Graphical abstract
Abbreviations: CI, confidence interval of the mean; mg/kg b.w., milligram per kilogram of body weight; MTD, maximum tolerated dose; NCE, normochromatic erythrocyte; PCE, polychromatic erythrocyte; Es, total erythrocytes; MN test, micronucleus test
Keywords: Pesticides, Triazoles, Maximum tolerated doses, Erythropoiesis, Micronucleus test, Mice
Highlights
-
•
Triazole pesticides exert an inhibitory effect on erythropoiesis in mammal bone marrow.
-
•
The effects of triazole pesticides may depend on the type and arrangement of substituents in molecules.
-
•
At the lower acceptable level of PCEs/(PCEs + NCEs) (20%) suppression of erythropoiesis masks cytogenetic effects.
-
•
The MTDs of 79 different technical materials of pesticide active ingredients for CD-1 mice have been determined.
Abstract
Effects of technical materials of pesticide active ingredients, belonging to various chemical classes, on erythropoiesis in mouse bone marrow were studied as part of the research on the pesticide mutagenic activity in micronucleus test. The purpose of the present study was to estimate the toxic action of the test substances on the target organ and the validity of the results of the micronucleus assay under conditions of erythropoiesis suppression.
It was demonstrated that intragastrically administrated triazole pesticides reached bone marrow (target organ where micronucleus induction was assessed) and exerted an inhibitory effect on erythropoiesis. The effects of triazole pesticides were enhanced in the following order: difenoconazole ≤ tebuconazole < cyproconazole < flutriafol. Furthermore, an association between structural features of molecules and specific target organ activity of the test pesticides was observed.
Based on the data on the general toxicity and the results of the evaluation of the effects on erythropoiesis, the maximum tolerated doses (MTDs) of 79 different technical materials of pesticides for CD-1 mice were determined.
1. Introduction
Erythrocytes of the mammalian bone marrow are widely used in cytogenetic methods for evaluation of potential mutagenic activity of pesticides in the context of toxicological and hygienic assessment in order to classify pesticides by mutagenic hazard. The micronucleus test on polychromatic erythrocytes (PCEs) is highly efficient for that purpose, since PCEs can be easily distinguished and have a short life cycle. When an erythroblast turns into a polychromatic erythrocyte, the nucleus is pushed out, but if a micronucleus have been formed (which is part of the original chromosomal material) it will remain in the cytoplasm. Therefore, it is easy to visualize micronuclei that appeared spontaneously or were induced by test agents in a PCE lacking the nucleus. An increase in the incidence of micronucleated PCEs in bone marrow of animals treated with test substances is an indicator of induced chromosomal lesions.
When conducting an experiment, it is important to choose the dose ranges correctly. Maximum Tolerated Dose (MTD), which is a dose that causes toxic effects (e.g. abnormal behavior, mild weight loss, mild cytotoxicity in target tissue, etc.) but does not lead to animal death or severe clinical signs of toxicity necessitating humane euthanasia and limiting the study, is chosen as the maximum dose of a toxic substance. For substances with low toxicity, the highest dose of 1000 mg/kg b.w. is used in experiments lasting 14 days or more. For an administration period of less than 14 days, the highest dose is 2000 mg/kg [1]. If the highest dose is based on the toxicity to the target organ, the dose that produces a reduction in the proportion of PCEs among total erythrocytes in the bone marrow to the level of 20–50% of negative control value (but not less than 20%) is chosen according to the OECD Guideline No. 474 [1].
Pesticides are toxic substances and can damage various organ systems. In particular, it was shown that pesticides, especially organochlorines and organophosphates, inhibit hemopoiesis [[2], [3], [4]], whereas in cases of synthetic pyrethroid and neonicotinoid exposure, an increase of oxidative stress marker levels and telomerase activity were found with additional increased inflammations of various organs, and inflicted genotoxicity [[5], [6], [7], [8]]. Furthermore, other pesticide groups, regarding the organophosphorus and carbamate type, have been also studied for their histopathological lesions, oxidative stress and genotoxic effects [9,10]. Biomonitoring of such pesticides has gained attention throughout the years as seen in studies focusing on neonicotinoids and organophosphorus pesticides [11,12] and ways of ascertaining information based on a “real-life human exposure” from on a long-term and low-dose exposure to a specific chemical mixture when not simulated as a real-life condition of exposure, are not clear, leading to “grey zones” in the interpretation of the results due to uprising limitations. Various studies [[13], [14], [15]] have clarified this and similar methods should be applied. Besides these aforementioned factors, genetic variations in xenobiotic metabolizing enzymes that can induce damage to vital organs should also be taken into account [16]. Another method of clarifying potential toxicity problems within compounds is via “structural alerts”. Such structural alerts can either be high chemical reactivity molecular fragments or fragments transformed, via bioactivation by human enzymes, into fragments with high chemical reactivity [17]. Measuring alterations in gene expression contributes also as a method for obtaining data on metabolic toxicity arising from chemical compounds such as commercial formulations of herbicides [18] and their respective damage on vital organs [19].
The purpose of this study was to evaluate the effects of various technical materials of pesticide active ingredients on erythropoiesis in mammalian bone marrow and to assess the validity of cytogenetic analysis conducted on bone marrow samples under the conditions of erythropoiesis suppression.
2. Materials and methods
The study of 79 technical materials of pesticides (52 different names) was conducted in CD-1 mice in accordance with the ethical guidelines of EU Directive 2010/63/EU and OECD TG 474 [1]. Mice were purchased from “Andreevka” Branch of the Federal Government Budgetary Establishment of Science “Scientific Center of Biomedical Technologies” of the Federal Bio-Medical Agency of the Russian Federation. The acclimation period was 7 days. Mice had access to drinking water and feed ad libitum, and were maintained under a 12:12-h light/dark photoperiod at 22–22,5 °C and 36–40% humidity.
For the assessment of mutagenic activity at least 5 groups of minimum 5 mice for each sex were used in each study, including a positive control group (40 mg/kg b.w. cyclophosphamide), a negative control group (vehicle), and 3 treatment groups. Test pesticides were administered intragastrically at three or more dose levels. The maximum dose in the main experiment was either 2000 mg/kg b.w. (for substances with low toxicity), or an MTD, determined in a preliminary dose-finding experiment. Pesticides were administered once per day for two subsequent days (with a 24-h interval) in the volume of 10 ml/kg b.w. using metal gavage needle.
Mice were sacrificed 22 h after the second administration by cervical dislocation. Then femurs were removed and bone marrow was harvested. Bone marrow smears were prepared on microscope slides (2 slides per animal), air-dried, fixed, stained with azure-eosin using “Leuсodif 200” kit (Erba Lachema s.r.o., CZ) and independently coded by a researcher not engaged in the cell counting process.
To assess the effects of pesticides on erythropoiesis, the ratio of PCEs to the sum of PCEs and normochromatic erythrocytes (NCEs) was determined by counting at least 500 cells (PCEs + NCEs) per animal (at least 250 cells per each slide) under a microscope (Nikon Eclipse Ci-L). At least 4000 PCEs were counted per animal, by two different researchers in order to assess the mutagenic activity of pesticides.
3. Statistical analysis
Statistical analysis was performed using SPSS Statistics v. 22.0 software (IBM Corporation, New York, USA). The statistical significance of the difference in the proportion of PCEs/(PCEs + NCEs) (polychromatic erythrocytes./total erythrocytes; measure of suppression of erythropoiesis) between the highest-dose group and the concurrent negative control group was evaluated using the independent samples t-test for each study.
4. Results
Table 1 shows the highest doses of the pesticides used for assessment of their genotoxic activity in micronucleus test. Indicated values were selected based on the known values of LD50 and the MTDs determined in preliminary dose-finding experiments.
Table 1.
Effects of technical materials of pesticides on erythropoiesis in bone marrow of CD-1 mice at the highest doses in the experiments.
| № | Pesticide name | Purity (%) | MTD/The highest dose (mg/kg b.w.) | Vehicle (negative control) | Proportion of PCEs among total erythrocytes (% of control value, ±SD) |
Suppression of erythropoiesis (p-value. α = 0,01)a |
||
|---|---|---|---|---|---|---|---|---|
| females (♀) | males (♂) | females (♀) | males (♂) | |||||
| 1 | Azoxystrobin | 98.0 | 2000 | 0.5% potato starch | 101.9 ± 5.6 | 92 ± 26 | – | – |
| 2 | Bentazone | 97.0 | 800 | 1% potato starch | 112 ± 12 | 96.1 ± 3.9 | – | – |
| 3 | Bispyribac acid | 95.2 | 2000 | 1% potato starch | 79 ± 16 | 86.3 ± 7.8 | – | – |
| 4 | Chlorothalonil | 99.0 | 2000 | 1% potato starch | 96.5 ± 8.8 | 108 ± 10 | – | – |
| 5 | Chlorothalonil | 98.6 | 2000 | 1% potato starch | 96 ± 19 | 78.4 ± 2.0 | – | – |
| 6 | Chlorsulfuron | 95.4 | 2000 | 1% potato starch | 94.2 ± 5.8 | 90.9 ± 5.5 | – | – |
| 7 | Clethodim | 94.1 | 500 | 55.9% methyl-beta-cyclodextrin | 91 ± 13 | 102.1 ± 6.4 | – | – |
| 8 | Clopyralid | 97.5 | 1000 | 1% potato starch | 72 ± 18 | 73 ± 20 | – | – |
| 9 | Clopyralid | 95.5 | 1000 | 1% potato starch | 90. ± 12 | 67 ± 22 | – | – |
| 10 | Clopyralid | 95.0 | 1000 | 1% potato starch | 102.2 ± 6.5 | 85 ± 28 | – | – |
| 11 | Clothianidin | 98.1 | 200 | 1% potato starch | 100.0 ± 7.4 | 104 ± 14 | – | – |
| 12 | Clothianidin | 97.2 | 180 | 1% potato starch | 98 ± 20 | 79 ± 11 | – | – |
| 13 | Cypermethrin | 92.2 | 63 | Sunflower oil | 72 ± 20 | 78 ± 13 | – | – |
| 14 | α-Cypermethrin | 98.0 | 30 - ♀, 20 - ♂ | Sunflower oil | 98 ± 21 | 98 ± 12 | – | – |
| 15 | Cyproconazole | 94.0 | 280 | 1% potato starch | 37 ± 18 | 29 ± 12 | + (0.000) | + (0.000) |
| 16 | 2,4-D acid | 98.8 | 125 | Sunflower oil | 91 ± 11 | 91.1 ± 8.9 | – | – |
| 17 | 2,4-D acid (2-ethylhexyl ester) | 95.4 | 450 | Sunflower oil | 82.6 ± 6.5 | 61 ± 22 | – | – |
| 18 | Desmedipham | 99.4 | 2000 | 1% potato starch | 92 ± 17 | 97 ± 10 | – | – |
| 19 | Desmedipham | 98.3 | 2000 | 1% potato starch | 87.3 ± 9.1 | 98 ± 14 | – | – |
| 20 | Dicamba acid | 98.3 | 600 | Sunflower oil | 88 ± 11 | 82 ± 14 | – | – |
| 21 | Dicamba acid | 98.1 | 600 | Sunflower oil | 92.2 ± 7.8 | 79.6 ± 9.3 | – | – |
| 22 | Dicamba acid (dimethylamine salt) | 98.3 | 600 | 1% potato starch | 98 ± 14 | 98.0 ± 9.8 | – | – |
| 23 | Difenoconazole | 97.9 | 1000 | 1% potato starch | 59 ± 17 | 62 ± 20 | + (0.001) | + (0.004) |
| 24 | Diflufenican | 98.7 | 2000 | 1% potato starch | 98 ± 17 | 106 ± 11 | – | – |
| 25 | Dimethoate | 98.0 | 60 | 0.5% potato starch | 90.2 ± 9.8 | 75.0 ± 5.0 | – | – |
| 26 | Dimethomorph | 98.2 | 2000 | 1% potato starch | 112.0 ± 8.0 | 94 ± 11 | – | – |
| 27 | Diquat dibromid | 40.0 | 40 - ♀, 20 - ♂ | water for injection | 96 ± 14 | 87.8 ± 4.1 | – | – |
| 28 | Diquat dibromid | 41.3 | 37.5 | Water for injection | 92 ± 10 | 84 ± 16 | – | – |
| 29 | Diquat dibromid | 40.0 | 40 | Water for injection | 82 ± 15 | 89 ± 18 | – | – |
| 30 | Dithianon | 95.2 | 150 | 0.5% potato starch | 93.8 ± 4.2 | 80.8 ± 3.9 | – | – |
| 31 | Ethametsulfuron-methyl | 97.8 | 2000 | 1% potato starch | 104 ± 14 | 91.5 ± 6.8 | – | – |
| 32 | Ethametsulfuron-methyl | 95.0 | 2000 | 1% potato starch | 95.8 ± 8.3 | 108 ± 19 | – | – |
| 33 | Ethofumesate | 97.3 | 2000 | 1% potato starch | 114 ± 10 | 109 ± 13 | – | – |
| 34 | Ethofumesate | 98.3 | 2000 | 1% potato starch | 80.0 ± 5.5 | 82 ± 20 | – | – |
| 35 | Fenoxaprop-P-ethyl | 98.0 | 2000 | 1% potato starch | 110.4 ± 4.2 | 82.7 ± 9.6 | – | – |
| 36 | Fipronil | 98.3 | 25 | Sunflower oil | 88 ± 19 | 100 ± 7.8 | – | – |
| 37 | Florasulam | 98.2 | 2000 | 1% potato starch | 94.0 ± 8.0 | 106 ± 19 | – | – |
| 38 | Flutriafol | 94.0 | 150b | 1% potato starch | 18.52 ± 7.4 | 14.3 ± 2.0 | + (0.000) | + (0.000) |
| 50 | 1% potato starch | 50 ± 20 | 41 ± 18 | + (0.001) | + (0.000) | |||
| 39 | Flutriafol | 97.6 | 300b | 1% potato starch | 14.0 ± 7.0 | 18 ± 10 | + (0.000) | + (0.000) |
| 50 | 1% potato starch | 40 ± 12 | 34 ± 16 | + (0.000) | + (0.000) | |||
| 40 | Flutriafol | 95.6 | 286b | Sunflower oil | 25 ± 12 | 13.7 ± 2.0 | + (0.000) | + (0.000) |
| 143 | Sunflower oil | 55.8 ± 5.8 | 53 ± 18 | + (0.000) | + (0.000) | |||
| 41 | Glyphosate | 95.7 | 2000 | 1% potato starch | 108.0 ± 8.0 | 101.9 ± 7.7 | – | – |
| 42 | Glyphosate | 98.3 | 2000 | 1% potato starch | 98.1 ± 5.8 | 78 ± 14 | – | – |
| 43 | Glyphosate | 95.1 | 2000 | 1% potato starch | 102 ± 10 | 102 ± 9.8 | – | – |
| 44 | Glyphosate | 95.8 | 2000 | 1% potato starch | 115 ± 11 | 115.7 ± 7.8 | – | – |
| 45 | Imazalil | 97.8 | 300 | Sunflower oil | 110 ± 15 | 112.2 ± 6.1 | – | – |
| 46 | Imazamox | 97.0 | 2000 | 1% potato starch | 113 ± 15 | 106 ± 14 | – | – |
| 47 | Imazamox | 98.0 | 2000 | 1% potato starch | 92.7 ± 7.3 | 112 ± 12 | – | – |
| 48 | Imazamox | 97.3 | 2000 | 1% potato starch | 87 ± 13 | 84 ± 22 | – | – |
| 49 | Imazethapyr | 97.9 | 2000 | 1% potato starch | 106 ± 11 | 88.2 ± 7.8 | – | – |
| 50 | Imidacloprid | 98.0 | 75 | 1% potato starch | 110 ± 16 | 107 ± 17 | – | – |
| 51 | Imidacloprid | 97.1 | 60 | 1% potato starch | 100 ± 15 | 72.9 ± 8.5 | – | – |
| 52 | Imidacloprid | 97.1 | 120 | 0.5% potato starch | 103.9 ± 7.8 | 94.4 ± 5.6 | – | – |
| 53 | Imidacloprid | 97.0 | 90 | 0.5% potato starch | 96.1 ± 5.9 | 94.3 ± 5.7 | – | – |
| 54 | Imidacloprid | 97.0 | 60 | 0.5% potato starch | 100 ± 12 | 114 ± 22 | – | – |
| 55 | Isoproturon | 97.2 | 2000 | 1% potato starch | 114 ± 17 | 98.2 ± 7.3 | – | – |
| 56 | Mefenpyr diethyl | 96.4 | 2000 | 1% potato starch | 102.0 ± 8.0 | 93 ± 11 | – | – |
| 57 | Mesotrione | 98.8 | 2000 | 1% potato starch | 122 ± 13 | 88 ± 20 | – | – |
| 58 | Mesotrione | 98.2 | 2000 | 1% potato starch | 106 ± 16 | 105.7 ± 5.7 | – | – |
| 59 | Metribuzin | 93.5 | 250 | 1% potato starch | 108.7 ± 6.5 | 110.6 ± 8.5 | – | – |
| 60 | Metribuzin | 99.7 | 250 | 1% potato starch | 98 ± 15 | 100 ± 11 | – | – |
| 61 | Metsulfuron-methyl | 99.4 | 2000 | 1% potato starch | 91.6 ± 8.3 | 90.4 ± 7.7 | – | – |
| 62 | Nicosulfuron | 95.5 | 2000 | 1% potato starch | 96 ± 11 | 102.0 ± 9.8 | – | – |
| 63 | Oxyfluorfen | 97.1 | 2000 | 1% potato starch | 90 ± 26 | 84 ± 32 | – | – |
| 64 | Pendimethalin | 95.7 | 2000 | 1% potato starch | 94.5 ± 9.1 | 107.8 ± 7.8 | – | – |
| 65 | Phenmedipham | 98.6 | 2000 | 1% potato starch | 94 ± 11 | 113.7 ± 5.9 | – | – |
| 66 | Picloram | 95.6 | 2000 | Sunflower oil | 82 ± 13 | 94 ± 18 | – | – |
| 67 | Pirimiphos-methyl | 93.0 | 150 | Sunflower oil | 92 ± 12 | 86 ± 13 | – | – |
| 68 | Prometryn | 97.3 | 2000 | 1% potato starch | 83 ± 14 | 78 ± 20 | – | – |
| 69 | Propisochlor | 95.1 | 1000 | Sunflower oil | 83 ± 23 | 83 ± 25 | – | – |
| 70 | Quizalofop-P-ethyl | 96.0 | 1000 | 1% potato starch | 96 ± 24 | 102 ± 12 | – | – |
| 71 | Rimsulfuron | 98.2 | 2000 | 1% potato starch | 110.0 ± 6.0 | 101.8 ± 7.1 | – | – |
| 72 | S-metolachlor | 97.0 | 1000 | Sunflower oil | 100 ± 17 | 86 ± 19 | – | – |
| 73 | S-metolachlor | 96.0 | 2000 | Sunflower oil | 80 ± 12 | 85.2 ± 7.4 | – | – |
| 74 | S-metolachlor | 98.6 | 2000 | Sunflower oil | 87.0 ± 7.4 | 108.2 ± 2.0 | – | – |
| 75 | Tebuconazole | 98.4 | 1000 | 1% potato starch | 57.1 ± 4.1 | 61.7 ± 8.5 | + (0.007) | + (0.000) |
| 76 | Terbuthylazine | 97.2 | 2000 | Water for injection | 109 ± 15 | 80 ± 30 | – | – |
| 77 | Thiamethoxam | 95.3 | 600 | 0.5% potato starch | 88 ± 17 | 104 ± 15 | – | – |
| 78 | Thifensulfuron-methyl | 97.1 | 2000 | 1% potato starch | 94.0 ± 6.0 | 98 ± 12 | – | – |
| 79 | Tribenuron-methyl | 97.5 | 2000 | 1% potato starch | 86.2 ± 6.9 | 76.9 ± 9.2 | – | – |
Independent samples t-test.
The highest dose in the preliminary dose-finding experiment.
For comparison of the effects of the various technical materials on erythropoiesis, the ratios of the PCEs to total erythrocytes (PCEs + NCEs) in bone marrow as percentage of the respective negative control ratios were used (Table 1). It should be noted that negative control values slightly varied from experiment to experiment, and historical negative control in laboratory was 0,50 ± 0,06 for females and 0,52 ± 0,06 for males. Cyclophosphamide did not cause a significant decrease in the proportion PCEs/(PCEs + NCEs) in comparison with the negative control.
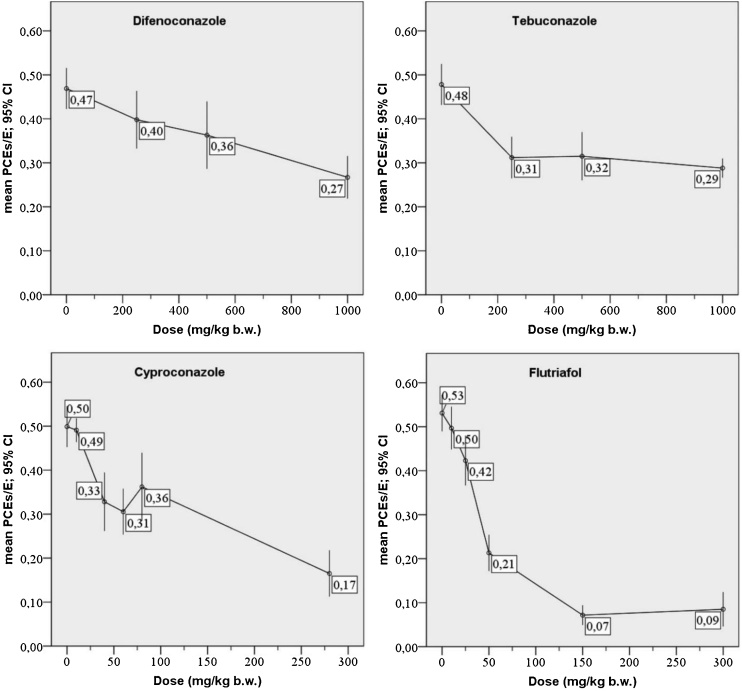
Among the tested technical materials, 4 pesticides caused significant inhibition of erythropoiesis in the bone marrow; all of them are derivatives of triazole - flutriafol, cyproconazole, tebuconazole, difenoconazole. Therefore, the MTD for those pesticides was determined not only on the basis of lethality and/or visible symptoms of toxicity, but also the effect on the hematopoietic system. In particular, the MTD for flutriafol was determined as 50 mg/kg b.w., at which no visible signs of toxicity were observed (Fig. 1).
Fig. 1.
Graphs illustrating suppression of erythropoiesis in bone marrow of CD-1 mice dosed with difenoconazole (97.9%), tebuconazole (98.4%), cyproconazole (94.0%), and flutriafol (97.6%). The Y axes: the mean ratio of PCEs to the sum of PCEs + NCEs (E). The X axes: doses of the respective pesticides administered to mice. The bars demonstrate 95% confidence intervals (CI) of the mean. In zero dose it is represented the ratio value for the negatice control.
A comparison of the effects of tebuconazole, difenoconazole, cyproconazole and flutriafol showed that the most prominent suppression of erythropoiesis was observed in the cases of flutriafol and cyproconazole. Tebuconazole and difenoconazole caused a milder decrease in PCE proportion.
Flutriafol was shown to cause a strong inhibition of erythropoiesis at the doses of 100–150 mg/kg b.w., while no visible signs of toxicity were observed (LD50 of flutriafol is 1140 mg/kg b.w. and 1480 mg/kg b.w. for male and female rats, respectively). At the dose of 150 and 300 mg/kg b.w., the decrease in PCE proportion to total erythrocytes was so strong that it was impossible to perform a microscopic analysis for evaluation of the micronucleus induction. This analysis was complicated even at the dose of 50 mg/kg b.w. due to the sharp decrease in the number of PCEs (proportion PCEs/(PCEs + NCEs) was 0.21, i.e. about 40% of negative control). In addition, there was a statistically significant decrease in the incidence of micronucleated PCEs by up to 50–70% in comparison with the negative control. This effect was probably due to the suppression of division of erythroid cells since micronuclei form during anaphase from lagging acentric chromosomes or chromatid fragments that appeared due to rupture of chromosomes (clastogenic effect) or lagging chromosomes (aneugenic effect).
In the study of cyproconazole the maximum dose of 280 mg/kg b.w. was used, and the proportion of PCEs decreased to 36.7% and 29.4% of the negative control level in females and males, respectively. As in the case of flutriafol, there was a decrease in the incidence of micronucleated PCEs by up to 50–60% in comparison to the negative control.
Therefore, the suppression of erythropoiesis by pesticides (even at the lower recommended level of 20% PCEs/(PCEs + NCEs) of the negative control value) decreases micronuclei incidence and interferes with the assessment of clastogenic and aneugenic effects. The lower level of PCEs/(PCEs + NCEs) should probably be higher than the value recommended by OECD TG474 for the validity of MN test.
Tebuconazole and difenoconazole inhibited erythropoiesis to a lesser extent. At the highest dose used in the experiment 1000 mg/kg b.w., the level of erythropoiesis decreased to 57–62% of negative control value.
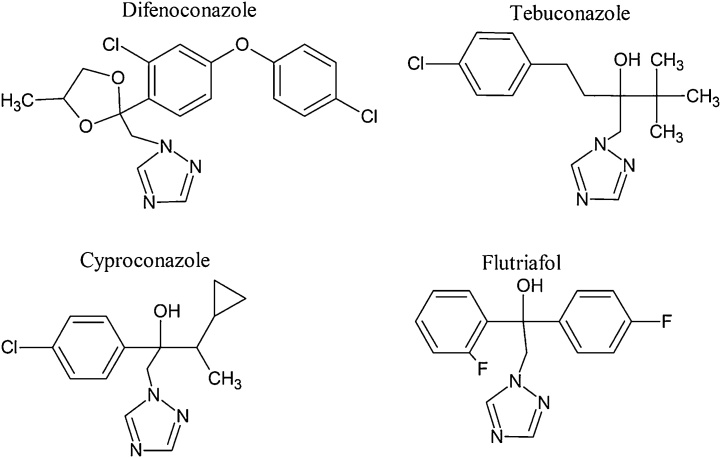
A comparison of molecular structures of tested triazole pesticides showed that erythropoiesis-inhibiting activity may depend on the position of halogenphenyl groups, the presence of hydroxy group and the type of halogen substituents. Three of four pesticides (flutriafol, cyproconazole and tebuconazole) share the common central fragment in the molecule: OH-C-CH2-triazole. In addition, the most active pesticides, flutriafol and cyproconazole, have the same structural feature - halogenphenyl group in the proximity of the fragment OH-C-CH2-triazole at 2-position (Fig. 2). The higher activity of flutriafol can be related to the presence of two halogenphenyl groups comprising fluorine atoms, which are more electronegative than chlorine atom present in the molecule of cyproconazole.
Fig. 2.
Structural formulas of 4 triazole pesticides that cause suppression of erythropoiesis in CD-1 mice bone marrow.
Like cyproconazole, tebuconazole has one chlorophenyl group, but in this case, chlorophenyl is positioned more distally from the common fragment OH-C-CH2-triazole.
In spite of difenoconazole having two chlorophenyl groups, its activity is moderate, probably due to the absence of OH-group as well as the presence of chlorophenyl groups as parts of the biphenyl radical.
5. Discussion and conclusions
MTDs for a number of technical materials of pesticide active substances were established based on the conducted experiments. Our data are useful in the practice of cytogenetic analysis in mammals since the LD50 values, which can serve as reference points for the choice of MTDs, are often provided in the literature for rats only. Therefore, additional experiments are often needed to find doses for other animal species. The above-mentioned data can help shorten the duration of in vivo studies and decrease the number of animals in experiments supporting the 3R principals.
The commonly accepted threshold value for the maximum tolerated dose in the micronucleus test selected based on the toxic effect on the target organ, namely the proportion of PCEs among total erythrocytes not less than 20% of negative control value [1], is not always suitable in practice. With such suppression of erythropoiesis, the incidence of micronucleated PCEs decreases up to 50–60% of negative control, probably due to the suppression of erythroid cell division. Therefore, it is advisable to choose lower doses of the test substance or to correctly evaluate the mutagenic effect of pesticides in the micronucleus test.
It was demonstrated that triazole pesticides inhibit erythropoiesis in mammal bone marrow. The effects were enhanced in the following order: difenoconazole ≤ tebuconazole < cyproconazole < flutriafol. The comparison of the molecular structures of tested triazole pesticides showed that their erythropoiesis-inhibiting activity may depend on the position of halogenphenyl groups, the presence of hydroxy group and the type of halogen substituents (F is more electronegative and active than Cl). Additional research is needed to determine the mechanisms of the effects of triazole pesticides on hemopoiesis. The results of the present study are consistent with the data obtained by Ditchenko et al. [20] who demonstrated the relationship between the presence of different di- and monochlorophenyl radicals in derivatives of 1,2,4-triazole and their effects on the conductivity of the plasmalemma.
The effects of triazole pesticides on erythropoiesis can be mediated by their anti-androgenic activity [21] since androgens are known to stimulate the production of red blood cells [22].
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2018.12.006.
Contributor Information
Nataliya Ilyushina, Email: Ilyushina-na@mail.ru.
Aristidis M. Tsatsakis, Email: aristsatsakis@gmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.OECDTestNo. 474: Mammalian Erythrocyte MicronucleusTest (2016).
- 2.Fleming L.E., Timmeny W. Aplastic anemia and pesticides. An etiologic association? J. Occup. Med. 1993;35(11):1106–1116. doi: 10.1097/00043764-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Prihartono N., Kriebel D., Woskie S., Thetkhathuek A., Sripaung N., Padungtod C., Kaufman D. Risk of aplastic anemia and pesticide and other chemical exposures. Asia Pac. J. Public Health. 2011;23(May (3)):369–377. doi: 10.1177/1010539511403605. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S., Basak P., Chaklader M., Das P., Pereira J.A., Chaudhuri S. Law S.PEsticide induced marrow toxicity and effects on marrow cell population and on hematopoietic stroma. Exp. Toxicol. Pathol. 2013;65(3):287–295. doi: 10.1016/j.etp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Vardavas A.I., Fragkiadaki P., Alegakis A.K., Kouretas D., Goutzourelas N., Tsiaoussis J., Tsitsimpikou C., Stivaktakis P.D., Carvalho F., Tsatsakis A.M. Downgrading the systemic condition of rabbits after long term exposure to cypermethrin and piperonyl butoxide. Life Sci. 2016;145:114–120. doi: 10.1016/j.lfs.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Vardavas A.I., Stivaktakis P.D., Tzatzarakis M.N., Fragkiadaki P., Vasilaki F., Tzardi M., Datseri G., Tsiaoussis J., Alegakis A.K., Tsitsimpikou C., Rakitskii V.N., Carvalho F., Tsatsakis A.M. Long-term exposure to cypermethrin and piperonyl butoxide cause liver and kidney inflammation and induce genotoxicity in New Zealand white male rabbits. Food Chem. Toxicol. 2016;94:250–259. doi: 10.1016/j.fct.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Vardavas A.I., Ozcagli E., Fragkiadaki P., Stivaktakis P.D., Tzatzarakis M.N., Alegakis A.K., Vasilaki F., Kaloudis K., Tsiaoussis J., Kouretas D., Tsitsimpikou C., Carvalho F., Tsatsakis A.M. The metabolism of imidacloprid by aldehyde oxidase contributes to its clastogenic effect in New Zealand rabbits. Mutat. Res. Toxicol. Environ. Mutagen. 2018;829–830:26–32. doi: 10.1016/j.mrgentox.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Stivaktakis P.D., Kavvalakis M., Tzatzarakis M.N., Alegakis A.K., Panagiotakis M.N., Fragkiadaki P., Vakonaki E., Ozcagli E., Hayes W.A., Rakitskii V.N., Tsatsakis A.M. Long-term exposure of rabbits to imidaclorpid as quantified in blood induces genotoxic effect. Chemosphere. 2016;149:108–113. doi: 10.1016/j.chemosphere.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Tsitsimpikou C., Tzatzarakis M.N., Fragkiadaki P., Kovatsi L., Stivaktakis P.D., Kalogeraki A., Kouretas D., Tsatsakis A.M. Histopathological lesions, oxidative stress and genotoxic effects in liver and kidneys following long term exposure of rabbits to diazinon and propoxur. Toxicology. 2013;307:109–114. doi: 10.1016/j.tox.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Stivaktakis P.D., Giannakopoulos E., Vlastos D., Matthopoulos D.P. Determination of genotoxic effects of methidathion alkaline hydrolysis in human lymphocytes using the micronucleus assay and square-wave voltammetry. Bioelectrochemistry. 2017;113:9–14. doi: 10.1016/j.bioelechem.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Kavvalakis M., Tzatzarakis M.N., Theodoropoulou E.P., Barbounis E.G., Tsakalof A.K., Tsatsakis A.M. Development and application of LC–APCI–MS method for biomonitoring of animal and human exposure to imidacloprid. Chemosphere. 2013;93(10):2612–2620. doi: 10.1016/j.chemosphere.2013.09.087. [DOI] [PubMed] [Google Scholar]
- 12.Katsikantami I., Colosio C., Tzatzarakis M.N., Vakonaki E., Rizos A.K., Sarigiannis D.A., Tsatsakis A.M. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data—the internal exposure approach. Food Chem. Toxicol. 2018;123:57–71. doi: 10.1016/j.fct.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 13.Tsatsakis A.M., Docea A.O., Tsitsimpikou C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi chemicals’ low dose exposure. Food Chem. Toxicol. 2016;96:174–176. doi: 10.1016/j.fct.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Tsatsakis A.M., Kouretas D., Tzatzarakis M.N., Stivaktakis P., Tsarouhas K., Golokhvast K.S., Tsitsimpikou C. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;36(6):554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
- 15.Docea A.O., Gofita E., Goumenou M., Calina D., Rogoveanu O., Varut M., Olaru C., Kerasioti E., Fountoucidou P., Taitzoglou I., Zlatian O., Rakitskii V.N., Hernandez A.F., Kouretas D., Tsatsakis A.M. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018;115:470–481. doi: 10.1016/j.fct.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez A.F., Gil F., Lacasana M., Rodríguez-Barranco M., Tsatsakis A.M., Raquena M., Parron T., Alarcon R. Pesticide exposure and genetic variation in xenobiotic-metabolizing enzymes interact to induce biochemical liver damage. Food Chem. Toxicol. 2013;61:144–151. doi: 10.1016/j.fct.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Limban C., Nuta D.C., Chirita C., Negres S., Arsene A.L., Goumenou M., Karakitsios S.P., Tsatsakis A.M., Sarigiannis D.A. The use of structural alerts to avoid the toxicity of pharmaceuticals. Toxicol. Rep. 2018;5:943–953. doi: 10.1016/j.toxrep.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesnage R., Biserni M., Wozniak E., Xenakis T., Mein C.A., Antoniou M.N. Comparison of transcriptome responses to glyphosate, isoxaflutole, quizalofop-p-ethyl and mesotrione in the HepaRG cell line. Toxicol. Rep. 2018;5:819–826. doi: 10.1016/j.toxrep.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozano V.L., Defarge N., Rocque L.M., Mesnage R., Hennequin D., Cassier R., Spiroux de Vendômois J., Panoff J.M., Seralini G.E., Amiel C. Sex-dependent impact of Roundup on the rat gut microbiome. Toxicol. Rep. 2018;5:96–107. doi: 10.1016/j.toxrep.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ditchenko T.I., Yurin V.M., Svirid S.D., Belskaya V.Y. Comparative assessment of membrane-tropic activity of fungicides, derivatives of 1,2,4-triazole. Yurin V.M., editor. Xenobiotics and the Living Systems, III International Science Conference. 2008:39–41. (in Russian) [Google Scholar]
- 21.Lv X., Pan L., Wang J., Lu L., Yan W., Zhu Y., Xu Y., Guo M., Zhuang S. Effects of triazole fungicides on androgenic disruption and CYP3A4 enzyme activity. Environ. Pollut. 2017;222:504–512. doi: 10.1016/j.envpol.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 22.Barcelo A.C., Olivera M.I., Bozzini C., Alippi R.M., Bozzini C.E. Androgens and erythropoiesis. Induction of erythropoietin-hypersecretory state and effect of finasteride on erythropoietin secretion. Comp. Haematol. Int. 1999;9(1):1–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.