = 25; 10 day = −46.2 ± 0.8, n = 31; Figure 3F), POMC neurons from exercised mice exhibited a depolarized resting membrane potential (1 day = −42.0 ± 0.9 mV, t(45) = 2.921, p < 0.05, n = 27; 5 day = −42.5 ± 0.8, t(57) = 3.746, p < 0.05, n = 34; 10 day = −42.9 ± 0.9, t (62) = 2.768, p < 0.05, n = 33; Figure 3F). The depolarized membrane potential observed in POMC neurons from exercised mice was associated with an increase in the action potential frequency and decreased input resistance (Figure 3G–H).
Figure 3.
Exercise activates arcuate POMC neurons. (A–D) Brightfield illumination (A) of POMC-hrGFP neuron from POMC-hrGFP::LepR-cre::tdtomato mice. (B) and (C) show the same neuron under FITC (hrGFP) and Alexa Fluor 350 illumination. Merged image of targeted POMC neuron is shown in (D). Arrow indicates the targeted cell. Scale bar = 50 μm. (E) Current-clamp recording of POMC-hrGFP neurons show the resting membrane potential from sedentary and exercised mice. (F–I) Current-clamp recording of POMC-hrGFP neurons displayed increased resting membrane potential (mV, F), increased action potential frequency (Hz, G), and decreased input resistance (GΩ, H) after 10 days exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, unpaired t test compared to controls. The number of GFP-positive neurons studied for each group is shown in parentheses.
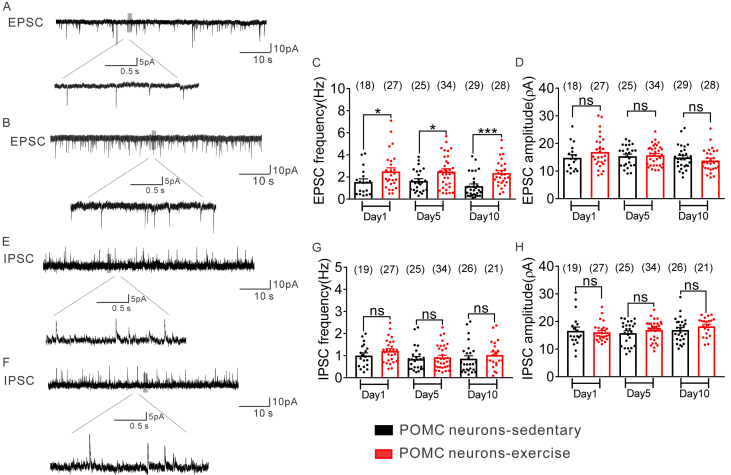
The frequency of sEPSCs in all POMC neurons (regardless of leptin receptor expression) from sedentary mice was: 1 day = 1.6 ± 0.3 Hz, n = 18; 5 day = 1.7 ± 0.27 Hz, n = 25; 10 day = 1.2 ± 0.2 Hz, n = 29; (Figure 4A–C). When compared to values obtained from POMC neurons of sedentary mice, exercise increased the frequency of sEPSCs to POMC neurons (1 day = 2.5 ± 0.3 Hz, t (43) = 2.093, p < 0.05, n = 27; 5 day = 2.5 ± 0.2 Hz, t (57) = 2.551, p < 0.05, n = 34; 10 day = 2.5 ± 0.3 Hz, t (55) = 3.556, p < 0.05, n = 28; Figure 4A–C), while the amplitude of sEPSCs remained unchanged (Figure 4D).
Figure 4.
Exercise enhances excitatory synaptic inputs but has no effect on inhibitory synaptic inputs to arcuate POMC neurons. (A–B) Voltage clamp recording of spontaneous excitatory postsynaptic currents (sEPSCs) observed in POMC-hrGFP neurons from POMC-hrGFP::LepR-cre::tdtomato mice after 1, 5, and 10 days of sedentary (upper) or exercise activity (lower). (Vm = −70 mV). (C–D) Plots indicating the increased sEPSC frequency (Hz) and no changed amplitude (pA) observed in the arcuate POMC-hrGFP neurons after exercise compared with sedentary group. (E–F) Voltage clamp recording of spontaneous inhibitory postsynaptic currents (sIPSCs) observed in POMC-hrGFP neuron from PLT after sedentariness (upper) and 10 days exercise (lower). (Vm = −15 mV; black bar: sedentariness; red bar: exercise). (G–H) Plots indicating no change of both sIPSC frequency (Hz) and amplitude (pA) observed in arcuate POMC neurons after exercise compared with sedentary group. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, ***p < 0.001, unpaired t test compared to controls. The number of GFP-positive neurons studied for each group is shown in parentheses.
The frequency of sIPSCs in all POMC neurons (regardless of leptin receptor expression) from sedentary mice was: 1 day = 1.0 ± 0.1 Hz, n = 19; 5 day = 0.8 ± 0.1 Hz, n = 25; 10 day = 0.9 ± 0.1 Hz, n = 26 (Figure 4E–G). When compared to values obtained from POMC neurons of sedentary mice, exercise mice showed the similar frequency of sIPSCs to POMC neurons (1 day = 1.2 ± 0.1 Hz, t (45) = 1.406, p > 0.05, n = 28; 5 day = 0.9 ± 0.1 Hz, t (57) = 0.5388, p > 0.05, n = 34; 10 day = 1.0 ± 0.1 Hz, t (45) = 0.8877, p > 0.05, n = 21; Figure 4E–G), while the amplitude of sIPSCs remained unchanged (Figure 4H).
Body weights were similar between sedentary and exercised POMC-hrGFP::LepR-cre::tdtomato mice from all exercise time points (Figure S3B).
3.3. Exercise effects on calcium dynamics of NPY/AgRP and POMC neurons
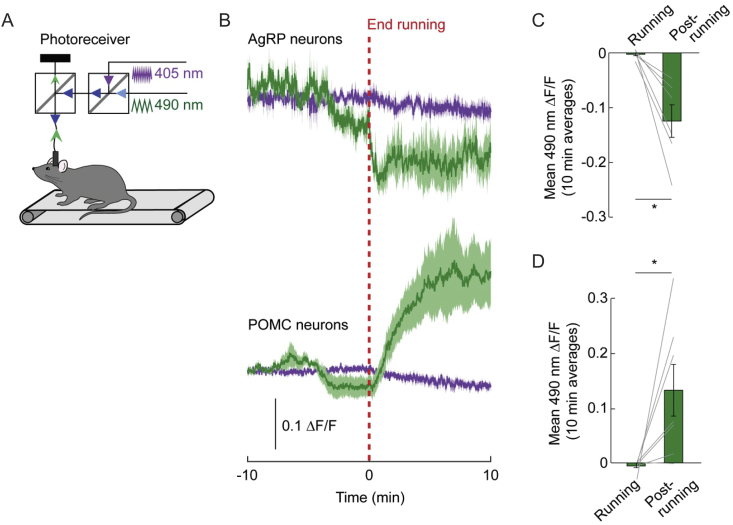
Whole-cell calcium dynamics of arcuate NPY/AgRP and POMC neurons following a single bout of HIIE was measured using in-vivo fiber photometry [1], [35], [58] (Figure 5A). A single bout of HIIE decreased whole cell calcium levels in arcuate AgRP neurons and increased calcium levels in arcuate POMC neurons (Figure 5B–D). These results support an inhibition of AgRP neurons and excitation of POMC neurons post-exercise. Importantly, the changes of calcium levels in AgRP and POMC neurons are consistent with the changes in membrane potential and synaptic activity observed in electrophysiological recordings. Although these changes in membrane potential, synaptic properties, as well as in-vivo calcium levels are from a distributed population of neurons (Figures S2 and S4), these data support a decrease in excitability of arcuate NPY/AgRP neurons concomitant with increased excitability of POMC neurons in response to exercise.
Figure 5.
Calcium Dynamics of AgRP and POMC neurons following HIIE running. (A) Dual-wavelength fiber photometry (FP) setup used to record calcium-dependent fluorescence (excited at 490 nm) and calcium-independent fluorescence (excited at 405 nm) in mice during and after HIIE running. (B) Average ΔF/F of GCaMP6s signals of ad libitum fed mice before and immediately after the end of HIIE running (top AgRP, n = 6; bottom POMC, n = 7). Signals are aligned to the end of HIIE running. Green: 490 nm signal; purple: 405 nm control signal. Darker lines represent means and lighter shaded areas represent SEMs. (C) 10 min averages of ΔF/F (490 nm signal) in AgRP neurons before and after the end of HIIE running (n = 6, paired t-test, *p < 0.05). (D) 10 min averages of ΔF/F (490 nm signal) in POMC neurons before and after the end of HIIE running (n = 7, paired t-test, *p < 0.05).
3.4. Leptin receptor expressing POMC neurons are more susceptible to the effects of exercise
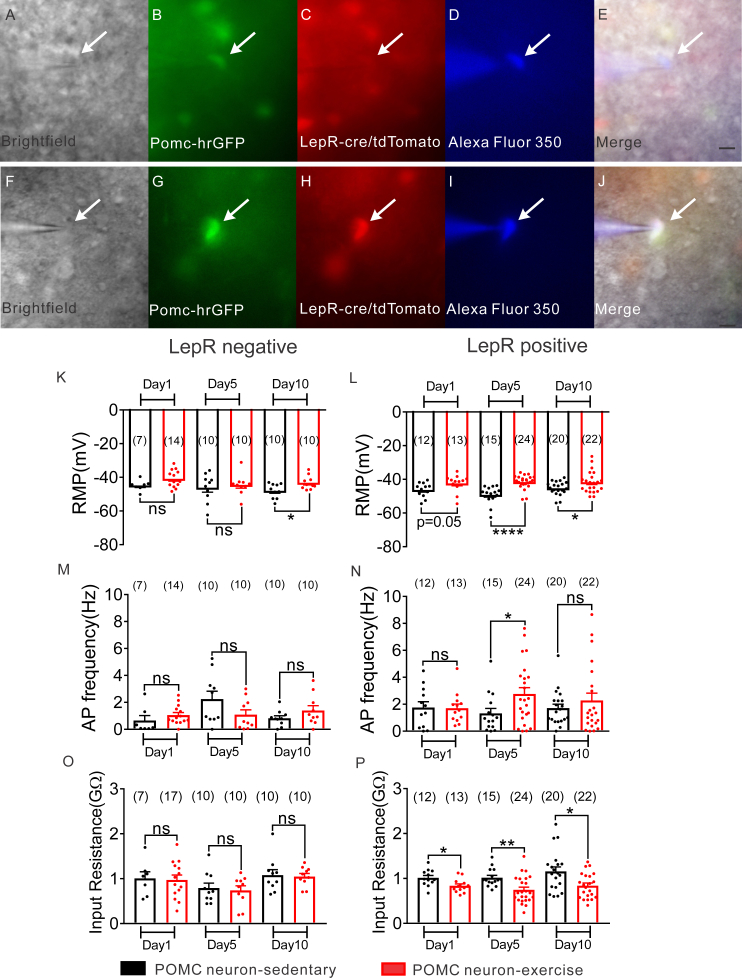
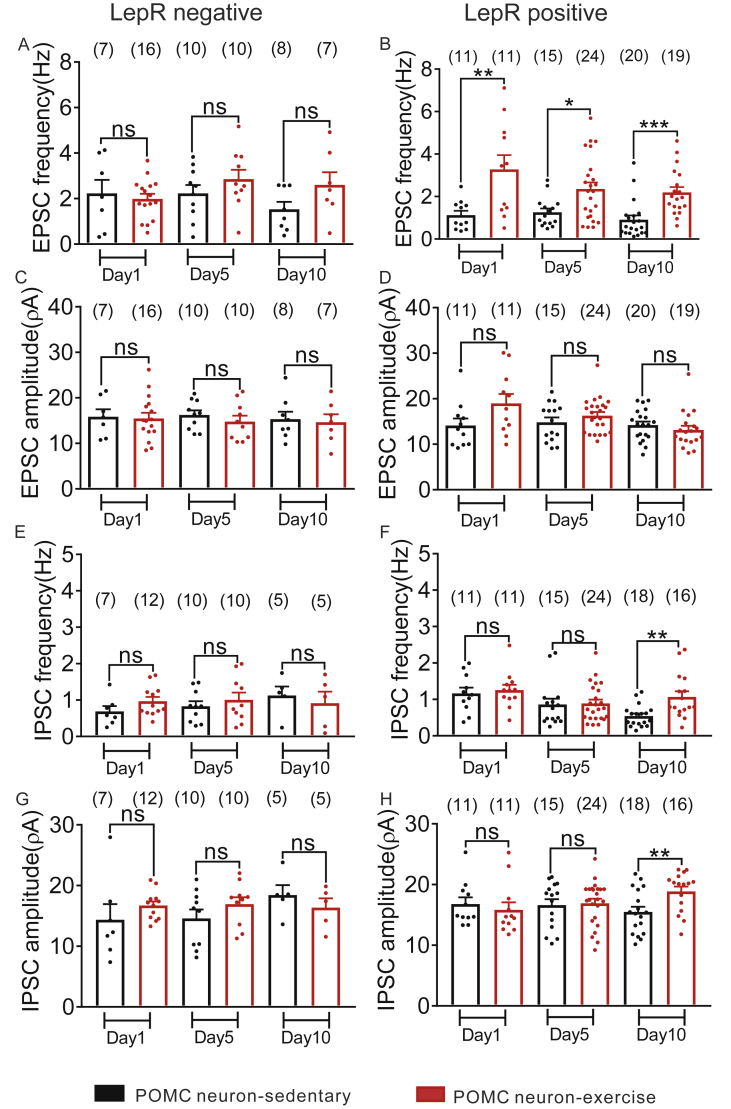
Recent work suggests that leptin plays an important role in synaptic (re)organization of POMC and NPY/AgRP neurons [49], [59]. In order to investigate the role of leptin receptor expression in the response to exercise, we took advantage of the ability to identify arcuate POMC or NPY neurons that do not express LepRs (green only, Figure 6A–E; Figure S5A–E) and POMC-hrGFP or NPY-hrGFP neurons which express LepRs (green/red, Figure 6F–J; Figure S5F–J) in POMC-hrGFP::LepR-cre::tdtomato and NPY-hrGFP::LepR-cre::tdtomato mice. We recently reported that more than half of NPY neurons restricted to the arcuate nucleus express LepRs [27]. Here, we show that 15.5% of arcuate leptin receptor neurons express POMC and 36.7% of arcuate POMC neurons express Leptin receptor (Table 1). Notably, exercise-induced changes in membrane potential, input resistance, and spontaneous EPSC frequency were observed within one day of exercise in POMC neurons which express leptin receptors (Figure 6K–P; Figure 7A–D). These effects persisted throughout the 10 days of experiment. Leptin receptor expressing POMC neurons also exhibited an increase in IPSC frequency and amplitude after 10 days of exercise (for IPSC frequency: t (32) = 3.222, p < 0.05, n (sedentary) = 18, n (exercise) = 16; for IPSC amplitude: t (32) = 2.83, p < 0.05; n (sedentary) = 18, n (exercise) = 16; Figure 7E–H). Opposite to the effects observed in POMC neurons, LepRs expressing or not expressing NPY neurons exhibited similar responses to exercise (Figure S5; Figure S6).
Figure 6.
Comparison of electrophysiological properties between LepR negative and LepR positive arcuate POMC neurons after 1, 5, or 10 days exercise. (A–E) LepR negative POMC-hrGFP neuron: Brightfield illumination (A) of POMC-hrGFP neuron from PLT mice. (B) and (C) show the same neuron under FITC (hrGFP) and Alexa Fluor 594 illumination to demonstrate the expression of hrGFP and no expression of LepR/tdTomato. (D) Image shows the complete dialysis of Alexa Fluor 350 from the intracellular pipette. Merged image of targeted LepR negative POMC-hrGFP neuron is shown in (E). Arrow indicates the targeted cell. Scale bar = 50 μm (F–J) LepR positive POMC-hrGFP neuron: Brightfield illumination (F) of POMC-hrGFP neuron from PLT mice. (G) and (H) show the same neuron under FITC (hrGFP) and Alexa Fluor 594 illumination to demonstrate the expression of both hrGFP and LepR/tdTomato. (I) Image shows the complete dialysis of Alexa Fluor 350 from the intracellular pipette. Merged image of targeted LepR positive POMC-hrGFP neuron is shown in (J). Arrow indicates the targeted cell. Scale bar = 50 μm. (K, M, O) Plots from current-clamp recording show resting membrane potential (K), action potential frequency (M), and input resistance (O) of LepR negative POMC-hrGFP neurons after 1, 5, or 10 days exercise. (L, N, P) Plots from current-clamp recording show resting membrane potential (L), action potential frequency (N), and input resistance (P) of LepR positive POMC-hrGFP neurons after 1, 5, or 10 days exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ****p < 0.0001, unpaired t test compared to controls. The number of neurons studied for each group is shown in parentheses.
Table 1.
Table shows the distribution of arcuate POMC neurons that express or do not express leptin receptors in POMC-hrGFP::LepR-icre::tdtomato mice. These data were studied from five male POMC-hrGFP::LepR-icre::tdtomato mice.
| Region | Area | LepR neurons expressing POMC (%) | POMC neurons expressing LepR (%) |
|---|---|---|---|
| RCA | Medial | 38.9 (13/33.4) | 27.4 (13/47.4) |
| Lateral | 37.7 (11.7/31) | 47.6 (11.7/24.6) | |
| Total | 38.4 (24.7/64.4) | 34.3 (24.7/72) | |
| Arc 1 | Medial | 44.5 (84.4/189.6) | 43.9 (84.4/192.4) |
| Lateral | 25.1 (20.2/80.4) | 25.7 (20.2/78.6) | |
| Total | 38.7 (104.6/270) | 38.6 (104.6/271) | |
| Arc 2 | Medial | 9.9 (72/730.6) | 31.4 (72/229.4) |
| Lateral | 29.6 (24/81.2) | 76.4 (24/31.4) | |
| Total | 11.8 (96/811.8) | 36.8 (96/260.8) | |
| Arc 3 | Medial | 1.6 (4.2/267.6) | 19.6 (4.2/21.4) |
| Lateral | 0.3 (0.2/63.4) | 25 (0.2/0.8) | |
| Total | 1.3 (4.4/331) | 19.8 (4.4/22.2) | |
| Total | Medial | 14.2 (173.6/1221.2) | 35.4 (173.6/490.6) |
| Lateral | 21.9 (56.1/256) | 41.4 (56.1/135.4) | |
| Total | 15.5 (229.7/1477.2) | 36.7 (229.7/626) |
Figure 7.
Leptin receptor dependent plasticity of arcuate POMC neurons in response to exercise. (A–D) Histograms from voltage-clamp recordings illustrate LepR positive POMC-hrGFP neurons but not LepR negative POMC-hrGFP neurons displayed significant increase of sEPSC frequency independent of changing amplitude after exercise. (E–H) Plots from voltage-clamp recordings indicating LepR positive POMC-hrGFP neurons, but not LepR negative POMC-hrGFP neurons, exhibited an increase in sIPSC frequency (Hz) and amplitude (pA) after exercise training compared with sedentary group. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, unpaired t test compared to controls. The number of neurons studied for each group is shown in parentheses.
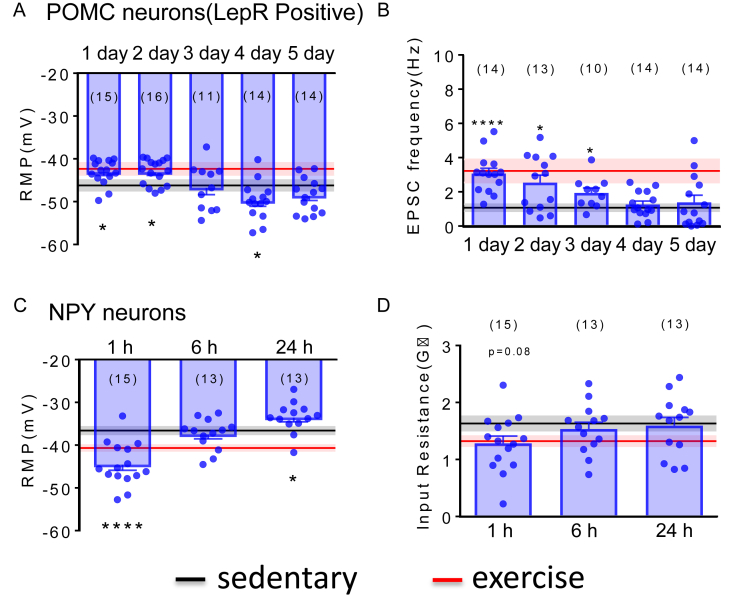
3.5. Temporal variability in the duration of exercise effects on POMC and NPY neurons
We next analyzed the effects of exercise on POMC and NPY neurons in the immediate hours and/or days after a single bout of HIIE. Arcuate POMC neurons that express leptin receptors remained activated (i.e. depolarized membrane potential and increased EPSC frequency) for 2 days after a single bout of HIIE (Figure 8A–B). Interestingly, we observed a hyperpolarization of POMC neurons which express leptin receptors on the 4th day after a single bout of HIIE before returning to baseline levels on the 5th day following HIIE (Figure 8A). In contrast, the effects of exercise in arcuate NPY neurons returned to baseline levels within 6 h after a single bout of HIIE (Figure 8C–D). Together, these data highlight a temporal variability in the effects of exercise on arcuate POMC and NPY neuronal activity.
Figure 8.
Temporal variability in the duration of exercise effects on POMC and NPY neurons. (A) Histogram from current-clamp recordings illustrates the resting membrane potential of POMC-hrGFP neurons after single bout of HIIE from the 1st to 5th day. (B) Histogram from voltage-clamp recordings shows the sEPSC frequency of POMC-hrGFP neurons from the 1st to 5th day after a single bout of HIIE. (C) Histogram from current-clamp recordings demonstrates the resting membrane potential of NPY-hrGFP neurons from 1 h to 24 h after a single bout of HIIE. (D) Histogram from current-clamp recordings illustrates the input resistance of NPY-hrGFP neurons from 1 h to 24 h after a single bout of HIIE. Black line: means of resting membrane potential, sEPSC frequency and Input resistance of Leptin receptor expressing POMC neurons or NPY neurons after sedentary on 1st day. Red line: means of resting membrane potential, sEPSC frequency and Input resistance of Leptin receptor expressing POMC neurons or NPY neurons after HIIE on 1st day. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, ****p < 0.0001, unpaired t test compared to controls. The number of neurons studied for each group is shown in parentheses.
3.6. High intensity interval exercise suppresses feeding in mice
Using a food deprivation/exercise protocol previously described [41], food intake measurements were studied after 1 day (single bout HIIE), 5 days, and 10 days of exercise training. Mice exhibited a decrease of food intake in the 6 h following high intensity interval exercise (Figure S7A), which persisted for 24 h (Figure S7B). The effects on feeding were comparable after 1 day (single bout HIIE), 5 days, and 10 days of exercise training.
Moreover, food intake was also suppressed after a single bout of HIIE, independent of food restriction (Figure S7C–D). These data support a correlative increase in melanocortin tone leading to suppression of food intake after HIIE in rodents.
4. Discussion
Exercise predominantly evoked an inhibitory effect on NPY-expressing neurons while eliciting an overall excitatory effect in adjacent POMC-expressing neurons. These effects, in combination with those from previous work, support an exercise-dependent enhancement of the melanocortin neural circuitry within the arcuate nucleus, which is congruent with a negative energy balance and improved glucose metabolism (summarized in Figure S8).
4.1. Membrane and synaptic effects of exercise on POMC and NPY neurons
Arcuate POMC neurons were depolarized while adjacent NPY neurons were hyperpolarized in response to a single bout of exercise. The changes in membrane potential were accompanied by a decrease in input resistance, which support an increased channel activity. These results are consistent with the hypothesis that exercise alters the membrane potential of both POMC and NPY neurons via activation of a resting conductance. Excitatory neurotransmission is mediated by glutamatergic ionotropic AMPA (AMPARs) and NMDA receptors (NMDARs) while the inhibitory neurotransmission is mediated by presynaptic GABAergic neurons. Glutamate and GABA are released subsequent to activation of respective neurons within the hypothalamus [33], [36], [57], and also likely arising from nuclei elsewhere in the brain [19], [53], [61]. Our results indicate exercise increased the frequency of spontaneous EPSCs onto POMC neurons, while suppressing the frequency of spontaneous EPSCs and increasing the frequency of spontaneous IPSCs onto NPY neurons. Moreover, whole cell calcium levels decreased in arcuate AgRP neurons while being increased in arcuate POMC neurons after a single bout of HIIE, consistent with the changes that we observed in the membrane potential of NPY and POMC neurons previously. Therefore, exercise increases melanocortin tone within the arcuate nucleus via pre- and post-synaptic activities.
Increased melanocortin signaling consistently results in improved energy balance and glucose homeostasis [13], [18], [48], [56]. In contrast, increased activity of NPY/AgRP neurons results in impaired energy balance and glucose metabolism [2], [32], [56]. In the current study, the activity profile exhibited by arcuate POMC and NPY/AgRP neurons in response to exercise is in accordance with improved energy balance and glucose metabolism. While the effects of exercise on feeding behavior have been controversial, recent evidence suggests that exercise, including high intensity exercise, can result in decreased food intake in the immediate hours after exercise [41], [55]. Similarly, acute bouts of exercise can result in significant improvements in peripheral insulin sensitivity and hepatic glucose production [8], [10], [22], [60], [64]. While currently unclear, the activity profile exhibited by NPY/AgRP and POMC neurons could possibly serve as a cellular correlate for these exercise-induced changes in metabolic physiology.
4.2. Synchronicity of exercise effects on cellular and synaptic activity in NPY/AgRP and POMC neurons
Another salient finding is that the duration of the exercise effects on the cellular and synaptic activity of melanocortin neurons is restricted to a variable temporal window. Single bouts of exercise have been shown to alter plasma hormone levels [22], [41], [47], [60], promote motor learning [40], [52], and induce neuroplasticity leading to improved cognitive function [26], [40], [42]. While single bouts of exercise can result in improvements of glucose metabolism, exercise training consistently results in enhanced effects on the regulation of metabolism [9], [17], [20], [22], [30], [45], [60]. Notably, we examined the exercise-induced time dependent changes in POMC and NPY neuronal characteristics. When compared to POMC neurons from sedentary mice, POMC neurons exhibited a depolarized membrane potential and increased frequency of spontaneous EPSCs after a single bout of exercise. These effects persisted and were enhanced throughout exercise training. Additionally, exercise training (5 and 10 days) led to additive effects on action potential frequency and input resistance. These data suggest that the rapid depolarization of POMC neurons after an acute bout of exercise may better correlate with increased excitatory synaptic inputs, rather than a change in post-synaptic ionic conductance or channel state. The activation of a putative conductance subsequent to exercise training may help to reinforce the effects of exercise to activate POMC neurons. After a single bout of exercise, NPY neurons exhibited a hyperpolarized membrane potential and decreased input resistance when compared to NPY neurons from sedentary mice. Similar to POMC neurons, these effects persisted and were enhanced throughout exercise training. The hyperpolarized membrane potential was accompanied by an increasing suppression of action potential frequency with additional exercise training. Interestingly, while NPY neurons were hyperpolarized, the synaptic activities onto NPY neurons were unaffected after an acute bout of exercise. The frequencies of spontaneous IPSCs were increased while spontaneous EPSC frequency decreased after 5 or 10 days of exercise training, respectively. The asynchronous changes in membrane potential and synaptic activity suggest that the hyperpolarized membrane potential of NPY neurons better correlates with changes of post-synaptic ionic conductance or channel state, rather than changes of synaptic activity. These data suggest the possibility of multiple mechanisms being involved in the remodeling of synaptic and cellular properties of NPY and POMC neurons in response to exercise. These data also support the idea that repeated bouts of exercise occurring during training may reinforce the remodeling of this neural circuit.
4.3. Leptin receptor dependent plasticity of arcuate POMC neurons in responses to exercise
Recent work has highlighted a plastic property of NPY/AgRP and POMC neuronal circuitry [36], [49], [59], [65]. Moreover, the plasticity of this circuit has been suggested to be highly sensitive to the levels of leptin [51], [61]. In particular, genetic models of leptin deficiency results in increased excitatory tone to NPY/AgRP neurons and increased inhibitory tone to POMC neurons [49], [59]. Similarly, fasting, which results in a fall of leptin levels, increased NPY/AgRP and decreased POMC neuronal activity [24], [36], [59], [65]. In order to better examine the role of leptin receptors in the exercise-induced plasticity of POMC and NPY neurons, we utilized a mouse model where we identified POMC and NPY neurons that express or do not express leptin receptors. Importantly, we found that the exercise-induced changes in cellular and synaptic properties of POMC neurons were enriched in POMC neurons that express leptin receptors. In particular, the increase in action potential frequency was concomitant with the depolarized membrane potential and increased EPSC frequency after a single bout of exercise. A similar rapid decrease of input resistance was observed in LepR positive POMC neurons after acute exercise. Importantly, all changes in cellular and synaptic properties of LepR positive POMC neurons persisted and were enhanced throughout exercise training. Contrary to the effects observed in LepR positive POMC neurons, LepR negative POMC neurons failed to incite similar changes. It should be noted that while the changes in LepR negative POMC neurons were not significant, these neurons trended toward an overall excitation during continued exercise training observed via changes of membrane potential, action potential frequency, and spontaneous EPSC frequency. These data highlight a novel and selective exercise-induced regulation in a subpopulation of arcuate POMC neurons that express leptin receptors; however, NPY neurons that express or do not express leptin receptors exhibited similar responses to exercise.
While leptin via leptin receptors may contribute to the exercise-induced effects on cellular activity in the current study, multiple nutrient sensing and peptidergic systems are also altered in response to exercise. Many of these systems have trophic properties and contribute to synaptic plasticity within the brain. While currently unclear, it is also possible that the leptin receptor expressing POMC neurons select for one of these mechanisms. This warrants future investigation. Moreover, previous models of leptin deficiency as well as observations in fasting conditions depict a cellular reorganization of the melanocortin circuit in response to perceived energy deprivation. In the current study we show that NPY/AgRP neurons are inhibited and POMC neurons are excited in response to exercise. Thus, exercise appears to be a metabolic challenge that is unique from energy deprivation, and the remodeling of this circuit is a specific response of exercise to improve metabolism.
4.4. Temporal variability in the duration of exercise effects on POMC and NPY neurons
Another interesting observation is that a single bout of exercise is sufficient to remodel the NPY or leptin expressing POMC neurons in the melanocortin circuit for hours to days. Synaptic plasticity occurs in multiple areas of the brain [12], [29], [36], [39]. While early reports of synaptic plasticity were characterized in classical components of cognitive learning and memory [4], [6], [37], [38], recent evidence suggests that cellular plasticity may occur in response to various challenges (including changing metabolic states) [23], [36], [49], [59]. In the current study, we postulate that the plasticity of the melanocortin circuit observed in response to exercise might be an analogous representation to learning and memory paradigms. However, in-lieu of altered cognition the melanocortin circuit is rewired into a circuit, which is beneficial for metabolic regulation creating a representation of a new metabolic “set point” [28], [34], [44], [51]. Also, it is notable that the persistence of these effects is transiently regulated on different time scales. In particular, the exercise induced excitation of POMC neurons which express leptin receptors persisted for 2 days after a single bout of exercise, while the inhibitory effects on NPY neurons reversed within hours. It should be noted that the timeline of events described in the current study are analogous to reports that acute bouts of exercise in humans can improve insulin sensitivity for up to 48 h [43]. Together, it is possible that the cellular rearrangement of the melanocortin circuit is at least in part a cellular correlate (cellular mechanism) to these earlier observations.
5. Conclusions
In summary, a single bout of exercise activated arcuate POMC neurons while inhibiting arcuate NPY neurons. Repeated bouts of exercise enhanced the respective activities of NPY and POMC neurons. These changes in activity were dependent upon temporally regulated alterations of both pre and post synaptic responses. Moreover, the activation of POMC neurons was predicated on the presence of leptin receptors, but not in NPY neurons. Together these data highlight multiple mechanisms in the remodeling of synaptic and cellular properties of NPY and POMC neurons in response to exercise. Altogether, providing a putative cellular mechanism by which exercise may improve metabolism via activity of melanocortin neurons.
Disclosure statement
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Joel K. Elmquist (of the Division of Hypothalamic Research, Department of Internal Medicine, UT Southwestern Medical Center, Dallas, Texas) for kindly providing us with the NPY-hrGFP and POMC-hrGFP mice. And we also thank the help from The Guangdong Provincial Clinical Medical Centre for Neurosurgery, No. 2013B020400005. This work was supported by NIH grants to K.W.W. (R01 DK100699 and R01 DK119169), J.N.B (1R01DK114104), A.L.A. (F32DK112561) and C.M.C. (K01 DK111644).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.08.011.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Plot showing the timeline of the exercise training. Food was removed at the beginning of exercise, and mice were refed at the end of exercise. For 1, 5, and 10 days exercise protocols, the speed and duration of the treadmill is indicated in the inset and described in Methods.
Drawings at three rostro caudal levels of the mouse hypothalamus showing the location where NPY-hrGFP neurons were targeted for the electrophysiological recording (A: 1 day exercise; B: 5 days exercise; C: 10 days exercise) 3V: third ventricle, arc: arcuate nucleus, d3V: dorsal third ventricle, DMH: dorsomedial hypothalamic nucleus, F: fornix, LV: lateral ventricle, ME: median eminence, opt: optic tract, PMD: premammillary nucleus, dorsal part, PMV: premammillary nucleus, ventral part, RCA: retrochiasmatic area, sox: supraoptic decussation, VMH: ventromedial hypothalamic nucleus. The number of neurons studied for each group is shown in parentheses.
Histograms showing the body weights of POMC-hrGFP::LepR-cre::tdtomato mice (A) and NPY-hrGFP::LepR-cre::tdtomato (B) mice before and after 1, 5, and 10 days of exercise training. Data are from male mice and are expressed as mean ± SEM. Unpaired t test compared to controls. The number of mice studied for each group is shown in parentheses.
Drawings at three rostro caudal levels of the mouse hypothalamus showing the location where POMC-hrGFP neurons were targeted for the electrophysiological recording (A: 1 day exercise; B: 5 days exercise; C: 10 days exercise) 3V: third ventricle, arc: arcuate nucleus, d3V: dorsal third ventricle, DMH: dorsomedial hypothalamic nucleus, F: fornix, LV: lateral ventricle, ME: median eminence, opt: optic tract, PMD: premammillary nucleus, dorsal part, PMV: premammillary nucleus, ventral part, RCA: retrochiasmatic area, sox: supraoptic decussation,VMH:ventromedial hypothalamic nucleus. The number of neurons studied for each group is shown in parentheses.
Comparison of the electrophysiological proprieties between LepR negative and LepR positive arcuate NPY neurons after 1, 5, or 10 days of exercise. (A–E) LepR negative NPY-hrGFP neuron: Brightfield illumination (A) of NPY-hrGFP neuron from PLT mice. (B) and (C) show the same neuron under FITC (hrGFP) and Alexa Fluor 594 illumination to demonstrate the expression of hrGFP and no expression of LepR/tdTomato. (D) Image shows the complete dialysis of Alexa Fluor 350 from the intracellular pipette. Merged image of targeted LepR negative NPY-hrGFP neuron is shown in (E). Arrow indicates the targeted cell. Scale bar = 50 μm. (F-J) LepR positive NPY-hrGFP neuron: Brightfield illumination (F) of NPY-hrGFP neuron from PLT mice. (G) and (H) show the same neuron under FITC (hrGFP) and Alexa Fluor 594 illumination to demonstrate the expression of both hrGFP and LepR/tdTomato. (I) Image shows the complete dialysis of Alexa Fluor 350 from the intracellular pipette. Merged image of targeted LepR positive NPY-hrGFP neuron is shown in (J). Arrow indicates the targeted cell. Scale bar = 50 μm. (K, M, O) Histograms from current-clamp recordings show the resting membrane potential (K), action potential frequency (M), and input resistance (O) of LepR negative NPY-hrGFP neurons after 1, 5, or 10 days of exercise. (L, N, P) Plots from current-clamp show the resting membrane potential (L), action potential frequency (N), and input resistance (P) of LepR positive NPY-hrGFP neurons after 1, 5, or 10 days of exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, unpaired t test compared to controls. The number of neurons studied for each group is shown in parentheses
Comparison of the synaptic properties between LepR negative and LepR positive arcuate NPY neurons after 1, 5, or 10 days of exercise. (A-D) Plots from voltage clamp recordings show spontaneous EPSC frequency and amplitude of LepR negative (A, C) and LepR positive (B, D) NPY-hrGFP neurons after 1, 5, or 10 days of exercise. (E-H) Plots from voltage clamp recordings show spontaneous IPSC frequency and amplitude of LepR negative (E, G) and LepR positive (F, H) NPY-hrGFP neurons after 1, 5, or 10 days of exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, ****p < 0.0001, unpaired t test compared to controls. The number of neurons studied for each group is shown in parentheses.
Effects of exercise on food intake in mice. (A) 6 h and (B) 24 h food intake following a 6 h period of food deprivation, during which HIIE was conducted in the 2nd hour. (C) 6 h and (D) 24 h food intake following a single bout of HIIE (without 6 h food deprivation). Black: sedentary; red: single bout HIIE (1day exercise); green: 5 days exercise; blue: 10 days exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, unpaired t test compared to controls. The number of mice studied for each group is greater than or equal to four.
Schematic showing the exercise-induced increase of excitatory synaptic activity on LepR expressing POMC neurons, while NPY neurons receive increased inhibitory input concomitant with decreased excitatory input following exercise. The effects of exercise on the cellular properties of POMC and NPY neurons reverse within hours to days after cessation of exercise (detraining).
References
- 1.Alhadeff A.L., Su Z., Hernandez E., Klima M.L., Phillips S.Z., Holland R.A. A neural circuit for the suppression of pain by a competing need state. Cell. 2018;173:140–152. doi: 10.1016/j.cell.2018.02.057. e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atasoy D., Betley J.N., Su H.H., Sternson S.M. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bear M.F., Malenka R.C. Synaptic plasticity: LTP and LTD. Current Opinion in Neurobiology. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 5.Betley J.N., Cao Z.F., Ritola K.D., Sternson S.M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 7.Bogardus C., Ravussin E., Robbins D.C., Wolfe R.R., Horton E.S., Sims E.A. Effects of physical training and diet therapy on carbohydrate metabolism in patients with glucose intolerance and non-insulin-dependent diabetes mellitus. Diabetes. 1984;33:311–318. doi: 10.2337/diab.33.4.311. [DOI] [PubMed] [Google Scholar]
- 8.Cartee G.D. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. American Journal of Physiology. Endocrinology and Metabolism. 2015;309:E949–E959. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartee G.D., Hepple R.T., Bamman M.M., Zierath J.R. Exercise promotes healthy aging of skeletal muscle. Cell Metabolism. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castorena C.M., Arias E.B., Sharma N., Cartee G.D. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes. 2014;63:2297–2308. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coggan A.R., Swanson S.C., Mendenhall L.A., Habash D.L., Kien C.L. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. American Journal of Physiology. 1995;268:E375–E383. doi: 10.1152/ajpendo.1995.268.3.E375. [DOI] [PubMed] [Google Scholar]
- 12.Collingridge G.L., Peineau S., Howland J.G., Wang Y.T. Long-term depression in the CNS. Nature Reviews Neuroscience. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- 13.Cowley M.A., Smart J.L., Rubinstein M., Cerdan M.G., Diano S., Horvath T.L. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 14.Cowley M.A., Smith R.G., Diano S., Tschop M., Pronchuk N., Grove K.L. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo R.A., Sherwin R.S., Kraemer N. Effect of physical training on insulin action in obesity. Diabetes. 1987;36:1379–1385. doi: 10.2337/diab.36.12.1379. [DOI] [PubMed] [Google Scholar]
- 16.Elmquist J.K., Elias C.F., Saper C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 17.Gabriel B.M., Zierath J.R. The limits of exercise physiology: from performance to health. Cell Metabolism. 2017;25:1000–1011. doi: 10.1016/j.cmet.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y., Yao T., Deng Z., Sohn J.W., Sun J., Huang Y. TrpC5 mediates acute leptin and serotonin effects via pomc neurons. Cell Reports. 2017;18:583–592. doi: 10.1016/j.celrep.2016.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garfield A.S., Shah B.P., Madara J.C., Burke L.K., Patterson C.M., Flak J. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metabolism. 2014;20:1030–1037. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodyear L.J., Kahn B.B. Exercise, glucose transport, and insulin sensitivity. Annual Review of Medicine. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 21.Hamada T., Arias E.B., Cartee G.D. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. Journal of Applied Physiology (1985) 2006;101:1368–1376. doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hawley J.A., Hargreaves M., Joyner M.J., Zierath J.R. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Horvath T.L. The hardship of obesity: a soft-wired hypothalamus. Nature Neuroscience. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- 24.Horvath T.L., Diano S. The floating blueprint of hypothalamic feeding circuits. Nature Reviews Neuroscience. 2004;5:662–667. doi: 10.1038/nrn1479. [DOI] [PubMed] [Google Scholar]
- 25.Horvath T.L., Sarman B., Garcia-Caceres C., Enriori P.J., Sotonyi P., Shanabrough M. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proceedings of the National Academy of Sciences of the U S A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotting K., Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neuroscience & Biobehavioral Reviews. 2013;37:2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y., He Z., Gao Y., Lieu L., Yao T., Sun J. PI3K is integral for the acute activity of leptin and insulin in arcuate NPY/AgRP neurons in males. J Endocrine Soc. 2018 doi: 10.1210/js.2018-00061. js.2018-00061-js.02018-00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy G.C. The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London B Biological Sciences. 1953;140:578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 29.Kessels H.W., Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konner A.C., Janoschek R., Plum L., Jordan S.D., Rother E., Ma X. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metabolism. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Journal of Clinical Investigation. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krashes M.J., Shah B.P., Madara J.C., Olson D.P., Strochlic D.E., Garfield A.S. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leibel R.L. Is obesity due to a heritable difference in 'set point' for adiposity? Western Journal of Medicine. 1990;153:429–431. [PMC free article] [PubMed] [Google Scholar]
- 35.Lerner T.N., Shilyansky C., Davidson T.J., Evans K.E., Beier K.T., Zalocusky K.A. Intact-brain analyses reveal distinct information carried by snc dopamine subcircuits. Cell. 2015;162:635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T., Kong D., Shah B.P., Ye C., Koda S., Saunders A. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malenka R.C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 38.Malenka R.C. Synaptic plasticity. Mucking up movements. Nature. 1994;372:218–219. doi: 10.1038/372218a0. [DOI] [PubMed] [Google Scholar]
- 39.Malenka R.C., Nicoll R.A. Long-term potentiation–a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 40.Mang C.S., Snow N.J., Campbell K.L., Ross C.J., Boyd L.A. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. Journal of Applied Physiology (1985) 2014;117:1325–1336. doi: 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mani B.K., Castorena C.M., Osborne-Lawrence S., Vijayaraghavan P., Metzger N.P., Elmquist J.K. Ghrelin mediates exercise endurance and the feeding response post-exercise. Molecular Metabolism. 2018;9:114–130. doi: 10.1016/j.molmet.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonnell M.N., Buckley J.D., Opie G.M., Ridding M.C., Semmler J.G. A single bout of aerobic exercise promotes motor cortical neuroplasticity. Journal of Applied Physiology (1985) 2013;114:1174–1182. doi: 10.1152/japplphysiol.01378.2012. [DOI] [PubMed] [Google Scholar]
- 43.Mikines K.J., Sonne B., Farrell P.A., Tronier B., Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. American Journal of Physiology. 1988;254:E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- 44.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 45.Neufer P.D., Bamman M.M., Muoio D.M., Bouchard C., Cooper D.M., Goodpaster B.H. Understanding the cellular and molecular mechanisms of physical activity-induced health benefits. Cell Metabolism. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Newton A.J., Hess S., Paeger L., Vogt M.C., Fleming Lascano J., Nillni E.A. AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice. Endocrinology. 2013;154:172–183. doi: 10.1210/en.2012-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Numao S., Kawano H., Endo N., Yamada Y., Konishi M., Takahashi M. Effects of a single bout of aerobic exercise on short-term low-carbohydrate/high-fat intake-induced postprandial glucose metabolism during an oral glucose tolerance test. Metabolism. 2013;62:1406–1415. doi: 10.1016/j.metabol.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Paeger L., Pippow A., Hess S., Paehler M., Klein A.C., Husch A. Energy imbalance alters Ca(2+) handling and excitability of POMC neurons. Elife. 2017;6 doi: 10.7554/eLife.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinto S., Roseberry A.G., Liu H., Diano S., Shanabrough M., Cai X. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 50.Plum L., Ma X., Hampel B., Balthasar N., Coppari R., Munzberg H. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. Journal of Clinical Investigation. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polidori D., Sanghvi A., Seeley R.J., Hall K.D. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity (Silver Spring) 2016;24:2289–2295. doi: 10.1002/oby.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roig M., Skriver K., Lundbye-Jensen J., Kiens B., Nielsen J.B. A single bout of exercise improves motor memory. PLoS One. 2012;7:e44594. doi: 10.1371/journal.pone.0044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawchenko P.E. Central connections of the sensory and motor nuclei of the vagus nerve. Journal of the Autonomic Nervous System. 1983;9:13–26. doi: 10.1016/0165-1838(83)90129-7. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz M.W., Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 55.Sim A.Y., Wallman K.E., Fairchild T.J., Guelfi K.J. High-intensity intermittent exercise attenuates ad-libitum energy intake. International Journal of Obesity (London) 2014;38:417–422. doi: 10.1038/ijo.2013.102. [DOI] [PubMed] [Google Scholar]
- 56.Steculorum S.M., Ruud J., Karakasilioti I., Backes H., Engstrom Ruud L., Timper K. AgRP neurons control systemic insulin sensitivity via myostatin expression in Brown adipose tissue. Cell. 2016;165:125–138. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sternson S.M., Shepherd G.M., Friedman J.M. Topographic mapping of VMH--> arcuate nucleus microcircuits and their reorganization by fasting. Nature Neuroscience. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 58.Su Z., Alhadeff A.L., Betley J.N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Reports. 2017;21:2724–2736. doi: 10.1016/j.celrep.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi K.A., Cone R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 60.Thyfault J.P., Wright D.C. “Weighing” the effects of exercise and intrinsic aerobic capacity: are there beneficial effects independent of changes in weight? Applied Physiology Nutrition and Metabolism. 2016;41:911–916. doi: 10.1139/apnm-2016-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang D., He X., Zhao Z., Feng Q., Lin R., Sun Y. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in Neuroanatomy. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams K.W., Elmquist J.K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature Neuroscience. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Atasoy D., Su H.H., Sternson S.M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young J.C., Enslin J., Kuca B. Exercise intensity and glucose tolerance in trained and nontrained subjects. Journal of Applied Physiology (1985) 1989;67:39–43. doi: 10.1152/jappl.1989.67.1.39. [DOI] [PubMed] [Google Scholar]
- 65.Zeltser L.M., Seeley R.J., Tschop M. Synaptic plasticity in circuits regulating energy balance. Nature Neuroscience. 2012 doi: 10.1038/nn.3219. [DOI] [PubMed] [Google Scholar]
- 66.Zhan C., Zhou J., Feng Q., Zhang J.E., Lin S., Bao J. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. Journal of Neuroscience. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plot showing the timeline of the exercise training. Food was removed at the beginning of exercise, and mice were refed at the end of exercise. For 1, 5, and 10 days exercise protocols, the speed and duration of the treadmill is indicated in the inset and described in Methods.
Drawings at three rostro caudal levels of the mouse hypothalamus showing the location where NPY-hrGFP neurons were targeted for the electrophysiological recording (A: 1 day exercise; B: 5 days exercise; C: 10 days exercise) 3V: third ventricle, arc: arcuate nucleus, d3V: dorsal third ventricle, DMH: dorsomedial hypothalamic nucleus, F: fornix, LV: lateral ventricle, ME: median eminence, opt: optic tract, PMD: premammillary nucleus, dorsal part, PMV: premammillary nucleus, ventral part, RCA: retrochiasmatic area, sox: supraoptic decussation, VMH: ventromedial hypothalamic nucleus. The number of neurons studied for each group is shown in parentheses.
Histograms showing the body weights of POMC-hrGFP::LepR-cre::tdtomato mice (A) and NPY-hrGFP::LepR-cre::tdtomato (B) mice before and after 1, 5, and 10 days of exercise training. Data are from male mice and are expressed as mean ± SEM. Unpaired t test compared to controls. The number of mice studied for each group is shown in parentheses.
Drawings at three rostro caudal levels of the mouse hypothalamus showing the location where POMC-hrGFP neurons were targeted for the electrophysiological recording (A: 1 day exercise; B: 5 days exercise; C: 10 days exercise) 3V: third ventricle, arc: arcuate nucleus, d3V: dorsal third ventricle, DMH: dorsomedial hypothalamic nucleus, F: fornix, LV: lateral ventricle, ME: median eminence, opt: optic tract, PMD: premammillary nucleus, dorsal part, PMV: premammillary nucleus, ventral part, RCA: retrochiasmatic area, sox: supraoptic decussation,VMH:ventromedial hypothalamic nucleus. The number of neurons studied for each group is shown in parentheses.
Comparison of the electrophysiological proprieties between LepR negative and LepR positive arcuate NPY neurons after 1, 5, or 10 days of exercise. (A–E) LepR negative NPY-hrGFP neuron: Brightfield illumination (A) of NPY-hrGFP neuron from PLT mice. (B) and (C) show the same neuron under FITC (hrGFP) and Alexa Fluor 594 illumination to demonstrate the expression of hrGFP and no expression of LepR/tdTomato. (D) Image shows the complete dialysis of Alexa Fluor 350 from the intracellular pipette. Merged image of targeted LepR negative NPY-hrGFP neuron is shown in (E). Arrow indicates the targeted cell. Scale bar = 50 μm. (F-J) LepR positive NPY-hrGFP neuron: Brightfield illumination (F) of NPY-hrGFP neuron from PLT mice. (G) and (H) show the same neuron under FITC (hrGFP) and Alexa Fluor 594 illumination to demonstrate the expression of both hrGFP and LepR/tdTomato. (I) Image shows the complete dialysis of Alexa Fluor 350 from the intracellular pipette. Merged image of targeted LepR positive NPY-hrGFP neuron is shown in (J). Arrow indicates the targeted cell. Scale bar = 50 μm. (K, M, O) Histograms from current-clamp recordings show the resting membrane potential (K), action potential frequency (M), and input resistance (O) of LepR negative NPY-hrGFP neurons after 1, 5, or 10 days of exercise. (L, N, P) Plots from current-clamp show the resting membrane potential (L), action potential frequency (N), and input resistance (P) of LepR positive NPY-hrGFP neurons after 1, 5, or 10 days of exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, unpaired t test compared to controls. The number of neurons studied for each group is shown in parentheses
Comparison of the synaptic properties between LepR negative and LepR positive arcuate NPY neurons after 1, 5, or 10 days of exercise. (A-D) Plots from voltage clamp recordings show spontaneous EPSC frequency and amplitude of LepR negative (A, C) and LepR positive (B, D) NPY-hrGFP neurons after 1, 5, or 10 days of exercise. (E-H) Plots from voltage clamp recordings show spontaneous IPSC frequency and amplitude of LepR negative (E, G) and LepR positive (F, H) NPY-hrGFP neurons after 1, 5, or 10 days of exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, ****p < 0.0001, unpaired t test compared to controls. The number of neurons studied for each group is shown in parentheses.
Effects of exercise on food intake in mice. (A) 6 h and (B) 24 h food intake following a 6 h period of food deprivation, during which HIIE was conducted in the 2nd hour. (C) 6 h and (D) 24 h food intake following a single bout of HIIE (without 6 h food deprivation). Black: sedentary; red: single bout HIIE (1day exercise); green: 5 days exercise; blue: 10 days exercise. Data are from male mice and are expressed as mean ± SEM. *p < 0.05, unpaired t test compared to controls. The number of mice studied for each group is greater than or equal to four.
Schematic showing the exercise-induced increase of excitatory synaptic activity on LepR expressing POMC neurons, while NPY neurons receive increased inhibitory input concomitant with decreased excitatory input following exercise. The effects of exercise on the cellular properties of POMC and NPY neurons reverse within hours to days after cessation of exercise (detraining).