Abstract
Objectives
To compare overall survival (OS) in locally advanced hypopharyngeal cancer treated with surgery or definitive chemoradiotherapy in the contemporary era.
Methods
From 2010 to 2015, data for patients diagnosed with hypopharyngeal cancer (T2‐T4aM0) and treated with total pharyngectomy with lymph node dissection (surgery group) or definitive radiotherapy and chemotherapy (chemoradiotherapy group) was retrieved from the SEER database. Multivariate analyses were performed in each subgroup divided according to T category (T2‐3 or T4a).
Results
The number of patients in the surgery and chemoradiotherapy groups was 209 and 648, respectively. Among them, the number of T4a patients was 111 and 126 in each group. Three‐year OS rate in the surgery and chemoradiotherapy groups was 37.9% and 44.1%, respectively (P = 0.178). The 3‐year OS rate for the T2‐3 patients was 46.5% and 48.7% (P = 0.598), and the 3‐year OS rate for the T4a patients was 29.9% and 26.1% in the surgery and chemoradiotherapy groups, respectively (P = 0.439). On multivariate analysis, the chemoradiotherapy group was not inferior to the surgery group in T2‐T4a patients (Hazard ratio [HR] for the chemoradiotherapy group 0.889, 95% confidence interval [CI] 0.699‐1.129, P = 0.334), in T2‐3 patients (HR 0.932, 95% CI 0.699‐1.297, P = 0.675), and in T4a patients (HR 0.880, 95% CI 0.617‐1.256, P = 0.481).
Conclusions
Chemoradiotherapy for locally advanced hypophagyngeal cancer showed a comparable OS rate to surgery. For patients with T4a category cancer with high possibility of preserving the laryngopharyngeal function, chemoradiotherapy may be a promising alternative treatment.
Keywords: chemoradiotherapy, locally advanced hypopharyngeal cancer, SEER, total pharyngectomy
1. INTRODUCTION
Hypopharyngeal cancer is a poor prognostic cancer.1 The 5‐year overall survival (OS) rate is approximately 35%.2 Although the survival rate has been significantly improved,3 the absolute survival rate has been strikingly restricted for decades compared to human papilloma virus‐related oropharyngeal cancer.4
Optimal treatment for locally advanced hypopharyngeal cancer is controversial, especially in T4a cancer. For T4a hypopharyngeal cancer, (chemo)radiotherapy showed a poor survival rate compared to the radical surgery,2, 5, 6 and the invasion of thyroid or cricoid cartilage were considered as to be difficult to preserve laryngopharyngeal function after chemoradiotherapy.7 Therefore, surgery is considered as the treatment of choice for T4a cancer. According to the National Comprehensive Cancer Network Categories of Evidence and Consensus, surgery is category 2A (uniform consensus) for T4a hypopharyngeal cancer, whereas chemoradiotherapy is category 3 (major disagreement).8 The EHNS‐ESMO‐ESTRO guideline suggests that patients who have massive larynx cartilage invasion are not suitable for organ‐preserving treatment.9
However, the use of chemoradiotherapy is increasing and have been the major treatments for locally advanced hypopharyngeal cancer.10 Recently, techniques of radiotherapy have been improved. Intensity‐modulated radiotherapy (IMRT) has been gradually generalized in clinic since early 2000,11 and IMRT and accelerated radiotherapy showed an improved local control rate compared to conventional 3‐dimensional conformal radiotherapy.12, 13 Combined chemotherapy also showed improved treatment outcomes.14 Therefore, the latest treatment outcomes of chemoradiotherapy for locally advanced hypopharyngeal cancer including T4a cancer might be improved compared to the historical reports.
In this study, we analyzed the latest population‐based database to compare chemoradiotherapy with surgery in locally advanced pharyngeal cancer.
2. METHODS AND MATERIALS
2.1. Patient population
SEER 18 registry were used to retrieve a list of patients. Inclusion criteria were as follows: (1) pathologically diagnosed hypopharynx squamous cell carcinoma between 2010 and 2015, (2) T2‐4aN0‐3M0 based on the 7th edition of the American Joint Committee on Cancer staging system, (3) having information of race, age, and tumor grade, (4) and treated with total pharyngectomy (code 32‐52) with 10 or more lymph nodes dissection (surgery group) or definitive external beam radiotherapy and chemotherapy without surgery or lymph node dissection (chemoradiotherapy group; Figure 1).
Figure 1.
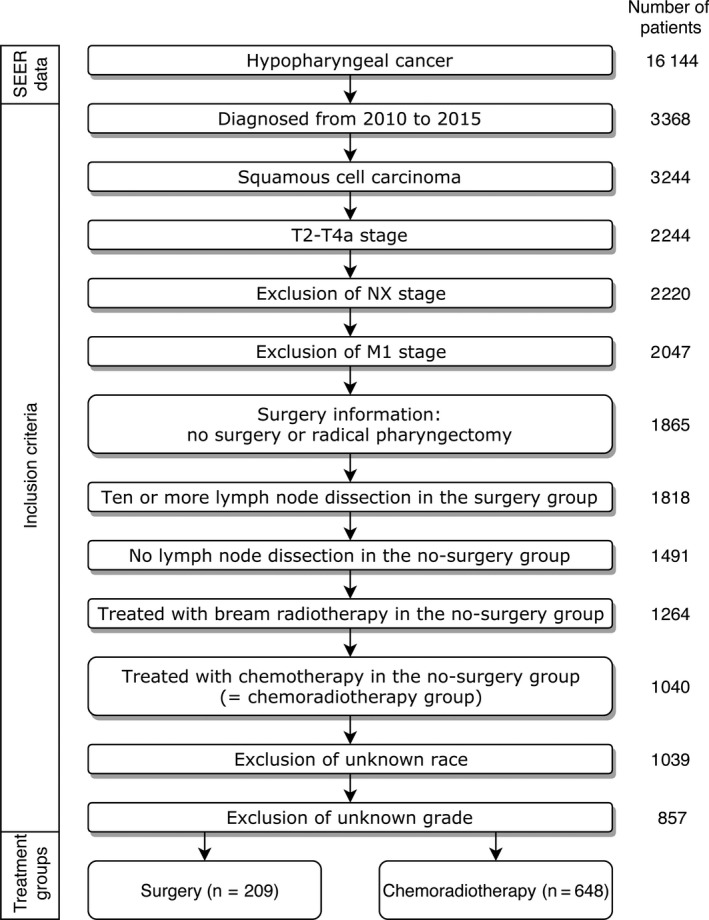
Flowchart of patient selection
Surgery codes from 32 to 52 include total pharyngectomy, pharyngectomy with laryngectomy or removal of contiguous bone tissue, radical pharyngectomy which includes total mandibular resection with or without laryngectomy. All treatments (surgery, radiotherapy, and chemotherapy) were the first course of treatment at the time of diagnosis.
2.2. Statistical analysis
Characteristics of the surgery and chemoradiotherapy groups were compared using the Pearson’s chi‐squared test. Three‐year OS rates of the surgery and chemoradiotherapy groups were calculated by the Kaplan‐Meier survival estimate. Univariate analyses were performed to evaluate prognostic significances of clinicopathologic variables on the OS rates using the Kaplan‐Meier method followed by a log‐rank test. Variables which were significantly prognostic in the univariate analysis, or significantly different between the surgery and chemoradiotherapy groups, or considered clinically important were incorporated in a multivariate analysis using the Cox’s proportional hazard model. Two‐sided P‐value <0.05 were considered as statistically significant. The R software ver 3.5.1 (https://www.r-project.org) was used for all analysis. Graphs were created using the “survminer” R package.
3. RESULTS
3.1. Patient characteristics
The characteristics of patients are summarized in Table 1. The number of the patients who were diagnosed with hypopharyngeal cancer from 2010 to 2015 was 16144. Finally, the number of the patients who were satisfied the inclusion criteria was 857 (Figure 1). Among them, 209 (24.4%) and 648 (75.6%) patients were included in the surgery and chemoradiotherapy groups, respectively, suggesting that definitive chemoradiotherapy were more chosen as a primary treatment for locally advanced hypopharyngeal cancer.
Table 1.
Patient characteristics
| Characteristics | All | T2‐T3 | T4a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Surgery | Chemoradiotherapy | P value* | Surgery | Chemoradiotherapy | P value* | Surgery | Chemoradiotherapy | P value* | |
| (N = 209) | (N = 648) | (N = 98) | (N = 522) | (N = 111) | (N = 126) | ||||
| Age | |||||||||
| <65 y | 100 (47.8%) | 358 (55.2%) | 0.074 | 38 (38.8%) | 287 (55.0%) | 0.005 | 62 (55.9%) | 71 (56.3%) | 1.000 |
| ≥65 y | 109 (52.2%) | 290 (44.8%) | 60 (61.2%) | 235 (45.0%) | 49 (44.1%) | 55 (43.7%) | |||
| Race | |||||||||
| White | 164 (78.5%) | 508 (78.4%) | 0.947 | 75 (76.5%) | 413 (79.1%) | 0.735 | 89 (80.2%) | 95 (75.4%) | 0.653 |
| Black | 30 (14.4%) | 97 (15.0%) | 15 (15.3%) | 77 (14.8%) | 15 (13.5%) | 20 (15.9%) | |||
| Others | 15 (7.2%) | 43 (6.6%) | 8 (8.2%) | 32 (6.1%) | 7 (6.3%) | 11 (8.7%) | |||
| Sex | |||||||||
| Male | 171 (81.8%) | 535 (82.6%) | 0.888 | 77 (78.6%) | 425 (81.4%) | 0.604 | 94 (84.7%) | 110 (87.3%) | 0.695 |
| Female | 38 (18.2%) | 113 (17.4%) | 21 (21.4%) | 97 (18.6%) | 17 (15.3%) | 16 (12.7%) | |||
| Subsite | |||||||||
| Pyriform sinus | 116 (55.5%) | 318 (49.1%) | 0.187 | 45 (45.9%) | 256 (49.0%) | 0.736 | 71 (64.0%) | 62 (49.2%) | 0.070 |
| Other sites | 38 (18.2%) | 152 (23.5%) | 23 (23.5%) | 126 (24.1%) | 15 (13.5%) | 26 (20.6%) | |||
| Not otherwise specified | 55 (26.3%) | 178 (27.5%) | 30 (30.6%) | 140 (26.8%) | 25 (22.5%) | 38 (30.2%) | |||
| Grade | |||||||||
| 1‐2 | 113 (54.1%) | 390 (60.2%) | 0.139 | 57 (58.2%) | 317 (60.7%) | 0.716 | 56 (50.5%) | 73 (57.9%) | 0.306 |
| 3‐4 | 96 (45.9%) | 258 (39.8%) | 41 (41.8%) | 205 (39.3%) | 55 (49.5%) | 53 (42.1%) | |||
| T category | |||||||||
| T2 | 36 (17.2%) | 282 (43.5%) | <0.001 | 36 (36.7%) | 282 (54.0%) | 0.002 | 0 (0.0%) | 0 (0.0%) | NA |
| T3 | 62 (29.7%) | 240 (37.0%) | 62 (63.3%) | 240 (46.0%) | 0 (0.0%) | 0 (0.0%) | |||
| T4a | 111 (53.1%) | 126 (19.4%) | 0 (0.0%) | 0 (0.0%) | 111 (100.0%) | 126 (100.0%) | |||
| N category | |||||||||
| N0 | 67 (32.1%) | 181 (27.9%) | 0.230 | 45 (45.9%) | 146 (28.0%) | 0.004 | 22 (19.8%) | 35 (27.8%) | 0.522 |
| N1 | 33 (15.8%) | 135 (20.8%) | 14 (14.3%) | 113 (21.6%) | 19 (17.1%) | 22 (17.5%) | |||
| N2 | 106 (50.7%) | 314 (48.5%) | 38 (38.8%) | 247 (47.3%) | 68 (61.3%) | 67 (53.2%) | |||
| N3 | 3 (1.4%) | 18 (2.8%) | 1 (1.0%) | 16 (3.1%) | 2 (1.8%) | 2 (1.6%) | |||
| Insurance | |||||||||
| Insured | 152 (72.7%) | 475 (73.3%) | 0.664 | 74 (75.5%) | 394 (75.5%) | 0.909 | 78 (70.3%) | 81 (64.3%) | 0.592 |
| Medicaid | 47 (22.5%) | 133 (20.5%) | 19 (19.4%) | 96 (18.4%) | 28 (25.2%) | 37 (29.4%) | |||
| Uninsured/unknown | 10 (4.8%) | 40 (6.2%) | 5 (5.1%) | 32 (6.1%) | 5 (4.5%) | 8 (6.3%) | |||
| Marriage | |||||||||
| Married | 91 (43.5%) | 315 (48.6%) | 0.231 | 48 (49.0%) | 259 (49.6%) | 0.995 | 43 (38.7%) | 56 (44.4%) | 0.449 |
| Others/unknown | 118 (56.5%) | 333 (51.4%) | 50 (51.0%) | 263 (50.4%) | 68 (61.3%) | 70 (55.6%) | |||
| No. of examined lymph nodes | |||||||||
| 0 | 0 (0.0%) | 648 (100%) | <0.001 | 0 (0.0%) | 522 (100%) | <0.001 | 0 (0.0%) | 126 (100%) | <0.001 |
| 10≤ | 209 (100%) | 0 (0.0%) | 98 (100%) | 0 (0.0%) | 111 (100%) | 0 (0.0%) | |||
| Radiotherapy | |||||||||
| No | 89 (42.6%) | 0 (0.0%) | <0.001 | 56 (57.1%) | 0 (0.0%) | <0.001 | 33 (29.7%) | 0 (0.0%) | <0.001 |
| Yes | 120 (57.4%) | 648 (100.0%) | 42 (42.9%) | 522 (100.0%) | 78 (70.3%) | 126 (100.0%) | |||
| Chemotherapy | |||||||||
| No | 121 (57.9%) | 0 (0.0%) | <0.001 | 70 (71.4%) | 0 (0.0%) | <0.001 | 51 (45.9%) | 0 (0.0%) | <0.001 |
| Yes | 88 (42.1%) | 648 (100.0%) | 28 (28.6%) | 522 (100.0%) | 60 (54.1%) | 126 (100.0%) | |||
| Radiotherapy + Chemotherapy | |||||||||
| No | 125 (59.8%) | 0 (0.0%) | <0.001 | 73 (74.5%) | 0 (0.0%) | <0.001 | 52 (46.8%) | 0 (0.0%) | <0.001 |
| Yes | 84 (40.2%) | 648 (100.0%) | 25 (25.5%) | 522 (100.0%) | 59 (53.2%) | 126 (100.0%) | |||
Pearson’s chi‐squared test.
Patients in the surgery group were older (P = 0.074) and had higher T stage (P < 0.001) than those in the chemoradiotherapy group. In the subgroup of T2‐3 categories, patients who underwent chemoradiotherapy had significantly high N stage (P = 0.004)—approximately half of the patients who received surgery were N0 category (45.9%). In the subgroup of T4a category, the proportion of pyriform sinus tumor among the subsites showed higher tendency in the surgery group (P = 0.070). Otherwise, there were no significant differences between the two treatment groups in the T4a category.
Among all patients in the surgery group, radiotherapy was performed in 120 (57.4%) patients and chemotherapy was performed in 88 (42.1%) patients, respectively. The number of patients in the surgery group who were treated with both radiotherapy and chemotherapy was 84 (40.2%).
3.2. Overall survival rate
Three‐year OS rate in the surgery and chemoradiotherapy groups was 37.9% and 44.1%, respectively (P = 0.178; Table 2; Figure 2A). No subgroup including T4a category showed statistically significant OS difference between the surgery and chemoradiotherapy group.
Table 2.
Univariate analysis for overall survival rate in locally advanced hypopharyngeal cancer
| Characteristics | Surgery | Chemoradiotherapy | P value* | ||
|---|---|---|---|---|---|
| 3‐y OS (%) | 95% CI | 3‐y OS (%) | 95% CI | ||
| All | 37.9 | 30.5‐47.2 | 44.1 | 39.8‐48.9 | 0.178 |
| Age | |||||
| <65 y | 39.8 | 29.4‐54.1 | 49.2 | 43.5‐55.7 | 0.109 |
| ≥65 y | 36.4 | 26.6‐49.8 | 37.5 | 31.3‐44.9 | 0.972 |
| P value* | 0.532 | <0.001 | <0.001 | ||
| Race | |||||
| White | 41.9 | 33.2‐52.9 | 45.9 | 41.0‐51.2 | 0.511 |
| Black | 28.1 | 14.3‐55.1 | 34.4 | 24.0‐49.3 | 0.188 |
| Others | 17.0 | 4.8‐60.0 | 42.7 | 29.3‐62.2 | 0.260 |
| P value* | 0.046 | 0.227 | 0.025 | ||
| Sex | |||||
| Male | 36.7 | 28.6‐47.2 | 44.4 | 39.7‐49.7 | 0.167 |
| Female | 43.5 | 28.1‐67.4 | 42.2 | 32.5‐54.8 | 0.827 |
| P value* | 0.722 | 0.716 | 0.872 | ||
| Subsite | |||||
| Pyriform sinus | 30.6 | 21.5‐43.5 | 46.0 | 40.0‐53.1 | 0.082 |
| Other sites | 47.5 | 32.2‐70.0 | 39.8 | 31.5‐50.5 | 0.878 |
| Not otherwise specified | 49.8 | 36.7‐67.6 | 44.2 | 36.5‐53.6 | 0.780 |
| P value* | 0.740 | 0.268 | 0.322 | ||
| Grade | |||||
| 1‐2 | 39.1 | 29.0‐52.8 | 46.8 | 41.4‐53.0 | 0.145 |
| 3‐4 | 36.6 | 26.5‐50.6 | 39.9 | 33.3‐47.9 | 0.770 |
| P value* | 0.926 | 0.167 | 0.194 | ||
| T category | |||||
| T2 | 39.8 | 23.4‐67.7 | 53.9 | 47.3‐61.4 | 0.167 |
| T3 | 49.9 | 37.7‐65.9 | 42.8 | 36.0‐50.9 | 0.580 |
| T4a | 29.9 | 20.5‐43.7 | 26.1 | 18.4‐37.0 | 0.439 |
| P value* | 0.200 | <0.001 | <0.001 | ||
| N category | |||||
| N0 | 51.7 | 39.3‐67.9 | 46.0 | 38.2‐55.5 | 0.633 |
| N1 | 43.2 | 25.7‐72.7 | 51.1 | 42.0‐62.2 | 0.895 |
| N2 | 28.3 | 19.0‐41.9 | 41.9 | 35.9‐48.8 | 0.061 |
| N3 | 33.3 | 6.7‐100.0 | 14.5 | 2.9‐72.0 | 0.355 |
| P value* | 0.216 | <0.001 | 0.003 | ||
| Insurance | |||||
| Insured | 43.9 | 34.8‐55.3 | 47.7 | 42.7‐53.3 | 0.450 |
| Medicaid | 19.9 | 10.0‐39.4 | 32.9 | 24.4‐44.4 | 0.149 |
| Uninsured/unknown | 43.8 | 18.9‐100.0 | 36.6 | 22.5‐59.3 | 0.654 |
| P value* | 0.019 | 0.010 | <0.001 | ||
| Marriage | |||||
| Married | 36.0 | 25.1‐51.6 | 52.1 | 45.9‐59.2 | 0.061 |
| Others/unknown | 35.4 | 25.9‐48.5 | 34.9 | 29.1‐41.9 | 0.536 |
| P value* | 0.629 | <0.001 | <0.001 | ||
| No. of examined lymph nodes | |||||
| 0 | NA | NA | 44.1 | 39.8‐48.9 | NA |
| 10≤ | 37.9 | 30.5‐47.2 | NA | NA | NA |
| P value* | NA | NA | 0.178 | ||
| Radiotherapy | |||||
| No | 36.1 | 26.1‐49.9 | NA | NA | NA |
| Yes | 39.1 | 29.2‐52.5 | 44.1 | 39.8‐48.9 | 0.921 |
| P value* | 0.081 | NA | 0.024 | ||
| Chemotherapy | |||||
| No | 37.2 | 28.0‐49.5 | NA | NA | NA |
| Yes | 39.0 | 27.9‐54.6 | 44.1 | 39.8‐48.9 | 0.914 |
| P value* | 0.203 | NA | 0.064 | ||
| Radiotherapy + Chemotherapy | |||||
| No | 36.7 | 27.6‐48.9 | NA | NA | NA |
| Yes | 40.0 | 28.6‐55.8 | 44.1 | 39.8‐48.9 | 0.951 |
| P value* | 0.139 | NA | 0.045 | ||
CI, confidence interval; NA, not applicable; OS, overall survival.
Kaplan‐Meier survival estimate compared by a log‐rank test.
Figure 2.
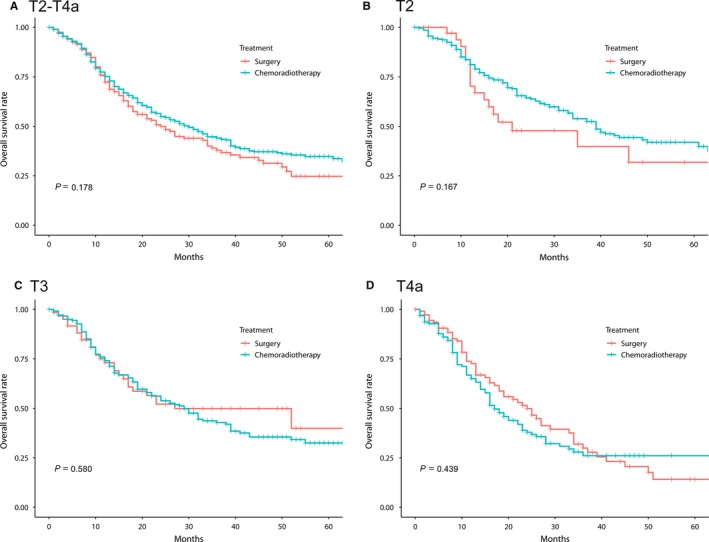
The Kaplan‐Meier overall survival estimate in locally advanced (T2‐T4a) hypopharyngeal cancer treated with surgery or definitive chemoradiotherapy. (A) T2‐T4a, (B) T2, (C) T3, and (D) T4a categories
Since the T category distribution between the treatment groups differed, we also calculated the survival rate of each subgroup by the T category. The 3‐year OS rates for the T2‐3 patients in the surgery and chemoradiotherapy groups were 46.5% and 48.7% (P = 0.598) (39.8% vs. 53.9% for T2 [P = 0.167] and 49.9% vs. 42.8% for T3 [P = 0.580], respectively; Figure 2B,C). The 3‐year OS rate for the T4a patients was 29.9% and 26.1% in the surgery and chemoradiotherapy groups, respectively (P = 0.439; Figure 2D).
As the distribution of subsites was different between the treatment groups in the T4a category, survival rate was separately calculated for pyriform sinus tumors in the T4a category. The 3‐year OS rates were 26.2% in the surgery group and 36.7% in the chemoradiotherapy groups (P = 0.517). Although there was no statistically significant difference in the survival rate between the treatment groups, the chemoradiotherapy group showed a stable survival rate after 36 months of survival (Figure 3).
Figure 3.
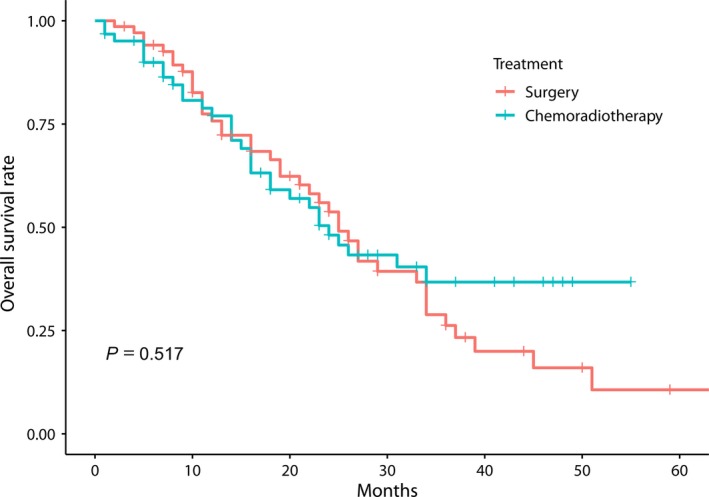
The Kaplan‐Meier overall survival estimate in T4a hypopharyngeal cancer at pyriform sinus treated with surgery or definitive chemoradiotherapy
On univariate analysis for the OS rate, patients who were aged ≥65 years (P = 0.001), non‐white race (P = 0.025), T4a category (P < 0.001), high N category (P = 0.003), Medicaid or uninsured (P < 0.001), and unmarried status (P < 0.001) were correlated with significantly dismal OS rates. Multivariate analysis was performed incorporating all variables—the treatment group (the surgery or chemoradiotherapy groups), age, race, sex, subsite, grade, T and N categories, insurance, and marital status—because all variables were statistically significant in the comparison between treatment group and/or the univariate analyses, or considered clinically important. On this multivariate analysis, the chemoradiotherapy group did not show a significantly adverse OS rate compared to the surgery group (hazard ratio [HR] 0.889, 95% confidence interval [CI] 0.699‐1.129, P = 0.334). Patients who were aged ≥65 years (P < 0.001), T4a (P < 0.001), N3 (P = 0.002), Medicaid (P = 0.002), and unmarried status (P = 0.002) were associated with poor OS rates after the multivariate analysis.
In subgroup analyses according to the T categories, the chemoradiotherapy group also showed similar OS rates compared to the surgery group. On multivariate analysis in the T2‐3 categories, HR of the chemoradiotherapy group compared to the surgery groups was 0.932 (95% CI 0.669‐1.297, P = 0.675). Even in the T4a category, a multivariate analysis incorporating all variables showed no significant OS difference between the surgery and chemoradiotherapy groups (HR of the chemoradiotherapy group 0.880, 95% CI 0.617‐1.256, P = 0.481). The subsites except pyriform sinus were a significantly adverse feature in the T4a category (HR 2.509, 95% CI 1.510‐4.170, P < 0.001; Table 3).
Table 3.
Multivariate analysis for OS rate according to T category in locally advanced hypopharyngeal cancer
| Characteristics | All | T2‐3 | T4a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value* | HR | 95% CI | P value* | HR | 95% CI | P value* | |
| Treatment | |||||||||
| Surgery | Reference | Reference | Reference | ||||||
| Chemoradiotherapy | 0.889 | 0.699‐1.129 | 0.334 | 0.932 | 0.669‐1.297 | 0.675 | 0.880 | 0.617‐1.256 | 0.481 |
| Age | |||||||||
| <65 y | Reference | Reference | Reference | ||||||
| ≥65 y | 1.779 | 1.431‐2.210 | <0.001 | 2.153 | 1.644‐2.820 | <0.001 | 1.488 | 0.991‐2.235 | 0.056 |
| Race | |||||||||
| White | Reference | Reference | Reference | ||||||
| Black | 1.216 | 0.928‐1.592 | 0.156 | 1.073 | 0.764‐1.505 | 0.686 | 1.370 | 0.844‐2.224 | 0.203 |
| Others | 1.16 | 0.791‐1.702 | 0.447 | 1.421 | 0.874‐2.310 | 0.157 | 0.952 | 0.504‐1.801 | 0.881 |
| Sex | |||||||||
| Male | Reference | Reference | Reference | ||||||
| Female | 1.041 | 0.807‐1.342 | 0.759 | 1.031 | 0.762‐1.395 | 0.843 | 1.148 | 0.686‐1.919 | 0.600 |
| Subsite | |||||||||
| Pyriform sinus | Reference | Reference | Reference | ||||||
| Other sites | 1.275 | 0.989‐1.644 | 0.061 | 1.013 | 0.751‐1.365 | 0.934 | 2.509 | 1.510‐4.170 | <0.001 |
| Not otherwise specified | 1.003 | 0.795‐1.265 | 0.979 | 0.847 | 0.637‐1.125 | 0.252 | 1.252 | 0.827‐1.896 | 0.288 |
| Grade | |||||||||
| 1‐2 | Reference | Reference | Reference | ||||||
| 3‐4 | 1.086 | 0.891‐1.324 | 0.415 | 1.302 | 1.021‐1.660 | 0.033 | 0.773 | 0.537‐1.112 | 0.165 |
| T category | |||||||||
| T2 | Reference | Reference | |||||||
| T3 | 1.259 | 0.992‐1.599 | 0.059 | 1.261 | 0.990‐1.604 | 0.060 | NA | ||
| T4a | 1.784 | 1.372‐2.320 | <0.001 | NA | |||||
| N category | |||||||||
| N0 | Reference | Reference | Reference | ||||||
| N1 | 0.999 | 0.736‐1.357 | 0.996 | 0.933 | 0.643‐1.355 | 0.715 | 1.121 | 0.637‐1.973 | 0.692 |
| N2 | 1.259 | 0.997‐1.588 | 0.053 | 1.390 | 1.054‐1.833 | 0.020 | 1.008 | 0.653‐1.555 | 0.972 |
| N3 | 2.472 | 1.409‐4.337 | 0.002 | 2.779 | 1.435‐5.381 | 0.002 | 2.942 | 0.923‐9.381 | 0.068 |
| Insurance | |||||||||
| Insured | Reference | Reference | Reference | ||||||
| Medicaid | 1.507 | 1.167‐1.945 | 0.002 | 1.574 | 1.133‐2.187 | 0.007 | 1.468 | 0.944‐2.282 | 0.088 |
| Uninsured/unknown | 1.340 | 0.866‐2.073 | 0.189 | 1.606 | 0.941‐2.741 | 0.082 | 0.945 | 0.421‐2.124 | 0.892 |
| Marriage | |||||||||
| Married | Reference | Reference | Reference | ||||||
| Others/unknown | 1.379 | 1.122‐1.694 | 0.002 | 1.362 | 1.058‐1.753 | 0.016 | 1.567 | 1.068‐2.299 | 0.022 |
CI, confidence interval; HR, hazard ratio; NA, not applicable; OS, overall survival.
Cox proportional hazards model.
4. DISCUSSION
In comparison with definitive chemoradiotherapy and surgery for locally advanced hypopharyngeal cancer, similar OS rates were observed. Even in the T4a category, chemoradiotherapy did not decrease the OS rate, suggesting that definitive chemoradiotherapy may be a treatment option for the T4a hypopharyngeal cancer without sacrificing the OS rate.
Several studiesdemonstrated that 3‐year locoregional control rate of IMRT for locally advanced (Stage III‐IV) hypopharyngeal cancer is approximately 68%‐85%15, 16, 17, 18 and 5‐year local control rate was 53%‐63%,18, 19 and these results are similar with those of surgery.19, 20 The major pattern of failure of head and neck cancer patients who were treated with IMRT has become distant metastases rather than locoregional failures.21
Despite developed treatment modalities, however, the prognosis of locally advanced hypopharyngeal cancer is poor. With this perception, multidisciplinary decision making is mandatory to select optimal treatments. The key consideration for selection of treatment is the estimated survival, survival benefit from treatment, adverse effect after treatment, quality of life, and patient expectations.22
CategoryT4a (AJCC 7th) denotes the invasion of thyroid or cricoid cartilage, hyoid bone, thyroid gland, or central compartment soft tissue. Among them, the involvement of thyroid or cricoid cartilage were considered as risk factors decreasing the organ‐preserving possibility after chemoradiotherapy.7, 23 On the contrary, the invasion of thyroid or cricoid cartilage might not always cause a decrease in laryngopharyngeal dysfunction.23, 24 If the involvement of thyroid or cricoid cartilage is minor, chemoradiotherapy may provide an opportunity to conserve laryngopharyngeal functions.25
On the other hand, some patients may not be appropriate for chemoradiotherapy. The patients who had a tumor at the posterior wall of hypopharynx or retropharyngeal node invasion, and who had initial swallowing dysfunctions have high risks of dysphagia after chemoradiotherapy.26, 27, 28
However, it is noticeable that majority of locally advanced hypopharyngeal cancer patients who received surgery also need additional radiotherapy or chemoradiotherapy,14 and surgery is also an invasive procedure. General condition which is needed for surgical treatment may not be acceptable for all patients. IMRT, image‐guided radiotherapy (IGRT), and adaptive radiotherapy can reduce toxicity and improve quality of life after treatment even in old age,29, 30, 31 indicating that definitive chemoradiotherapy might be as beneficial as surgery if the patients are selected carefully.
Onepopulation‐based study using the National Cancer Data Base (NCDB) from 1998 to 2011 demonstrated that the treatment outcome of chemoradiotherapy for hypopharyngeal cancer are comparable between surgery with chemoradiotherapy and surgery with radiotherapy.14 In this NCDB study, T4a category was 29.2%, and subgroup analysis for locally advanced hypopharyngeal cancer was not performed. Therefore, the results from this NCDB study were not enough to confirm that chemoradiotherapy is comparable to surgery even in T4a category cancer.
Several studies insisted that (chemo)radiotherapy for T4a hypopharyngeal cancer showed a poor survival rate compared to surgery.2, 5, 6 On the other hand, a multi‐institutional study which performed a matched‐pair analysis between surgery and chemoradiotherapy between 2006 and 2008 showed no significant differences in survival or local control rates between the two treatment groups even in T4a category patients.32
We analyzed 791 of hypopharyngeal cancer patients diagnosed from 2004 to 2009 in the SEER database (T2‐4aN0‐3M0, AJCC 6th), resulting in the OS rate of T4a cancer patients who were treated with surgery was significantly superior to that of patients received chemoradiotherapy (data now shown). Therefore, the non‐inferiority of chemoradiotherapy compared to the surgery in T4a category might be an emerging result in this contemporary period.
One recent review study demonstrated that surgery and chemoradiotherapy showed similar survivorship in advanced hypopharyngeal carcinoma after reviewing two randomized trials and 11 observational studies33—in these included studies, considerable number of patients were T4 category, and especially the studies that covered the period of 2010s did not show a significant difference in OS between surgery and chemoradiotherapy.20, 25, 33, 34
Larynx preservation rate after chemoradiotherapy using IMRT for locally advanced hypopharyngeal cancer is reported as 89%‐96% at 2 years and 60% at 5 years.12, 17, 18 Therefore, if IMRT is performed for patients who were carefully selected based on the possibility of organ preservation, a considerable number of patients may be able to expect larynx preservation.
Several trials for hypopharyngeal cancer have started to study effective treatments for this disease. Neoadjuvant chemotherapy (NCT01312350), BKM120 (NCT02113878), and WEE1 Inhibitor (NCT03028766) with cisplatin‐based chemoradiotherapy, adaptive radiotherapy (NCT03096808), and upfront neck dissection with definitive chemoradiotherapy (NCT02918955) are evaluated. Also, effectiveness of laser therapy for mucositis induced by a chemoradiotherapy (NCT01772706) and swallowing rehabilitation on quality of life after radiotherapy (NCT02892487) are also investigated.
The limitation of this study mainly comes from the retrospective nature. All patients were real clinical data, and majority of the patients would be treated based on the multidisciplinary decisions. Even though T4a category patients are included in the same category, the severity of tumor might be more aggressive in the surgery group considering a low possibility of preserving the laryngopharyngeal function. Conversely, patients whose medical condition were not appropriate for surgical treatment may be treated with chemoradiotherapy.
Population database, but relatively small patient number owing to the rarity of the disease as well as the confined study period and short follow‐up period can be another limitation. However, because of the poor survival rate especially for T4a hypopharyngeal cancer, the follow‐up period may be sufficient to reach statistical significance if there is a significant difference.
Information of comorbidity, locoregional control rate, and radiotherapy regimen (total dose, fractionation, and fraction size), and radiotherapy modality (conventional or IMRT) were also not available. IMRT has been a major modality of radiotherapy for head and neck cancer.11, 35 Therefore, most of the patients in our study are supposed to be treated with IMRT.
All treatments in the SEER database are the first course of treatment after diagnosis. Induction chemotherapy and definitive concurrent chemotherapy could not be divided. However, based on the historical outcomes demonstrating no significant difference between the induction and concurrent regimens,14, 36, 37 the chemoradiotherapy group was considered as one integrated treatment group (“non‐surgical treatment” group).
In conclusion, definitive chemoradiotherapy for locally advanced hypopharyngeal cancer including T4a category showed a non‐inferior OS rate compared to surgery. For patients with T4a category cancer with high possibility of preserving the laryngopharyngeal function or with inoperable condition, chemoradiotherapy may be a promising alternative treatment.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
This study was supported by 2017 Young Medical Science Researcher Grants from Ewha Womans University College of Medicine.
Kim Y‐J, Lee R. Surgery vs. radiotherapy for locally advanced hypopharyngeal cancer in the contemporary era: A population‐based study. Cancer Med. 2018;7:5889–5900. 10.1002/cam4.1811
In the contemporary era from 2010 to 2015, chemoradiotherapy for locally advanced hypopharyngeal cancer (T2–4a) showed a comparable OS rate to surgery. For patients with T4a category cancer with high possibility of preserving the laryngopharyngeal function or with inoperable condition, chemoradiotherapy may be a promising alternative treatment.
REFERENCES
- 1. Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long‐term survivors of head and neck cancer. Cancer. 2014;120:1507‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petersen JF, Timmermans AJ, van Dijk B, et al. Trends in treatment, incidence and survival of hypopharynx cancer: a 20‐year population‐based study in the Netherlands. Eur Arch Otorhinolaryngol. 2018;275(1):181‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newman JR, Connolly TM, Illing EA, Kilgore ML, Locher JL, Carroll WR. Survival trends in Hypopharyngeal cancer: A population‐based review. Laryngoscope. 2015;125:624‐629. [DOI] [PubMed] [Google Scholar]
- 4. Wendt M, Romanitan M, Näsman A, et al. Presence of human papillomaviruses and p16 expression in hypopharyngeal cancer. Head Neck. 2014;36:107‐112. [DOI] [PubMed] [Google Scholar]
- 5. Shirai K, Saitoh J‐I, Musha A, et al. Clinical outcomes of definitive and postoperative radiotherapy for stage I‐IVB hypopharyngeal cancer. Anticancer Res. 2016;36:6571‐6578. [DOI] [PubMed] [Google Scholar]
- 6. Cheng CT, Lin CY, Hung‐Chun Cheng S, et al. Survival benefit of surgical approach for advanced oropharyngeal and hypopharyngeal cancer: A retrospective analysis. Head Neck. 2017;39:2104‐2113. [DOI] [PubMed] [Google Scholar]
- 7. Department of Veterans Affairs Laryngeal Cancer Study Group , Wolf GT, Fisher SG, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324: 1685‐1690. [DOI] [PubMed] [Google Scholar]
- 8. NCCN Clinical Practice Guidelines in Oncology . Head and Neck Cancers Version 2 .2018. https://www.nccn.org/professionals/physician_gls/default.aspx#head-and-neck. Accessed November 15, 2018
- 9. Grégoire V, Lefebvre JL, Licitra L, et al. Squamous cell carcinoma of the head and neck: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2010;21: v184‐v186. [DOI] [PubMed] [Google Scholar]
- 10. Kuo P, Chen MM, Decker RH, Yarbrough WG, Judson BL. Hypopharyngeal cancer incidence, treatment, and survival: temporal trends in the United States. Laryngoscope. 2014;124:2064‐2069. [DOI] [PubMed] [Google Scholar]
- 11. Mell LK, Roeske JC, Mundt AJ. A survey of intensity‐modulated radiation therapy use in the United States. Cancer. 2003;98:204‐211. [DOI] [PubMed] [Google Scholar]
- 12. Miah AB, Bhide SA, Guerrero‐Urbano MT, et al. Dose‐escalated intensity‐modulated radiotherapy is feasible and may improve locoregional control and laryngeal preservation in laryngo‐hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2012;82:539‐547. [DOI] [PubMed] [Google Scholar]
- 13. Karasawa K, Kunogi H, Hirai T, et al. Radiotherapy with fraction size of 2.25 Gy in T1–2 laryngeal and hypopharyngeal cancer. J Radiat Res. 2013;54: 684‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuo P, Sosa JA, Burtness BA, et al. Treatment trends and survival effects of chemotherapy for hypopharyngeal cancer: Analysis of the national cancer data base. Cancer. 2016;122:1853‐1860. [DOI] [PubMed] [Google Scholar]
- 15. Daly ME, Le QT, Jain AK, et al. Intensity‐modulated radiotherapy for locally advanced cancers of the larynx and hypopharynx. Head Neck. 2011;33:103‐111. [DOI] [PubMed] [Google Scholar]
- 16. Katsoulakis E, Riaz N, Hu M, et al. Hypopharyngeal squamous cell carcinoma: Three‐dimensional or Intensity‐modulated radiotherapy? A single institution's experience. Laryngoscope. 2016;126:620‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mok G, Gauthier I, Jiang H, et al. Outcomes of intensity‐modulated radiotherapy versus conventional radiotherapy for hypopharyngeal cancer. Head Neck. 2015;37:655‐661. [DOI] [PubMed] [Google Scholar]
- 18. Liu W‐S, Hsin C‐H, Chou Y‐H, et al. Long‐term results of intensity‐modulated radiotherapy concomitant with chemotherapy for hypopharyngeal carcinoma aimed at laryngeal preservation. BMC cancer. 2010;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang W‐Y, Jen Y‐M, Chen C‐M, et al. Intensity modulated radiotherapy with concurrent chemotherapy for larynx preservation of advanced resectable hypopharyngeal cancer. Radiation Oncology. 2010;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim JW, Kim MS, Kim SH, et al. Definitive chemoradiotherapy versus surgery followed by adjuvant radiotherapy in resectable stage III/IV hypopharyngeal cancer. Cancer Res Treat. 2016;48:45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao M, Lu M, Savvides PS, et al. Distant metastases in head‐and‐neck squamous cell carcinoma treated with intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:684‐689. [DOI] [PubMed] [Google Scholar]
- 22. Forastiere AA, Weber RS, Trotti A. Organ preservation for advanced larynx cancer: issues and outcomes. J Clin Oncol. 2015;33:3262‐3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wagner MM, Curé JK, Caudell JJ, et al. Prognostic significance of thyroid or cricoid cartilage invasion in laryngeal or hypopharyngeal cancer treated with organ preserving strategies. Radiat Oncol. 2012;7:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li B, Bobinski M, Gandour‐Edwards R, Farwell D, Chen A. Overstaging of cartilage invasion by multidetector CT scan for laryngeal cancer and its potential effect on the use of organ preservation with chemoradiation. Br J Radiol. 2011;84:64‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jang JY, Kim E‐H, Cho J, et al. Comparison of oncological and functional outcomes between initial surgical versus non‐surgical treatments for hypopharyngeal cancer. Ann Surg Oncol. 2016;23:2054‐2061. [DOI] [PubMed] [Google Scholar]
- 26. Bhide S, Newbold K, Harrington K, Nutting C. Clinical evaluation of intensity‐modulated radiotherapy for head and neck cancers. Br J Radiol. 2012;85:487‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhayani MK, Hutcheson KA, Barringer DA, Roberts DB, Lewin JS, Lai SY. Gastrostomy tube placement in patients with hypopharyngeal cancer treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck. 2013;35:1641‐1646. [DOI] [PubMed] [Google Scholar]
- 28. Barry P, Henson AL, Goodwin NE, et al. Factors predicting suitability of organ preservation with radiation therapy in laryngeal and hypopharyngeal cancer. J Solid Tumors. 2014;4:9. [Google Scholar]
- 29. Nguyen N, Vock J, Chi A, et al. Impact of intensity‐modulated and image‐guided radiotherapy on elderly patients undergoing chemoradiation for locally advanced head and neck cancer. Strahlenther Onkol. 2012;188(8):677‐685. [DOI] [PubMed] [Google Scholar]
- 30. Modesto A, Laprie A, Vieillevigne L, et al. Intensity‐modulated radiotherapy for laryngeal and hypopharyngeal cancer. Strahlenther Onkol. 2015;191:225‐233. [DOI] [PubMed] [Google Scholar]
- 31. Bhide SA, Davies M, Burke K, et al. Weekly volume and dosimetric changes during chemoradiotherapy with intensity‐modulated radiation therapy for head and neck cancer: a prospective observational study. Int J Radiat Oncol Biol Phys. 2010;76:1360‐1368. [DOI] [PubMed] [Google Scholar]
- 32. Iwae S, Fujii M, Hayashi R, et al. Matched‐pair analysis of patients with advanced hypopharyngeal cancer: surgery versus concomitant chemoradiotherapy. Int J Clin Oncol. 2017;22:1001‐1008. [DOI] [PubMed] [Google Scholar]
- 33. Habib A. Management of advanced hypopharyngeal carcinoma: systematic review of survival following surgical and non‐surgical treatments. J Laryngol Otol. 2018;132:385‐400. [DOI] [PubMed] [Google Scholar]
- 34. Harris BN, Biron VL, Donald P, et al. Primary surgery vs chemoradiation treatment of advanced‐stage hypopharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2015;141:636‐640. [DOI] [PubMed] [Google Scholar]
- 35. Marta GN, Silva V, de Andrade CH, et al. Intensity‐modulated radiation therapy for head and neck cancer: systematic review and meta‐analysis. Radiother Oncol. 2014;110:9‐15. [DOI] [PubMed] [Google Scholar]
- 36. Hitt R, Grau JJ, Lopez‐Pousa A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25:216‐225. [DOI] [PubMed] [Google Scholar]
- 37. Prades J‐M, Lallemant B, Garrel R, et al. Randomized phase III trial comparing induction chemotherapy followed by radiotherapy to concomitant chemoradiotherapy for laryngeal preservation in T3M0 pyriform sinus carcinoma. Acta Oto‐Laryngologica. 2010;130:150‐155. [DOI] [PubMed] [Google Scholar]


