Abstract
Background and Aim
The diagnosis of Hirschsprung's disease (HD) relies on anorectal manometry and rectal biopsy. The role of endoscopic biopsy is uncertain for the diagnosis of HD in children. In this study, we evaluated the adequacy of biopsies procured by endoscopic mucosal resection (EMR) for the diagnosis of HD.
Methods
Consecutive children with suspected HD from January 2013 to January 2018 were enrolled in the study. EMR was performed using the standard band ligation device at a distance of about 3 cm from dentate line in rectum. All samples were assessed macroscopically and microscopically. An adequate sample was defined as those measuring >3 mm and including adequate submucosa.
Results
A total of 132 children underwent evaluation for constipation in the study period. Of these, 10 children (median age, 4.25 years) underwent EMR using the band ligation device for the evaluation of HD. EMR was performed with and without submucosal lifting injection in four and six children, respectively. All the samples were adequate macroscopically (>3 mm). Absence of ganglion cells was noted in six children. Of these, three children underwent full‐thickness rectal biopsy followed by surgery. Three children did not undergo surgery. Ganglion cells were identified in four children, thereby excluding the diagnosis of HD.
Conclusion
Rectal biopsy using EMR with a band ligation device is feasible, safe, and provides adequate sample for the evaluation of HD in children.
Keywords: child, endoscopic mucosal resection, Hirschsprung's disease
Introduction
Hirschsprung's disease (HD) is a rare disease with an incidence of about 1.8 per 10 000 live births.1 HD is characterized by the absence of ganglion cells in the rectosigmoid region, which may or may not extend more proximally.
The diagnosis of HD is aided by clinical history as well as a battery of tests, including barium enema, anorectal manometry, and rectal biopsy. Of these, rectal biopsy remains the gold standard for confirming the diagnosis of HD.2 Among the various techniques used for rectal biopsy, suction biopsy is the most commonly used. However, suction biopsy may not be diagnostic in older children and adults due to increased thickness of rectal mucosa.3, 4 Recently, endoscopic mucosal resection (EMR) has been shown to be safe and feasible for acquiring rectal biopsy specimens in adult patients.4 EMR has not been evaluated in children with suspected HD. In this study, we analyzed the feasibility and safety of EMR in the evaluation of suspected HD in children.
Methods
The data of all children who underwent evaluation for HD from January 2013 to January 2018 were analyzed retrospectively. Inclusion criteria were (i) age < 18 years; (ii) suspected HD with the presence of least one of the two following parameters: absence of rectoanal inhibitory reflex on anorectal manometry or radiological findings consistent with HD; and (iii) children who underwent the acquisition of rectal tissue endoscopically. Exclusion criteria were failure to give consent, coagulation disorder, children in whom biopsy specimens were obtained surgically, and absence of manometry and radiological features suggestive of HD.
Preprocedure evaluation
All children underwent a standard set of investigations prior to the procedure. These included complete hemogram, prothrombin time, partial thromboplastin time, serum calcium, thyroid‐stimulating hormone, barium enema, and anorectal manometry.
Bowel preparation was performed either with weight‐based PEG‐3350 regimen (4 g/kg/day) or pediatric enema alone.
Equipment and accessories
The equipment used for obtaining an endoscopic biopsy included an endoscope with integrated waterjet function (outer diameter 9.9 mm) (GIF HQ 190; Olympus Corp., Tokyo, Japan), electrosurgical unit (VIO300D; ERBE, Tübingen, Germany), multiband ligator (MBL‐U‐4, Cook Medical), and oval polypectomy snare (SnareMaster, Olympus).
Procedure details
We have recently described the technique of band‐EMR for acquiring rectal tissue specimens in a case with suspected HD.5 In brief, the technique is as subsequently described.
The procedure was performed in left lateral position under conscious sedation supervised by a pediatric anesthetist.
First, any residual fecal matter was thoroughly washed with a water jet prior to the procedure.
An appropriate site was chosen at a distance of about 2–3 cm from anal verge along the posterior rectal wall. Subsequently, band‐EMR was performed with or without prior submucosal lifting injection. The decision to inject a submucosal saline solution mixed with indigo carmine was left to the discretion of the endoscopist performing the procedure. After mounting the band ligation device on the gastroscope, suction was applied at the preselected site to suck the rectal mucosa inside the cap of band ligator device. Thereafter, the band was deployed by rotating the ligator handle clockwise until band release. The rectal tissue within the band was cut using a polypectomy snare that was positioned below the band. The resected specimen was retrieved with the same snare (Fig. 1) (see Video, Supplemental Digital Content 1). All children were observed for 4–6 h after the procedure and discharged on the same day.
Figure 1.
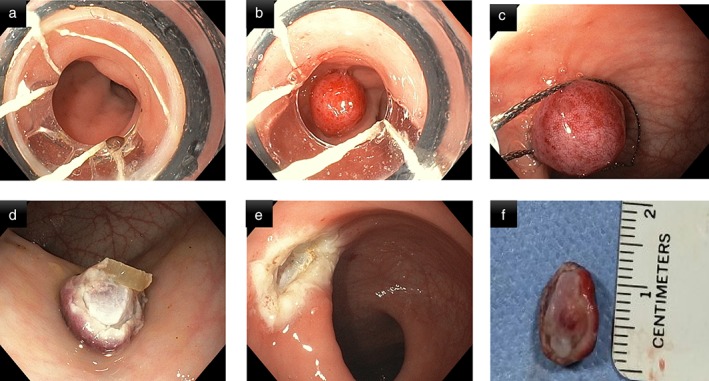
Technique of rectal biopsy using band ligation‐assisted endoscopic mucosal resection. (a) Mounting of band ligator on gastroscope and selection of appropriate site for endoscopic mucosal resection (EMR); (b) band ligation of rectal mucosa at the selected site; (c) resection of the suctioned rectal mucosa with polypectomy snare; (d) sample of rectal tissue after band—EMR; (e) inspection of EMR site for bleeding or perforation; (f) measurement of dimensions of specimen obtained.
The dimensions of the tissue obtained were measured using a scale, and the sample was sent for histopathological examination. The adequacy of sample was determined by the pathologist according to the extent of submucosa included. Immunohistochemistry using calretinin, NSE, and bcl2 was performed in addition to routine hematoxylin and eosin staining to identify the presence or absence of ganglion cells (Fig. 2).
Figure 2.
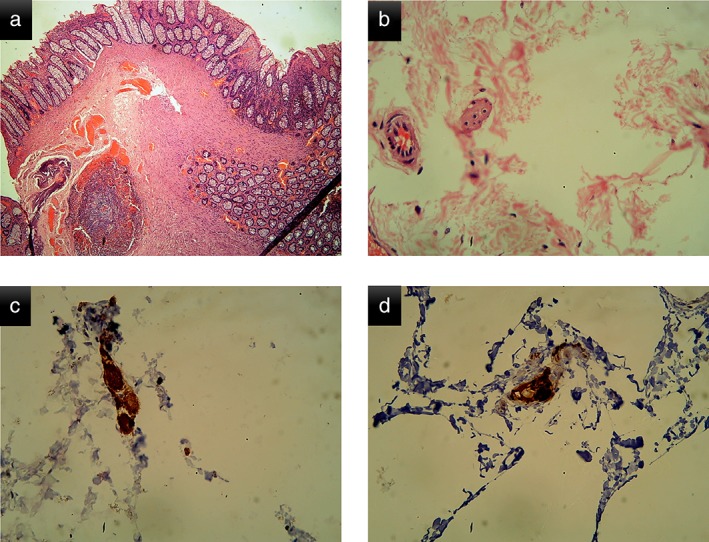
Histopathological features of the resected specimen in one of the children. (a) Low‐power microscopic view of the rectal biopsy (note the presence of submucosa in the sample); (b) high‐power view of the rectal biopsy showing the presence of ganglion cell; (c) immunohistochemistry using calretinin confirming the presence of ganglion cell; and (d) immunohistochemistry using bcl2 confirming the presence of ganglion cell.
In children with aganglionosis on endoscopic biopsy, full‐thickness biopsy was offered prior to surgery to confirm the diagnosis. Histopathological evaluation of the resected specimen was also performed in children who underwent surgery.
Results
A total of 132 children were evaluated for chronic constipation. Of these, HD was suspected in 23 children based on findings of barium enema and anorectal manometry. Ten children (median age 4.25 years, 6 boys, range 3–15) underwent endoscopic rectal tissue acquisition using band‐EMR and were included in the final analysis (Fig. 3).
Figure 3.
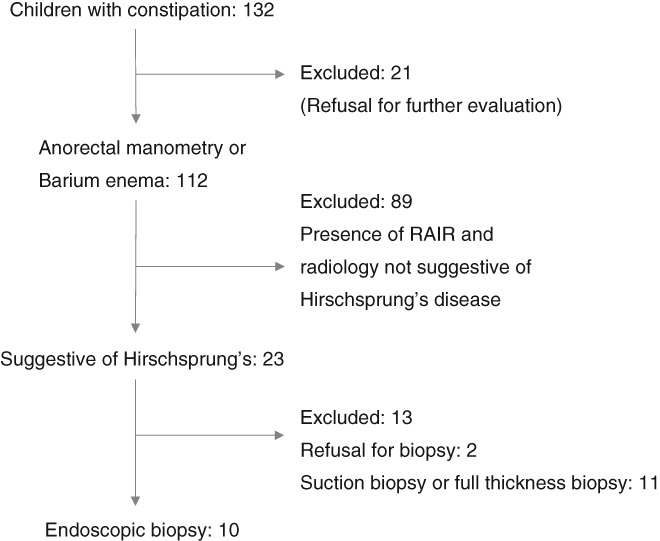
Analysis flow chart of children presenting with constipation.
The median duration of symptoms was 3.5 years (0.5–8 years) (Table 1). Anorectal manometry was performed in nine children, and absence of rectoanal inhibitory reflex was documented in eight (80%).
Table 1.
Demographic characteristics of children with suspected Hirschsprung's disease
| Cases | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 3 | 5 | 6.5 | 3 | 15 | 4 | 4.5 | 3.5 | 3 | 8 |
| Age of onset of constipation | 2.5 years | 1 years | 1 month | 6 months | 8 years | 9 months | 1 months | 5 months | 1.5 year | 1 months |
| Duration of constipation | 6 months | 4 years | 6.5 years | 2.5 years | 7 years | 3 years | 4.5 years | 3 years | 1.5 years | 8 years |
| Other symptoms | Abdominal distention, FTT | Anemia | Abdominal distention, FTT | None | None | FTT, abdominal distention | Abdominal distention | Abdominal distention | Abdominal distention | FTT, abdominal distention |
| Barium enema | Dilated colon | Dilated colon | Reversal of rectosigmoid ratio with transition zone | Normal | Reversal of rectosigmoid ratio with transition zone | Dilated colon | Reversal of rectosigmoid ration with transition zone | Normal | Dilated colon | Reversal of rectosigmoid ration with transition zone |
| Anorectal manometry | Absent RAIR | Incomplete RAIR | Absent RAIR | Absent RAIR | Absent RAIR | Absent RAIR | Absent RAIR | RAIR present | — | Absent RAIR |
FTT, failure to thrive; RAIR, rectoanal inhibitory reflex.
In four children (age < 4 years), submucosal injection was given prior to band EMR. In the other six children (age 4–15 years), band‐EMR was performed without the saline lifting injection (Table 2). The procedure was successfully completed in all the children. There were no immediate or delayed complications like bleeding or perforation in either group. Endoclips were applied in one case at the resection site due to suspicion of deep resection. In this case band‐EMR was performed without submucosal injection. None of the children required hospitalization after the procedure.
Table 2.
Characteristics of rectal biopsy samples obtained by band‐assisted endoscopic mucosal resection
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Submucosal injection | Yes | No | No | Yes | No | No | No | Yes | Yes | No |
| Histopathology (H and E) (ganglion cells in submucosa) | Present | Present | Absent | Present | Absent | Absent | Absent | Present | Absent | Absent |
| IHC (S 100, calretinin and NSE) | Positive | Positive | Negative | Positive | Negative | Negative | Negative | Negative | Negative | Negative |
| Final diagnosis—Hirschsprung's disease | No | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
H and E, Hematoxylin and eosin; IHC, immunohistochemistry; NSE, neuron‐specific enolase.
The adequacy of specimens as analyzed by the pathologist was documented in all the specimens. Adequate submucosa was obtained in both the groups, that is, with and without submucosal injection. The diagnosis of HD was established in six children with the absence of ganglion cells on histological examination. Of these six children, three underwent full‐thickness surgical biopsy for confirmation of aganglionosis prior to surgery. The histopathological evaluation of resected specimens was concordant with endoscopic and full‐thickness rectal biopsy in these three cases. The remaining three children with aganglionosis on endoscopic biopsy did not undergo full‐thickness biopsy and are awaiting surgery.
In four children, ganglion cells were identified on endoscopic biopsy specimen, and the diagnosis of HD could be reliably excluded (Table 2).
Discussion
In this study, we demonstrated that EMR using the band ligation device is safe and effective in obtaining biopsies for the evaluation of suspected HD in children. Adequate tissue samples can be obtained with this technique as judged macroscopically and microscopically. To our knowledge, this is the first study that describes the use of EMR for the diagnosis of HD in a pediatric population.
The diagnostic evaluation of HD consists of a contrast enema, anorectal manometry, and rectal biopsy. Of these, contrast enema and anorectal manometry are not confirmatory, and rectal biopsy is required to confirm the diagnosis.6, 7, 8, 9, 10 In our study, rectoanal inhibitory reflex (RAIR) was absent in a majority of the children undergoing rectal biopsy. Of these, the diagnosis of HD could be confirmed in three children who underwent both endoscopic and surgical biopsy. Importantly, the diagnosis of HD was refuted in four children with absence of RAIR but in whom ganglion cells were identified on biopsy. Therefore, objective documentation of absence of ganglion cells is mandatory before concluding the diagnosis of HD based on anorectal manometry alone.
Various techniques have been described to procure rectal biopsy. These include suction biopsy, full‐thickness rectal biopsy, and biopsy with a jumbo forceps.6 Rectal suction biopsy is the most preferred sampling technique for the diagnosis of HD. Although suction biopsy may be adequate in neonates and infants, the same may provide an insufficient sample in older children due to more fibrous submucosa.11 On the other hand, full‐thickness biopsy provides sufficient tissue for the confirmation of diagnosis. However, this technique is more invasive and requires general anesthesia and an operating theater. For the same reason, full‐thickness biopsy is not usually preferred as the initial method of procuring a sample in children with suspected HD.
In the present study, we evaluated a novel minimally invasive endoscopic technique for procuring biopsies in children with suspected HD. The endoscopic procedure using a band ligation device could be successfully performed in all the children. There were no episodes of bleeding, perforation, and sepsis. In addition, all the procedures could be performed in outpatient settings. EMR using multiband ligator or cap‐assisted snaring is not new and has been reported in patients with Barrett's esophagus.12 However, band‐assisted EMR for procuring rectal tissue has not been described previously. In a recent prospective study including 17 adult patients (mean age: 35.8 years), the use of EMR has been described to obtain rectal tissue. In contrast to our study, the authors used a special transparent plastic cap with a prelooped snare (Cook Ireland Ltd., Limerick, Ireland) to cut lesions that are suctioned into the cap.4 The other important difference in our study was that we used a submucosal injection in four children. The rectal wall is thinner in young children compared to older children and adults. Therefore, submucosal injection may help in avoiding perforations and adds to the safety of the procedure. On the contrary, submucosal injection may compromise the adequacy of biopsy specimen in older children and adults. In our opinion, the technique to obtain biopsy specimens needs to be individualized according to the age of the child.
Adequate submucosa was obtained in all the 10 specimens as observed by the pathologist. We did not need to repeat the procedure in any of our children. The main issue with rectal suction biopsy is an inadequate sample in a sizeable proportion of patients. Therefore, multiple biopsies may be required. In a recent study, the sample volumes were significantly lower in the suction biopsy group compared to EMR group (0.023 cm3 vs 0.26 cm3, P = 0.001).4 In the same study, adequate submucosal tissue was absent in about half of the patients in the rectal suction biopsy group.4 In other studies, about 10–20% of rectal suction biopsies proved to be inadequate either due to small size or insufficient inclusion of submucosa.13, 14, 15, 16, 17 Therefore, endoscopic sampling using band‐EMR can be a useful alternative to the conventional biopsy techniques.
The advantages of using EMR‐band ligation are manifold: (i) the technique is easy and does not require special expertise; (ii) the band ligation device is universally available; (iii) the small size of device allows its use in children as well; (iv) multiple biopsies can be obtained in the same session if the initial sample is considered inadequate; and (v) the procedure can be performed in outpatient settings.
The strengths of our study include the following: this is the first study evaluating the role of EMR for tissue acquisition in the diagnosis of HD in children. We also performed immunohistochemical staining using calretinin, bcl2, and NSE for the confirmation of diagnosis. However, certain drawbacks are noteworthy. These include a small number of patients and the lack of a control group. We included children most likely to be benefitted with rectal biopsy, that is, absence of RAIR and findings suggestive of HD on barium enema. For these reasons, the sample size was small. There were no children in the infancy age group (<1 year), and therefore, the results cannot be applied to that population. Full‐thickness rectal biopsy and surgery were performed in only three of the six children with absence of ganglion cells in initial histology. However, a second rectal biopsy may not be required if the initial specimen is considered adequate by the pathologist. The documentation of ganglion cells in four children helped in ruling out HD, and no further investigations were required.
In conclusion, EMR with a band ligation device can be safely and reliably used for obtaining rectal biopsy in children with suspected HD. Large prospective and randomized trials are required to establish the results of present study. In addition, comparison of both the techniques, that is, with and without submucosal injection, is required.
Supporting information
Video S1 Supplemental Digital Content 1. Technique of band‐assisted endoscopic mucosal resection
Financial disclosures: None.
Declaration of conflict of interest: None.
References
- 1. Bradnock TJ, Knight M, Kenny S, Nair M, Walker GM, British Association of Paediatric Surgeons Congenital Anomalies Surveillance System . Hirschsprung's disease in the UK and Ireland: incidence and anomalies. Arch. Dis. Child. 2017; 102: 722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Lorijn F, Reitsma JB, Voskuijl WP et al Diagnosis of Hirschsprung's disease: a prospective, comparative accuracy study of common tests. J. Pediatr. 2005; 146: 787–92. [DOI] [PubMed] [Google Scholar]
- 3. Croffie JM, Davis MM, Faught PR et al At what age is a suction rectal biopsy less likely to provide adequate tissue for identification of ganglion cells? J. Pediatr. Gastroenterol. Nutr. 2007; 44: 198–202. [DOI] [PubMed] [Google Scholar]
- 4. Barshop K, Willingham FF, Brugge WR, Zukerberg LR, Kuo B. EMR is superior to rectal suction biopsy for analysis of enteric ganglia in constipation and dysmotility. Gastrointest. Endosc. 2018; 87: 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nabi Z, Chavan R, Shava U, Sekharan A, Reddy DN. A novel endoscopic technique to obtain rectal biopsy specimens in children with suspected Hirschsprung's disease. Video GIE. 2018; 3: 157–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takawira C, D'Agostini S, Shenouda S, Persad R, Sergi C. Laboratory procedures update on Hirschsprung disease. J. Pediatr. Gastroenterol. Nutr. 2015; 60: 598–605. [DOI] [PubMed] [Google Scholar]
- 7. Low PS, Quak SH, Prabhakaran K, Joseph VT, Chiang GS, Aiyathurai EJ. Accuracy of anorectal manometry in the diagnosis of Hirschsprung's disease. J. Pediatr. Gastroenterol. Nutr. 1989; 9: 342–6. [DOI] [PubMed] [Google Scholar]
- 8. Loening‐Baucke V, Pringle KC, Ekwo EE. Anorectal manometry for the exclusion of Hirschsprung's disease in neonates. J. Pediatr. Gastroenterol. Nutr. 1985; 4: 596–603. [DOI] [PubMed] [Google Scholar]
- 9. Huang Y, Zheng S, Xiao X. Preliminary evaluation of anorectal manometry in diagnosing Hirschsprung's disease in neonates. Pediatr. Surg. Int. 2009; 25: 41–5. [DOI] [PubMed] [Google Scholar]
- 10. Emir H, Akman M, Sarimurat N, Kilic N, Erdogan E, Soylet Y. Anorectal manometry during the neonatal period: its specificity in the diagnosis of Hirschsprung's disease. Eur. J. Pediatr. Surg. 1999; 9: 101–3. [DOI] [PubMed] [Google Scholar]
- 11. Kapur RP. Practical pathology and genetics of Hirschsprung's disease. Semin. Pediatr. Surg. 2009; 18: 212–23. [DOI] [PubMed] [Google Scholar]
- 12. Chennat J, Konda VJ, Ross AS et al Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high‐grade dysplasia and intramucosal carcinoma—an American single‐center experience. Am. J. Gastroenterol. 2009; 104: 2684–92. [DOI] [PubMed] [Google Scholar]
- 13. Meinds RJ, Kuiper GA, Parry K et al Infant's age influences the accuracy of rectal suction biopsies for diagnosing of Hirschsprung's disease. Clin. Gastroenterol. Hepatol. 2015; 13: 1801–7. [DOI] [PubMed] [Google Scholar]
- 14. Friedmacher F, Puri P. Current practice patterns of rectal suction biopsy in the diagnostic work‐up of Hirschsprung's disease: results from an international survey. Pediatr. Surg. Int. 2016; 32: 717–22. [DOI] [PubMed] [Google Scholar]
- 15. Keyzer‐Dekker CM, Sloots CE, Schokker‐van Linschoten IK, Biermann K, Meeussen C, Doukas M. Effectiveness of rectal suction biopsy in diagnosing Hirschsprung disease. Eur. J. Pediatr. Surg. 2016; 26: 100–5. [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi H, Li Z, Yamataka A, Lane GJ, Miyano T. Rectal biopsy: what is the optimal procedure? Pediatr. Surg. Int. 2002; 18: 753–6. [DOI] [PubMed] [Google Scholar]
- 17. Alizai NK, Batcup G, Dixon MF, Stringer MD. Rectal biopsy for Hirschsprung's disease: what is the optimum method? Pediatr. Surg. Int. 1998; 13: 121–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Supplemental Digital Content 1. Technique of band‐assisted endoscopic mucosal resection


