Abstract
Objective
A novel dual GIP and GLP-1 receptor agonist, LY3298176, was developed to determine whether the metabolic action of GIP adds to the established clinical benefits of selective GLP-1 receptor agonists in type 2 diabetes mellitus (T2DM).
Methods
LY3298176 is a fatty acid modified peptide with dual GIP and GLP-1 receptor agonist activity designed for once-weekly subcutaneous administration. LY3298176 was characterised in vitro, using signaling and functional assays in cell lines expressing recombinant or endogenous incretin receptors, and in vivo using body weight, food intake, insulin secretion and glycemic profiles in mice.
A Phase 1, randomised, placebo-controlled, double-blind study was comprised of three parts: a single-ascending dose (SAD; doses 0.25–8 mg) and 4-week multiple-ascending dose (MAD; doses 0.5–10 mg) studies in healthy subjects (HS), followed by a 4-week multiple-dose Phase 1 b proof-of-concept (POC; doses 0.5–15 mg) in patients with T2DM (ClinicalTrials.gov no. NCT02759107). Doses higher than 5 mg were attained by titration, dulaglutide (DU) was used as a positive control. The primary objective was to investigate safety and tolerability of LY3298176.
Results
LY3298176 activated both GIP and GLP-1 receptor signaling in vitro and showed glucose-dependent insulin secretion and improved glucose tolerance by acting on both GIP and GLP-1 receptors in mice. With chronic administration to mice, LY3298176 potently decreased body weight and food intake; these effects were significantly greater than the effects of a GLP-1 receptor agonist.
A total of 142 human subjects received at least 1 dose of LY3298176, dulaglutide, or placebo. The PK profile of LY3298176 was investigated over a wide dose range (0.25–15 mg) and supports once-weekly administration. In the Phase 1 b trial of diabetic subjects, LY3298176 doses of 10 mg and 15 mg significantly reduced fasting serum glucose compared to placebo (least square mean [LSM] difference [95% CI]: −49.12 mg/dL [−78.14, −20.12] and −43.15 mg/dL [−73.06, −13.21], respectively). Reductions in body weight were significantly greater with the LY3298176 1.5 mg, 4.5 mg and 10 mg doses versus placebo in MAD HS (LSM difference [95% CI]: −1.75 kg [−3.38, −0.12], −5.09 kg [−6.72, −3.46] and −4.61 kg [−6.21, −3.01], respectively) and doses of 10 mg and 15 mg had a relevant effect in T2DM patients (LSM difference [95% CI]: −2.62 kg [−3.79, −1.45] and −2.07 kg [−3.25, −0.88], respectively.
The most frequent side effects reported with LY3298176 were gastrointestinal (vomiting, nausea, decreased appetite, diarrhoea, and abdominal distension) in both HS and patients with T2DM; all were dose-dependent and considered mild to moderate in severity.
Conclusions
Based on these results, the pharmacology of LY3298176 translates from preclinical to clinical studies. LY3298176 has the potential to deliver clinically meaningful improvement in glycaemic control and body weight. The data warrant further clinical evaluation of LY3298176 for the treatment of T2DM and potentially obesity.
Keywords: Glucagon-like peptide-1, Glucose-dependent insulinotropic polypeptide, LY3298176, Obesity, Type 2 diabetes mellitus
Abbreviations: ADA, Antidrug antibodies; CI, Confidence interval; CNS, Central nervous system; DIO, Diet-induced obesity; DU, Dulaglutide; ECG, Electrocardiogram; GIP, Glucose-dependent insulinotropic polypeptide; GLP-1 RA, Glucagon-like peptide-1 receptor agonist; HS, Healthy subjects; ipGTT, Intraperitoneal glucose tolerance test; LSM, Least square mean; LY, LY3298176; MAD, Multiple-ascending dose; MMRM, Mixed model for repeated measures; PL, PPlacebo; OGTT, Oral glucose tolerance test; POC, Proof-of-concept; SAD, Single-ascending dose; SMBG, Self-monitored blood glucose; T2DM, Type 2 diabetes mellitus
Highlights
-
•
LY3298176 activates both GIP and GLP-1 receptor signaling in vitro.
-
•
LY3298176 lowers blood glucose in mice through actions on both incretin receptors.
-
•
LY3298176 reduced fasting glucose in humans with type 2 diabetes.
-
•
Weight loss was greater with LY3298176 than the selective GLP-1 receptor agonist, dulaglutide in healthy humans.
-
•
Tolerability of LY3298176 was comparable to GLP-1 receptor agonists.
1. Introduction
Treatment of type 2 diabetes mellitus (T2DM) with glucagon-like peptide-1 receptor agonists (GLP-1RAs) leads to better glycaemic control, reduced body weight, and improvement in several cardiovascular risk factors, which has been demonstrated to be accompanied by improved micro- and macrovascular outcomes [1]. These benefits are mediated by the GLP-1R, a member of the class B family of G protein-coupled receptors, that is expressed in pancreatic beta-cells, various cell types of the gastrointestinal tract, and neurons throughout both the central (CNS) and the peripheral nervous systems [2]. Activation of GLP-1R signaling by GLP-1RAs improves glucose control by enhancing glucose-stimulated insulin secretion [3], [4], delaying gastric transit [5], [6], and decreasing plasma glucagon levels [7], and reduces body weight by activating anorexigenic pathways in the brain [8]. Due to the glucose-dependence of beta-cell activation, which involves a number of intracellular mediators, includiing calcium and Epac2 [9], [10], GLP-1RAs are not associated with increased risk of hypoglycaemia [11]. While the broad metabolic benefits of GLP-1RAs have established this class in the T2DM treatment paradigm, many patients do not reach their glycaemic targets, and weight loss achieved with these agents remains well below what can be attained with bariatric surgery, the most potent clinical intervention for obesity [12]. Thus, there are significant opportunities to improve upon the existing GLP-1RA class.
One emerging approach is to combine foundational GLP-1RA therapy with pharmacological strategies targeting additional pathways implicated in nutrient and energy metabolism, such as glucose-dependent insulinotropic polypeptide (GIP) [13]. GIP is an incretin that is secreted from K cells in the upper small intestine in response to food [14]. Postprandial GIP levels are approximately 4-fold higher compared to GLP-1 under normal physiological conditions [15]. GIP is responsible for the majority of the insulinotropic incretin effect in man [14], [16] and has important additional functions that are distinct from GLP-1. Unlike GLP-1, GIP is both glucagonotropic and insulinotropic in a glycaemic-dependent manner, dose-dependently stimulating glucagon secretion under hypoglycaemic conditions and insulin under hyperglycaemic conditions [17], [18], [19], [20], [21]. Although both GIPR and GLP-1R are present in beta-cells, GIPR expression is distributed differently in extra-pancreatic tissues as GIPR is abundant in adipose tissue [22] and is found in many non-overlapping areas of the CNS [23]. The biological activity of GIP on adipocytes has been investigated for some time, and although rather disparate, GIP is implicated in adipose tissue carbohydrate and lipid metabolism by its actions to regulate glucose uptake [24], lipolysis [25], and lipoprotein lipase activity [26], [27], [28], some of which support a role of GIP in fat accumulation that align with studies indicating GIPR null mice are resistant to obesity. Further, acute GIP infusion to humans increases adipose tissue blood flow [29], suggesting additional mechanisms of actions. Chronically elevating GIP levels in a transgenic mouse model has been shown to reduce diet-induced obesity (DIO) and improve insulin sensitivity, glucose tolerance, and beta-cell function [30]. These findings suggest that pharmacological activation of GIPR may have a therapeutic benefit on peripheral energy metabolism. In support of this, studies in DIO mice showed the body weight lowering effects of GLP-1RA are enhanced upon co-administration of a long-acting GIP analogue [31]. However, the role of GIP in the regulation of body weight has been controversial. Observations from genetically modified mice are confounding as both GIPR-deficient [27] and GIP-overexpressing mice [30] seem to be protected from obesity. In addition, chronic administration of a long-acting GIP molecule in rodents has no effects on body weight [31]. In the brain, GIP appears to activate neurons distinct from GLP-1, and central infusion of GIP in mice can inhibit food intake in a manner that is additive to GLP-1 [32]. Historically, the therapeutic utility of GIP has been limited by the fact that the incretin response to GIP is severely blunted in T2DM, possibly due to downregulation of the GIPR by high circulating glucose. A substantial body of data suggests, however, that GIP resistance can be largely overcome by agents that lower circulating glucose levels [33], [34], paving the way for considerations of GIP as add-on to glucose-lowering therapies, like GLP-1.
Recently, unimolecular, multi-functional peptides that combine GLP-1RA activity with glucagon and/or GIP activity have been suggested as new therapeutic agents for glycaemic and weight control. In rodents, dual GIP and GLP-1 receptor agonists achieve significantly better glucose control and weight loss compared to selective GLP-1RAs, such as exenatide or liraglutide [31]. To date, clinical data have been reported for two GIPR/GLP-1R co-agonist [31], [35], [36], [37]. A PEGylated dual agonist enhanced insulin secretion, improved glycaemic control, and induced weight loss without causing relevant GI side effects in subjects with T2D [31]. The authors suggest that these results demonstrate the potential of GIP to enhance the pharmacology of selective GLP-1 RAs by strengthening the inherent efficacy and broadening their therapeutic range however, the studies were small and of short duration. Here, we describe a novel, single-peptide, dual GIP and GLP-1 receptor agonist, LY3298176. The dual functionality of the peptide is described in preclinical in vitro and in vivo models, and clinical assessment demonstrates that administration of LY3298176 results in glucose lowering and substantial body weight lowering efficacy in healthy individuals during a multiple ascending dose study, and in a randomized, 4-week, Phase 1b trial in T2DM patients. Based on these findings, this molecule was evaluated in a 26-week, randomized study in T2DM, the results of which are forthcoming [38].
2. Material and methods
2.1. Preclinical methodology
GIP, GLP-1, LY3298176 (C225H348N48O68), semaglutide [39], a long-acting GIPR agonist (LA-GIPRA), and [d-Ala2]GIP [40] were synthesized at Eli Lilly and Company using traditional peptide chemistry methods and solubilised in PBS, with the exception of [d-Ala2]GIP which was formulated in Tris–HCl, pH 8. HEK293 cells expressing either human GIPR (NP_000155) or GLP-1R (NP_002053), pancreatic human beta ECN90 cells and primary human adipocytes were used for whole cell cAMP accumulation assays [41]. All in vitro binding and cAMP assays in HEK293 cells were performed in the absence of albumin to allow direct comparison to native peptides without the confounding influence of albumin binding. Isolation of pancreatic islets from mice and insulin secretion assays were performed as previously reported [42]. Glucose tolerance tests were performed in wild-type, GIPR, and GLP-1R null C57BL/6 mice (Taconic). Effects on body weight, food intake, and energy expenditure were determined in DIO C57/Bl6 mice (Taconic). Additional experimental details are provided in the Supplemental Appendix.
2.2. Clinical study design and subjects
A Phase 1, randomised, placebo-controlled, double-blind study was comprised of three parts: single-ascending dose (SAD), and 4-week multiple-ascending dose (MAD) protocols in healthy subjects (HS), followed by a 4-week multiple-dose Phase 1b proof-of-concept (POC) in patients with T2DM. This study was conducted from May 11, 2016 through June 26, 2017 at 2 study sites (USA and Singapore). Inclusion and exclusion criteria are provided in the Supplemental Appendix.
2.3. Randomisation, masking, and study design
Subjects were randomly assigned to receive LY3298176 (LY), placebo (PL), or dulaglutide (DU) in prespecified ratios (SAD, 3LY:1 PL; MAD, 6LY:1 PL:1DU; POC, 4LY:1 PL) (Supplemental Fig. 2). The SAD portion of the study evaluated doses of LY3298176 ranging from 0.25 to 8 mg in 6 cohorts of healthy subjects. The MAD part used 4 once-weekly doses given to healthy subjects on Days 1, 8, 15, and 22. MAD testing consisted of 3 non titrated arms, investigating dose levels from 0.5 to 4.5 mg, and an arm in which doses were titrated up to 10 mg (5/5/8/10 mg); Dulaglutide was given to one group of subjects in a dose of 1.5 mg/wk as a control. Finally Phase 1b POC study tested two fixed doses (0.5 and 5 mg) and two titration schedules that increased dose levels to 10 mg (5/5/10/10 mg) and 15 mg (5/5/10/15 mg). The investigator and subject remained masked to treatment assignment throughout the study. Subjects received LY3298176 as vials containing 5 mg of LY3298176. Doses ≤1 mg were reconstituted with sterile saline (0.9% sodium chloride). Doses >1 mg were reconstituted with sterile water, US Pharmacopeia or British Pharmacopeia. Placebo was provided as normal saline, the volume matching the investigational drug at each dose level. Dulaglutide was provided as a 1.5 mg/0.5 mL single-use pen.
2.4. Clinical outcomes
The primary objective was to investigate the safety and tolerability following single and multiple doses of LY3298176 in healthy subjects and in patients with T2DM. Secondary objectives were to characterise the pharmacokinetics and pharmacodynamics of LY3298176 in healthy subjects and patients with T2DM by assessing changes in HbA1c, fasting glucose, fasting insulin, body weight, 7-point self-monitored blood glucose (SMBG) profiles, and glucose- and insulin response following an oral glucose tolerance test (OGTT). The OGTT was performed on day 23 at the Cmax of LY3298176. Most other objectives where followed to Day 29. Plasma samples obtained during this tudy were analyzed for LY3298176 concentration using a validated HRAM LC/MS method. Additional methods are described in more detail in the Supplemental Appendix. Safety assessments were comprised of treatment-emergent adverse events, hypoglycaemia events (defined in the Supplemental Appendix), laboratory parameters, vital signs, electrocardiogram (ECG) parameters, and LY3298176 antidrug antibodies (ADAs).
2.5. Ethics statement
Animals were studied and maintained in accordance with the Institutional Animal Care and Use Committee of Eli Lilly and Company and the Guide for the Use and Care of Laboratory Animals by the National Institutes of Health. The clinical trial (ClinicalTrials.gov, NCT02759107) was conducted in accordance with the Declaration of Helsinki, the Council for International Organizations of Medical Sciences International Ethical Guidelines, and the International Conference on Harmonisation Good Clinical Practices Guideline. Local institutional review boards approved the protocol. All subjects provided written informed consent.
2.6. Statistical analysis
Preclinical in vivo data are presented as mean ± SEM with 95% confidence intervals (CI) for treatment differences compared to placebo and were compared by one-way ANOVA using GraphPad Prism 7. In chronic in vivo studies, data were analysed by using two-way ANOVA repeated measures, followed by Tukey's multiple comparison test in order to evaluate treatment versus time effect. For energy balance, analysis of covariance (ANCOVA) similar to that previously described [43], [44] was used to assess potential effects on energy expenditure, independent of changes in body weight. Briefly, average energy expenditure was calculated for each animal daily, and results were analyzed in SAS® (Version 9.4) using a mixed model with treatment group, time, and their interaction, as model terms, plus body weight as a covariate to identify body weight independent treatment effects. Observations from each animal at different times were treated as repeated measurements using a Toeplitz (TOEP) covariance structure, which was identified as the best fit for the for the results. Comparisons of interest were drawn from the modeling output by a model-based T-test or appropriate contrast statements. The test p values were not adjusted for multiple testing. The null hypothesis was rejected at P < 0.05.
In the clinical trial, the sample size was not based on any statistical inferences. It was customary for this type of study, and deemed adequate to evaluate the primary objective. Dropouts were replaced so that the targeted numbers of patients/subjects for safety review and data collection would be achieved; replacement subjects were assigned to receive the treatment of the dropout. PK and PD analyses were performed in the “PK/PD” population (subjects receiving at least 1 dose of the study drug according to the treatment the subjects actually received) and safety analyses were performed in the “Safety” population (all enrolled patients/subjects whether or not they completed all protocol requirements). PK parameters for LY3298176 were computed by standard non-compartmental methods of analysis using Pharsight WinNonlin® and were summarized by study part and dose group. Change from baseline in HbA1c was analysed using ANCOVA with treatment as a fixed effect, and baseline as a covariate. Change from baseline in fasting glucose, fasting insulin, AUC(0–2 h) for glucose, AUC(0–2 h) for insulin, and body weight were analyzed separately using a mixed model for repeated measures (MMRM) with treatment, day, and treatment-by-day interaction as fixed effects and patient/subject as random effect. The number of subjects (n), least square mean (LSM), LSM difference compared to placebo, and 95% (CI) of the difference were reported. PK parameters are presented as geometric mean (coefficient of the variable CV %), median (minimum, maximum), or as geometric mean (minimum, maximum). Baseline values are presented as n (%) or mean ± SD. 7-Point SMBG profile data are presented on Days −2 (prior to administration of study drug), 8 (after the second dose), 15 (after the third dose), and 22 (after the fourth dose). Data analyses were performed using SAS®.
2.7. Role of the funding source
The study sponsor was involved in the study design, data collection, data review, data analysis and drafting of the report. All authors had full access to the data related to these studies and approved the report for publication.
3. Results
3.1. Chemical structure of LY3298176
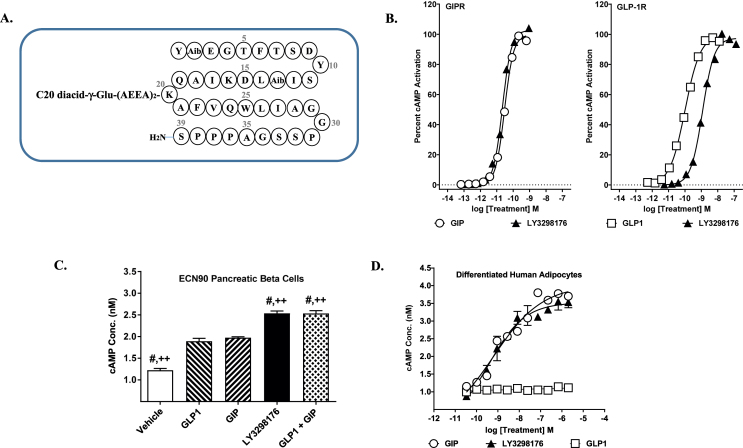
LY3298176 is a 39 amino acid linear peptide conjugated to a C20 fatty diacid moiety via a linker connected to the lysine residue at position 20. The peptide sequence of LY3298176 also contains two non-coded amino acid residues at positions 2 and 13 (Aib, α-amino isobutyric acid), and the C-terminus is amidated (Figure 1A). The acylation technology enables albumin binding, which provides a once-weekly dosing regimen in humans (Table 2). The molecular weight of LY3298176 is 4810.52 Da.
Figure 1.
Discovery and Characterization of LY3298176, a GIP-based Dual Incretin Receptor Agonist. (A) Structure schematic of the dual GIP and GLP-1 receptor agonist, LY3298176. (B) Representative concentration response curves for stimulation of cAMP accumulation by GIP, GLP-1 or LY3298176 in HEK293 cells expressing human GIPR or GLP-1R. (C) cAMP accumulation in human pancreatic ECN90 beta-cells in response to treatment with GLP-1, GIP, the combination of GLP-1 plus GIP, or LY3298176. P < 0.05 using one-way ANOVA versus GLP-1 (#) or GIP (++). (D) cAMP accumulation in human adipocytes. All data are expressed as mean ± SEM.
Table 2.
Pharmacokinetics of LY3298176.
| LY3298176 0.25 mg N = 6 |
LY3298176 0.5 mg N = 12 |
LY3298176 1.0 mg N = 5 |
LY3298176 2.5 mg N = 6 |
LY3298176 5.0 mg N = 5 |
LY3298176 8.0 mg N = 7 |
|
|---|---|---|---|---|---|---|
| SAD cohorts SC Route | ||||||
| Cmax, ng/mL | 26.0 (29) | 57.7 (37) | 108 (14) | 231 (40) | 397 (23) | 874 (19) |
| Tmax, ha | 48 (48,48) | 48 (24, 96) | 24 (8, 48) | 24 (24, 96) | 24 (24, 72) | 48 (24, 72) |
| AUC0-inf, ng.h/mL | 5760 (22) | 12000 (24) | 22600 (14) | 53200 (36) | 90500 (15) | 169000 (8) |
| T1/2, hb | 116 (94.6, 132) | 124 (94.4, 163) | 106 (92.9, 117) | 120 (102, 137) | 123 (99.9, 147) | 111 (99.6, 121) |
| CL/F, L/h | 0.0434 (22) | 0.0416 (24) | 0.0443 (14) | 0.0470 (36) | 0.0553 (15) | 0.0472 (8) |
| Vz/F, L | 7.26 (23) | 7.46 (28) | 6.76 (18) | 8.15 (35) | 9.80 (7) | 7.55 (4) |
Data are Geometric Mean (coefficient of variability CV %), unless otherwise noted. aMedian (minimum, maximum), bGeometric mean (minimum, maximum), Abbreviations: SAD = single ascending dose, Cmax = maximum observed drug concentration, Tmax = time of Cmax, AUC0-inf = area under the concentration time curve from time 0 extrapolate to infinity, CL/F = apparent total body clearance of drug following subcutaneous administration, Vz/F = apparent volume of distribution of drug during terminal phase following subcutaneous administration, N = number of subjects, T1/2 = half-life associated with the terminal rate constant in non-compartmental analysis.
3.2. Preclinical characterization of LY3298176
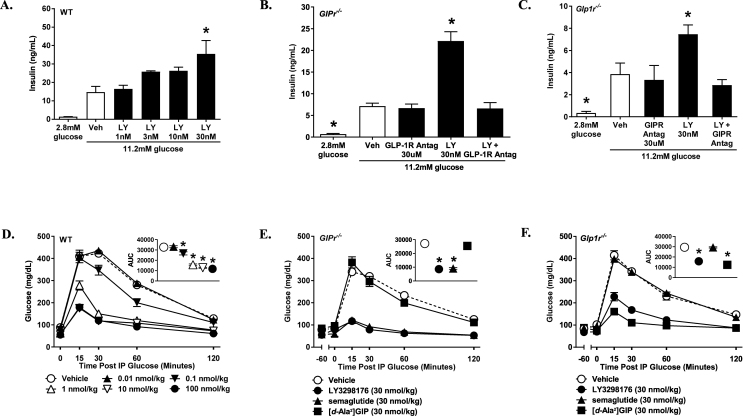
LY3298176 was designed to be potent at both the GIPR and GLP-1R to allow significant target engagement of both receptors. In receptor binding studies, LY3298176 binds either receptor with high affinity (GIPR Ki = 0.135, SEM = 0.020 nM; GLP-1R Ki = 4.23, SEM = 0.23 nM); the affinity is comparable to native GIP for the GIPR and approximately 5-fold weaker than native GLP-1 for the GLP-1R (Supplemental Table 1). In signaling studies using cell lines with recombinantly expressed GIPR or GLP-1R, LY3298176 potently stimulates cAMP accumulation by either receptor (GIPR EC50 = 0.0224, SEM = 0.0053 nM; GLP-1R EC50 = 0.934, SEM = 0.068 nM) (Figure 1B); the potency of LY3298176 is similar to native GIP and approximately 13-fold weaker than GLP-1 in these assays (Figure 1B and Supplemental Table 1). LY3298176 is less potent than the selective GLP-1RA semaglutide (GLP-1R Ki = 1.97, SEM = 0.47 nM; cAMP EC50 = 0.0571, SEM = 0.0117 nM), and has minimal activity on the closely related glucagon receptor (Supplemental Table 1). To assess signaling in cells expressing endogenous levels of these receptors, we utilised a human pancreatic beta-cell line (ECN90) that expresses both receptors, and responds to either GIP or GLP-1 with similar cAMP increases (Figure 1C); and cultures of differentiated human adipocytes that express only the GIPR, and respond to only GIP (Figure 1D). LY3298176 elicited a cAMP response in ECN90 cells that was significantly higher than that observed for either GLP-1 or GIP alone (Figure 1C), while cAMP accumulation in human adipocytes was comparable to GIP alone (Figure 1D). The effects of LY3298176 on beta-cell function and glucose control were examined using wild-type (WT) mice that express both incretin receptors, and transgenic mice lacking either the GIPR (GIPR−/−) or the GLP-1R (GLP-1R−/−). LY3298176 stimulated glucose-dependent insulin secretion in islets isolated from all three genotypes (Figure 2A–C). In the knockout islets, antagonists specific for the expressed receptor blocked insulin secretion as predicted (exendin-4(9-39) in islets from GIPR−/− mice and a modified GIPR antagonist, GIP(3-30)NH2 [45], in islets from GLP-1PR−/− mice). This demonstrates specificity of LY3298176 for the GIPR and GLP-1R, and activity at both incretin receptors. Glycaemic control in vivo was assessed using intraperitoneal glucose tolerance tests (ipGTT) in normal and receptor-deficient mice. LY3298176 improved the glucose excursions in all three genotypes (Figure 2D–F), and the response was comparable to that observed for semaglutide (Figure 2E) and the DPP4-resistant GIP analogue [d-Ala2]GIP (Figure 2F) in GIPR−/− and GLP-1R−/− mice, respectively. To further assess the specific pharmacodynamic effect of LY3298176 at the GIPR, additional ipGTT experiments were performed in wild-type mice that were co-administered the GLP-1R antagonist Jant-4 [46]. Here, GLP-1R blockade abolished glucose lowering by semaglutide but only marginally affected the efficacy of LY3298176 (Supplemental Fig. 1). Together, these results indicate that LY3298176 can induce glucose-dependent insulin secretion in vitro and in vivo through either the GIPR or the GLP-1R.
Figure 2.
LY3298176 Enhances Islet Insulin Secretion and Improves Glucose Tolerance in Mice. Glucose-stimulated insulin secretion by GIP, GLP-1, or LY3298176 in isolated islets from wild-type (A), GIPR null (B), and GLP-1R null mice (C). Antagonists to GLP-1R (exendin-4(9-39)) and GIPR (modified GIP(3-30)NH2) were used to assess receptor specificity. P < 0.05 using one-way ANOVA versus vehicle treatment in high glucose (*). Glucose excursions from intraperitoneal glucose tolerance tests in wild-type (D), GIPR null (E), and GLP-1R null (F) mice administered LY3298176 or the selective agonists semaglutide or [d-Ala2]GIP. Glucose was administered 18 h after a single injection of LY3298176 or semaglutide and 1 h following [d-Ala2]GIP; glucose AUC(0–120 min, mg. min/dL) is depicted in the upper right panels of (D, E, and F). Data are presented as Mean ± SEM of 6 mice per group. P < 0.05 using one-way ANOVA versus vehicle (*).
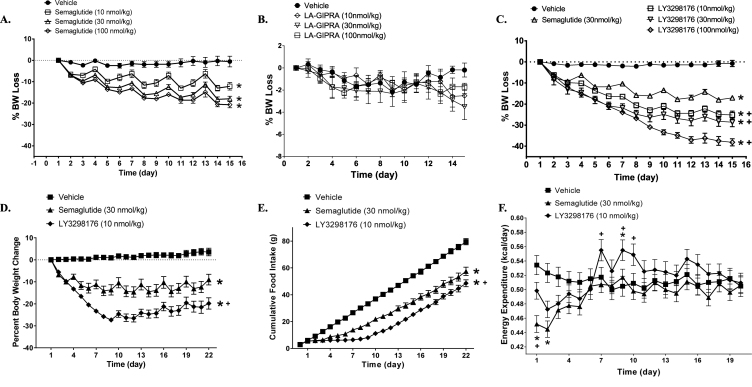
The effects of LY3298176 on body weight and food intake were examined in a DIO mouse model. Pharmacokinetic studies in mice and rats demonstrated that LY3298176 is a long-acting dual GIP and GLP-1 receptor agonist (data not shown). Chronic treatment of DIO mice with semaglutide caused a dose-related loss of body weight primarily due to reduced fat mass (Supplemental Fig. 2A), which appeared to reach its maximum effect at the 30 nmoL/kg dose (Figure 3A); only a minor decrease in plasma glucose was observed (Supplemental 2 B). In comparison, administration of a LA-GIPRA had no effect on body weight or composition (Figure 3B and Supplemental Fig. 2C) but was efficacious at lowering fasting blood glucose concentrations with all doses tested (Supplemental Fig. 2D). Chronic treatment with LY3298176 resulted in a significant dose-dependent decrease in body weight that was more pronounced than that observed with semaglutide (Figure 3CandD). Weight loss with LY3298176 treatment was statistically significant compared to semaglutide treatment and was primarily driven by loss of fat mass (Supplemental Fig. 2E). It appears that LY3298176 mainly produced a larger and more prolonged reduction in food intake during the first 7–10 days of dosing (Figure 3E), resulting in more fat oxidation (Supplemental Fig. 3A). In addition, a slight but significant increase in energy expenditure that started after 7 days of treatment was noted in animals administered LY3298176 but not in mice treated with semaglutide; this increase may contribute to the maintenance of lower body weight at later time points (Figure 3F).
Figure 3.
LY3298176 Lowers Body Weight in Obese Mice. The starting body weight of DIO mice was around 45 g. Body weight loss in DIO mice chronically administered semaglutide (A), LA-GIPRA (B), or LY3298176 (C). Metabolic effects of LY3298176 (♦) or semaglutide (▲) compared to vehicle treatment (■) on body weight (D), cumulative food intake (E), and energy expenditure (F) in DIO mice. For body weight and food consumption differences, P < 0.05 using one-way ANOVA repeated measures versus the vehicle (*) or semaglutide (+) treated groups. For energy expenditure, data presented are least square means for each treatment over time, which were adjusted for body weight as indicated in the statistical model as described in the methods. P < 0.05 using ANCOVA versus the vehicle (*) or semaglutide (+) treated groups. All data are expressed as mean ± SEM.
Overall, the preclinical data demonstrate that LY3298176 is a potent dual GIP and GLP-1 receptor agonist that activates both receptors in vitro and in vivo and provides better weight loss compared to selective GLP-1RA therapy in mice. Based on these results, LY3298176 was advanced into clinical testing.
LY3298176 in Healthy Subjects and Patients with T2DM
3.2.1. Study subjects
A total of 146 subjects were enrolled (4 subjects discontinued before receiving the study drug) and 142 subjects received at least 1 dose of LY3298176, placebo, or dulaglutide (SAD part, N = 56; MAD part, N = 33, POC part, N = 53) (Supplemental Fig. 3) Baseline demographics and clinical characteristics are described in Table 1.
Table 1.
Baseline Demographics and Clinical Characteristics.
| SAD N = 56 |
MAD N = 33 |
POC N = 53 |
|
|---|---|---|---|
| Baseline Demographics | |||
| Age, years | 39.4 ± 10.3 | 40.3 ± 10.9 | 56.8 ± 6.9 |
| Sex | |||
| Men | 53 (94.6) | 33 (100.0) | 28 (52.8) |
| Women | 3 (5.4) | 0 (0.0) | 25 (47.2) |
| Race | |||
| American Indian or Alaska Native | 0 (0.0) | 0 (0.0) | 1 (1.9) |
| Asian | 55 (98.2) | 32 (97.0) | 7 (13.2) |
| Black or African American | 0 (0.0) | 0 (0.0) | 4 (7.5) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 1 (1.8) | 1 (3.0) | 41 (77.4) |
| Clinical Characteristics | |||
| Body weight, kg | 71.9 ± 11.1 | 71.1 ± 9.4 | 86.0 ± 15.9 |
| Body mass index, kg/m2 | 24.7 ± 3.2 | 24.3 ± 2.7 | 31.2 ± 4.0 |
| HbA1c, % | N/A | N/A | 8.4 ± 0.8 |
| HbA1c, mmol/mol | N/A | N/A | 68.30 ± 8.74 |
| Fasting glucose, mg/dL | 86.40 ± 7.20 | 83.34 ± 7.02 | 184.50 ± 42.48 |
| Fasting glucose mmol/L | 4.8 ± 0.4 | 4.63 ± 0.39 | 10.25 ± 2.36 |
| Systolic blood pressure, mmHg | 113.0 ± 12.1 | 113.9 ± 13.6 | 124.4 ± 17.8 |
| Diastolic blood pressure, mmHg | 69.1 ± 9.9 | 68.6 ± 10.1 | 76.3 ± 6.4 |
| Pulse rate, bpm | 55.8 ± 7.1 | 55.8 ± 7.8 | 70.6 ± 9.3 |
Data presented as n (%) and mean ± SD. All data presented at baseline, except HbA1c (Screening). N/A = not available.
3.2.2. PK of LY3298176
The maximum LY3298176 concentration observed occurred within 24–48 h after dosing. The mean half-life was approximately 5 days (116.7 h), thus supporting a once-weekly dosing regimen (Table 2). The mean steady-state apparent clearance and volume of distribution were 0.056 L/h and 9.5 L, respectively. The pharmacokinetics of LY3298176 appeared dose-proportional over the dose range studied. The intersubject variability for Cmax and AUC (AUC0-inf for SAD, AUC0-τ for MAD) was ≤30%. The average accumulation following four weekly doses was estimated to be 1.58. PK parameters in patients with T2DM appeared comparable to corresponding parameters from healthy subjects (Supplemental Table 2).
3.2.3. Glycaemic control
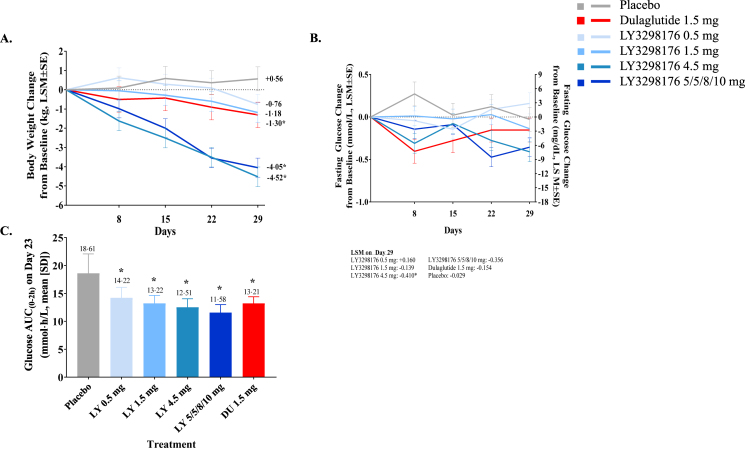
Healthy subjects treated in the MAD study had significant reductions in fasting glucose with LY3298176 4.5 mg compared to placebo on Day 29; fasting insulin did not differ significantly among the LY3298176 doses and placebo (Figure 4B and Supplemental Table 3). A significant decrease in glucose response, measured by OGTT AUC(0–2 h), was observed across all LY3298176 doses, and dulaglutide 1.5 mg, compared with placebo on Day 23 (Figure 4C). Insulin AUC(0–2 h) during the OGTT, did not differ between any LY3298176 dose and dulaglutide compared with placebo (Supplemental Table 3). The 7-point SMBG profiles are presented in Supplemental Fig. 6.
Figure 4.
Body Weight Change and Glucose Response to a OGTT in Healthy Subjects (MAD Part) *Statistically significant at a 5% confidence level compared to placebo. (A) Change from baseline in body weight over time. Data presented as LSM (SE) (B) Treatment differences in body weight on Day 29. Data presented as LSM difference (95% CI) for LY3298176 versus placebo (C) Glucose AUC(0–2 h) on Day 23. Data presented as mean ± SD. Subjects in the 5/5/8/10 mg group received 5 mg LY3298176 on Day 1 and Day 8, 8 mg LY3298176 on Day 15, and 10 mg LY3298176 on Day 22. DU = dulaglutide; LY = LY3298176.
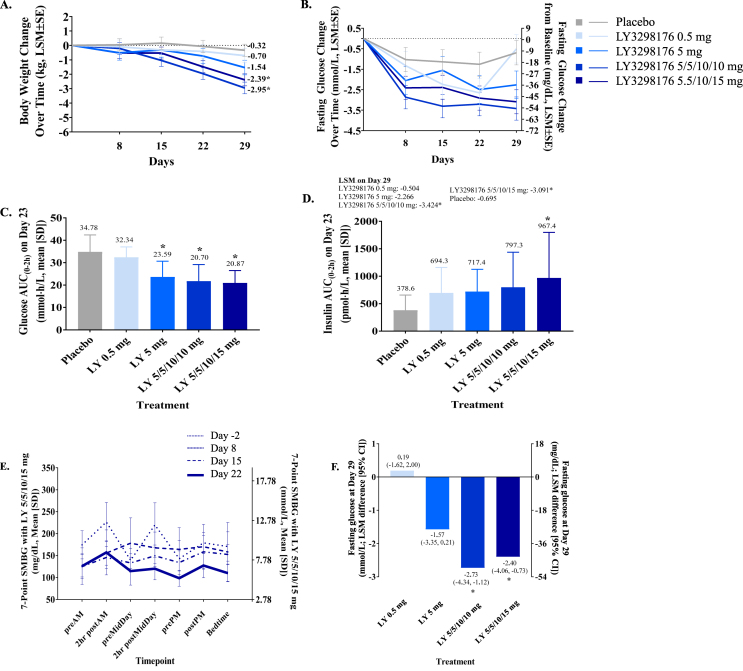
In patients with T2DM treated with LY3298176 in the Phase 1 b POC study, HbA1c was decrease in a dose-dependent manner from baseline compared to placebo, with significant treatment differences observed in the 5/5/10/10 mg and 5/5/10/15 mg titration groups on Day 29 (LSM differences [95% CI]: −0.84% [−1.17, −0.52] and −0.58% [−0.92, −0.24], respectively) (Supplemental Table 3). Fasting glucose and fasting serum insulin were significantly decreased in subjects treated with the 5/5/10/10 mg and 5/5/10/15 mg titration doses compared to placebo on Day 23 (Figure 5B, Figure 5F and Supplemental Table 2). The glucose response during the OGTT, summed as AUC(0–2 h), was significantly decreased with all LY3298176 doses except 0.5 mg compared with placebo (Figure 5C). The insulin response during the OGTT, as AUC(0–2 h), significantly increased with LY3298176 in the 5/5/10/15 mg compared to placebo (Figure 5D). Consistent with these findings, the 7-point SMBG showed dose-dependent reduction of postprandial glucose levels following weekly administration in patients with T2DM (Figure 5E and Supplemental Fig. 5).
Figure 5.
Body Weight Change, Glucose and Insulin Response to a OGTT and Fasting Glucose Response in Patients with T2DM (Phase 1 b POC Part) *Statistically significant at 5% significance level compared to placebo (A) Change from baseline in body weight over time. Data presented as LSM (SE) (B) Treatment differences in body weight on Day 29. Data presented as LSM difference (95% CI) for LY3298176 versus placebo (C) Glucose AUC(0–2 h) on Day 23. Data presented as mean ± SD (D) Insulin AUC(0–2 h) on Day 23. Data presented as arithmetic mean ± SD (E) 7-Point SMBG on Days −2 (baseline), 8 (2nd dose), 15 (3rd dose) and 22 (4 t h dose). Data presented as mean ± SD (F) Treatment differences in fasting glucose on Day 29. Data presented as LSM difference (95% CI) for LY3298176 versus placebo. Subjects in the 5/5/10/10 mg group received 5 mg LY3298176 on Day 1 and Day 8, and 10 mg LY3298176 on Day 15 and Day 22. Subjects in the 5/5/10/15 mg group received 5 mg LY3298176 on Day 1 and Day 8, 10 mg LY3298176 on Day 15, and 15 mg LY3298176 on Day 22. LY = LY3298176.
3.2.4. Body weight
In healthy subjects (MAD part), a dose- and time-dependent decrease in body weight from baseline was observed (Figure 4A). The decrease in body weight was statistically significant for all treatment groups compared with placebo except the 0.5 mg group. The weight loss was greatest with LY3298176 4.5 mg and 5/5/8/10 mg titration groups (−4.52 and −4.05 kg change form baseline). This weight loss was greater than what was observed for dulaglutide 1.5 mg (−1.3 kg change from baseline) on Day 29. The differences were statistically significant for the two LY3298176 dose groups (4.5 mg and 5/5/8/10 mg) compared with dulaglutide (Supplemental Fig. 4A). In patients with T2DM (Phase 1b POC part), a dose- and time-dependent decrease in body weight from baseline to Day 29 with a smaller effect size compared to healthy subjects (MAD part) was also observed (Figure 5A and Supplemental Fig. 4B).
3.2.5. Safety profile
No deaths occurred during the study. One SAE occurred in a dulaglutide-treated subject (Supplemental Table 4). Five subjects discontinued due to AEs (decreased appetite, vomiting, supraventricular tachycardia, diarrhoea, and increased pancreatic enzymes (Supplemental Fig. 3). Gastrointestinal AEs (nausea, vomiting, diarrhoea, decreased appetite, abdominal distension) were the most frequently reported events by both healthy subjects and patients with T2DM (Table 3 and Supplemental Table 4). All events were mild to moderate in severity. In the SAD study, decreased appetite was the most frequently reported gastrointestinal AE. The high incidence of gastrointestinal AEs, notably vomiting, was considered to be dose-limiting at the 8 mg dose, therefore the 5 mg dose was considered as the single maximum tolerated dose. Higher dose levels could be achieved with titration in both healthy subjects (MAD part) and patients with T2DM (Phase 1b POC part). The gastrointestinal AEs (nausea, vomiting, diarrhoea and decreased appetite) were still the most frequently reported events and were dose-limiting because of the limited titration time. Overall, patients with T2DM presented with less gastrointestinal AEs than healthy subjects, and tolerated higher doses. Higher dose levels were better tolerated using a dose titration scheme of administration and could extensively increase exposure compared to the single-ascending dose study.
Table 3.
Adverse events in Patients with T2DM.
| LY3298176 0.5 mg N = 9 |
LY3298176 5 mg N = 9 |
LY3298176 5/5/10/10 mg N = 12 |
LY3298176 5/5/10/15 mg N = 12 |
Placebo N = 11 |
All N = 53 |
|
|---|---|---|---|---|---|---|
| Any TEAE | 5 (55.6) | 7 (77.8) | 10 (83.3) | 11 (91.7) | 3 (27.3) | 36 (67.9) |
| Mild, events | 15 | 17 | 54 | 87 | 6 | 179 |
| Moderate, events | 0 | 0 | 1 | 9 | 0 | 10 |
| Severe, events | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious adverse events | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Study discontinuation due to adverse events | 0 (0.0) | 3 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any TEAEs in order of frequency | ||||||
| Vomiting | 1 (11.1) | 0 (0.0) | 1 (8.3) | 9 (75.0) | 0 (0.0) | 11 (20.8) |
| Decreased appetite | 2 (22.2) | 6 (66.7) | 5 (41.7) | 11 (91.7) | 1 (9.1) | 25 (47.2) |
| Diarrhoea | 1 (11.1) | 1 (11.1) | 3 (25.0) | 5 (41.7) | 1 (9.1) | 11 (20.8) |
| Abdominal distension | 0 (0.0) | 1 (11.1) | 2 (16.7) | 7 (58.3) | 0 (0.0) | 10 (18.9) |
| Nausea | 1 (11.1) | 1 (11.1) | 1 (8.3) | 6 (50.0) | 0 (0.0) | 9 (17.0) |
| Gastrooesophageal reflux disease | 0 (0.0) | 1 (11.1) | 1 (8.3) | 5 (41.7) | 0 (0.0) | 7 (13.2) |
| Eructation | 0 (0.0) | 0 (0.0) | 1 (8.3) | 4 (33.3) | 0 (0.0) | 5 (9.4) |
| Weight decrease | 0 (0.0) | 0 (0.0) | 2 (16.7) | 2 (16.7) | 0 (0.0) | 4 (7.5) |
| Dyspepsia | 0 (0.0) | 0 (0.0) | 3 (25.0) | 0 (0.0) | 0 (0.0) | 3 (5.7) |
| Headache | 1 (11.1) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 3 (5.7) |
| Abdominal pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 2 (3.8) |
| Dermatitis allergic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 2 (3.8) |
| Hepatic enzyme increased | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (8.3) | 0 (0.0) | 2 (3.8) |
| Pancreatic enzyme increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (9.1) | 2 (3.8) |
| Other adverse events | ||||||
| Total hypoglycaemia (≤70 mg/dL) | 0 (0.0) | 0 (0.0) | 2 (16.7) | 1 (8.3) | 1 (9.1) | 4 (7.5) |
| Severe hypoglycaemia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Acute pancreatitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection site reaction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Data presented as n (%), unless otherwise noted. TEAE = treatment-emergent adverse event.
The incidence of hypoglycaemic episodes (includes documented symptomatic, probable, and asymptomatic events) was low across all treatment groups. There was no case of severe hypoglycaemia (Table 3 and Supplemental Table 4). No clinically relevant changes in systolic or diastolic blood pressure were observed (Supplemental Table 4). An increase in pulse rate was detected for both healthy subjects and patients with T2DM treated with LY3298176 or healthy subjects treated with dulaglutide (Supplemental Table 5). There was no signal of drug-related QT prolongation from the ECG analysis.
Increases from baseline in mean values of lipase and amylase within the normal range were observed with LY3298176 compared to baseline in healthy subjects and patients with T2DM (Supplemental Table 6). There were no AEs of acute pancreatitis reported. There was no apparent evidence of treatment- or dose-related trends in clinical chemistry; however, values outside the reference ranges were noted for some hepatic parameters in a few subjects (Supplemental Table 6).
No events of hypersensitivity were reported. Two healthy subjects (MAD part) reported injection site reactions, which were mild to moderate in severity and resolved without treatment (Supplemental Table 6). No injection site reaction was reported in patients with T2DM (Table 3). Four subjects developed either treatment-emergent or treatment-boosted ADAs; 2 of whom developed ADAs with titres up to 1:160. Those four subjects did not report hypersensitivity events and PK of LY3298176 was within the expected range for their dose levels.
4. Discussion
Based on functional studies in cell culture systems and specific murine gene knockout models, LY3298176 is a dual GIP and GLP-1 receptor agonist. The molecule has greater potency for the GIPR in vitro, but in vivo activity through both incretin pathways is apparent in the glucose lowering by LY3298176 in mice expressing only one or the other receptor. In human subjects with T2DM, LY3298176 had potent effects on both fasting and postprandial glycaemia; the 7-point SMBG profile revealed a nearly flat and well-controlled glucose curve for the highest LY3298176 dose after only four weeks of treatment. Moreover, the significant insulin response seen with LY3298176 during an OGTT speaks to a robust incretin effect. Comparison of these glycaemic results with data from Phase 1 studies of selective GLP-1RAs [47], [48], [49] raises the possibility of additional effects by GIP to overall efficacy. These findings suggest that the combined incretin action of LY3298176 is not subject to the attenuation of GIP-stimulated insulin secretion typically seen in T2DM, and support enhanced glucose-lowering when both the GIPR and GLP-1R are activated [50], [51].
An important finding of these studies is that obese mice administered LY3298176 lost more weight than animals treated with a selective GLP-1RA. There is limited information on the consequences of selective GIPR activation, but available data as well as our own study presented here suggest that chronic administration of a long-acting GIPRA does not reduce body weight [52]. It therefore appears that GIP activation acts synergistically with GLP-1 receptor activation to allow greater weight loss in mice than what can be achieved with single GLP-1 receptor agonism. This observed weight loss appears to be due to both enhanced suppression of calorie intake and a slight but significant increase in energy expenditure. Although additional mechanistic studies are required to fully understand the effects of LY3298176 on energy balance, it seems likely that the synergistic actions of the GIP and GLP-1 receptors occurs at the level of the CNS [32]. The data in this report are consistent with previous findings from combination and dual agonist studies, which show the interaction of GLP-1RA and GIPRA yields better weight control [31], [35]. Our results in obese mice offer further support that the addition of GIPR agonism to GLP-1 enhances weight loss via modulating energy metabolism, an effect that has not been previously reported.
The findings in mice were echoed in the human studies, in which the effects of higher doses of LY3298178 caused greater weight loss than the selective GLP-1RA dulaglutide. In patients with T2DM, LY3298176 demonstrated meaningful weight loss after only four weeks of treatment. This was consistent with subject reports of decreased appetite during treatment with the dual agonist. Although several factors likely contribute, the beneficial effects of LY3298176 may in part be attributed to its greater potency at the GIPR versus GLP-1R. While further investigation is needed to determine the optimal properties of a dual agonist, this profile differentiates LY3298176 from another dual GIP-GLP-1 receptor agonist, NNC0090-2746, that displays balanced activity at the receptors [31], [35]. Other functional dual agonists, which combine a GLP-1RA with glucagon activity, have recently reported four week data on weight loss; in comparison with these previous studies, LY3298176 appears to provide similar (SAR425899) or greater weight loss (MEDI0384) than these compounds [53], [54].
The safety characteristics of LY3298176 were similar to selective GLP-1RAs. The most frequently reported AEs were gastrointestinal (nausea, vomiting, and diarrhoea) that were of mild to moderate in severity. Gastrointestinal AEs with LY3298176 were dose limiting, similar to what has been universally observe with other drugs with potent GLP-1RA activity. However, titration decreased gastrointestinal AEs, and the simple titration regimens tested in these studies helped subjects tolerate higher doses (up to 15 mg) during the MAD Phase 1 b PoC and Phase 2 b studies [38]. It is important to note that our titration paradigm was not optimised, and slower titration with smaller steps may enable an even more favorable tolerability profile. Similar to effects seen with GLP-1RAs, increases in mean concentrations of lipase and amylase with the dual agonist were observed. The frequency of reporting of hypoglycaemia, hypersensitivity events, and injection site reactions was low across the treatment groups, and we did not observe important differences between LY3298176 and placebo or dulaglutide. Although increased pulse rate is a known effect of GLP-1RA therapy, there was not a consistent dose effect and the maximum increase observed with LY3298176 was similar to dulaglutide.
Typical limitations of Phase 1 studies include short duration and a small sample size, and both are applicable here. While the observed effects on weight and glycaemic efficacy for a 4 week trial are significant, there is still uncertainty in the effect size of these important parameters. It is worth noting that the ethnic background of HS and T2DM differed significantly, and although ethnicity has not had a demonstrated impact on other incretin based drugs, this will need to be verified in larger studies. Although an active comparator aided in the interpretation of this study, an active comparator was studied only in the healthy subject cohort to more fully evaluate tolerability. Larger studies with longer treatment duration are warranted to evaluate the full safety and efficacy of this novel dual agonist. In addition, a dedicated titration study will evaluate whether and by how much the gastrointestinal AE profile can be improved via titration (NCT03131687). The improved insulin secretion with LY3298176 is consistent with a strong incretin effect, however, the trial design did not enable discerning the contribution of GIP versus GLP-1.
5. Conclusion
In summary, we report that LY3298176 is a dual GIP and GLP-1 receptor agonist. The PK profile enables once weekly dosing in humans. The large effect size of glycaemic efficacy in T2DM and the large effect size of weight loss shown in healthy subjects and patients with T2DM within only 4 weeks of treatment are promising. On the basis of these results, combined GLP-1R and GIPR stimulation with LY3298176 appears to offer the potential of improved effectiveness compared to selective GLP-1RA therapy, raising the possibility that it will be an effective treatment for patients with T2DM. The results of this trial provide support for a thorough evaluation of LY3298176 in a more definitive Phase 2 program.
Author contributions
J.A-F. was responsible for molecule design. T.C. was responsible for design, data collection and analysis in DIO mice studies. D.A.B. was responsible for data collection and analysis in preclinical ipGTT studies. O.C. was responsible for data collection and analysis in islet studies. W.C.R. was responsible for data collection in preclinical adipose studies. J.S.M. and K.W.S. were responsible for data collection and analysis of in vitro characterization studies. X.C. was responsible for the statistical considerations in the analysis. C.L. and S.U. were responsible for data analysis and interpretation. R.E.G., T.C. and A.H. are the guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in critical review and interpretation of the data for the manuscript. All authors had full access to the data related to these studies and approved the final version submitted for publication. A portion of the preclinical data was presented at the 53rd Annual Meeting of the European Association for the Study of Diabetes in Lisbon, Portugal, held September 11–15, 2017.
Data sharing statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request in a timely fashion after the indication studied has been approved in the US and EU and after primary publication acceptance. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
Acknowledgments
The authors would like to thank principal investigator Edward John Pratt, MD (Lilly-NUS Centre for Clinical Pharmacology, Singapore) and study investigator Martha Hernandez-Illas MD (QPS-MRA) along with site staff, and trial participants and their families. We thank Zvonko Milicevic, MD for reviewing the studies, Yongming Qu, PhD for the statistical review, Parag Garhyan, PhD, for pharmacokinetics review, Melissa Thomas, MD, PhD for discussions on biomarkers, and Chrisanthi Karanikas, MS and Oralee Varnado, PhD for writing and editorial support (all from Eli Lilly and Company). We also thank Jude Onyia, PhD (Eli Lilly and Company) for scientific discussions and continued support throughout the course of this project. Technical support was provided by Robert Cummins, BS, Hongchang Qu, PhD, Libbey O'Farrell, BS, Meghan Hayes, MS, Alexis Bennett, MS, James Ficorilli, BS, Jennifer Martin, MS, Aaron Showalter, MS, Xiaoping Ruan, BS, Ajit Regmi, MS, Wenzhen Ma, MA, Alexander Efanov, PhD, Charity Zink, BS, Brad Wainscott, MS, Francis Willard, PhD, Steve Kahl, MS, and Hui-Rong Qian, PhD (all from Eli Lilly and Company). Funding was provided by Eli Lilly and Company.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.09.009.
Declaration of interest
T.C., K.W.S, C.L., J. A-F, S.U., X.C. D.A.B., O.C., W.C.R., J.S.M., C.T.B., R.E.G., and A.H. are employees and shareholders of Eli Lilly and Company. K.B.B. and U.K. are former employees of Eli Lilly and Company. D.A.D. is a member of the Eli Lilly Scientific Advisory Board.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Andersen A. Glucagon-like peptide 1 in health and disease. Nature Reviews Endocrinology. 2018;14(7):390–403. doi: 10.1038/s41574-018-0016-2. [DOI] [PubMed] [Google Scholar]
- 2.Richards P. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holst J.J. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Letters. 1987;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- 4.Kreymann B. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 5.Imeryuz N. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. American Journal of Physiology. 1997;273(4 Pt 1):G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 6.Nauck M.A. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. American Journal of Physiology. 1997;273(5 Pt 1):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 7.Schirra J. Mechanisms of the antidiabetic action of subcutaneous glucagon-like peptide-1(7-36)amide in non-insulin dependent diabetes mellitus. Journal of Endocrinology. 1998;156(1):177–186. doi: 10.1677/joe.0.1560177. [DOI] [PubMed] [Google Scholar]
- 8.Turton M.D. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 9.Holz G.G. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37) Journal of Biological Chemistry. 1999;274:14147–14156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang G., Chepurny O.G., Holz G.G. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. Journal of Physiology. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilsboll T. No reactive hypoglycaemia in Type 2 diabetic patients after subcutaneous administration of GLP-1 and intravenous glucose. Diabetic Medicine. 2001;18(2):144–149. doi: 10.1046/j.1464-5491.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 12.English W.J., Williams D.B. Metabolic and bariatric surgery: an effective treatment option for obesity and cardiovascular disease. Progress in Cardiovascular Diseases. 2018 doi: 10.1016/j.pcad.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metabolism. 2018;27(4):740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Nauck M.A., Meier J.J. Incretin hormones: their role in health and disease. Diabetes, Obesity and Metabolism. 2018;20(Suppl 1):5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 15.Vollmer K. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57(3):678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 16.Dupre J. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. The Journal of Cinical Endocrinology and Metabolism. 1973;37:826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 17.Meier J.J. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46(6):798–801. doi: 10.1007/s00125-003-1103-y. [DOI] [PubMed] [Google Scholar]
- 18.Christensen M.B. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. The Journal of Cinical Endocrinology and Metabolism. 2014;99(3):E418–E426. doi: 10.1210/jc.2013-3644. [DOI] [PubMed] [Google Scholar]
- 19.Christensen M. Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes. Diabetes. 2015;64(1):72–78. doi: 10.2337/db14-0440. [DOI] [PubMed] [Google Scholar]
- 20.Christensen M. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pederson R.A., Brown J.C. Interaction of gastric inhibitory polypeptide, glucose, and arginine on insulin and glucagon secretion from the perfused rat pancreas. Endocrinology. 1978;103:610–615. doi: 10.1210/endo-103-2-610. [DOI] [PubMed] [Google Scholar]
- 22.Yip R.G. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139(9):4004–4007. doi: 10.1210/endo.139.9.6288. [DOI] [PubMed] [Google Scholar]
- 23.Nyberg J. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. Journal of Neuroscience Research. 2007;85(10):2099–2119. doi: 10.1002/jnr.21349. [DOI] [PubMed] [Google Scholar]
- 24.Song D.H. Glucose-dependent insulinotropic polypeptide enhances adipocyte development and glucose uptake in part through Akt activation. Gastroenterology. 2007;133(6):1796–1805. doi: 10.1053/j.gastro.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Getty-Kaushik L. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity. 2006;14(7):1124–1131. doi: 10.1038/oby.2006.129. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.J., Nian C., McIntosh C.H. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes. A role for a protein kinase B, LKB1, and AMP-activated protein kinase cascade. Journal of Biological Chemistry. 2007;282(12):8557–8567. doi: 10.1074/jbc.M609088200. [DOI] [PubMed] [Google Scholar]
- 27.Miyawaki K. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nature Medicine. 2002;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 28.Eckel R.H., Fujimoto W.Y., Brunzell J.D. Gastric inhibitory polypeptide enhanced lipoprotein lipase activity in cultured preadipocytes. Diabetes. 1979;28(12):1141–1142. doi: 10.2337/diab.28.12.1141. [DOI] [PubMed] [Google Scholar]
- 29.Asmar M. Glucose-dependent insulinotropic polypeptide increases blood flow in adipose tissue of humans by recruiting capillaries. The Journal of Cinical Endocrinology and Metabolism. 2018 doi: 10.1210/jc.2018-00389. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Kim S.J. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finan B. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science Translational Medicine. 2013;5(209):209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 32.NamKoong C. Central administration of GLP-1 and GIP decreases feeding in mice. Biochemical and Biophysical Research Communications. 2017;490(2):247–252. doi: 10.1016/j.bbrc.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Hojberg P.V. Near normalisation of blood glucose improves the potentiating effect of GLP-1 on glucose-induced insulin secretion in patients with type 2 diabetes. Diabetologia. 2008;51(4):632–640. doi: 10.1007/s00125-008-0943-x. [DOI] [PubMed] [Google Scholar]
- 34.Hojberg P.V. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 35.Frias J.P. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metabolism. 2017;26(2):343–352.e2. doi: 10.1016/j.cmet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Portron A. Pharmacodynamics, pharmacokinetics, safety and tolerability of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 after single subcutaneous administration in healthy subjects. Diabetes, Obesity and Metabolism. 2017;19(10):1446–1453. doi: 10.1111/dom.13025. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt C. Pharmacodynamics, pharmacokinetics and safety of multiple ascending doses of the novel dual glucose-dependent insulinotropic polypeptide/glucagon-like peptide-1 agonist RG7697 in people with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 2017;19(10):1436–1445. doi: 10.1111/dom.13024. [DOI] [PubMed] [Google Scholar]
- 38.Frias J.P. Efficacy and safety of LY3298176, a novel GIP and GLP-1 receptor dual agonist in patients with type 2 diabetes: a 26-week, randomised, placebo- and active comparator-controlled trial. The Lancet. 2018 doi: 10.1016/S0140-6736(18)32260-8. [in press] [DOI] [PubMed] [Google Scholar]
- 39.Lau J. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. Journal of Medicinal Chemistry. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 40.Hinke S.A. Dipeptidyl peptidase IV-resistant [D-Ala2]Glucose-Dependent insulinotropic polypeptide (GIP) improves glucose tolerance in normal and obese diabetic rats. Diabetes. 2002;51(3):652–661. doi: 10.2337/diabetes.51.3.652. [DOI] [PubMed] [Google Scholar]
- 41.Auld D.S. Receptor binding assays for HTS and drug discovery. In: Sittampalam G.S., editor. Assay guidance manual. 2004. Bethesda (MD) [Google Scholar]
- 42.Bueno A.B. Positive allosteric modulation of the glucagon-like peptide-1 receptor by diverse electrophiles. Journal of Biological Chemistry. 2016;291(20):10700–10715. doi: 10.1074/jbc.M115.696039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tschop M.H. A guide to analysis of mouse energy metabolism. Nature Methods. 2011;9(1):57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speakman J.R., Fletcher Q., Vaanholt L. The '39 steps': an algorithm for performing statistical analysis of data on energy intake and expenditure. Dis Model Mech. 2013;6(2):293–301. doi: 10.1242/dmm.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen L.S. N-terminally and C-terminally truncated forms of glucose-dependent insulinotropic polypeptide are high-affinity competitive antagonists of the human GIP receptor. British Journal of Pharmacology. 2016;173(5):826–838. doi: 10.1111/bph.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patterson J.T. A novel human-based receptor antagonist of sustained action reveals body weight control by endogenous GLP-1. ACS Chemical Biology. 2011;6(2):135–145. doi: 10.1021/cb1002015. [DOI] [PubMed] [Google Scholar]
- 47.Barrington P. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2011;13(5):426–433. doi: 10.1111/j.1463-1326.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 48.Barrington P. LY2189265, a long-acting glucagon-like peptide-1 analogue, showed a dose-dependent effect on insulin secretion in healthy subjects. Diabetes, Obesity and Metabolism. 2011;13(5):434–438. doi: 10.1111/j.1463-1326.2011.01365.x. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt W.E. Early clinical studies with liraglutide. Int J Clin Pract Suppl. 2010;(167):12–20. doi: 10.1111/j.1742-1241.2010.02500.x. [DOI] [PubMed] [Google Scholar]
- 50.Asmar M. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes. 2010;59(9):2160–2163. doi: 10.2337/db10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finan B. Reappraisal of GIP pharmacology for metabolic diseases. Trends in Molecular Medicine. 2016;22(5):359–376. doi: 10.1016/j.molmed.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Norregaard P.K. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes, Obesity and Metabolism. 2018;20(1):60–68. doi: 10.1111/dom.13034. [DOI] [PubMed] [Google Scholar]
- 53.Lindauer K. Early clinical development of the dual GLP-1/glucagon receptor agonist SAR425899 and establishment of a population PK/PD model. Diabetologia. 2016;59(Suppl 1):1–581. [Google Scholar]
- 54.Ambery P. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. The Lancet. 2018;391(10140):2607–2618. doi: 10.1016/S0140-6736(18)30726-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.