Abstract
Background and Aims
The aims of this study were to examine changes in the proportion of decompensated hepatitis C virus (HCV) cirrhosis patients with ascites, hepatic encephalopathy, and variceal bleeding at pretreatment compared to 3 and 12 months post‐sustained virological response (SVR) and to compare pretreatment and post‐SVR model of end‐stage liver disease and Child‐Pugh scores and alpha‐fetoprotein levels.
Methods
Electronic medical records of 64 decompensated HCV cirrhosis patients who received direct‐acting antivirals were reviewed. The McNemar–Bowker test and the Wilcoxon‐Signed Rank test were used to compare patient outcomes.
Results
Ascites was resolved in 29% of patients 3 months post‐SVR (65% vs 36%, P < 0.01) and in 35% of patients 12 months post‐SVR (65% vs 30%, P = 0.07). Hepatic encephalopathy was resolved in 54% of patients 3 months post‐SVR (70% vs 16%, P < 0.01) and in 48% of patients 12 months post‐SVR (70% vs 22% P = 0.03). Variceal bleeding was absent in 32% of patients 3 months post‐SVR (35% vs 3%, P < 0.01) and in 27% of patients 12 months post‐SVR (35% vs 8%, P < 0.01). Alpha‐fetoprotein levels were significantly reduced post‐SVR, but model of end‐stage liver disease and Child‐Pugh scores were not.
Conclusions
Decompensated HCV cirrhosis patients who achieved SVR with direct‐acting antiviral treatment had significant reductions in manifestations of hepatic decompensation sustainable up to 1 year post‐SVR.
Keywords: decompensated cirrhosis, direct acting antivirals, hepatitis C, treatment
Introduction
Over 185 million people are infected with hepatitis C virus (HCV) worldwide,1, 2, 3, 4 and approximately 12% of these individuals have manifestations of decompensated cirrhosis (e.g. ascites, hepatic encephalopathy, and variceal bleeding) caused by HCV.5 The cumulative probability of developing decompensated cirrhosis after diagnosis is 12%, 18%, and 29% in years 3, 5, and 10, respectively.6 After the development of decompensated cirrhosis in HCV patients, the probability of survival is 51% at 5 years.7
To curb the increased likelihood of mortality associated with decompensated HCV cirrhosis, clinicians can now treat and cure decompensated HCV cirrhosis patients of HCV.8, 9, 10 Until recently, treatment was futile in decompensated HCV cirrhosis patients. With the recent advent of direct‐acting antivirals (DAAs), the American Association for the Study of Liver Disease (AASLD), the Infectious Disease Society of America (ISDA), and the European Association for the Study of the Liver (EASL) now have guidelines that recommend the use of all‐oral DAA regimens for decompensated HCV cirrhosis patients.11, 12 In a recent study conducted by Lawitz et al.,13 100% of decompensated HCV cirrhosis patients achieved a sustained virological response (SVR). Furthermore, a large‐scale trial conducted by Charlton et al.14 demonstrated decompensated that HCV cirrhosis patients with Child‐Pugh (CP) class B and class C achieved SVR rates greater than 85% with DAA treatment.
Although DAA treatment is safe, tolerable, and efficacious in decompensated HCV cirrhosis patients,9, 15 data beyond SVR rates are lacking. To date, research on post‐SVR liver functioning in decompensated HCV cirrhosis patients has been limited to only a few studies.13, 16 Findings from these respective studies demonstrated that DAA treatment reduced the model of end‐stage liver disease (MELD) and CP scores in decompensated HCV cirrhosis patients post‐SVR. Surprisingly, to date, no studies have examined sustainable reductions in specific forms of decompensation in HCV cirrhosis patients. Hence, the primary aim of this study was to examine changes in the proportion of decompensated HCV cirrhosis patients with ascites, hepatic encephalopathy, and variceal bleeding at pretreatment compared to 3 and 12 months post‐SVR. The secondary aim of this study was to compare pretreatment and post‐SVR MELD and CP scores and alpha‐fetoprotein (AFP) levels.
Patients and methods
Design
This retrospective cohort study was conducted at a single tertiary medical center. Electronic medical records of 64 decompensated HCV cirrhosis patients who received DAA treatment between November 2014 and January 2016 were reviewed for study inclusion. Decompensated HCV cirrhosis patients who achieved SVR post‐treatment were included in the study, and those who did not achieve SVR were excluded from the study. Patients with hepatocellular carcinoma (HCC), prior liver transplantation, and human immunodeficiency virus (HIV) coinfection were also excluded from the study. Fifteen patients were excluded due to a diagnosis of HCC (regardless of diagnosis prior to the initiation of DAA treatment, during the treatment course, or after achieving SVR), three patients were excluded due to a liver transplant prior to treatment, one patient was excluded due to coinfection with HIV, and eight patients were excluded due to failure to achieve SVR. Altogether, a sample of 37 decompensated HCV cirrhosis patients who achieved SVR met this study's inclusion criteria. Data extraction included age, race, gender, genotype, prior treatment history, and current treatment duration and regimen. The study was approved by the institutional review board at the University of Alabama at Birmingham.
Outcomes
Primary outcomes were the proportion of decompensated HCV cirrhosis patients with ascites, hepatic encephalopathy, and variceal bleeding at 3 and 12 months post‐SVR. Secondary outcomes were post‐SVR MELD and CP scores and AFP levels.
Statistical analysis
Measures of central tendency and frequency distributions were used for univariate analysis. Pretreatment proportions of patients with ascites, hepatic encephalopathy, and variceal bleeding were compared to 3 and 12 months post‐SVR proportions using the McNemar–Bowker test. Changes in median MELD and CP scores and AFP levels were compared using the Wilcoxon‐signed rank test.
Results
Patient characteristics
The majority of patients were male (57%), Caucasian (84%), treatment naïve (60%), genotype 1 (78%), and with a median age of 60 years (Table 1). Of the patients, 65% received 12 weeks of treatment, followed by 32% and 3% receiving 24 weeks and 16 weeks of treatment, respectively. A total of 81% of patients were treated with ledipasvir/sofosbuvir, and those who did not receive ledipasvir/sofosbuvir were treated with sofosbuvir + ribavirin (14%), sofosbuvir + daclatasvir (3%), and sofosbuvir + simperivir (3%). Treatment regimens were chosen according to AASLD/ISDA guidelines and/or provider preference. DAA treatment was well tolerated, and to our knowledge, there were no direct adverse effects from treatment.
Table 1.
Characteristics of decompensated HCV cirrhosis patients
| n | 37 (100%) |
| Age, median (IQR) | 60 (43–73) |
| Male | 21 (57%) |
| Caucasian | 31 (84%) |
| Genotype 1a | 25 (68%) |
| Genotype 1b | 4 (10%) |
| Genotype 2b | 5 (14%) |
| Genotype 3 | 2 (5%) |
| Genotype 4 | 1 (3%) |
| Treatment naïve | 22 (60%) |
| 12 week duration of treatment | 24 (65%) |
| 16 week duration of treatment | 1 (3%) |
| 24 week duration of treatment | 12 (32%) |
| Treatment with ledipasvir/sofosbuvir | 30 (81%) |
| Treatment with sofosbuvir + ribavirin | 5 (13%) |
| Treatment with sofosbuvir + daclatasvir | 1 (3%) |
| Treatment with sofosbuvir + simperivir | 1 (3%) |
| Ascites | 24 (65%) |
| Hepatic encephalopathy | 26 (70%) |
| Variceal bleeding | 13 (35%) |
| MELD score (n = 32)a, median (IQR) | 15 (8–28) |
| CP score (n = 32), median(IQR) | 7 (5–11) |
| AFP level (n = 27), median (IQR) | 6.45 (0.81–39.70) |
AFP, alpha‐fetoprotein; CP, Child‐Pugh; HCV, hepatitis C virus; IQR, interquartile range; MELD, model of end‐stage liver disease.
Ascites
Compared to pretreatment patients with ascites, ascites was resolved in 29% of patients 3 months post‐SVR (65% vs 36%, P < 0.01) and in 35% of patients 12 months post‐SVR (65% vs 30%, P = 0.07) (Table 2).
Table 2.
Proportion of decompensated HCV cirrhosis patients with ascites, hepatic encephalopathy, and variceal bleeding at pretreatment compared to 3 and 12 months post‐SVR
| Pretreatment n (%) |
3 Months post‐SVR n (%) |
% Change | P‐value | 12 Months post‐SVR n (%) |
% Change | P‐value | |
|---|---|---|---|---|---|---|---|
| Ascites | 24 (65%) | 9 (36%) | 29 | <0.01 | 8 (30%) | 35 | 0.07 |
| Hepatic encephalopathy | 26 (70%) | 5 (16%) | 54 | <0.01 | 5 (22%) | 48 | 0.03 |
| Variceal bleeding | 13 (35%) | 1 (3%) | 32 | <0.01 | 2 (8%) | 27 | <0.01 |
HCV, hepatitis C virus; SVR, sustained virological response.
Hepatic encephalopathy
Compared to pretreatment patients with hepatic encephalopathy, hepatic encephalopathy was resolved in 54% of patients 3 months post‐SVR (70% vs 16%, P < 0.01) and in 48% of patients 12 months post‐SVR (70% vs 22%, P = 0.03).
Variceal bleeding
Compared to pretreatment patients with variceal bleeding, variceal bleeding was absent in 32% of patients 3 months post‐SVR (35% vs 3%, P < 0.01) and in 27% of patients 12 months post‐SVR (35% vs 8%, P < 0.01) (Fig. 1).
Figure 1.
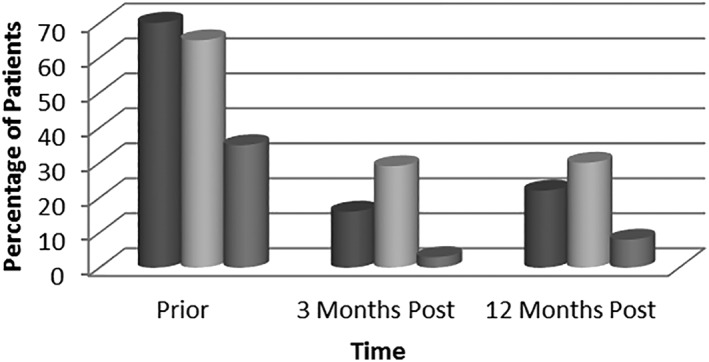
Change in the manifestations of decompensated cirrhosis prior to and post‐treatment with direct‐acting antiviral.  , HE;
, HE;  Ascites;
Ascites;  , VB.
, VB.
MELD and CP scores
There were no differences in statistical significance between pretreatment and post‐SVR median (interquartile range [IQR]) MELD scores, 15 (8–28) versus 15 (6–36) (P = 0.20), and median CP scores, 7 (5–11) versus 7 (5–15) (P = 0.60).
Alpha‐fetoprotein levels
Compared to pretreatment median AFP levels, 6.45 (0.81–39.70), post‐SVR median AFP levels, 4.24 (0.6–15.7), were significantly reduced (P < 0.01), with a median decrease of 2.21 (Fig. 2).
Figure 2.
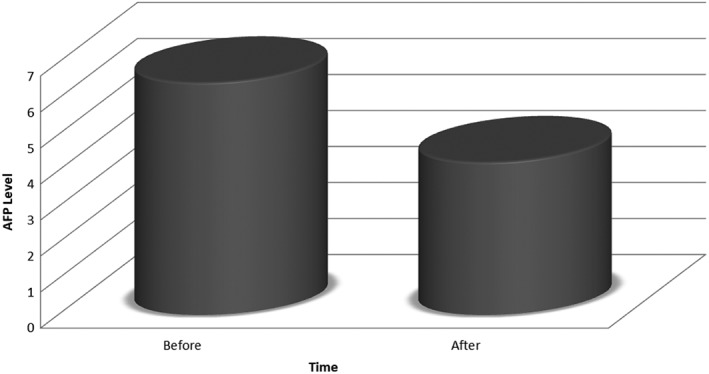
Change in AFP level prior to and post‐treatment with direct‐acting antiviral.
Discussion
Decompensated HCV cirrhosis patients who achieved SVR had fewer cases of ascites, hepatic encephalopathy, and variceal bleeding 3 months post‐SVR, and patients had fewer cases of hepatic encephalopathy and variceal bleeding 12 months post‐SVR. Significant improvements in MELD and CP scores were not observed. However, AFP levels were significantly reduced.
Along with existing evidence demonstrating the safety and efficacy of DAA treatment with decompensated HCV cirrhosis patients,8, 17, 18, 19 the findings of this study suggest that DAAs can attenuate some advanced forms of hepatic decompensation by resolving ascites and hepatic encephalopathy and decreasing the amount of variceal bleeds in patients who achieve SVR. Ascites and hepatic encephalopathy are the most common reasons for hospital admission among patients with advanced liver disease,20 and advanced liver disease is associated with poor quality of life.21 Furthermore, several studies have demonstrated that HCV patients who achieve SVR with DAAs are likely to experience significant improvements in patient‐reported outcomes (e.g. physical and social functioning, work productivity, mental health, bodily pain, and general health).22 Although largely understudied,23 it is plausible that the resolution of decompensation can improve the quality of life of decompensated HCV cirrhosis patients as well as curb hospital admission rates.
Provided many patients in this study achieve a reduction in the manifestations of hepatic decompensation, DAA treatment in patients currently on liver transplantation waitlists could potentially improve patient survival while waiting for transplantation or delist patients from transplantation waitlists. Several patients currently on liver transplantation waitlists have decompensated cirrhosis, and the literature is mixed on whether decompensated HCV cirrhosis patients should be treated with DAAs and be cured of HCV before or after liver transplantation.24 Irrespective of the mixed literature, findings from this study suggest that the benefit of DAA treatment extends beyond SVR to include a potential for patients to receive relief from hepatic decompensation while waiting for a liver transplantation that is often indeterminable.
The few studies that have been published on the impact of DAA treatment on post‐SVR MELD and CP scores with decompensated HCV cirrhosis patients largely observed improvements,14, 16 whereas this study did not. Our study did not exclude patients based on baseline bilirubin or creatinine levels or creatinine clearance, whereas in the SOLAR‐1 trial, patients with a total bilirubin greater than 10 mg/dL, a creatinine <2.5 times the upper limit of normal, and a creatinine clearance <40 mL/min were excluded. We thus postulate that the lack of MELD and CP score improvement in our study was due to worsened hepatic and renal dysfunction in our patient population at baseline.
As previously reported, the MELD score is poorly correlated with disease—specifically the quality of life scores. As encephalopathy and ascites were important factors for patients when considering quality of life, manifestations are notably absent in the MELD score.19 Although there were no improvements in MELD and CP scores in this study, they did not worsen with DAA treatment. Clinicians may be able to use DAAs in decompensated HCV cirrhosis patients with limited concern of an adverse effect on MELD and CP scores; however, more research is needed to confirm the effects of DAAs on MELD and CP scores in this patient population.
It has been previously described that patients with advanced HCV in the absence of HCC have serum AFP elevations.25 There is a paucity of data that describes changes in AFP levels after treatment of HCV with DAAs. To our knowledge, there is only one other study by Nguyen et al. 26 that found a decrease in AFP levels following DAA treatment. This study corroborates these data. Current guidelines do not recommend the use of AFP alone for routine HCC screening in cirrhosis patients,27 but increased AFP has been shown to be associated with carcinogenesis.28, 29, 30, 31 If future studies replicate post‐SVR reductions in AFP with DAA treatment, perhaps the clinical utility of AFP as a screening modality for HCC could have improved sensitivity and specificity. This must be studied further but is important for clinicians.
This study had some noteworthy limitations and strengths. The study was retrospective, lacked racial diversity, and had a modest sample size. Notwithstanding, the study was able to detect statistical differences between baseline compared to months 3 and 12, and the study had a similar sample size as a study recently published by Lawitz et al.13 in this clinical area of research. The study's exclusion criteria were minimal and included treatment‐experienced patients. A high percentage of patients included in the study had ascites, hepatic encephalopathy, and variceal bleeding prior to the start of DAA treatment.
It is well described that DAAs are effective in achieving SVR in decompensated HCV cirrhosis patients. This study demonstrates patients who achieve SVR with DAAs can also achieve reductions in the manifestations of hepatic decompensation, notably ascites, hepatic encephalopathy, and variceal bleeding. Further studies are needed to assess the sustained benefit of these reductions over a longer study period. This is of clinical importance because resolution of advanced forms hepatic decompensation can lead to fewer hospital admissions, improved patient quality of life, and increased patient survival while waiting for liver transplantation.
Declaration of conflict of interest: Dr. Omar Sims has received research support from the National Institute on Alcohol and Alcoholism. Dr. Omar Massoud has received grants from Gilead Sciences. The remaining authors have nothing to disclose.
References
- 1. Gower E, Estes C, Blach S, Razavi‐Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014; 61: S45–57. [DOI] [PubMed] [Google Scholar]
- 2. Dore GJ, Ward J, Thursz M. Hepatitis C disease burden and strategies to manage the burden (Guest Editors Mark Thursz, Gregory Dore and John Ward). J. Viral Hepat. 2014; 21: 1–4. [DOI] [PubMed] [Google Scholar]
- 3. Lim S, Vos T, Flaxman A et al A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012; 380: 2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age‐specific antibody to HCV seroprevalence. Hepatology. 2013; 57: 1333–42. [DOI] [PubMed] [Google Scholar]
- 5. Davis GL, Alter MJ, El‐Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)‐infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010; 138: 513–21. [DOI] [PubMed] [Google Scholar]
- 6. Fattovich G, Giustina G, Degos F et al Morbidity and mortality in compensated cirrhosis type C: A retrospective follow‐up study of 384 patients. Gastroenterology. 1997; 112: 463–72. [DOI] [PubMed] [Google Scholar]
- 7. Planas R, Balleste B, Alvarez MA et al Natural history of decompensated hepatitis C virus‐related cirrhosis. A study of 200 patients. J. Hepatol. 2004; 40: 823–30. [DOI] [PubMed] [Google Scholar]
- 8. Saxena V, Nyberg L, Pauly M et al Safety and efficacy of simeprevir/sofosbuvir in hepatitis C‐Infected patients with compensated and decompensated cirrhosis. Hepatology. 2015; 62: 715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Modi A, Nazario H, Trotter J et al Safety and efficacy of simeprevir plus sofosbuvir with or without ribavirin in patients with decompensated genotype 1 hepatitis C cirrhosis. Liver Transpl. 2016; 22: 281–6. [DOI] [PubMed] [Google Scholar]
- 10. Chau J, Kottilil S. Sofosbuvir and velpatasvir: a stellar option for patients with decompensated hepatitis C virus (HCV) cirrhosis. Ann. Transl. Med. 2016; 4(Suppl. 1): S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (IDSA). Recommendations for testing, managing, and treating hepatitis C. Online February 2016.
- 12.European Association for the Study of the Liver (EASL). Recommendations on treatment of hepatitis C. Online February 2016. [DOI] [PubMed]
- 13. Lawtiz E, Poordad F, Guiterrez JA et al Simeprevir, daclatasvir and sofosbuvir for hepatitis C virus‐infected patients with decompensated liver disease. J. Viral Hepat. 2017; 24: 287–94. [DOI] [PubMed] [Google Scholar]
- 14. Charlton M, Everson G, Flamm S, Kumar P, Landis C, Brown R Jr. Ledipasvir and Sofosbubvir plus Ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015; 149: 649–59. [DOI] [PubMed] [Google Scholar]
- 15. Cury MP, O'Leary JG, Bzowej N et al Sofosbuvir and Velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 2015; 31: 2618–28. [DOI] [PubMed] [Google Scholar]
- 16. Foster GR, Irving WL, Cheung MC et al Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016; 64: 1224–31. [DOI] [PubMed] [Google Scholar]
- 17. Samuel D, Manns M, Forns X et al Ledipasvir/sofosbuvir with ribavirin is safe in >600 decompensated and post liver transplantation patients with HCV infection: an integrated safety analysis of the solar 1 and solar 2 trials. J. Hepatol. 2015; 62(Suppl. 2): S260–1. [Google Scholar]
- 18. Curry MP, O'Leary JG, Bzowej N et al Sofosbuvir and Velpatasvir for HCV in patients with decompensated cirrhosis. N. Engl. J. Med. 2015; 373: 2618–28. [DOI] [PubMed] [Google Scholar]
- 19. Poordad F, Schiff ER, Vierling JM et al Daclatasvir with sofosbuvir and ribavirin for HCV infection with advanced cirrhosis or post‐Liver transplant recurrence. Hepatology. 2016; 63: 1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Pascoli M, Ceranto E, De Nardi P et al Hospitalizations due to cirrhosis: clinical aspects in a large cohort of Italian patients and cost analysis report. Dig. Dis. 2017; 35: 433–8. [DOI] [PubMed] [Google Scholar]
- 21. Younossi ZM, Stepanova M, Feld J et al Sofosbuvir/velpatasvir improves patient‐reported outcomes in HCV patients: results from Astral‐1 placebo‐controlled trial. J. Hepatol. 2016; 65: 33–9. [DOI] [PubMed] [Google Scholar]
- 22. Younossi Z, Brown A, Buti M et al Impact of eradicating hepatitis C virus on the work productivity of chronic hepatitis C (CH‐C) patients: an economic model from five European countries. J. Viral Hepat. 2016; 23: 217–26. [DOI] [PubMed] [Google Scholar]
- 23. Younossi Z, Stepanova M, Charlton M et al Patient‐reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus‐related decompensated cirrhosis: an exploratory analysis from the randomized, open‐label ASTRAL‐4 phase 3 trial. Lancet Gastroenterol. Hepatol. 2016; 1: 122–32. [DOI] [PubMed] [Google Scholar]
- 24. Bunchorntavakul C, Reddy KR. Treat chronic hepatitis C virus infection in decompensated cirrhosis – pre‐ or post‐liver transplantation? The ironic conundrum in the era of effective and well‐tolerated therapy. J. Viral Hepat. 2016; 23: 408–18. [DOI] [PubMed] [Google Scholar]
- 25. Di Bisceglie AM, Sterling RK, Chung RT et al Serum alpha‐fetoprotein levels in patients with advanced hepatitis C: results from the HALT‐C Trial. J. Hepatol. 2005; 43: 434–41. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen K, Jimenez M, Moghadam N et al Decrease of alpha – fetoprotein in patients with cirrhosis treated with direct – acting antivirals. J. Clin. Transl. Hepatol. 2017; 5: 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Association for the Study of Liver Diseases (AASLD). Guidelines for the treatment of hepatocellular carcinoma. Online January 2017.
- 28. Chen TM, Huang PT, Tsai MH et al Predictors of alpha‐fetoprotein elevation in patients with chronic hepatitis C, but not hepatocellular carcinoma, and its normalization after pegylated interferon alfa 2a‐ribavirin combination therapy. J. Gastroenterol. Hepatol. 2007; 22: 669–75. [DOI] [PubMed] [Google Scholar]
- 29. Yamada R, Hiramatsu N, Oze T et al Impact of alpha‐fetoprotein on hepatocellular carcinoma development during entecavir treatment of chronic hepatitis B virus infection. J. Gastroenterol. 2015; 50: 785–94. [DOI] [PubMed] [Google Scholar]
- 30. Kim GA, Seock CH, Park JW et al Reappraisal of serum alpha‐foetoprotein as a surveillance test for hepatocellular carcinoma during entecavir treatment. Liver Int. 2015; 35: 232–9. [DOI] [PubMed] [Google Scholar]
- 31. Tamura Y, Yamagiwa S, Aoki Y, Kurita S, Suda T, Ohkoshi S. Serum alpha‐fetoprotein levels during and after interferon therapy and the development of hepatocellular carcinoma in patients with chronic hepatitis C. Dig. Dis. Sci. 2009; 54: 2530–7. [DOI] [PubMed] [Google Scholar]


