Abstract
Background
Esophageal small cell carcinoma (ESCC) is a rare malignancy for which there is no consensus management approach. This is the largest known analysis of nonmetastatic ESCC patients to date, evaluating national practice patterns and outcomes of surgical‐based therapy vs chemoradiotherapy (CRT) vs chemotherapy alone.
Methods
The National Cancer Data Base was queried for esophageal cancer patients with histologically confirmed nonmetastatic ESCC. Univariable and multivariable logistic regression ascertained factors associated with receipt of surgical‐based management. Kaplan‐Meier analysis evaluated overall survival (OS) and the log‐rank test is used to compare OS between groups; Cox univariate and multivariate analyses determined variables associated with OS.
Results
Altogether, 323 patients were analyzed; 64 (20%) patients underwent surgical‐based therapy, 211 (65%) CRT, and 48 (15%) chemotherapy alone. On multivariable analysis, no single factor significantly predicted for administration of surgery. Despite no OS differences between the surgery‐based (median OS 21 months) and CRT arms (18 months), both were superior to CT alone (10 months) (P < 0.001). Among other factors, receiving any local therapy independently predicted for higher OS over chemotherapy alone on Cox multivariate analysis (P < 0.001).
Conclusions
This study of a large, contemporary national database demonstrates that most ESCC is treated with CRT in the United States; adding local therapy to systemic therapy may be beneficial to these patients, although individualized multidisciplinary management is still recommended.
Keywords: chemotherapy, esophageal cancer, radiation therapy, small cell carcinoma, surgery
1. INTRODUCTION
Esophageal small cell carcinoma (ESCC) is a rare but highly aggressive malignancy that accounts for less than 2% of esophageal neoplasms.1, 2 Due to its rarity, there are many accepted treatment paradigms. One approach is to treat as esophageal squamous cell or adenocarcinoma, with definitive chemoradiotherapy (CRT), CRT followed by surgery, or surgery alone.3 Another is to utilize nonsurgical treatment (eg chemotherapy (CT) with or without radiotherapy (RT)) similar to that used for small cell carcinoma of the lung. National guidelines have not delineated the optimal treatment strategy for this malignancy.3
The somewhat conflicting literature, largely consisting of lower‐volume reports, may also contribute to the lack of consensus. One study of 64 patients (26 nonmetastatic cases) at Memorial Sloan‐Kettering Cancer Center suggested that combined surgery/CT was most associated with reducing recurrences,4 with other data (121 nonmetastatic cases) suggesting the addition of RT increases survival.5 Both of the aforementioned studies inherently have notable biases, such as the increased proportion of metastatic disease in the former and the lack of chemotherapy records in the latter. A literature review of 199 patients (93 nonmetastatic cases) demonstrated that addition of CT to local therapy (surgery and/or RT) improved outcomes, with no difference in efficacy between either surgery or RT when used as local therapy.6 However, a major limitation of that investigation was the lack of comparing local therapy/CT with chemotherapy alone. Lastly, another larger study of 126 patients (85 nonmetastatic cases) demonstrated similar survival with surgery/CT (with or without RT) as compared to CRT; although this was numerically higher than CT alone (no statistical comparisons), the study lumped nonmetastatic and metastatic cases together, and nearly all patients with metastatic disease received CT alone.7
Hence, owing to the rarity of this neoplasm, there are virtually no higher‐volume studies specifically evaluating management of nonmetastatic ESCC. As such, national databases may be of high utility to evaluate practice patterns and outcomes. This investigation of purely nonmetastatic ESCC patients, the largest report to date, utilized the contemporary National Cancer Data Base (NCDB), which is estimated to capture 70% of the United States cancer population.8
2. MATERIALS AND METHODS
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de‐identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 All pertinent cases are reported regularly from CoC‐accredited centers and compiled into a unified dataset, which is then validated. The NCDB contains information not included in the Surveillance, Epidemiology, and End Results database, including details regarding use of systemic therapy. The data used in the study were derived from a de‐identified NCDB file (2004‐2014). The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de‐identified, this study was exempt from institutional review board evaluation.
Inclusion criteria for this study were patients with newly‐diagnosed, histologically‐confirmed and nonmetastatic ESCC. Histologic criteria referred to the International Classification of Disease for Oncology codes of 8041‐8045 or 8073 (representing small cell or oat cell carcinoma). All patients were clinically without metastasis (M0). Exclusion criteria were unknown M classifications or those patients receiving no therapy or palliative treatments. Patients were divided into three primary groups for further analysis: those receiving CT alone, CRT, and surgical‐based treatment (with or without CT and/or RT).
In accordance with the variables in NCDB files, information collected on each patient broadly included demographic, clinical, and treatment data. All statistical tests were two‐sided, with a threshold of P < 0.05 for statistical significance, and were performed using SAS (version 9.4, Cary, NC). Univariable and multivariable (stepwise) logistic regression modeling was utilized to determine characteristics that were predictive for receipt of surgical‐based therapy. The Kaplan‐Meier method was used for survival analysis, and comparisons between groups were performed with the log‐rank test. Overall survival (OS) was defined as the interval between the date of diagnosis and the date of death, or censored at last contact. Cox univariate and multivariate (stepwise) analyses were performed to determine factors associated with overall survival.
3. RESULTS
A complete flow diagram of patient selection is provided in Figure 1. In total, 323 patients with nonmetastatic, pathologically‐proven ESCC met study criteria (Table 1). Of these, 64 (20%) patients underwent surgical‐based treatment, 211 (65%) CRT, and 48 (15%) CT alone.
Figure 1.
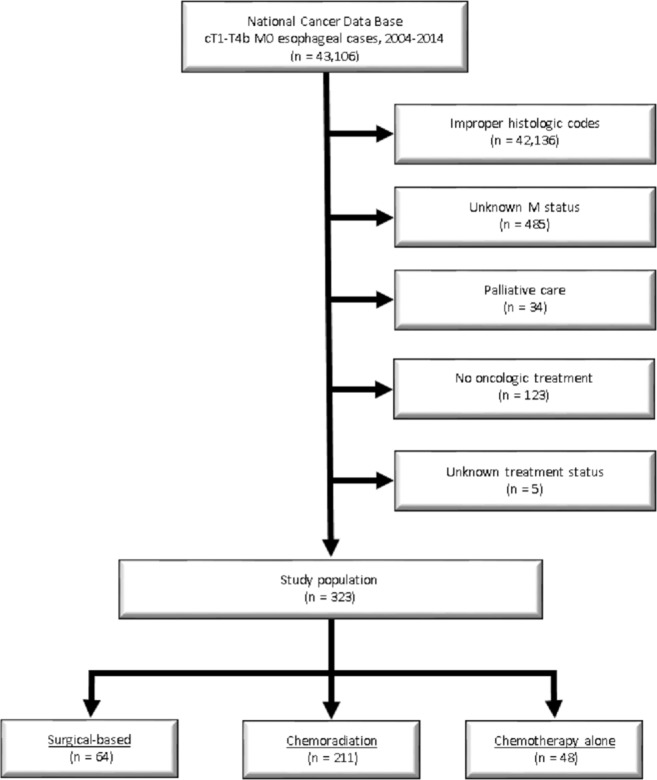
Patient selection diagram
Table 1.
Characteristics of the overall cohort and factors associated with receiving surgery
| Parameter | Surgery (N = 64) | CRT (N = 211) | CT Alone (N = 48) | Univariable | |
|---|---|---|---|---|---|
| OR (95% CI) | P‐value | ||||
| Age (years) | |||||
| Median (range) | 63 (25‐86) | 67 (35‐90) | 68 (48‐87) | 0.981 (0.959‐1.004) | 0.101 |
| Gender | |||||
| Male | 44 (69%) | 139 (66%) | 29 (60%) | REF | ‐ |
| Female | 20 (31%) | 72 (34%) | 19 (40%) | 0.839 (0.467‐1.509) | 0.558 |
| Race | |||||
| White | 57 (89%) | 175 (83%) | 40 (86%) | REF | ‐ |
| Black | 7 (11%) | 27 (13%) | 4 (8%) | 0.617 (0.264‐1.444) | 0.266 |
| Other | 0 (0%) | 9 (4%) | 3 (6%) | ||
| Charlson Deyo scorea | |||||
| 0 | 52 (81%) | 151 (72%) | 40 (83%) | REF | ‐ |
| 1 | 8 (13%) | 43 (20%) | 6 (13%) | 0.600 (0.267‐1.345) | 0.422 |
| ≥2 | 4 (6%) | 17 (8%) | 2 (4%) | 0.773 (0.252‐2.372) | 0.998 |
| Insurance type | |||||
| Uninsured | 3 (5%) | 4 (2%) | 3 (6%) | REF | ‐ |
| Private | 28 (44%) | 69 (33%) | 11 (23%) | 0.817 (0.198‐3.376) | 0.084 |
| Medicaid/Other Government | 1 (2%) | 23 (11%) | 2 (4%) | 0.093 (0.008‐1.043) | 0.048 |
| Medicare | 31 (48%) | 111 (53%) | 31 (65%) | 0.509 (0.125‐2.081) | 0.691 |
| Unknown | 1 (2%) | 4 (2%) | 1 (2%) | ‐ | ‐ |
| Income (US dollars/year) | |||||
| <$30 000 | 10 (16%) | 46 (22%) | 4 (8%) | REF | ‐ |
| $30 000‐$34 999 | 18 (28%) | 49 (23%) | 15 (31%) | 1.406 (0.597‐3.313) | 0.588 |
| $35 000‐$45 999 | 18 (28%) | 54 (26%) | 18 (38%) | 1.250 (0.533‐2.933) | 0.962 |
| ≥$46 000 | 17 (27%) | 56 (27%) | 8 (17%) | 1.328 (0.560‐3.152) | 0.766 |
| Unknown | 1 (2%) | 6 (3%) | 3 (6%) | ‐ | ‐ |
| Location | |||||
| Metro | 51 (80%) | 162 (77%) | 31 (65%) | REF | ‐ |
| Urban | 9 (14%) | 38 (18%) | 12 (25%) | 0.681 (0.314‐1.477) | 0.057 |
| Rural | 3 (5%) | 3 (1%) | 0 (0%) | 3.784 (0.724‐19.311) | 0.070 |
| Unknown | 1 (2%) | 8 (4%) | 5 (10%) | ‐ | ‐ |
| Percentage of adults in zip code without high school diploma | |||||
| ≥21% | 11 (17%) | 44 (21%) | 3 (6%) | REF | ‐ |
| 13‐20.9% | 18 (28%) | 48 (23%) | 19 (40%) | 1.148 (0.497‐2.653) | 0.687 |
| 7‐12.9% | 24 (38%) | 71 (34%) | 16 (33%) | 1.179 (0.531‐2.615) | 0.578 |
| <7% | 10 (16%) | 42 (20%) | 7 (15%) | 0.872 (0.339‐2.244) | 0.535 |
| Unknown | 1 (2%) | 6 (3%) | 3 (6%) | ‐ | ‐ |
| Facility type | |||||
| Community | 29 (45%) | 117 (55%) | 27 (56%) | REF | ‐ |
| Academic | 34 (53%) | 89 (42%) | 21 (44%) | 1.535 (0.882‐2.671) | 0.130 |
| Unknown | 1 (2%) | 5 (2%) | 0 (0%) | ‐ | ‐ |
| Facility location | |||||
| Northeast | 13 (20%) | 55 (26%) | 10 (21%) | REF | ‐ |
| South | 22 (34%) | 62 (29%) | 17 (35%) | 1.392 (0.651‐2.978) | 0.584 |
| Midwest | 17 (27%) | 52 (25%) | 17 (35%) | 1.232 (0.555‐2.735) | 0.999 |
| West | 11 (17%) | 37 (18%) | 4 (8%) | 1.341 (0.549‐3.276) | 0.761 |
| Unknown | 1 (2%) | 5 (2%) | 0 (0%) | ‐ | ‐ |
| Distance to treating facility (mi) | |||||
| Median (range) | 17 (1‐1028) | 9 (0‐1018) | 8 (0‐66) | 1.001 (0.999‐1.003) | 0.346 |
| Clinical T classification | |||||
| 1 | 12 (19%) | 25 (12%) | 12 (25%) | REF | ‐ |
| 2 | 6 (9%) | 26 (12%) | 2 (4%) | 0.661 (0.221‐1.977) | 0.739 |
| 3 | 23 (36%) | 77 (36%) | 4 (8%) | 0.606 (0.275‐1.335) | 0.416 |
| 4 | 0 (0%) | 29 (14%) | 7 (15%) | ||
| Unknown | 23 (36%) | 54 (26%) | 23 (48%) | ‐ | ‐ |
| Clinical N classification | |||||
| 0 | 25 (39%) | 67 (32%) | 13 (27%) | REF | ‐ |
| 1 | 19 (30%) | 103 (49%) | 10 (21%) | 0.538 (0.278‐1.043) | 0.343 |
| 2 | 3 (5%) | 7 (3%) | 3 (6%) | 0.640 (0.171‐2.392) | 0.835 |
| 3 | 0 (0%) | 5 (2%) | 0 (0%) | ||
| Unknown | 17 (27%) | 29 (13%) | 22 (46%) | ‐ | ‐ |
| Year of diagnosis | |||||
| 2004‐2008 | 40 (63%) | 99 (47%) | 28 (58%) | REF | ‐ |
| 2009‐2014 | 24 (38%) | 112 (53%) | 20 (42%) | 0.577 (0.329‐1.012) | 0.055 |
Statistically significant P‐values are in bold. Only values included in the final multivariable model are shown. CI, confidence interval; CRT, chemoradiotherapy; CT, chemotherapy; OR, odds ratio.
The Charlson‐Deyo index is a weighted score of comorbidities as defined by several medical codes.
Due to the controversial role of surgery,29 univariable logistic regression analysis was performed to evaluate factors associated with receiving surgery. Patients with Medicaid/other (non‐Medicare) governmental insurance were less likely to undergo surgery (P = 0.048). However, patients with private insurance trended towards receipt of surgery (P = 0.084), along with those living in rural (P = 0.070) and nonurban (P = 0.057) areas. Treatment at more recent time periods also trended toward decreased use of surgery (P = 0.055). Likely related to sample size issues, no single factor significantly predicted for administration of surgery on multivariable analysis.
Median follow‐up was 48 months (range, 1‐141 months). As shown in Figure 2A, there were no OS differences between the surgery‐based and CRT arms, but both were superior to CT alone (P < 0.001). The median OS for the surgery‐based cohort was 21 months (95% CI, 16‐33 months), as compared to 18 months (95% CI, 15‐23 months) for CRT, and 10 months (95% CI, 6‐12 months) for CT alone.
Figure 2.
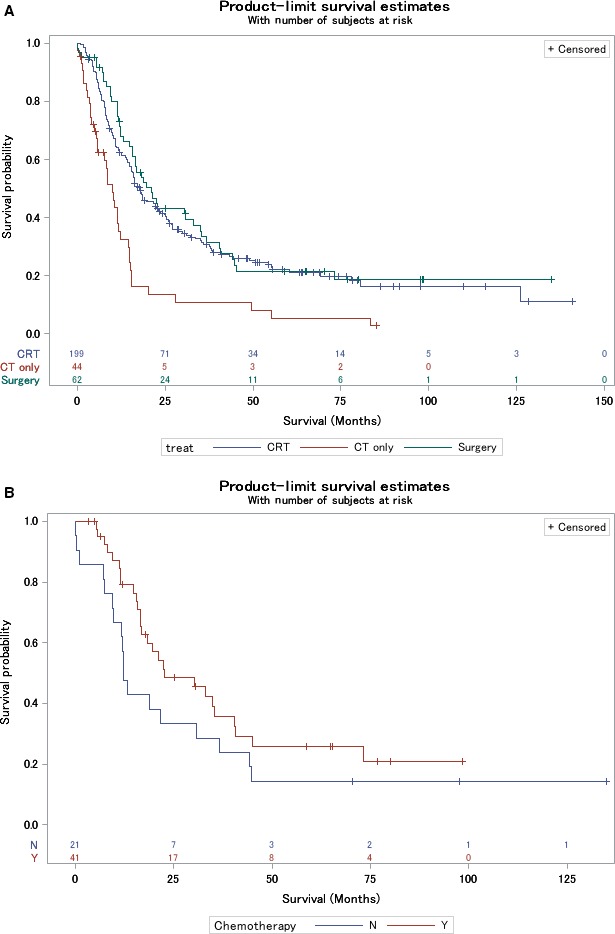
Kaplan‐Meier overall survival curves comparing surgery‐based treatment, chemoradiotherapy, and chemotherapy alone (A). Kaplan‐Meier overall survival curves of the surgery cohort stratified for delivery of additional chemotherapy (B)
As part of additional subgroup analysis to investigate the potential impact of adjuvant CT after surgery, the 64 patients in the surgery cohort were subdivided into 43 patients that received CT and 21 that did not (39 underwent RT in any capacity, and 36 were given both CT and RT). Despite these small sample sizes, the impact of additional chemotherapy in the surgical cohort was assessed, with a nonsignificant difference between groups (Figure 2B; P = 0.143).
Owing to the similar survival between both groups involving local therapy (surgical‐based and CRT), these groups were combined for Cox multivariate analysis. Receipt of any local therapy independently predicted for higher OS (P < 0.001). Other factors independently associated with poorer OS included advancing age, increasing T and N classification, treatment at a community facility, and residence in an area with lower educational status (P < 0.05 for all) (Table 2).
Table 2.
Univariate and multivariate Cox proportional hazards model for overall survival
| Parameter (comparator vs reference) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Treatment group (CT alone vs surgery/CRT) | 2.276 | 1.602‐3.235 | <0.001 | 2.824 | 1.711‐4.661 | <0.001 |
| Age (continuous) | 1.013 | 1.002‐1.025 | 0.025 | 1.019 | 1.005‐1.034 | 0.028 |
| Gender (male vs female) | 0.928 | 0.706‐1.220 | 0.592 | |||
| Race (others vs white) | 0.993 | 0.690‐1.430 | 0.971 | |||
| Charlson‐Deyo score (0 vs 2) | 0.582 | 0.362‐0.937 | 0.026 | |||
| Charlson‐Deyo score (1 vs 2) | 0.648 | 0.370‐1.134 | 0.129 | |||
| Distance to treating facility (continuous) | 0.999 | 0.998‐1.001 | 0.390 | |||
| Insurance (private vs uninsured) | 0.806 | 0.403‐1.612 | 0.542 | |||
| Insurance (Medicaid/other government vs uninsured) | 1.336 | 0.608‐2.936 | 0.471 | |||
| Insurance (Medicare vs uninsured) | 1.069 | 0.543‐2.105 | 0.847 | |||
| Income ($30 000‐$34 999 vs <$30 000) | 1.312 | 0.883‐1.949 | 0.179 | |||
| Income ($35 000‐$45 999 vs <$30 000) | 0.966 | 0.649‐1.438 | 0.864 | |||
| Income (≥$46 000 vs <$30 000) | 0.978 | 0.652‐1.465 | 0.912 | |||
| Location (urban vs metro) | 1.108 | 0.783‐1.569 | 0.563 | |||
| Location (rural vs metro) | 1.031 | 0.423‐2.514 | 0.947 | |||
| Percentage of adults in zip code without high school diploma (13‐20.9% vs ≥21%) | 0.788 | 0.512‐1.211 | 0.277 | 0.881 | 0.535‐1.450 | 0.618 |
| Percentage of adults in zip code without high school diploma (7‐12.9% vs ≥21%) | 0.968 | 0.658‐1.424 | 0.868 | 0.735 | 0.464‐1.165 | 0.190 |
| Percentage of adults in zip code without high school diploma (<7% vs ≥21%) | 0.715 | 0.494‐1.036 | 0.076 | 0.530 | 0.342‐0.823 | 0.005 |
| Facility type (academic vs community) | 0.698 | 0.537‐0.907 | 0.007 | 0.525 | 0.382‐0.721 | <0.001 |
| Facility location (South vs Northeast) | 1.008 | 0.715‐1.421 | 0.964 | |||
| Facility location (Midwest vs Northeast) | 1.034 | 0.720‐1.486 | 0.855 | |||
| Facility location (West vs Northeast) | 0.894 | 0.587‐1.363 | 0.603 | |||
| T stage (T2 vs T1) | 0.848 | 0.498‐1.444 | 0.544 | |||
| T stage (T3/4 vs T1) | 1.135 | 0.773‐1.667 | 0.518 | |||
| N stage (N1 vs N0) | 1.392 | 1.020‐1.898 | 0.037 | 1.568 | 1.134‐2.167 | 0.007 |
| N stage (N2/3 vs N0) | 2.620 | 1.403‐4.894 | 0.003 | 3.834 | 1.982‐7.418 | <0.001 |
| Year of diagnosis (2009‐2014 vs 2004‐2008) | 0.945 | 0.725‐1.232 | 0.678 | |||
Statistically significant P values are in bold. Only values included in the final multivariate model are shown. CI, confidence interval; HR, hazard ratio.
4. DISCUSSION
The study of rare neoplasms such as ESCC is highly limited by sample size and heterogeneity in existing reports; it is hence essential to perform large‐volume investigations of homogeneous patients. Our study of a contemporary national database, the largest study to date, demonstrates that most nonmetastatic ESCC is treated with CRT in the United States. As compared to CT alone, delivery of additional local therapy in the form of RT or surgery is associated with improved survival. The findings of this study corroborate elements from smaller studies.6, 7
It is important to mention that patients coded as undergoing palliative treatment were excluded from this study, so it is less likely that the CT alone group experienced poor survival from receiving palliative CT. The improved OS with the addition of RT to CT (also observed by Song et al.5) indicates that ESCC should be treated similar to limited‐stage small cell lung cancer, in which numerous prospective investigations have shown an OS benefit to adding RT to CT.30 However, this investigation also describes that treatment with an esophageal cancer paradigm may also be appropriate, in the sense that surgical management (with or without CT) is also associated with improved OS over CT alone. By this token, owing to the numerically higher OS with surgery/CT over surgery alone (Figure 2B), it may be logically stated that surgery/CT would also have superior OS as compared to CT alone. Although efforts were made to evaluate whether surgery/CT was superior to surgery alone (Figure 2B), sample size issues likely contributed to a statistically insignificant comparison. It is thus also logical that similar OS would exist between surgery/CT (n = 43) and CRT (n = 211). The reason that these conservative interpretations were posited (instead of formal analyses) is that continually constructing more subgroups split sample sizes greatly, and thus do not make for statistically adequate direct comparisons.
When delivered with systemic therapy, the issue of surgical‐based treatment vs definitive RT is also important to address. This question has been addressed with randomized trials in esophageal squamous cell and adenocarcinomas, displaying improvements in local control but no differences in OS between groups.31, 32 The results of those studies may be less applicable to the modern era for several reasons, however, including use of old surgical and RT techniques, split‐course RT paradigms, and treatment based on responders to chemotherapy. Nevertheless, when applied to NCDB data, which do not carry information on local control, operative complications, and stent placement, the comparative value of either modality remains inconclusive. Although it is possible that CRT patients were not “fit” enough to undergo surgery, it is also possible that surgery was delivered to bulkier and “higher‐risk” disease (in spite of the many unknown values in T/N classification). Taken together, we recommend that the choice of local therapy be tailored individually, including use of multidisciplinary discussion and patient input.
The sample sizes in this investigation were still relatively small, and this is likely the cause of the inconclusive findings on multivariable logistic regression analysis. However, this relative balance between groups also indicated that propensity matching was not statistically prudent, which would further decrease sample sizes. Likewise, this study also cannot assess the utility of surgery/CT or surgery/RT vs surgery/CRT, because few patients received the former.
The independent association between treatment at an academic center and higher OS as found on Cox multivariate analysis has far‐reaching implications on patient counseling and management by both oncologists and referring providers. There are many potential reasons for this, not limited to greater multimodality coordination, streamlined and thorough diagnostic processes and multidisciplinary discussion, technical expertise, ancillary staff for closer clinical monitoring, and potentially the availability of salvage treatments (or clinical trials). Nevertheless, these findings could warrant revisions in patterns of patient education, and it is recommended that patients with rare tumors such as ESCC should be treated at academic institutions.
Although the NCDB provides a unique platform with which to study this rare disease, this investigation is not without additional shortcomings to those discussed above. First, this study is not powered to address optimal sequencing of systemic and local therapies, which would result in splitting the cohort's subgroups into even smaller sample sizes and highly inaccurate OS comparisons. To this end, performing a meta‐analysis of available studies, such as those discussed previously,4, 5, 6, 7 would be useful; however, the marked heterogeneity in the available literature (eg merging metastatic and nonmetastatic patients, accounting for chemotherapy, etc.) is a major roadblock to doing so. It is also a major source of bias insofar as patients receiving local therapy may have been carefully selected based on response to induction chemotherapy, which cannot be quantified in the NCDB. Second, the NCDB does not keep track of several other factors, including chemotherapy cycles/agents, performance/functional status, toxicities, postoperative complications, toxicity‐related deaths, or RT field design/volumes/techniques. No information is also provided regarding whether proper workup was performed in each case to rule out a lung primary (eg PET‐CT). Third, it is additionally of particular concern whether the tumor was a mixed small cell tumor or a “pure” small cell neoplasm, although published studies addressing this question in lung cancer differ on the prognostic/predictive impact.33, 34 Fourth, the NCDB does not allow for an assessment of subsequent lines of treatment (eg re‐irradiation, further systemic and/or targeted therapy), which could impact OS. Lastly, the NCDB also does not provide genomic information, which has proven to be of great utility in other esophageal neoplasms.35, 36 Nevertheless, the known shortcomings of a national large‐volume database, the first of its kind to date, do not diminish the necessity for further investigation.
5. CONCLUSIONS
This is the largest study to date evaluating patterns of care and outcomes of ESCC. In the United States, most nonmetastatic ESCC is treated with CRT. As compared to CT alone, delivery of additional local therapy in the form of RT or surgery is associated with improved survival.
CONFLICT OF INTEREST
This has never been presented/published before in any form. All authors declare no conflicts of interest.
Verma V, Sleightholm RL, Fang P, Ryckman JM, Lin C. National Cancer Database report of nonmetastatic esophageal small cell carcinoma. Cancer Med. 2018;7:6365–6373. 10.1002/cam4.1712
REFERENCES
- 1. Nichols GL, Kelsen DP. Small cell carcinoma of the esophagus: The Memorial Hospital experience 1970 to 1987. Cancer 1989;64:1531‐1533. [DOI] [PubMed] [Google Scholar]
- 2. Huncharek M, Muscat J. Small cell carcinoma of the esophagus. The Massachusetts General Hospital experience, 1978 to 1993. Chest 1995;107:179‐181. [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network . Esophageal and Esophagogastric Junction Cancers. Version 4.2017. https://www.nccn.org/professionals/physician_gls/pdf/esophgeal.pdf. Accessed December 22, 2017.
- 4. Brenner B, Shah MA, Gonen M, et al. Small‐cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer 2004;90:1720‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Song Y, Wang W, Tao G, et al. Survival benefit of radiotherapy to patients with small cell esophagus carcinoma – an analysis of Surveillance Epidemiology and End Results (SEER) data. Oncotarget. 2016;7:15474‐15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casas F, Ferrer F, Farrus B, Casals J, Biete A. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80:1366‐1372. [PubMed] [Google Scholar]
- 7. Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol. 2008;3:1460‐1465. [DOI] [PubMed] [Google Scholar]
- 8. Bilimoria K, Stewart A, Winchester D, Ko C. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bott MJ, Patel AP, Verma V, et al. Patterns of care in hilar node‐positive (N1) non‐small cell lung cancer: A missed treatment opportunity? J Thorac Cardiovasc Surg. 2016;151:1549‐1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11‐16. [DOI] [PubMed] [Google Scholar]
- 11. Haque W, Verma V, Butler EB, Teh BS. Patterns of care and outcomes of multi‐agent versus single‐agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol. 2017;133:369‐375. [DOI] [PubMed] [Google Scholar]
- 12. Haque W, Verma V, Butler EB, Teh BS. National practice patterns and outcomes for T4b urothelial cancer of the bladder. Clin Genitourin Cancer. 2017;16:42‐49. 10.1016/j.clgc.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 13. Moreno AC, Verma V, Hofstetter WL, et al. Patterns of care and treatment outcomes of elderly patients with stage I esophageal cancer: analysis of the National Cancer Data Base. J Thorac Oncol. 2017;12:1152‐1160. [DOI] [PubMed] [Google Scholar]
- 14. McMillan MT, Ojerholm E, Verma V, et al. Radiation treatment time and overall survival in locally advanced non‐small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98:1142‐1152. [DOI] [PubMed] [Google Scholar]
- 15. Verma V, Ryckman JM, Simone CB 2nd, Lin C. Patterns of care and outcomes with the addition of chemotherapy to radiation therapy for stage I nasopharyngeal cancer. Acta Oncol. 2017;57:257‐261. 10.1080/0284186x.2017.1351039. [DOI] [PubMed] [Google Scholar]
- 16. Verma V, Ahern CA, Berlind CG, et al. National Cancer Data Base report on pneumonectomy versus lung‐sparing surgery for malignant pleural mesothelioma. J Thorac Oncol. 2017;12:1704‐1714. [DOI] [PubMed] [Google Scholar]
- 17. Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: analysis of the National Cancer Data Base. Gynecol Oncol. 2017;144:28‐33. [DOI] [PubMed] [Google Scholar]
- 18. Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non‐small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:146‐154. [DOI] [PubMed] [Google Scholar]
- 19. Haque W, Verma V, Butler EB, Teh BS. Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol. 2017;135:173‐181. 10.1007/s11060-017-2563-0. [DOI] [PubMed] [Google Scholar]
- 20. Haque W, Verma V, Butler EB, Teh BS. Radical cystectomy versus chemoradiation for musle‐invasive bladder cancer: impact of treatment facility and sociodemographics. Anticancer Res. 2017;37:5603‐5608. [DOI] [PubMed] [Google Scholar]
- 21. Haque W, Verma V, Butler EB, Teh BS. Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol. 2018;9:80‐89. 10.21037/jgo.2017.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verma V, Simone CB, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40:696‐706. doi:/10.1002/hed.24978. [DOI] [PubMed] [Google Scholar]
- 23. Haque W, Verma V, Butler EB, Teh BS. Addition of chemotherapy to hypofractionated radiotherapy for glioblastoma: practice patterns, outcomes, and predictors of survival. J Neurooncol. 2018;136:307‐315. 10.1007/s11060-017-2654-y. [DOI] [PubMed] [Google Scholar]
- 24. Verma V, Allen PK, Simone CB 2nd, Gay HA, Lin SH. Association of treatment at high‐volume facilities with survival in patients receiving chemoradiotherapy for nasopharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2017. 10.1001/jamaoto.2017.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verma V, Allen PK, Simone CB 2nd, Gay HA, Lin SH. Addition of definitive radiotherapy to chemotherapy in patients with newly diagnosed metastatic nasopharyngeal cancer. J Natl Compr Canc Netw. 2017;15:1383‐1391. [DOI] [PubMed] [Google Scholar]
- 26. Haque W, Verma V, Butler EB, Teh BS. Chemotherapy Versus Chemoradiation for Node‐Positive Bladder Cancer: Practice Patterns, Outcomes from the National Cancer Data Base. Bladder Cancer. 2017;3:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haque W, Verma V, Bernicker E, Butler EB, Teh BS. Management of pathologic node‐positive disease following initial surgery for clinical T1‐2 N0 esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. Acta Oncol. 2018;57:782‐789. 10.1080/0284186x.2017.1409435. [DOI] [PubMed] [Google Scholar]
- 28. Haque W, Lewis GD, Verma V, Darcourt JG, Butler EB, Teh BS. The role of adjuvant chemotherapy in locally advanced bladder cancer. Acta Oncol. 2018;57:509‐515. 10.1080/0284186x.2017.1415461. [DOI] [PubMed] [Google Scholar]
- 29. Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small‐cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22:2730‐2739. [DOI] [PubMed] [Google Scholar]
- 30. Pignon JP, Arriagada R, Ihde DC, et al. A meta‐analysis of thoracic radiotherapy for small‐cell lung cancer. N Engl J Med. 1992;327:1618‐1624. [DOI] [PubMed] [Google Scholar]
- 31. Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310‐2317. [DOI] [PubMed] [Google Scholar]
- 32. Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160‐1168. [DOI] [PubMed] [Google Scholar]
- 33. Radice PA, Matthews MJ, Ihde DC, et al. The clinical behavior of “mixed” small cell/large cell bronchogenic carcinoma compared to “pure” small cell subtypes. Cancer. 1982;50:2894‐2902. [DOI] [PubMed] [Google Scholar]
- 34. Mangum MD, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Combined small‐cell and non‐small‐cell lung cancer. J Clin Oncol. 1989;7:607‐612. [DOI] [PubMed] [Google Scholar]
- 35. Wu C, Hu Z, Jia W, et al. Genome‐wide association study identifies three new susceptibility loci for esophageal squamous‐cell carcinoma in Chinese populations. Nat Genet. 2011;43:679‐684. [DOI] [PubMed] [Google Scholar]
- 36. Chang J, Zhong R, Tian J, et al. Exome‐wide analyses identify low‐frequency variant in CYP26B1 and additional coding variants associated with esophageal squamous cell carcinoma. Nat Genet. 2018;50:338‐343. [DOI] [PubMed] [Google Scholar]


