Abstract
Single nucleotide polymorphisms (SNPs) in 3′UTR of key DNA repair enzyme genes are associated with inter‐individual differences of DNA repair capacity (DRC) and susceptibility to a variety of human malignancies such as lung cancer. In this study, seven candidate SNPs in 3′UTR of DRC‐related genes including ERCC1 (rs3212986, rs2336219, and rs735482), OGG1 (rs1052133), MLH3 (rs108621), CD3EAP (rs1007616), and PPP1R13L (rs6966) were analyzed in 300 lung cancer patients and controls from the northeast of China. Furthermore, we introduced ERCC1 (CDS+3′UTR) or CD3EAP (CDS) cDNA clone to transfect HEK293T and 16HBE cells. Cell viability between different genotypes of transfected cells exposed to BPDE was detected by CCK‐8 assay, while DNA damage was visualized using γH2AX immunofluorescence and the modified comet assay. We found that minor A‐allele of rs3212986 could reflect a linkage with increasing risk of NSCLC. Compared with CC genotype, AA genotype of ERCC1 rs3212986 was a high‐risk factor for NSCLC (OR = 3.246; 95%CI: 1.375‐7.663). Particularly stratified by smoking status in cases and controls, A allele of ERCC1 rs3212986 also exhibited an enhanced risk to develop lung cancer in smokers only (P < 0.05). Interestingly, reduced repair efficiency of DNA damage was observed in 293T ERCC1(AA) and 16HBE ERCC1(AA), while no significant difference was appeared in two genotypes of CD3EAP (3′ adjacent gene of ERCC1) overexpressed cells. Our findings suggest that rs3212986 polymorphism in 3ʹUTR of ERCC1 overlapped with CD3EAP may affect the repair of the damage induced by BPDE mainly via regulating ERCC1 expression and become a potential biomarker to predict smoking‐related lung cancer.
Keywords: CD3EAP, DNA repair capacity, ERCC1C8092A (rs3212986), lung cancer, polymorphism in 3'UTR
1. INTRODUCTION
Lung cancer has high morbidity and mortality and becomes the leading cause of cancer death worldwide. As the most common malignant tumors in developing countries, nearly 85% of lung cancer cases are diagnosed as nonsmall cell lung cancer (NSCLC), which is mainly classified into adenocarcinoma (AC) and squamous cell carcinoma, and have various and complex pathogenesis.1, 2, 3 Many recent studies indicated that accumulated DNA damage caused by environmental carcinogen exposure and reduced individual DNA repair efficiency is associated with an increased risk of NSCLC development.4, 5, 6, 7
DNA repair system can maintain genome stability and reduce cancer risk by removal of diversified DNA damages via three DNA excision repair pathways, namely nucleotide excision repair (NER), base excision repair, (BER) and mismatch repair (MMR). Previous studies reported that the polymorphisms of DNA repair genes, especially those in DNA excision repair pathways involving in the restoration of DNA adducts after exposure to UV light or benzo[a]pyren ediol epoxide (BPDE, a metabolite of benzo[a]pyrene), have observably linked with the occurrence and development of lung cancer.8, 9, 10, 11 ERCC1 (excision repair cross‐complementation group 1), OGG1 (8‐oxoguanine DNA glycosylase), and MLH3 (mutL homolog 3) are three key rate‐limiting enzymes involving in NER, BER, and MMR pathways, respectively. Single‐nucleotide polymorphisms (SNPs) in these genes are associated with the risk of cancers mainly due to the alerted DNA repair activity of respective enzymes.12, 13, 14, 15 However, previous epidemiological investigation and genome‐wide association studies usually focus on the significance of polymorphisms in open reading frame (ORF), while the supporting data of “regulatory SNPs” on noncoding regions especially 3ʹUTR and its relation with lung cancer have been barely reported so far. In eukaryotes, 3ʹUTR plays an important role in cellular location and mRNA regulation of gene expression. Mutations in 3ʹUTR of key DNA repair enzyme genes can contribute to interindividual differences in DNA repair efficiency, which has a crucial role in hereditary susceptibility to cancer risk and chemotherapy resistance.16 In our previous study, two SNPs in ERCC1 were investigated used HPLC and modified comet assay to evaluate the individual capacity of repairing BPDE‐DNA adduct from 117 randomly selected healthy participants. The conclusion indicated that the minor A allele in rs3212986, which was located at 3ʹUTR (3ʹ untranslated region) of ERCC1 and overlapped with coding region of CD3EAP (CD3e molecule, epsilon‐associated protein, antisense ERCC1), resulted in a diminished capacity of repairing BPDE‐DNA adducts.17 CD3EAP, known as a nucleoprotein and reverse complementary to ERCC1, may serve as a component of the RNA polymerase I transcription complex implicating in cell proliferation18 and coordinately overlaps with PPP1R13L (protein phosphatase 1, regulatory subunit 13 like, RelA‐associated inhibitor), which is an inhibitor of p53 and NF‐κB affecting the regulation of apoptotic pathway and inflammatory response.19, 20, 21 It is reported that the polymorphisms of overlapping gene ERCC1, CD3EAP, and PPP1R13L on the chromosomal region 19q13.3 were associated with individual DNA repair capacity (DRC) and lung cancer susceptibility,22 which suggested that this exceptional overlapping structure of three genes may have potential functions in carcinogenesis. However, the functional properties based on neighboring SNPs in haplotypes of crucial DNA repair enzymes and DRC‐related proteins as well as the predictive value and the related mechanism of DNA excision repair gene polymorphisms in 3ʹUTR are still largely uncertainty.
In this case‐control study, seven candidate SNPs in 3ʹUTR of DRC‐related genes, including ERCC1 (rs3212986, rs2336219, and rs735482), OGG1 (rs1052133), MLH3 (rs108621), CD3EAP (rs1007616), and PPP1R13L (rs6966), were analyzed in well‐characterized series of 300 lung cancer patients matched with 300 healthy controls from the northeast of China to prospectively evaluate the associations between DRC‐related polymorphisms in 3ʹUTR and the risk of lung tumorigenesis. Furthermore, the effects of the target SNPs on the capacity of repairing BPDE‐DNA adducts were also investigated using an in vitro transfected cell model. Data from the presented study further indicated that rs3212986 polymorphism in 3ʹUTR of ERCC1 overlapped with CD3EAP may affect the repair of the damage induced by BPDE mainly via regulating ERCC1 expression and become a potential biomarker to predict smoking‐related lung cancer.
2. MATERIAL AND METHODS
2.1. Study subjects
In this study, we recruited 300 patients suffering from lung cancer from the First Affiliated Hospital of China Medical University as our cases. The cases were diagnosed from 2013 to 2015 by the local authorized diagnosing pathologists. Control subjects were healthy volunteers who visited the hospital for physical examination. The controls had no history of cancers and were matched to the cases by age (±3 years), gender, and ethnicity and recruited in the same region and period. The protocol and consent form were approved by the Institutional Review Board of China Medical University. All activities involving human subjects were done under full compliance with government policies and the Helsinki Declaration. Informed consent was obtained from each of the participants after a detailed explanation of the nature and possible consequences of the study. Each participant donated 5 mL venous blood, their demographic data were recorded in questionnaires in detail, and the study participant consents were obtained prior to the study. Those who smoked at least one cigarette per day for more than 1 year were considered regular smokers.
2.2. DNA extraction and TaqMan® SNP Genotyping Assays
Genomic DNA was extracted as our published method.17 ERCC1(rs3212986, C>A, assay ID C_2532948_10; rs735482, A>C, assay ID C_341729_10; rs2336219, G>A, assay ID C_16204465_10), MLH3(rs108621, T>C, assay ID C_2178406_10), OGG1(rs1052133, G>C, assay ID C_3095552_1), PPP1R13L(rs6966, T>A, assay ID C_2615637_10), and CD3EAP (rs1007616, C>T, assay ID AH6RTHI) were purchased from ABI Company (ABI, US, Stagapore) and analyzed by TaqMan® genotyping on a LightCycler 480 Real‐time PCR system (Roche, Foster City, CA, USA). All PCR reagents were purchased from Roche Company (Roche).
The PCRs were performed in a 20 μL reaction mixture: 10 μL of probe Mix, 5 μL (1×) of assay mix, and 2 μL of DNA (25 ng/μL). The PCR included an initial step at 95°C for 10 minutes; 40 cycles of denaturation at 95°C for 10 second, extension at 60°C for 1 minutes and 72°C for 1 second; and at the end, cooling at 40°C for 30 second.
2.3. Chemicals and plasmids construction
Benzo[a]pyrene 7, 8‐diol 9, 10‐epoxide was purchased from National Cancer Institute Chemical Carcinogen Repository (Midwest Research Institute, Kansas City, MO, USA).
Plasmids expressing the ORFs of rs3212986 C or A allele in ERCC1 [pLenti‐EGFP‐CMV‐ERCC1 (CDS+3′UTR)] or CD3EAP [pLenti‐mcherry‐CMV‐CD3EAP (CDS)] and the firefly luciferase reporter plasmid were constructed by Obio Technology CO (Shanghai, China).
2.4. Cell culture and treatment
The immortalized 16HBE cell line, kindly provided by Prof. Wen Chen (Sun Yat‐Sen University, China), was cultured in MEM (Hyclone, Waltham USA). HEK293T and A549 cells were purchased from the Cell Bank of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, and cultured in DMEM/F‐12 (Hyclone) and DMEM (Hyclone), respectively. Both cells were supplemented with 10% fetal bovine serum (Hyclone) and maintained at 37°C, 5% CO2 in a humidified incubator.
All experiments were conducted using the cells at a logarithmic stage of growth curve. In accordance with the following treatments before experiments of cell viability and DNA repair assay, cells were seeded and 24 hours later the plasmids were introduced to the HEK293T or 16HBE with Lipofectamine™ 3000 Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendation, and the exponentially growing transfected cells were exposed to BPDE after 24 hours.
2.5. Cell Counting Kit‐8 assay
Cell viability was evaluated using Cell Counting Kit‐8 (Dojindo, Japan). The transfected HEK293T cells were incubated with various concentrations of BPDE (0.1, 0.5, 1, 1.5, 2, 4, 8, 16 µmol/L) for 24 hours and an additional 24 hours for DNA repair after treatment. Then, 10 µL of the Cell Counting Kit‐8 (CCK‐8) reagent was added to each well and incubated for another 3.5 hours. The absorbance (value) at 450 nm was measured using a scanning microplate reader (Biotek, Winooski, VT, USA).
2.6. Comet assay
Damage and repair of DNA adducts was assessed by alkaline comet assay with some modifications.18 Cells were harvested for the following procedures: precoated microscope slides were covered by dropping 100 µL 1% normal melting point agarose and 100 µL gel mixture of cells and 1% low melting point agarose. Gel slides were soaked in cell lysis solution (100 mmol/L disodium EDTA, 2.5 M NaCl, 10 mM Tris‐HCl pH 10.5, adding 1% Triton X‐100 before using) for 60‐90 minutes and immersed in the electrophoresis solution (1 mol/L disodium EDTA, 300 mmol/L NaOH, pH = 13) for 30 minute to denature DNA. After electrophoresis at 20 V (100 mA) for 20 minute, glasses were rinsed three times with 0.4 mol/L Tris‐HCl (pH 7.5). Due to the background color of the transfected cells, propidium Iodide or Goldview (green fluorescence) was used for staining. Approximately 100‐200 comets were analyzed per experiment. Results were expressed as mean tail moment.
2.7. Immunofluorescence microscopy
γH2AX proteins are concentrated at the damaged DNA strand and can be visualized as foci by immunofluorescence. Cells were fixed in 4% paraformaldehyde and permeabilized with 0.5% Triton X‐100/PBS. Coverslips were blocked with 1% BSA for 30 minutes and then incubated overnight with γH2AX‐antibody (Abcam, Cambridge, UK, ab2893, 1:200). Cells were incubated with secondary antibody Alexa Fluor 488 or 594 conjugated goat anti‐rabbit IgG (proteintech, 1:150) for 1 hour at room temperature. Next, cells were washed with PBS and incubated with DAPI (Beyotime, Shanghai, China) for nuclei staining. Foci on each slide were visualized by fluorescence microscope, and cells containing at least 10 foci were denoted as positive controls. At least 200 cells per slide were scored.
2.8. ERCC1 mRNA secondary structure predictions and optimal free energy calculations
Secondary structures for either rs3212986 C allele or A allele of ERCC1 mRNA sequences and the minimum optimal free energies were predicted by using RNA structure 5.3 software (Mathew Lab, Rochester, NY, USA). The predictions and calculations were performed according to the instructions and the default setting of the program.
2.9. Statistical analysis
All data obtained were analyzed by IBM SPSS 19.0 (IBM Company, Armonk, NY, USA) and GraphPad Prism 5.0 software (GraphPad Software Inc, San Diego, CA, USA). The Hardy‐Weinberg equilibrium for three SNPs in ERCC1, four SNPs in overlapping regions of CD3EAP and ERCC1, and the level of linkage disequilibrium (LD) were analyzed by Haploview 4.2 Software (Broad Institute, Cambridge, UK). Whether rs3212986 variants could affect ERCC1 mRNA secondary structure was predicted by RNA structure 5.3 software. The chi‐square test and logistic regression were used to evaluate the association between genetic polymorphisms and the risk of lung cancer. t Test was used to estimate the differences among the groups. A two‐tailed P‐value <0.05 was considered statistically significant.
3. RESULTS
3.1. Genetic polymorphisms in ERCC1, OGG1, MLH3, PPP1R13L, and CD3EAP and the risk of lung cancer
We initially screened the SNPs of selected genes to predict the risk of lung cancer using NCBI database combining with the minor allele frequency (MAF) (Table S1). According to MAF and sample size of our current study, we chose the following target SNPs: ERCC1 (rs3212986, rs2336219, and rs735482), MLH3 (rs108621), OGG1 (rs1052133), PPP1R13L (rs6966), and CD3EAP (rs1007616).
The study population consisted of 300 lung cancer cases and 300 cancer‐free controls, which had the same composition ratio of 188 men and 112 women (Table S2). The lung cancer cases’ ages ranged from 35 to 84 years (mean, 59.54 years and median, 60 years). Controls were characterized by a median age of 59 (range: 33‐85 and mean, 60.04 years). ERCC1 rs3212986 AA genotype revealed a higher risk of lung cancer compared to CC genotype (OR = 2.061, 95%CI: 1.057‐4.017) (Table 1). After stratifying by gender (Table 2), rs3212986 AA genotype was also at higher risk of lung cancer among men (OR = 3.246, 95%CI: 1.375‐7.663). No association was observed in female. In addition, the OGG1 rs1052133 CC genotype (OR = 2.588, 95%CI: 1.035‐6.474) exhibited a higher risk of lung cancer development in females. The analysis of age stratification showed that ERCC1 rs735482 AC genotype had a protective role on lung cancer (OR = 0.560, 95%CI: 0.336‐0.934) (Table 3).
Table 1.
Genotypes for genetic polymorphisms in ERCC1, OGG1, MLH3, PPP1R13L, and CD3EAP and their effects on the risk of lung cancer
| SNPs | Cases | Control | OR (95%CI) | χ2 | P [Link] | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| ERCC1 rs3212986 | 5.344 | 0.069 | |||||
| CC | 152 | 50.7 | 174 | 58.0 | 1.000 | ||
| CA | 121 | 40.3 | 111 | 37.0 | 1.248 (0.891‐1.748) | 1.658 | 0.198 |
| AA | 27 | 9.0 | 15 | 5.0 | 2.061 (1.057‐4.017) | 4.645 | 0.031 |
| ERCC1 rs735482 | 1.387 | 0.500 | |||||
| AA | 92 | 30.7 | 79 | 26.3 | 1.000 | ||
| AC | 150 | 50.0 | 160 | 53.3 | 0.805 (0.554‐1.170) | 1.292 | 0.256 |
| CC | 58 | 19.3 | 61 | 20.4 | 0.816 (0.511‐1.305) | 0.720 | 0.369 |
| ERCC1 rs2336219 | 0.885 | 0.642 | |||||
| GG | 92 | 30.7 | 83 | 27.7 | 1.000 | ||
| GA | 151 | 50.3 | 162 | 54.0 | 0.841 (0.581‐1.218) | 0.841 | 0.359 |
| AA | 57 | 19.0 | 55 | 18.3 | 0.935 (0.582‐1.503) | 0.077 | 0.781 |
| MLH3 rs108621 | 1.590 | 0.452 | |||||
| TT | 190 | 63.3 | 201 | 67.0 | 1.000 | ||
| TC | 96 | 32.0 | 90 | 30.0 | 1.128 (0.796‐1.600) | 0.460 | 0.498 |
| CC | 14 | 4.7 | 9 | 3.0 | 1.646 (0.696‐3.891) | 1.310 | 0.252 |
| OGG1 rs1052133 | 1.388 | 0.499 | |||||
| GG | 96 | 32.0 | 106 | 35.3 | 1.000 | ||
| GC | 154 | 51.3 | 153 | 51.0 | 1.111 (0.799‐1.586) | 0.339 | 0.560 |
| CC | 50 | 16.7 | 41 | 13.7 | 1.347 (0.819‐2.213) | 1.382 | 0.240 |
| PPP1R13L rs6966 | 1.595 | 0.451 | |||||
| TT | 80 | 26.7 | 85 | 28.3 | 1.000 | ||
| TA | 145 | 48.3 | 130 | 43.4 | 1.185 (0.805‐1.744) | 0.743 | 0.389 |
| AA | 75 | 25.0 | 85 | 28.3 | 0.938 (0.607‐1.449) | 0.084 | 0.771 |
| CD3EAP rs1007616 | 2.451 | 0.294 | |||||
| CC | 159 | 53.0 | 178 | 59.4 | 1.000 | ||
| CT | 115 | 38.3 | 100 | 33.3 | 1.287 (0.914‐1.814) | 2.089 | 0.148 |
| TT | 26 | 86.7 | 22 | 7.3 | 1.323 (0.721‐2.427) | 0.821 | 0.365 |
P‐value <0.05 was considered statistically significant (in bold).
Pearson chi‐square test for difference in distributions between the case and control groups.
Table 2.
The relationship between gender status and genetic polymorphisms in five selected genes
| Groups | Male | OR (95% CI) | Female | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | Cases (%) | Cases (%) | |||
| ERCC1 rs3212986 | ||||||
| CC | 93 (49.5) | 115 (61.2) | 1.000 | 59 (52.7) | 59 (52.7) | 1.000 |
| CA | 74 (39.4) | 65 (34.6) | 1.408 (0.915‐2.166) | 47 (42.0) | 46 (41.1) | 1.022 (0.593‐1.760) |
| AA | 21 (11.1) | 8 (4.2) | 3.246 (1.375‐7.663)[Link] | 6 (5.3) | 7 (6.2) | 0.857 (0.272‐2.703) |
| ERCC1 rs735482 | ||||||
| AA | 64 (34.0) | 45 (23.9) | 1.000 | 28 (25.0) | 34 (30.4) | 1.000 |
| AC | 89 (47.3) | 100 (53.2) | 0.626 (0.389‐1.008) | 61 (54.5) | 60 (53.6) | 1.235 (0.668‐2.282) |
| CC | 35 (18.6) | 43 (22.9) | 0.572 (0.318‐1.029) | 23 (20.5) | 18 (16.0) | 1.522 (0.701‐3.433) |
| ERCC1 rs2336219 | ||||||
| GG | 64 (34.0) | 47 (25.0) | 1.000 | 28 (25.0) | 36 (32.1) | 1.000 |
| GA | 90 (47.9) | 103 (54.8) | 0.642 (0.401‐1.208) | 61 (54.5) | 59 (52.7) | 1.329 (0.722‐2.446) |
| AA | 34 (18.1) | 38 (20.2) | 0.657 (0362‐1.193) | 23 (20.5) | 17 (15.2) | 1.739 (0.783‐3.864) |
| MLH3 rs108621 | ||||||
| TT | 123 (65.4) | 125 (66.5) | 1.000 | 67 (59.8) | 76 (67.9) | 1.000 |
| TC | 55 (29.3) | 58 (30.9) | 0.964 (0.618‐1.504) | 41 (36.6) | 32 (28.6) | 1.453 (0.824‐2.563) |
| CC | 10 (5.3) | 5 (2.7) | 2.033 (0.675‐6.118) | 4 (6.6) | 4 (3.5) | 1.134 (0.273‐4.713) |
| OGG1 rs1052133 | ||||||
| GG | 62 (33.0) | 62 (33.0) | 1.000 | 34 (30.4) | 34 (39.3) | 1.000 |
| GC | 94 (50.0) | 94 (50.0) | 1.000 (0.635‐1.574) | 60 (53.6) | 59 (52.7) | 1.316 (0.741‐2.336) |
| CC | 32 (17.0) | 32 (17.0) | 1.000 (0.547‐1.828) | 18 (16.1) | 9 (8.0) | 2.588 (1.035‐6.474)[Link] |
| PPP1R13L rs6966 | ||||||
| TT | 51 (27.1) | 54 (28.7) | 1.000 | 29 (25.9) | 31 (27.7) | 1.000 |
| TA | 89 (47.3) | 80 (42.6) | 1.178 (0.723‐1.918) | 56 (50.0) | 50 (44.6) | 1.197 (0.635‐2.257) |
| AA | 48 (25.6) | 54 (28.7) | 0.941 (0.545‐1.624) | 27 (24.1) | 31 (27.7) | 0.931 (0.452‐1.918) |
| CD3EAP rs1007616 | ||||||
| CC | 102 (54.3) | 116 (61.7) | 1.000 | 57 (50.9) | 62 (55.3) | 1.000 |
| CT | 66 (35.1) | 61 (32.4) | 1.230 (0.794‐1.907) | 49 (43.8) | 39 (34.8) | 1.367 (0.786‐2.377) |
| TT | 20 (10.6) | 11 (5.9) | 2.068 (0.946‐4.521) | 6 (5.3) | 11 (9.9) | 0.593 (0.206‐1.709) |
χ2 = 7.824 P = 0.005.
χ2 = 4.273 P = 0.039.
Table 3.
The relationship between age status and genetic polymorphisms in five selected genes
| Groups | Age < 60 (y) | OR (95% CI) | Age ≥ 60 (y) | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Cases (%) | Controls (%) | Cases (%) | Controls (%) | |||
| ERCC1 rs3212986 | ||||||
| CC | 67 (45.0) | 85 (53.8) | 1.000 | 85 (56.3) | 89 (62.7) | 1.000 |
| CA | 68 (45.6) | 63 (39.9) | 1.369 (0.857‐2.189) | 53 (35.1) | 48 (33.8) | 1.248 (0.891‐1.748) |
| AA | 14 (9.4) | 10 (6.3) | 1.776 (0.742‐4.250) | 13 (8.6) | 5 (3.5) | 2.722 (0.931‐7.964) |
| ERCC1 rs735482 | ||||||
| AA | 53 (35.6) | 40 (25.3) | 1.000 | 39 (25.8) | 39 (27.5) | 1.000 |
| AC | 72 (48.3) | 97 (61.4) | 0.560 (0.336‐0.934)[Link] | 78 (51.7) | 63 (44.4) | 1.238 (0.711‐2.155) |
| CC | 24 (16.1) | 21 (13.3) | 0.863 (0.422‐1.764) | 34 (22.5) | 40 (28.1) | 0.850 (0.449‐1.607) |
| ERCC1 rs2336219 | ||||||
| GG | 53 (35.6) | 42 (26.6) | 1.000 | 39 (25.8) | 41 (28.9) | 1.000 |
| GA | 74 (49.7) | 95 (60.1) | 0.617 (0.372‐1.024) | 77 (51.0) | 67 (47.2) | 1.208 (0.699‐2.088) |
| AA | 22 (14.8) | 21 (13.3) | 0.830 (0.403‐1.709) | 35 (23.2) | 34 (23.9) | 1.082 (0.568‐2.061) |
| MLH3 rs108621 | ||||||
| TT | 96 (64.4) | 100 (63.3) | 1.000 | 94 (62.3) | 101 (71.1) | 1.000 |
| TC | 46 (30.9) | 55 (34.8) | 0.871 (0.538‐1.410) | 50 (33.1) | 35 (24.6) | 1.535 (0.917‐2.570) |
| CC | 7 (4.7) | 3 (1.9) | 2.431 (0.611‐9.673) | 7 (4.6) | 6 (4.3) | 1.254 (0.407‐3.865) |
| OGG1 rs1052133 | ||||||
| GG | 41 (27.5) | 61 (38.6) | 1.000 | 55 (36.4) | 45 (31.7) | 1.000 |
| GC | 85 (57.0) | 78 (49.4) | 1.621 (0.982‐2.676) | 69 (45.7) | 75 (52.8) | 0.753 (0.451‐1.256) |
| CC | 23 (15.4) | 19 (12.0) | 1.801 (0.872‐3.719) | 27 (17.9) | 22 (15.5) | 1.004 (0.505‐1.996) |
| PPP1R13L rs6966 | ||||||
| TT | 37 (24.8) | 43 (27.2) | 1.000 | 43 (28.5) | 42 (29.6) | 1.000 |
| TA | 70 (47.0) | 68 (43.1) | 1.196 (0.689‐2.077) | 75 (49.6) | 62 (43.7) | 1.182 (0.687‐2.032) |
| AA | 42 (28.2) | 47 (29.7) | 1.039 (0.567‐1.902) | 33 (21.9) | 38 (26.7) | 0.848 (0.451‐1.594) |
| CD3EAP rs1007616 | ||||||
| CC | 83 (55.7) | 96 (60.8) | 1.000 | 76 (50.3) | 82 (57.7) | 1.000 |
| CT | 53 (35.6) | 55 (34.8) | 1.115 (0.691‐1.798) | 62 (41.1) | 45 (31.7) | 1.487 (0.906‐2.438) |
| TT | 13 (8.7) | 7 (4.4) | 2.148 (0.819‐5.636) | 13 (8.6) | 15 (10.6) | 0.935 (0.418‐2.093) |
χ2 = 4.976, P = 0.026.
3.2. Association between the risk of lung cancer and ERCC1 rs3212986 under smoking status
Cancers are likely caused by the interactions among environmental exposure, genetic polymorphisms, and lifestyle behaviors,23 such as tobacco exposure is one of the major environmental high‐risk factors to develop lung cancer.24 We further matched 186 lung cancer cases with 186 controls (Table S3) that smoking status was clearly identified, to further explore whether there is a potential interaction between rs3212986 and the risk of lung cancer under tobacco smoking exposure. Table 4 shows that for smokers, the A allele (AA and CA + AA genotypes) of rs3212986 exhibited an enhanced risk to develop lung cancer (P < 0.05). No association among nonsmokers was found in the presented study.
Table 4.
The relationship between smoking status and genetic polymorphisms in ERCC1
| Groups | Smoking | OR (95% CI) | No smoking | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||
| ERCC1 rs3212986 | ||||||
| CC | 40 | 54 | 1.000 | 55 | 60 | 1.000 |
| CA | 28 | 22 | 1.718 (0.860‐3.433) | 45 | 38 | 1.292 (0.734‐2.276) |
| AA | 13 | 5 | 3.510 (1.157‐10.645)[Link] | 5 | 7 | 0.779 (0.234‐2.599) |
| CA + AA | 41 | 27 | 2.050 (1.086‐3.868)[Link] | 50 | 45 | 1.212 (0.703‐2.089) |
χ2 = 5.335, P = 0.021.
χ2 = 4.967, P = 0.026.
3.3. Effects of haplotypes in ERCC1 and CD3EAP on the risk of lung cancer
The association between inferred haplotypes and lung cancer risk is shown in Table 5. Three SNPs (rs3212986, rs735482, and rs2336219) of ERCC1 in 3ʹUTR were differentiated into four haplotypes on account of significant linkage disequilibrium (Figure 1). Compared with CAG haplotype as the reference, CCG haplotype was presented a protective effect against lung cancer risk (OR = 0.291, 95%CI: 0.062‐0.769), while AAG haplotype including the minor A allele of ERCC1rs3212986 was noted for a trend toward increased risk of lung cancer (OR = 1.350, 95%CI: 0.989‐1.841) with critical P value. In addition, an integrated haplotype analysis of 5 SNPs in ERCC1, CD3EAP, and PPP1R13L on 19q13 was performed to explore higher LD with the cancer‐related SNP. However, four SNPs (CD3EAP rs1007616, ERCC1rs3212986, ERCC1rs735482, and ERCC1 rs2336219) were actually accepted into haplotype blocks analysis besides PPP1R13L rs6966, which was in weaker LD with others (Figure 1). Compared with wild‐type haplotype block, haplotype block CCAC including both the minor C allele of ERCC1 rs735482 and A allele of ERCC1 rs2336219 (OR = 14.323, 95%CI: 6.503‐31.547); haplotype block AAGC including the minor A allele of ERCC1 rs3212986 (OR = 17.692, 95%CI: 7.909‐39.576); haplotype block CAGT including the minor T allele of CD3EAP rs1007616 (OR = 22.646, 95%CI: 10.051‐51.026); haplotype block AAGT including both the minor A allele of ERCC1 rs3212986 and T allele of CD3EAP rs1007616 (OR = 5.000, 95%CI: 1.562‐16.005) were significantly associated with the high risk of lung cancer. From the above, it was implied that polymorphisms of ERCC1 and its overlapping gene CD3EAP were in strong LD. Particularly, minor A allele of rs3212986 in overlapping region of two genes may be a potentially functional SNP contributing to the high risk of lung cancer and low efficiency of DNA repair.
Table 5.
Association between the haplotypes and the risk of lung cancer
| Groups | Frequency[Link] | Group | OR (95% CI) | P [Link] | |
|---|---|---|---|---|---|
| Cases (n) | Controls (n) | ||||
| ERCC1 rs3212986 C>A; ERCC1 rs735482 A>C; ERCC1 rs2336219 G>A | |||||
| CAG | 0.283 | 162 | 177 | 1.000 | |
| CCA | 0.435 | 258 | 264 | 1.068 (0.812, 1.404) | 0.639 |
| AAG | 0.254 | 168 | 136 | 1.350 (0.989, 1.841) | 0.058 |
| CCG | 0.015 | 3 | 15 | 0.219 (0.062, 0.769) | 0.010 |
| ERCC1 rs3212986 C>A; ERCC1 rs735482 A>C; ERCC1 rs2336219 G>A; CD3EAP rs1007616 C>T | |||||
| CAGC | 0.081 | 7 | 90 | 1.000 | |
| CCAC | 0.402 | 254 | 228 | 14.323 (6.503‐31.547) | <0.05 |
| AAGC | 0.232 | 161 | 117 | 17.692 (7.909‐39.576) | <0.05 |
| CAGT | 0.202 | 155 | 88 | 22.646 (10.051‐51.026) | <0.05 |
| CCAT | 0.033 | 4 | 35 | 1.469 (0.405‐5.333) | 0.810 |
| AAGT | 0.021 | 7 | 18 | 5.000 (1.562, 16.005) | <0.05 |
| CCGC | 0.013 | 3 | 12 | 3.214 (0.731, 14.128) | 0.259 |
P‐value <0.05 was considered statistically significant (in bold).
A frequency of <0.01 is not included in the Table.
The P value was obtained using the chi‐square test.
Figure 1.
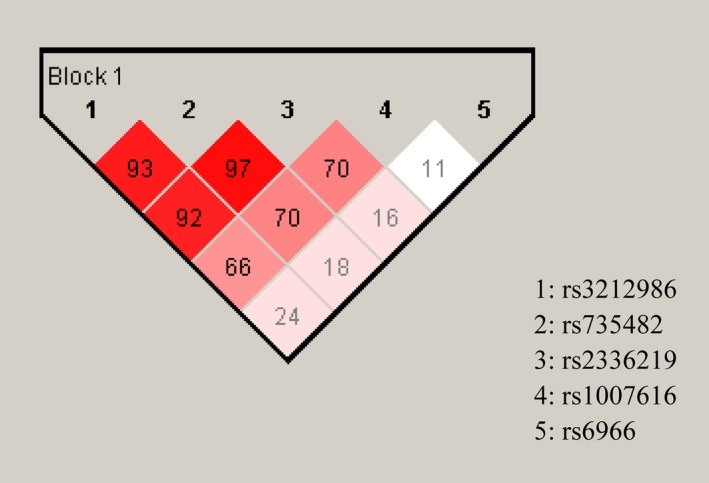
Analysis of haplotype blocks in the overlapping regions of ERCC1, CD3EAP, and PPP1R13L
3.4. Design plasmids according to rs3213986 location and obtain transfected cells
Rs3213986 is located in the 3′UTR of ERCC1, but also in the opposite orientated, adjacent coding region of CD3EAP (Figure 2). We first introduced full‐length ERCC1 (CDS+3′‐UTR) or CD3EAP (CDS) cDNA clone containing rs3213986 CC or AA genotype. The cDNA clone of different polymorphisms transfected into the tool cells HEK293T which showed high transfection efficiency or human bronchial epithelial cells 16HBE that could better exhibit the human cell state under exogenous chemical stimulation. The obtained transfected HEK293T and 16HBE cells were designated as 293TERCC1/CD3EAP(CC) and 293TERCC1/CD3EAP(AA), or 16HBEERCC1/CD3EAP(CC) and 16HBEERCC1/CD3EAP(AA). ERCC1/CD3EAP(EV) represented the cells transfected with empty vector that not expressing ERCC1 or CD3EAP, but just show colors. ERCC1 (CDS+3′‐UTR) vector showed green fluorescence while CD3EAP (CDS) displayed red one. The mock and empty vectors as control expressed significantly lower ERCC1 or CD3EAP protein levels than overexpressed cells.
Figure 2.
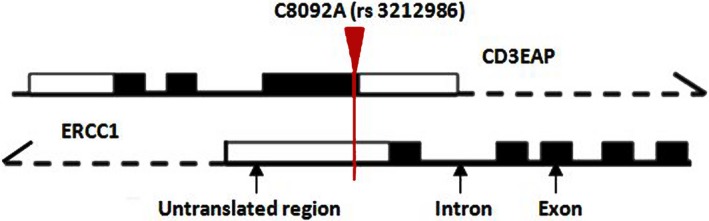
The diagram of positional relationship between ERCC1 and CD3EAP. ERCC1 C8092A (rs3213986) polymorphism located in the overlapping region of ERCC1 3′‐untranslated region and CD3EAP coding region
3.5. Rs3212986 A allele decreased cell viability after BPDE treatment via ERCC1
After 24 hour BPDE treatment, 293TERCC1(CC) showed more resistant to BPDE compared to 293TERCC1(AA), and the differences in survival rate between those became more pronounced after an additional 24 hour incubation(Figure 3A,B). In 16HBE transfected cells, ERCC1 overexpression with rs3212986 CC genotype could enhance the cell resistance to BPDE. After an additional 24 hour of incubation to allow DNA repair, the survival rate of 16HBEERCC1(CC) significantly relieved at 2, 4 µmol/L BPDE treatment compared to 16HBEERCC1(AA) (Figure 3E,F). In addition, there were no statistical differences between these two genotypes of CD3EAP transfected 293T and 16HBE cells, except under some sensitive points (P < 0.05) (Figure 3C,D,G,H).
Figure 3.
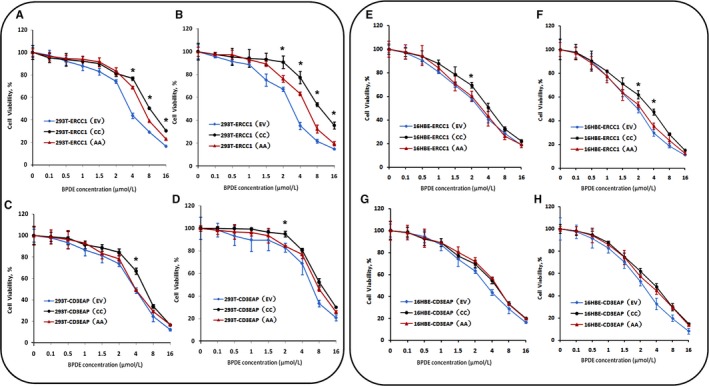
Comparison of cell viability in transfected cells after BPDE treatment. Cells survival rate of the transfected cells at 0.1, 0.5, 1, 1.5, 2, 4, 8, 16 μmol/L BPDE treatment. ERCC1 cDNA with either C allele or A allele of rs3212986 was transfected to HEK293T (A) or 16HBE (E) cells and treated with BPDE for 24 h, and another 24 h repair after 24 h treatment (B, F). CD3EAP cDNA with either C allele or A allele of rs3212986 was transfected to HEK293T (C) or 16HBE (G) cells and treated with BPDE for 24 h, and another 24 h repair after 24 h treatment (D, H). * P < 0.05 compared between ERCC1(CC) and ERCC1(AA), or CD3EAP(CC) and CD3EAP(AA) transfected cells
In summary, it suggested that different genotypes of rs3212986 can affect survival rates via ERCC1 under BPDE treatment in our cell model. According to the survival curve of all transfected cells, 1, 2, and 4 µmol/L BPDE treatment were shown a distinguished alteration and selected as the target concentrations for the following experiment for DNA damage and repair.
3.6. A allele of rs3212986 inhibited DNA repair of BPDE‐induced damage via ERCC1
BPDE reacts with many biological macromolecules including DNA to cause covalent BPDE‐DNA adducts and oxidative damage.25 To further clarify the underlying function on DRC of rs3212986, a comet assay and immunofluorescence microscopy of γH2AX used to quantify DNA damage level induced by BPDE. For the concerning time points, the comet tail moment (OTM, as an index of DNA damage level) increased in a dose‐dependent way in the cell lines, and by additional 24 hour incubation, cell damage was recovered except for 4 µmol/L BPDE treatment. The comet tails in 293TERCC1(CC) were statistically smaller than that in 293TERCC1(AA) at 12 hour of 2 µmol/L and 6 hour, 12 hour of 4 µmol/L BPDE treatment (P < 0.05) (Figure 4A). No clear differences between comets in 293TCD3EAP(CC) and 293TCD3EAP(AA) were found. However, the longest comet tails were observed in 293TCD3EAP(EV) at 24 hour of 2 µmol/L and 12 hour of 4 µmol/L BPDE treatment (P < 0.05) (Figure 4B).
Figure 4.
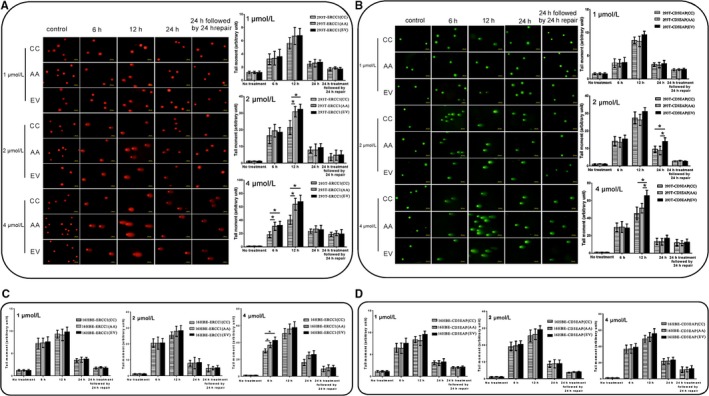
Comet assay. Comet assay of HEK293T (A,B) or 16HBE (C,D) transfected cells, either untreated (control) or exposed to 1, 2, 4 μmol/L BPDE for 6, 12, 24 h, or 24 h treatment followed by 24 h repair. The tail moment of 200 comets was analyzed for each experiment, n = 3. *P < 0.05
BPDE‐DNA adducts and DNA double‐strand breaks induced by BPDE could induce phosphorylation of H2AX serine 139 (S139).21 Here, we analyzed the induction of γH2AX foci by immunofluorescence response to DNA damage in cells treated with 1, 2, or 4 μmol/L BPDE. The γH2AX foci had a dose‐dependent relationship with BPDE concentrations. Between 6 and 12 hour of BPDE treatment, γH2AX foci formation was at the maximum. The 293TERCC1(CC) cells showed a better repair capacity than 293TERCC1(AA) (P < 0.05) (Figure 5A). Compared with the CD3EAP transfected HEK293T cells, the γH2AX foci percentage of 293TCD3EAP(EV) was observed statistically higher at 24 hour of 2 µmol/L and 12 hour of 4 µmol/L BPDE treatment (P < 0.05) (Figure 5B).
Figure 5.
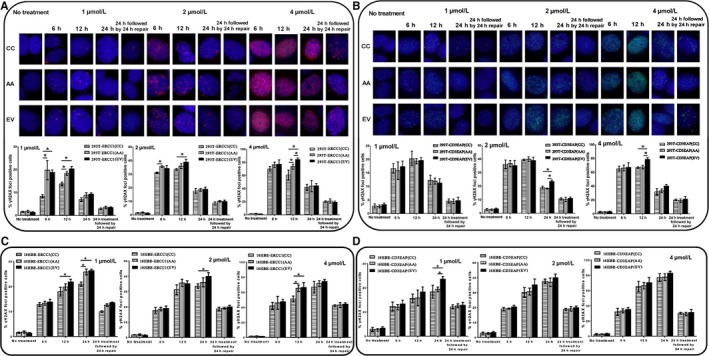
Immunofluorescence results of nuclear γH2AX foci in transfected cells in response to DNA damage. γH2AX foci of HEK293T (A,B) or 16HBE (C,D) transfected cells, either untreated (control) or exposed to 1, 2, 4 μmol/L BPDE for 6, 12, 24 h or 24 h treatment followed by 24 h repair. A cell with more than ten distinct foci in the nucleus was considered to be positive. Depicted is the average and standard deviation of three independent experiments. *P < 0.05
For 16HBE transfected cells, ERCC1 overexpression with rs3212986 CC genotype could enhance the DRC to BPDE (Figures 4C and 5C). In addition, the CD3EAP transfected 16HBE cells showed better DRC than CD3EAP (EV) cells after BPDE treatment (P < 0.05), but no clear differences between 16HBECD3EAP(CC) and 16HBECD3EAP(AA)(Figures 4D and 5D). The above results further confirmed that rs3212986 mainly takes actions via affecting the function of ERCC1, and AA genotype of rs3212986 had a worse DRC to the environmental carcinogen BPDE compared with CC genotype.
4. DISCUSSION
In present years, it has become increasingly clear that individuals’ susceptibility to the risk of carcinogenesis is highly related to interdifferent DRC. Many epidemiological investigations have demonstrated the relationship between polymorphisms with DNA repair genes and cancer risk.26, 27, 28 Although it was reported that SNPs in 3ʹUTR or haplotypes have relevance to the risk of several cancers, the conclusions were still quite controversial. The present study focuses on the association between lung cancer risk and 3ʹUTR polymorphisms of DNA repair genes based on a case‐control study.
ERCC1 is a lead enzyme for a well functional NER, which can remove DNA damage in the global genome.29 Some ERCC1 SNPs alert DNA repair capacity and therefore are considered as susceptibility biomarkers in predicting the cancer risk including NSCLC.13, 30, 31, 32, 33, 34 It was revealed that the minor allele of ERCC1 rs3212986 may be a risk factor to alter DNA repair capacity, enhance genetic susceptibility to lung cancer,30, 31 shorten the carriers’ overall survival, and decrease the activity of platinum in advanced NSCLC.32, 33, 34 In this case‐control study, compared with ERCC1 rs3212986 CC, AA genotype was shown to be a high‐risk factor for NSCLC (OR = 3.246; 95%CI: 1.375‐7.663). In agreement with this result, minor A allele of rs3212986 could also reflect a linkage with an increasing risk of NSCLC in haplotype analysis on chromosome 19. Besides hereditary factors, other factors such as gender, age, and smoking status also correlated with the formation of DNA adducts leading to distinct cancer susceptibility. Our stratified analysis showed that, compared with each wild type, AA genotype of ERCC1 rs3212986 increased the risk of NSCLC in male population (OR = 3.246; 95% CI: 1.375‐7.663), meanwhile CC genotype of OGG1 rs1052133 in female ones(OR = 2.588; 95% CI: 1.035‐6.474). OGG1 is a key enzyme of BER to identify and excise 8‐oxoG lesions that induced transversions from G:C to T:A.35, 36, 37 Similarly, Liu et al14 observed that C allele site of OGG1 rs1052133, jointed effects with a smoking habit, exerted an adverse influence on lung cancer risk in Asia. Moreover, the analysis after age stratification (Age < 60) indicated that AC genotype of ERCC1 rs735482 had a protective role for lung cancer (OR = 0.560, 95%CI: 0.336‐0.934). However, Jones et al38 found no association between ERCC1 rs735482 and lung cancer susceptibility in Caucasians. The inconsistent findings were possibly due to ethnic differences and limiting sample size. Furthermore, we found that the A allele (AA and CA + AA genotypes) of ERCC1 rs3212986 also exhibited an enhanced risk to develop lung cancer in smokers only (P < 0.05), but not in never smokers. As we well known, gene‐smoking interactions have associations with the risk of lung cancer. Zhou et al30 consistently showed that the AA genotype of ERCC1 rs3212986 polymorphism (or ERCC1 haplotypes) had significant interaction between cumulative cigarette smoking and lung cancer risk. No significant association was observed between ERCC1 rs2336219, MLH3 rs108621, PPP1R13L rs6966, or CD3EAP rs1007616 polymorphisms and the risk of lung cancer.
A potentially intriguing aspect of ERCC1 rs3212986 polymorphism is that this SNP is also located in the coding region of CD3EAP.23 Our previous research using cultured lymphocytes from healthy population found that the minor A allele of ERCC1 rs3212986 in genotypes and haplotypes remarkably increased BPDE‐induced DNA adducts, and AA homozygous cells also showed a reduced CD3EAP mRNA level in comparison with CC individuals after BPDE exposure.17 In this study, there was a strong LD between candidate SNPs of ERCC1 and CD3EAP. And high NSCLC risk was observed within the haplotype blocks embracing A allele of ERCC1 rs3212986. To analyze how rs3212986 polymorphism involved in DNA repair by affecting ERCC1 or CD3EAP, ERCC1(CDS+3′UTR) cDNA, or CD3EAP(CDS) cDNA clones containing different genotypes of rs3212986 was introduced into human cell lines to magnify and illuminate this affection by inducing BPDE‐DNA adducts. We found that AA genotype was associated with lower cell survival rate, which may be representative for a less effective DNA repair and a higher level of DNA damage. In order to compare the DRC of damage caused by BPDE treatment in different transfected cells, DNA damage induced by BPDE‐DNA adduct was intuitively detected by γH2AX immunofluorescence and the modified comet assay. As a result, reduced DRC was observed in 293TERCC1(AA) and 16HBEERCC1(AA), whereas no significant difference in DRC was observed in 293TCD3EAP(CC)/16HBECD3EAP(CC) and 293TCD3EAP(AA)/16HBECD3EAP(AA), which suggested that the variant genotypes of rs3212986 modulated DNA repair efficiency via modifying 3ʹUTR of ERCC1, but not CD3EAP. Previous investigations have been reported that there was high LD between ERCC1 rs3212986 and other polymorphisms of gene or polymorphisms of other adjacent genes on 19q13 such as XRCC1 (X‐ray repair complementing defective repair in Chinese hamster cells 1) or ERCC2/XPD (excision repair cross‐complementation group 2/xeroderma pigmentosum D) resulting in an increased risk to develop lung cancer,39, 40 which may affect the mRNA stability of ERCC1 and therefore be associated with a lower DNA repair efficiency.41 In addition, we predicted ERCC1 mRNA secondary structure between two genotypes of rs3212986 using bioinformatics software. The expected results were showed that genetic variants in rs3212986 may play a critical effect on DRC by altering the folded stem‐loop structure consisting of six repeats of "GCT," which can impact 18‐base sequence in ERCC1 3ʹUTR (Figure 6). Moreover, enzyme expression may be regulated by miRNAs which can also be affected by polymorphism in target complementary sequence. As either oncogenes or tumor suppressors, miRNAs might have a synergistic effect of SNPs on individual DRC in relation to cancerous susceptibility.42, 43 Since located in the ERCC1 3′UTR, rs3212986 was likely involved in post‐transcriptional regulation of ERCC1 by the specific binding of miRNAs as miR‐15a.15 Taken together the above results, although rs3213986 polymorphism mapping in overlapping genes ERCC1 and CD3EAP on chromosome 19 was involved in expression of two genes, the major effect of DRC was driven by regulating ERCC1 in 3′UTR, given the higher LD, unstable secondary structure, and posttranscriptional regulation of binding miRNAs. On the other hand, although we did not see significant difference in DNA repair efficiency between CD3EAP transfected cells with different rs3212986 genotypes after exposure to BPDE, we will not deny the importance of CD3EAP in DNA repair. It was noteworthy to find in the present study, contrasted to the empty vector transfected cells, the survival rates can be increased in overexpressed CD3EAP cells after the exposure of various BPDE concentrations. CD3EAP may probably be related to cell proliferation involving in the RNA polymerase I transcription complex.18, 44 In another previous study, we confirmed that co‐expression patterns were consisted in overlapping genes as ERCC1, CD3EAP, and PPP1R13L. Particularly, there was a significant association between CD3EAP exon 3 and ERCC1 exon 11, while CD3EAP exon 1 and PPP1R13L exon 1.45 The potential influence of CD3EAP expression was in response to the regulation of genetic networks known to be associated with DRC and lung cancer risk.
Figure 6.
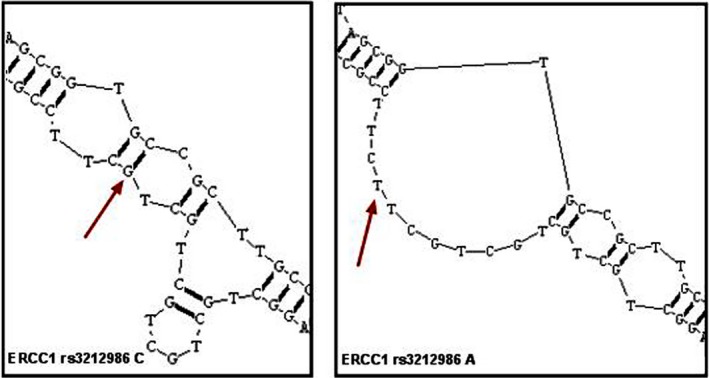
Rs3212986 variations might change ERCC1 mRNA structure and the stem‐loop structure formed by its upstream sequence would be unfolded. The RNA secondary structure was predicted by RNA structure Software. Only the most stable secondary structures with the lowest free energy are depicted
In conclusion, as one part of our series complementary assays for lung cancer risk assessment project, the present study demonstrated that ERCC1 C8092A (rs3213986) in 3ʹUTR of ERCC1 was associated with an increased risk of lung cancer, especially in smoking population, and therefore could be used as a valuable tumor marker. Although it was also located within the ORF of CD3EAP gene, its effects on the DRC toward BPDE induced DNA damage mainly via modulating ERCC1 expression, but not CD3EAP. However, further studies involving more polymorphisms, conditional gene knockout model cell lines, and a larger sample size are still needed to clarify the detailed function of SNPs in 3ʹUTR.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant nos. 81273118 and 81773470).
Yu T, Xue P, Cui S, et al. Rs3212986 polymorphism, a possible biomarker to predict smoking‐related lung cancer, alters DNA repair capacity via regulating ERCC1 expression. Cancer Med. 2018;7:6317–6330. 10.1002/cam4.1842
Tao Yu and Ping Xue contributed to this work equally.
REFERENCES
- 1. Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2014;64:252‐271. [DOI] [PubMed] [Google Scholar]
- 3. Yang L, Yang X, Tang Y, et al. Inhibition of DNA‐PK activity sensitizes A549 cells to X‐ray irradiation by inducing the ATM‐dependent DNA damage response. Mol Med Rep. 2018;17:7545‐7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng YL, Loffredo CA, Yu Z, et al. Bleomycin‐induced chromosome breaks as a risk marker for lung cancer: a case‐control study with population and hospital controls. Carcinogenesis. 2003;24:269‐274. [DOI] [PubMed] [Google Scholar]
- 5. Zienolddiny S, Skaug V. Single nucleotide polymorphisms as susceptibility, prognostic, and therapeutic markers of nonsmall cell lung cancer. Lung Cancer (Auckl). 2011;29:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pérez‐Ramírez C, Cañadas‐Garre M, Alnatsha A, et al. Impact of DNA repair, folate and glutathione gene polymorphisms on risk of non small cell lung cancer. Pathol Res Pract. 2018;214:44‐52. [DOI] [PubMed] [Google Scholar]
- 7. Miller EC. Some current perspectives on chemical carcinogenesis in humans and experimental animals: presidential address. Cancer Res. 1978;38:1479‐1496. [PubMed] [Google Scholar]
- 8. Slováková P, Majerová L, Matáková T, Skereňová M, Kavcová E, Halašová E. Mismatch repair gene polymorphisms and association with lung cancer development. Adv Exp Med Biol. 2015;833:15‐22. [DOI] [PubMed] [Google Scholar]
- 9. Zienolddiny S, Campa D, Lind H, et al. Polymorphisms of DNA repair genes and risk of non‐small cell lung cancer. Carcinogenesis. 2006;27:560‐567. [DOI] [PubMed] [Google Scholar]
- 10. Li C, Wang L‐E, Wei Q. DNA repair phenotype and cancer susceptibility—a mini review. Int J Cancer. 2009;124:999‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park CH, Sancar A. Formation of a ternary complex by human XPA, ERCC1, and ERCC4 (XPF) excision repair proteins. Proc Natl Acad Sci USA. 1994, 24:5017–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai Q, Luo H, Li XP, Huang J, Zhou TJ, Yang ZH. XRCC1 and ERCC1 polymorphisms are related to susceptibility and survival of colorectal cancer in the Chinese population. Oxford J. 2015;30:441–449. [DOI] [PubMed] [Google Scholar]
- 13. Pintarelli G, Cotroneo CE, Noci S, et al. Genetic susceptibility variants for lung cancer: replication study and assessment as expression quantitative trait loci. Sci Rep. 2017;7:42185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu CJ, Hsia TC, Tsai RY, et al. The joint effect of hOGG1 single nucleotide polymorphism and smoking habit on lung cancer in Taiwan. Anticancer Res. 2010;30:4141–4145. [PubMed] [Google Scholar]
- 15. Zhang Q, Zheng X, Li X, et al. The polymorphisms of miRNA‐binding site in MLH3 and ERCC1 were linked to the risk of colorectal cancer in a case‐control study. Cancer Med. 2018;7:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Moor CH, Meijer H, Lissenden S. Mechanisms of translational control by the 3' UTR in development and differentiation. Semin Cell Dev Biol. 2005;16:49–58. [DOI] [PubMed] [Google Scholar]
- 17. Yu T, Liu Y, Lu X, et al. Excision repair of BPDE‐adducts in human lymphocytes: diminished capacity associated with ERCC1 C8092A (rs3212986) polymorphism. Arch Toxicol. 2013;87:699–709. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto K, Yamamoto M, Hanada K, Nogi Y, Matsuyama T, Muramatsu M. Multiple protein‐protein interactions by RNA polymerase I‐associated factor PAF49 and role of PAF49 in rRNA transcription. Mol Cell Biol. 2004;24:6338–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergamaschi D, Samuels Y, O'Neil NJ, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33:162–167. [DOI] [PubMed] [Google Scholar]
- 20. Voge U, Laros I, Jacobsen NR, et al. Two regions in chromosome 19q13.2‐3 are associated with risk of lung cancer. Mutat Res. 2004;546:65–74. [DOI] [PubMed] [Google Scholar]
- 21. Yang JP, Hori M, Sanda T, Okamoto T. Identification of a novel inhibitor of nuclear factor‐kappaB, RelA‐associated inhibitor. J Biol Chem. 1999;274:15662–15670. [DOI] [PubMed] [Google Scholar]
- 22. Jeon H‐S, Jin G, Kang H‐G, et al. A functional variant at 19q13.3, rs967591G>A, is associated with shorter survival of early‐stage lung cancer. Clin Cancer Res. 2013;19:4185–4195. [DOI] [PubMed] [Google Scholar]
- 23. Skjelbred CF, Saebø M, Nexø BA, et al. Effects of polymorphisms in ERCC1, ASE‐1 and RAI on the risk of colorectal carcinomas and adenomas: a case control study. BMC Cancer. 2006;6:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei Q, Cheng L, Hong WK, Spitz MR. Reduced DNA repair capacity in lung cancer patients. Cancer Res. 1996;56:4103–4107. [PubMed] [Google Scholar]
- 25. Sullivan A, Lu X. ASPP: a new family of oncogenes and tumor suppressor genes. Br J Cancer. 2007;96:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das R, Kundu S, Laskar S, Choudhury Y, Ghosh SK. Assessment of DNA repair susceptibility genes identified by whole exome sequencing in head and neck cancer. DNA Repair (Amst). 2018;26:66–67. [DOI] [PubMed] [Google Scholar]
- 27. Qiao L, Feng X, Wang G, Zhou B, Yang Y, Li M. Polymorphisms in BER genes and risk of breast cancer: evidences from 69 studies with 33760 cases and 33252 controls. Oncotarget. 2018;9:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Özgöz A, HekimlerÖztürk K, Yükseltürk A,Şamlı H, Başkan Z, Mutlu İçduygu F et al. Genetic variations of DNA repair genes in breast cancer. Pathol Oncol Res. 2017. doi: 10.1007/s12253-017-0322-3. [DOI] [PubMed] [Google Scholar]
- 29. Holcomb N, Goswami M, Han SG, et al. Inorganic arsenic inhibits the nucleotide excision repair pathway and reduces the expression of XPC. DNA Repair (Amst). 2017;52:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou W, Liu G, Park S, et al. Gene‐smoking interaction associations for the ERCC1 polymorphisms in the risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:491–496. [DOI] [PubMed] [Google Scholar]
- 31. Gao H, Ge RC, Liu HY, Wang Y, Yan S. Effect of ERCC1 polymorphism on the response to chemotherapy and clinical outcome of non‐small cell lung cancer. Genet Mol Res. 2014;13:8997–9004. [DOI] [PubMed] [Google Scholar]
- 32. Isla D, Sarrie C, Rosell R, et al. Single nucleotide polymorphisms and outcome in docetaxel‐cisplatin‐treated advanced non‐small‐cell lung cancer. Ann Oncol. 2004;15:1194–1203. [DOI] [PubMed] [Google Scholar]
- 33. Pérez‐Ramírez C, Cañadas‐Garre M, Alnatsha A. et al. Pharmacogenetics of platinum‐based chemotherapy: impact of DNA repair and folate metabolism gene polymorphisms on prognosis of non‐small cell lung cancer patients. Pharmacogenomics J. 2018. doi: 10.1038/s41397-018-0014-8. [DOI] [PubMed] [Google Scholar]
- 34. Takenaka T, Yano T, Kiyohara C, et al. Effects of excision repair cross‐complementation group 1 (ERCC1) single nucleotide polymorphisms on the prognosis of non‐small cell lung cancer patients. Lung Cancer. 2010;67:101–107. [DOI] [PubMed] [Google Scholar]
- 35. Qin H, Zhu J, Zeng Y. Aberrant promoter methylation of hOGG1 may be associated with increased risk of non‐small cell lung cancer. Oncotarget. 2017;8:8330–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klungland A, Rosewell I, Hollenbach S, et al. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA. 1999;96:13300–13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenquist TA, Zharkov DO, Grollman AP. Cloning and characterization of a mammalian 8‐oxoguanine DNA glycosylase. Proc Natl Acad Sci USA. 1997;94:7429–7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones NR, Spratt TE, Berg AS, Muscat JE, Lazarus P, Gallagher CJ. Association studies of excision repair cross‐complementation group 1 (ERCC1) haplotypes with lung and head and neck cancer risk in a Caucasian population. Cancer Epidemiol. 2011;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhan P, Wang Q, Wei SZ et al. ERCC2/XPD Lys751Gln and Asp312Asn gene polymorphism and lung cancer risk: a meta‐analysis involving 22 case‐control studies. Thorac Oncol. 2010;5:1337–1345. [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Yang H, Li H, et al. Association between X‐ray repair cross complementing group 1 codon 399 and 194 polymorphisms and lung cancer risk: a meta‐analysis. Cancer Lett. 2009;285:134–140. [DOI] [PubMed] [Google Scholar]
- 41. Whitehouse CJ, Taylor RM, Thistlethwaite A, et al. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single‐strand break repair. Cell. 2001;104:107–117. [DOI] [PubMed] [Google Scholar]
- 42. Wang K, Diskin SJ, Zhang H, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y, Zhou L, Chen J, et al. Association of the 3'UTR FOXO3a polymorphism rs4946936 with an increased risk of childhood acute lymphoblastic leukemia in a Chinese population. Cell Physiol Biochem. 2014;34:325–332. [DOI] [PubMed] [Google Scholar]
- 44. Xiao M, Xiao S, Straaten TV, et al. Genetic polymorphisms in 19q13.3 genes associated with alteration of repair capacity to BPDE‐DNA adducts in primary cultured lymphocytes. Mutat Res. 2016;812:39–47. [DOI] [PubMed] [Google Scholar]
- 45. Zhang G, Xue P, Cui S, et al. Different splicing isoforms of ERCC1 affect the expression of its overlapping genes CD3EAP and PPP1R13L, and indicate a potential application in non‐small cell lung cancer treatment. Int J Oncol. 2018;52:2155–2165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


