Abstract
Background/Aim
Many studies have investigated risk factors other than antibiotic resistance linked to Helicobacter pylori (H. pylori) eradication failure. The aim of this study was to study the effect of serum levels of 25‐hydroxy‐vitamin D (25[OH]D) on eradication rates of H. pylori infection.
Methods
This study included 150 patients diagnosed with H. pylori gastritis using magnifying narrow‐band imaging endoscopy supported by stool antigen test. Serum 25‐OH vitamin D levels were measured via the Enzyme‐Linked Immune Sorbent assay (ELISA) method before starting eradication therapy of H. pylori infection. All patients were treated with clarithromycin‐based triple therapy for 14 days. H. pylori eradication was determined via a stool antigen test performed 4 weeks after the end of therapy. According to the serum level of 25‐OH vitamin D levels, the patients were divided into two groups: group I (sufficient) had a vitamin D level of ≥20 ng/mL, while group II (deficient) had a vitamin D level of <20 ng/mL.
Results
Our results revealed that eradication was successful in 105 (70%) patients and failed in 45 (30%) patients. The mean 25[OH]D level was significantly lower in the eradication failure group compared to the successful treatment group (14.7 ± 4.5 vs 27.41 ± 7.1; P < 0.001). Furthermore, there were significantly more patients with deficient 25[OH]D levels in the failed treatment group, 30 (66.6%), compared to the successful group, 10 (9.5%) (P < 0.001).
Conclusions
Our results demonstrated that 25‐OH vitamin D deficiency may be considered a risk factor related to eradication failure of H. pylori infection. In addition, a further randomized trial to evaluate the effect of vitamin D supplementation in H. pylori eradication is mandatory.
Keywords: Helicobacter pylori eradication, magnifying narrow band, vitamin D
Introduction
Infection by Helicobacter pylori has been established as a major cause of chronic gastritis. It affects 50% of the world's population, and it is important in the pathogenesis of other gastrointestinal diseases such as peptic ulcer disease, gastric adenocarcinoma, and gastric lymphoma.1 Up to 76–95% of gastric cancers and 90% of duodenal ulcers are associated with H. pylori infection.2 The National Institute of Health Consensus Development Conference concluded that patients with H. pylori infection should receive antimicrobial therapy as the risk of ulcer recurrence and associated complications do not diminish unless H. pylori infection is cured.3 Although triple therapy with a proton pump inhibitor (PPI), clarithromycin, and amoxicillin or metronidazole has been used as the first‐line treatment for H. pylori infections, the American College of Gastroenterology suggested that the cure rates were 70–85% in 2007.4 In addition, recent systematic review showed that the cure rates of sequential and standard triple therapy were 84.1 and 75.1%, respectively.5The most important factor affecting H. pylori cure rates is the antibiotic resistance of H. pylori strains. The number of H. pylori strains that are resistant to antibiotics is increasing.6 In addition, the successful treatment of H. pylori infection depends on host genetic factors such as cytochrome P450 2C19 (CYP2C19), interleukin 1B (IL‐1B), and multidrug‐resistant transporter‐1 (MDR1). 7 Host immunity also plays an important role against an infectious disease such as H. pylori infection. Vitamin D is an important secosteroid hormone with pleiotropic effects. While its role in the regulation of calcium and bone homeostasis is well established, recently, there is increasing recognition that vitamin D has immunomodulatory, anti‐inflammatory and antifibrotic properties and plays an important role in the regulation of cell proliferation and differentiation.8 Recent studies have demonstrated that vitamin D regulates the expression of specific endogenous antimicrobial peptides (AMPs) in immune cells; this action leads to a potential role for vitamin D in modulating the immune response to various infectious diseases. In the vitamin D deficiency state, the infected macrophage is unable to produce sufficient 1,25‐(OH)2D to upregulate the production of AMP cathelicidin.9 Although this antimicrobial mechanism of vitamin D has been demonstrated only in macrophages infected with mycobacterium tuberculosis, it is also well known that cathelicidin has broad‐spectrum activity against a wide variety of other pathogens, including Gram‐negative and Gram‐positive bacteria, viruses, and fungi.10 Evidence exists that vitamin D has a potential antimicrobial activity, and its deficiency has deleterious effects on general well‐being and longevity. Vitamin D may reduce the risk of infection through multiple mechanisms; Vitamin D boosts innate immunity by modulating the production of AMPs and cytokine response.11 Moreover, Vitamin D helps in boosting the activity of monocytes and macrophages, thereby contributing to a potent systemic antimicrobial effect. A vitamin D replete state appears to benefit most infections.12
Materials and methods
This study was conducted at gastrointestinal endoscopy unit, Internal Medicine Department of El‐Hussein University Hospital, AL‐Azhar University, Cairo, Egypt, between March 2018 and June 2018. In this study, 150 sequential Egyptian patients with dyspeptic symptoms for at least 1 month were consecutively enrolled. For each patient, full medical history along with complete physical examination was conducted. Informed consent was obtained from all patients. Diagnostic esophagogastroduodenoscopy using magnifying narrow‐band imaging (ME‐NBI) was performed for all patients afterward; if the patient had endoscopic findings suggesting H. pylori chronic gastritis, a stool antigen test for the detection of H. pylori infection was conducted to support endoscopic diagnosis. Serum 25‐OH vitamin D levels were measured using the ELISA method before beginning eradication therapy for the H. pylori infection. According to the serum level of 25‐OH vitamin D levels, the patients were divided into two groups: group I (sufficient) had a vitamin D level of ≥20 ng/mL, while group II (deficient) had a vitamin D level of <20 ng/mL. All patients were treated with clarithromycin‐based triple therapy for 14 days. H. pylori eradication was determined via a stool antigen test performed 4 weeks after the end of therapy.
Inclusion criteria
A total of 150 sequential patients diagnosed with H. pylori chronic gastritis by ME‐NBI along with a positive stool antigen test for H. pylori infection
Age 18–80 years
Provision of written consent
Exclusion criteria
Current Use of PPIs or Vitamin D supplement
Known hypersensitivity to PPI or antibiotics
Patients who had previously received H. pylori eradication treatment, corticosteroids/immunosuppressive treatment, antibiotics, and anti‐inflammatory and acid suppressive treatment in the prior 2 months
A history of systemic inflammatory or autoimmune disorders, gastric surgery, renal failure, liver cirrhosis, and malignancies
Endoscopic evaluation
The instruments used in this study were a videoendoscope and an electronic endoscopic system (Olympus EVIS Lucera Ellit CV‐290; Olympus Medical Systems, Tokyo, Japan) equipped with three imaging modes: high‐resolution white light endoscopy (WLE), autofluorescence imaging (AFI), and narrow‐band imaging (NBI). Mode switch from WLE to NBI or AFI was controlled by buttons on the control head. Combining magnifying endoscopy and the NBI system can lead to visualizing microscopic mucosal structures and their capillary patterns more clearly. All patients were offered conscious sedation with intravenous midazolam (2.5–5 mg) and/or propofol (40–200 mg) and then underwent endoscopic assessment for the presence or absence of regular arrangement of collecting venules (RAC) as well as mucosal and vascular patterns of the gastric body. All of the endoscopic examinations were performed by one senior endoscopist with more than 10 years of experience in diagnostic and therapeutic endoscopy. The entire endoscopic procedure was recorded on a digital video disc (DVD). NBI endoscopic diagnosis was performed on two occasions. In the first occasion, diagnosis was made during real‐time endoscopy, while in the second occasion, it was made on the basis of the pictures and videos obtained, which was then confirmed. Using ME‐NBI, the normal gastric body mucosal pattern was determined by small, round pits surrounded by subepithelial capillary networks creating a honeycombing pit pattern, with the presence of regular arrangement of collecting venules (RAC‐positive) (Fig. 1), while diagnosis of H. pylori chronic gastritis was made according to the presence of an obviously enlarged oval or prolonged pit with increased density of irregular vessels as well as the presence of a well‐demarcated oval or tubulovillous pit with clearly visible coiled or wavy subepithelial capillary networks along with the absence of regular arrangement of collecting venules (RAC‐negative) (Fig. 2).
Figure 1.
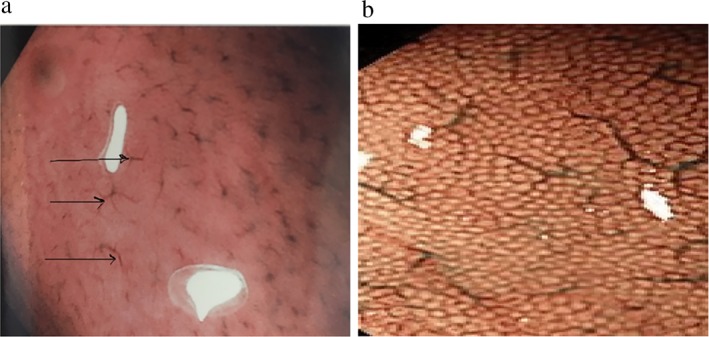
Narrow‐band imaging of normal gastric corpus mucosal and vascular pattern. (a) Regular arrangement of collecting venules (spider‐like pattern) (black arrows); (b) normal: small, round pits surrounded by subepithelial capillary networks (honeycombing like pit pattern).
Figure 2.
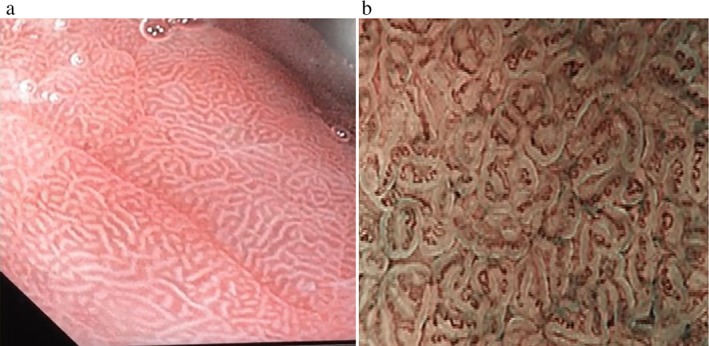
Narrow‐band imaging of H. pylori chronic gastritis. (a) Obviously enlarged oval or prolonged pit. (b) Well‐demarcated oval or tubulovillous pit with clearly visible coiled or wavy subepithelial capillary networks.
Treatment protocol
All patients were treated with clarithromycin‐based triple therapy (clarithromycin 500 mg, amoxicillin 1000 mg, and esomeperazol 20 mg) twice daily for 14 days.
Laboratory measurements
Stool antigen test for H. pylori infection
Small piece of stool samples (~5 mm in diameter; ~150 mg) were added and diluted into 1 mL of sample treatment solution in a test tube and mixed thoroughly. H. pylori infection was detected by specific antigens in the stool sample (fecal antigen test) and by ELISA (commercially available). A purified H. pylori antibody was coated on the surface of microcells. An aliquot of the diluted stool sample was added to wells, and the H. pylori antigens, if present, bind to the antibody. Unbound materials were washed. After adding enzyme conjugate, it binds to the antibody–antigen complex. Excess enzyme conjugate was washed, and TMB Chromogenic substrate was added. The enzyme conjugate catalytic reaction stopped at a specific time, and the color‐generated intensity was proportional to the antigen amount and read by a microwell reader compared to in a parallel manner with calibrator and controls. Interpretation: negative <15 ng/mL, positive >20 ng/mL, borderline: 15–20 ng/mL.
Detection of 25 OH vitamin D by ELISA
The ELISA kit is a solid‐phase enzyme‐linked immunosorbent assay based on the principle of competitive binding. In the first step, samples have to be pretreated in separate vials with a denaturation buffer to extract the analyte as most circulating 25‐OH vitamin D is bound to vitamin D‐binding protein (VDBP) in vivo. After neutralization, biotinylated 25‐OH vitamin D (enzyme conjugate) and peroxidase‐labeled streptavidin (enzyme complex) are added. After careful mixing, the solution is transferred to the walls of the microtiter plate. Endogenous 25‐OH vitamin D of the patient sample competes with a 25‐OH vitamin D3‐biotin conjugate to bind to the VDBG that is immobilized on the plate. Binding of 25‐OH vitamin D‐biotin is detected by peroxidase‐labeled streptavidin. Incubation is followed by a washing step to remove unbound components. The color reaction is started by the addition of enzyme substrate and stopped after a defined time. The color intensity is inversely proportional to the concentration of 25‐OH vitamin D in the sample. Status of 25OH vitamin D was evaluated as follows: vitamin D deficiency was defined as a 25(OH) D < 20 ng/mL and sufficiency as a 25(OH) D ≥ 20 ng/mL.13
Statistical analysis
The data obtained from the history, clinical examination, and investigations were tabulated and statistically analyzed using the GraphPad Prism program version 6. Mean standard deviations (SDs) were used to identify the data related to the continuous variables, and categorical variables were provided as percentages. The comparison of the variables with normal distribution was tested with an unpaired t‐test, and the comparison of the variables without normal distribution was tested with a Mann–Whitney U test. The categorical variables were compared with Pearson's χ 2 test, and a P‐value < 0.05 was considered statistically significant.
Results
The present study included 150 patients diagnosed with H. pylori chronic gastritis by ME‐NBI plus stool antigen test, who were divided into two groups according to the vitamin D level measured before starting eradication therapy for H. pylori infection: Group I, 110 (73.3%) patients with sufficient vitamin D level (≥20 ng/mL), and Group II, 40 (26.6%) patients with deficient vitamin D levels (<20 ng/mL). There was no significant differences between the two groups regarding age or gender distribution (P = 0.747 and P = 0.507, respectively) (Table 1). Our results revealed that H. pylori infection was eradicated successfully in 105 (70%) patients, while in 45 (30%) patients, eradication failed. At the end of therapy, all patients’ compliance with the drug protocol was excellent. According to our results, the mean vitamin 25(OH)D levels were significantly lower in the eradication failure group compared to the successful treatment group 14.7 ± 4.5 versus 27.41 ± 7.1 (P < 0.001). In addition, all patients had an overall 26.6% vitamin 25(OH)D deficiency. We found 30 (66.6%) patients in the failed treatment group and 10 (9.5%) patients in the successful treatment group to be vitamin 25(OH)D‐deficient. Therefore, vitamin 25(OH)D deficiency was significantly higher in the failed treatment group compared to the successful treatment group (P < 0.001) (Table 2 and Fig. 3).
Table 1.
Demographic and laboratory data of Group I (sufficient vitamin 25(OH) D level) and Group II (deficient vitamin 25(OH) D level)
| Variable | Group 1 (n = 110) | Group 2 (n = 40) | P‐value |
|---|---|---|---|
| Gender | |||
| Male | 66 (60%) | 28 (70%) | 0.507 |
| Female | 44 (40%) | 12 (30%) | |
| Age (years) | 38.65 ± 15.52 | 40.20 ± 14.59 | 0.747 |
| BMI (kg/m2) | 24.90 ± 2.56 | 24.97 ± 2.40 | 0.941 |
| WC (cm) | 81.30 ± 8.22 | 79.00 ± 6.48 | 0.369 |
| Hb (g/dL) | 12.20 ± 0.92 | 11.77 ± 1.19 | 0.302 |
| WBC (103/mm3) | 6.10 ± 1.85 | 5.85 ± 1.54 | 0.675 |
| DM | 35 (31.81%) | 15 (37.5%) | 0.104 |
| Smoking | 40 (36.36%) | 15 (37.5%) | 0.362 |
| Alcohol | 5 (4.54%) | 3 (7.5%) | 0.665 |
| ALT (U/L) | 20.0 ± 7.70 | 24 ± 5.80 | 0.232 |
| AST (U/L) | 24.0 ± 8.10 | 27 ± 6.89 | 0.210 |
| GGT (IU/L) | 41.00 ± 1.41 | 34.92 ± 17.81 | 0.132 |
| ALP (IU/L) | 114.90 ± 55.30 | 125.07 ± 50.04 | 0.591 |
| Cholesterol (mg/dL) | 174.29 ± 37.97 | 200.0 ± 56.75 | 0.196 |
| Creatinine (mg/dL) | 0.8 ± 0.26 | 0.82 ± 0.19 | 0.759 |
No significant differences between the two groups regarding laboratory data and demographic characteristics.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; GGT, gamma‐glutamyl transferase; Hb, hemoglobin; WBC, white blood cells; WC, waist circumference.
Table 2.
Comparison between the mean vitamin 25(OH) D level as well as the number of patients with deficient vitamin 25(OH) D between successful and failed eradication groups
| Successful (n = 105) (70%) | Failed (n = 45) (30%) | P‐value | |
|---|---|---|---|
| Vitamin D level (ng/mL) (mean ± SD) | 27.41 ± 7.1 | 14.7 ± 4.5 | <0.001 |
| Number of patients with deficient vitamin 25(OH) D | 10 (9.5%) | 30 (66.6%) | <0.001 |
The mean of vitamin 25(OH) D level was significantly lower in the eradication failure group compared to the successful treatment group (P < 0.0001).
Vitamin 25(OH) D deficiency was significantly higher in the failed treatment group compared to the successful treatment group (P < 0.001).
Figure 3.
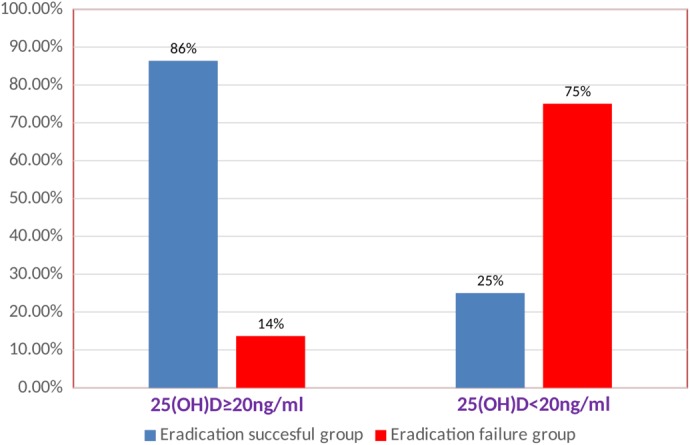
Comparison between eradication success and eradication failure rates of Helicobacter pylori eradication in relation to the vitamin 25(OH)D levels.
Discussion
In the present study, we have evaluated the possible association between vitamin D levels and H. pylori infection. Our study primarily investigates the relationship between H. pylori eradication rates and low vitamin D levels. In this study, we found the H. pylori eradication rates to be significantly lower in patients with low vitamin D levels. In addition, the number of patients with deficient vitamin D levels was significantly higher in the eradication failure group compared to the successful group. A potential pathogenic mechanism explaining the observed association between vitamin D status and eradication rates is the impairment of the vitamin D signal immune function, which may lead to inadequate immune response. Vitamin D deficiency may be a risk factor associated with H. pylori infection treatment failure and may lead to a need for supplementation of vitamin D before H. pylori eradication therapy. The link between vitamin D deficiency and susceptibility to infection has been suggested for longer than a century, with the early observation that children with nutritional rickets were more likely to experience infections of the respiratory system, leading to the coining of the phrase “rachitic lung”.14 Recently, one of the most interesting reports on vitamin D by Guo et al. 15 has shown that vitamin D demonstrates an antimicrobial effect against H. pylori. Guo et al.15 found that vitamin D plays an important role in gastric mucosa homeostasis and host protection from H. pylori infection. Besides its effect on bone metabolism, vitamin D may reduce inflammatory markers such as CRP, TNF‐α, IL‐6, and IL‐18, and the level of anti‐inflammatory cytokine IL‐10 may increase.16 Vitamin D is also known to regulate the expression of AMPs cathelicidin and β‐defensin, which kill the bacteria. Although the effect of cathelicidin has been demonstrated only in macrophages infected with Mycobacterium tuberculosis, antibacterial action against Gram‐negative and Gram‐positive bacteria has also been reported.17 In a vitamin D‐deficient state, the infected macrophage is unable to produce sufficient 1,25‐(OH)D2 to upregulate the production of cathelicidin and β‐defensin, thus rendering them unable to kill the H. pylori strains. More recently, epidemiological studies have demonstrated strong associations between seasonal variations in vitamin D levels and the incidence of various infectious diseases, including septic shock.18 Emerging evidence suggests that vitamin D might be a good prophylactic and possibly therapeutic antimicrobial agent for the control and eradication of H. pylori. More prospectively designed clinical trials considering pretreatment vitamin D levels are needed to further evaluate the relationship between vitamin D status and H. pylori infection, and some investigators propose that vitamin D in combination with standard antimicrobial therapeutics could improve the eradication rates of drug‐resistant H. pylori. There is considerable interest in the diagnostic methods for H. pylori infection both before and after treatment. Generally, H. pylori infection can be diagnosed by invasive (endoscopy and biopsy) and noninvasive techniques (e.g., serology, urea breath test [UBT], stool test). In recent years, various new endoscopic techniques have been developed that allow a clear visualization of minute mucosal structures. The new detailed images have enabled endoscopists to observe microscopic structures, such as gastric pit patterns, microvessels, cell morphology, and even microbes. NBI is a novel endoscopic technique that may enhance the accuracy of diagnosis by using narrow‐bandwidth filters in a red‐green‐blue (R/G/B) sequential illumination system.19 In the present study, we diagnose H. pylori gastritis by using magnifying NBI supported by a stool antigen test for H. pylori infection as the diagnostic gold standard. NBI diagnosis was based on the examination of micro surface structures of the gastric corpus, including both the gastric pit pattern and vascular pattern. Diagnosis of H. pylori gastritis was made according to the presence of obviously enlarged oval or prolonged pit with increased density of irregular vessels as well the presence of well‐demarcated oval or tubulovillous pit with clearly visible coiled or wavy subepithelial capillary networks along with the absence of regular arrangement of collecting venules. Magnifying endoscopy using NBI has two distinct applications: the analysis of the surface architecture of the epithelium (pit pattern) and the analysis of the vascular network.20 According to Yagi's classification, the normal body mucosa shows collecting venules and true capillaries that form a network surrounding gastric pits with a pinhole‐like appearance (Z0 pattern), while the other three types (Z1, Z2, and Z3) corresponded to the H. pylori‐positive mucosa. The Z0 pattern had 93.8% sensitivity and 96.2% specificity for predicting normal gastric mucosa without H. pylori infection.21 Similarly, Nakagawa's classification divided the morphology of collecting venules into three patterns: regular (R), irregular (I), and obscured (O). The sensitivity and specificity of R pattern gastric mucosa as an indicator of the absence of H. pylori infection were 63.9 and 100%, respectively22. Anagnostopoulos et al.23 tested the feasibility of magnifying endoscopy in a Western population, and the gastric body was categorized into four types: type 1, regular arrangement of the majority of the studies to evaluate gastric mucosal patterns has been conducted by Japanese investigators collecting venules and regular, round pits; type 2, regular, round pits, but loss of collecting venules; type 3, loss of normal collecting venules, with enlarged white pits surrounded by erythema; and type 4, loss of normal round pits, with irregular arrangement of collecting venules. The sensitivity and specificity of types 2 and 3 patterns for predicting H. pylori infection were 100 and 92.7%, respectively. The type 4 pattern corresponded to atrophic gastritis with a sensitivity of 90% and a specificity of 96%. A prospective study of 129 patients performed in Turkey confirmed that high‐resolution magnifying endoscopy is superior to standard endoscopy for the diagnosis of H. pylori‐associated gastritis.24 In the present study, we combined both ME‐NBI to diagnose H. pylori chronic gastritis supported with a stool antigen test as a gold standard to increase the sensitivity and specificity for the detection of H. pylori infection. The stool antigen test is a sensitive and specific noninvasive test for the diagnosis of H. pylori infection. It is inexpensive25 and easy to perform and is highly accurate in patients untreated for H. pylori infection. Although some authors have reported a high number of false‐positive results in posteradication assessment using the H. pylori stool antigen test, the majority of studies suggest that the test is very accurate, and in a recent posttherapy follow‐up study involving 10 dedicated European centers, the sensitivity and specificity of H. pylori Stool antigen test (93.8 and 96.9%) and UBT (90.6 and 99.2%) were very similar.26
Conclusions
Our results demonstrated that 25‐OH vitamin D deficiency may be considered a risk factor related to the eradication failure of H. pylori infection. In addition, a further randomized trial to evaluate the effect of vitamin D supplementation in H. pylori eradication is mandatory.
Declaration of conflict of interest: The authors declared that there is no conflict of interest.
References
- 1. Sokwala A, Shah MV, Devoni S, Youga G. Helicobacter pylori: a randomized comparative trial of 7‐day versus 14‐day triple therapy. S Afr Med J. 2012; 102: 368–71. [DOI] [PubMed] [Google Scholar]
- 2. Ramesh R, Sheng LW, Li J, Ying XW, Qian WR, Chang QY. Helicobacter pylori infection: A recent approach to diagnosis and management. J. Biomed. 2017; 2(1): 45–56. [Google Scholar]
- 3. NIH Consensus Conference . Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994; 272: 65–9. [PubMed] [Google Scholar]
- 4. Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology . American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 2007; 102: 1808–25. [DOI] [PubMed] [Google Scholar]
- 5. Feng L, Wen MY, Zhu YJ, Men RT, Yang L. Sequential therapy or standard triple therapy for Helicobacter pylori infection: an updated systematic review. Am. J. Ther. 2016; 23(3): e880–93. [DOI] [PubMed] [Google Scholar]
- 6. Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta‐analysis of sequential therapy. BMJ. 2013; 347: f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment. Pharmacol. Ther. 1999; 13(Suppl 3): 27–36. [DOI] [PubMed] [Google Scholar]
- 8. Kitson MT, Roberts SK. D‐livering the message: the importance of vitamin D status in chronic liver disease. J. Hepatol. 2012; 57(4): 897–909. [DOI] [PubMed] [Google Scholar]
- 9. Liu PT, Stenger S, Li H et al Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science. 2006; 311: 1770–3. [DOI] [PubMed] [Google Scholar]
- 10. Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002; 4: 361–72. [DOI] [PubMed] [Google Scholar]
- 11. Youssef DA, WT Miller C, El‐Abbassi AM et al Antimicrobial implications of vitamin D. Dermato Endocrinol. 2011; 3(4): 220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25‐dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000; 374(2): 334–8. [DOI] [PubMed] [Google Scholar]
- 13. Dumitrescu G, Mihai C, Dranga M, Prelipcean CC. Serum 25‐hydroxyvitamin D concentration and inflammatory bowel disease characteristics in Romania. World J Gastroenterol. 2014; 20: 2392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khajavi A, Amirhakimi GH. The rachitic lung: pulmonary findings in 30 infants and children with malnutritional rickets. Clin. Pediatr. (Phila). 1977; 16: 36–8. [DOI] [PubMed] [Google Scholar]
- 15. Guo L, Chen W, Zhu H et al Helicobacter pylori induces increased expression of the vitamin d receptor in immune responses. Helicobacter. 2014; 19(1): 37–47. [DOI] [PubMed] [Google Scholar]
- 16. Izquierdo MJ, Cavia M, Muñiz P et al Paricalcitol reduces oxidative stress and inflammation in hemodialysis patients. BMC Nephrol. 2012; 13: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang TT, Nestel FP, Bourdeau V et al Cutting edge: 1,25‐dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004; 173: 2909–12. [DOI] [PubMed] [Google Scholar]
- 18. Danai PA, Sinha S, Moss M, Haber MJ, Martin GS. Seasonal variation in the epidemiology of sepsis. Crit. Care Med. 2007; 35: 410–5. [DOI] [PubMed] [Google Scholar]
- 19. Tajiri H, Matsuda K, Fujisaki J. What can we see with the endoscope? Present status and future perspectives. Dig. Endosc. 2002; 14: 131–7. [Google Scholar]
- 20. Kiesslich R, Jung M. Magnification endoscopy: does it improve mucosal surface analysis for the diagnosis of gastrointestinal neoplasias? Endoscopy. 2002; 34: 819–22. [DOI] [PubMed] [Google Scholar]
- 21. Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002; 34: 376–81. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa S, Kato M, Shimizu Y et al Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest. Endosc. 2003; 58: 71–5. [DOI] [PubMed] [Google Scholar]
- 23. Anagnostopoulos GK, Yao K, Kaye P et al High‐resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori‐associated gastritis, and gastric atrophy. Endoscopy. 2007; 39: 202–7. [DOI] [PubMed] [Google Scholar]
- 24. Gonen C, Simsek I, Sarioglu S, Akpinar H. Comparison of high resolution magnifying endoscopy and standard videoendoscopy for the diagnosis of Helicobacter pylori gastritis in routine clinical practice: a prospective study. Helicobacter. 2009; 14(1): 12–21. [DOI] [PubMed] [Google Scholar]
- 25. Vakil N, Rhew D, Soll A, Ofman JJ. The cost‐effectiveness of diagnostic testing strategies for Helicobacter pylori . Am. J. Gastroenterol. 2000; 95: 1691–8. [DOI] [PubMed] [Google Scholar]
- 26. Vaira D, Malfertheiner P, Mégraud F et al Diagnosis of Helicobacter pylori infection with a new non‐invasive antigen‐based assay. HpSA European Study Group. Lancet. 1999; 354: 30–3. [DOI] [PubMed] [Google Scholar]


