Abstract
Aim: No meta-analysis has examined the effect of regular aquatic endurance exercise on lipid and lipoprotein levels. The purpose of the current work was to perform a meta-analysis to evaluate the effects of regular aquatic endurance exercise on lipid and lipoprotein levels.
Methods: The inclusion criteria of the randomized controlled trials were healthy adults in an exercise group performing regular aquatic exercise and a control group not exercising, with a description of the serum high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol, or triglyceride levels provided. The net change in the lipid and lipoprotein levels was calculated from each trial, and the changes in the lipid and lipoprotein levels were pooled using a random effects model.
Results: The meta-analysis examined 10 trials involving aquatic endurance exercise and 327 subjects. The pooled net changes in HDL-C, LDL-C, and total cholesterol improved significantly (HDL-C, 4.6 mg/dL; LDL-C, −10.1 mg/dL; total cholesterol, −8.5 mg/dL). When trials were limited to those involving only women, the pooled net changes in HDL-C, LDL-C, and total cholesterol improved significantly. When trials were limited to those involving subjects with a mean age < 60 years, the pooled net changes in HDL-C, total cholesterol, and triglyceride improved significantly. When trials were limited to those with dyslipidemia, the pooled net changes in HDL-C, LDL-C, total cholesterol, and triglyceride improved significantly.
Conclusions: Aquatic endurance exercise improved the lipid and lipoprotein levels and benefited women, middle-aged subjects, and patients with dyslipidemia in particular.
Keywords: Aquatic exercise, Lipids and lipoproteins, Meta-analysis
Introduction
Dyslipidemia is a major risk factor for cardiovascular disease. Several cohort studies have reported that adults with low levels of high-density lipoprotein cholesterol (HDL-C) or extremely high levels of HDL-C had a high risk of cardiovascular disease and a high mortality rate regardless of cause1–3). In addition, low-density lipoprotein cholesterol (LDL-C) was strongly associated with cardiovascular disease or mortality when LDL-C was ≥ 190 mg/dL2, 3). The major risk factors for dyslipidemia are known to depend heavily on lifestyle, and several meta-analyses have indicated that lifestyle modifications improve the lipid and lipoprotein levels4–16). Exercise is one way of improving these levels, and a number of studies have noted the beneficial effects of regular exercise on the lipid and lipoprotein levels. Metaanalyses of studies involving exercise have mainly examined the effects of endurance exercise10–13) and resistance training14), but recent analyses have examined the effects of other forms of exercise such as tai chi15) and yoga16) to promote health.
Aquatic exercise is a type of exercise that has recently increased in popularity17) and involves characteristics specific to water, such as water temperature, resistance, hydrostatic pressure, buoyancy, and viscosity; as such, the physiological properties of aquatic exercise differ from those of land-based exercise. For example, aquatic exercise poses less of a physical burden when stationary or when moving at a low or moderate speed18). Therefore, aquatic exercise can be effective as a form of exercise therapy for patients with obesity or orthopedic disease. Previous meta-analyses have suggested that aquatic exercise exerts a beneficial effect on health by lowering blood pressure19) and alleviating knee pain20), but no meta-analysis has examined the effects of regular aquatic exercise on the lipid and lipoprotein levels. In addition, land-based exercise and aquatic exercise result in similar levels of energy expenditure in metabolic equivalents, according to the guidelines of the American College of Sports Medicine21). This suggests that aquatic exercise may be as effective as landbased exercise in terms of improving the lipid and lipoprotein levels. On the basis of this hypothesis, the purpose of the current work was to perform a metaanalysis to evaluate the effects of regular aquatic exercise on the lipid and lipoprotein levels.
Methods
A meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement22) and was registered in the International Prospective Register of Systematic Reviews (PROSPERO: https://www.crd.york.ac.uk/PROSPERO, registration number: CRD42017 075556).
Data Sources and Study Selection
Databases were searched for literature published until October 2017. MEDLINE, PubMed, SPORTDiscus, the Cochrane library, and Google Scholar were searched using terms such as dyslipidemia, hyperlipidemia, lipid, lipoprotein, cholesterol, exercise, exercise therapy, physical fitness, swimming, water, aquatic exercise, and water-based exercise. The literature was comprehensively searched using different combinations of these terms. Listed references of original articles, reviews, and textbooks were also searched. The inclusion criteria for this meta-analysis were as follows: subjects had no cardiovascular or other diseases with a mean age of 20 years or older; subjects were randomly divided into an aquatic exercise group and a control group (i.e., a randomized controlled trial), with the aquatic exercise group only performing regular exercise in water and the control group not exercising, and neither group receiving intervention such as an improved diet or a change in lifestyle; the intervention lasted four weeks or longer (based on previous meta-analyses8, 9, 14, 15, 19, 23)); and studies described the mean HDL-C, LDL-C, total cholesterol (TC), or triglyceride (TG) levels and their standard deviation (SD) in the exercise and control groups before and after the intervention. Studies identified from a literature search were limited to those involving aquatic exercise and those examining the effect of this exercise on the lipid and lipoprotein levels. Once these studies were identified, two authors (Y.I. and Y.N) determined whether or not the study should be included in this meta-analysis.
Data Extraction
The data and details of the intervention (mean HDL-C, LDL-C, TC or TG, and SD in the serum lipids and lipoproteins, number of subjects, type of aquatic exercise, intensity, time, frequency, details of the control group, and duration of the intervention) were extracted from studies for the meta-analysis. Some serum lipid and lipoprotein data were converted from mmol/L to mg/dL cholesterol by multiplying the values by 38.7, and TG values were multiplied by 88.7. The body mass index (BMI) was selected as a secondary outcome.
The study quality was assessed based on the Physiotherapy Evidence Database (PEDro)24), with 10 terms scored at 1 point each. However, 2 points of the usual PEDro score depend on how a study is blind of subjects in groups and blind of therapists. In general, blinding of subjects is difficult in studies of exercise interventions23). These 2 points were therefore excluded in this meta-analysis, and PEDro was assessed based on eight terms scored at 1 point each.
The two authors independently extracted and checked the aforementioned data and assessed the quality of the studies using the modified PEDro score.
Statistical Analyses
The net change in HDL-C, LDL-C, TC, TG, and BMI was examined for each trial. The change was defined as the difference (values for the exercise group minus values for the control group) in changes (final values minus baseline values) in the mean. Pooled net changes were calculated with a random-effects model using the DerSimonian–Laird method and weighted by the inverse variance of differences from the baseline to the final assessment in each trial25). The correlation coefficient between the baseline and the final assessment was assumed to be 0.5026).
Cochran Q statistics were calculated, and the heterogeneity of pooled net change was examined among trials. The I2 statistic was calculated using the formula (Q−df)/Q, where df is the degree of freedom (obtained by subtracting 1 from the number of trials). The level of heterogeneity was assessed using the I2 statistic: low risk (less than 25%), moderate risk (25% to 75%), and high risk (greater than 75%)27).
Subgroup analyses of pooled net changes in the HDL-C, LDL-C, TC, and TG levels regarding the type of aquatic exercise were performed by classifying trials into endurance exercise, no endurance exercise, swimming, and no swimming. In addition, trials were limited to aquatic endurance exercise, and subgroup analyses were performed for eight categories: women only, women and men, mean age (≥ 60 years and < 60 years), intervention duration (≥ 8 weeks and ≥ 12 weeks), dyslipidemia, and no dyslipidemia. On the basis of the American Heart Association and the American College of Sports Medicine guidelines on physical activity to maintain health28), endurance exercise was defined as exercise that was moderate to vigorous in activity and that lasted a minimum of 30 min per session. “Dyslipidemia” was defined based on the Japan Atherosclerosis Society guidelines29), and trials with a mean HDL-C < 40 mg/dL, mean LDL-C ≥ 140 mg/dL, or mean TG ≥ 150 mg/dL of subjects were designated as having dyslipidemic subjects.
Two methods were used to evaluate any publication bias, i.e. the symmetry of funnel plots produced by the net changes in serum lipids or lipoproteins (x-axis) and the inverse of the standard error (y-axis) were assessed. First, the trim and fill method of Duval and Tweedie was used to estimate the number of missing trials30). If the results suggested that trials were missing, then pooled net changes in lipids and lipoproteins were adjusted in light of the effect of these trials. Second, Egger's regression test was used to evaluate the asymmetry of funnel plots31).
The results for baseline variables were expressed as the mean ± SD weighted by the number of subjects, and comparisons of the exercise group and control group were carried out by the Mann–Whitney test. In all the statistical tests, a P value < 0.05 was considered to be statistically significant. The results of net changes were expressed as the 95% confidence intervals (CI). The Comprehensive Meta-Analysis soft program (Version 2.2; Biostat, Inc., Englewood, NJ, USA) was used to perform the meta-analysis and assess the publication bias. Other statistical tests were performed using the SPSS soft program (Version 21.0; IBM Inc., Armonk, NY, USA).
Results
Study Selection
As a result of a literature search, 42 studies were identified as involving aquatic exercise and describing lipid or lipoprotein data. Of these, 31 studies did not meet the selection criteria and were excluded from the meta-analysis (Fig. 1). As a result, 12 trials in 11 articles (1 article included 2 trials)32–42) were ultimately analyzed.
Fig. 1.
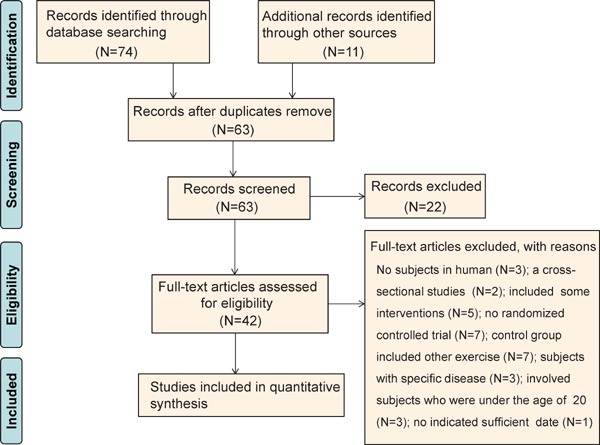
PRISMA flow diagram regarding article selection for meta-analysis.
Trial Characteristics
All trials had a parallel design and were in English. Table 1 shows the characteristics of each included trial. The trials involved 393 subjects in total: 208 subjects in the exercise groups and 185 subjects in the control groups. Eleven trials32–34, 36–42) reported the number of subjects by sex. These trials involved 16 males (4.5%) and 337 females (95.5%). Ten trials32–34, 36, 37, 39–42) reported the mean age of subjects, which ranged from 46 to 73 years (median: 62 years). Ten trials32, 34–39, 41, 42) carried out endurance exercise, one trial carried out endurance exercise plus resistance training33), and one trial carried out exercise under 30 min40). Three trials34, 40) carried out only swimming. Five trials34, 35, 38, 41, 42) used the heart rate to gauge the intensity of exercise. All trials involved a set exercise time and frequency. An exercise session ranged from 15 to 60 min. The frequency of exercise was 1–4 sessions per week. The water temperature ranged from 26 to 36°C according to the six trials32, 35–37, 39, 41) that reported the temperature. The duration of intervention ranged from 6 to 24 weeks (median: 12 weeks). Supplementary Table 1 shows the PEDro on each trial, and the score ranged from 3 to 7 points (median: 5 points).
Table 1. Characteristics of included trials.
| Study name | Subjects | Female (%) | Mean age (years) | Exercise intervention | ||||
|---|---|---|---|---|---|---|---|---|
| Type and intensity | Time (min) | Frequency (sessions/week) | Duration (weeks) | WT (°C) | ||||
| Takeshima et al32), 2002, Japan | 30 | 100 | 69 | Endurance-type exercise (walking and dancing) and resis-tance exercise, and V̇O2 at LT | 40 | 3 | 12 | 30 |
| Colado et al33), 2009, Spain | 25 | 100 | 54 | 8 exercises on circuit, 20 repetitions per set on 1 – 3 sets (week 1–12), 10 exercises on circuit and 20 repetitions/set on 3 sets (13–18), and 8 exercises on circuit and 15 repetitions/set on 2 sets of supersets (week 19–24), and OMNI scale of 5 (week 1–4) and OMNI scale of 7 (week 5–24) | 35–60 | 2–3 | 24 | NR |
| Nualnim et al34), 2012, United States | 43 | 74 | 60 | Swimming, and less than 60 HRmax (first few weeks) and 70–75% HRmax | 15–45 | 3–4 | 12 | NR |
| Nuttamonwarakul et al35), 2012, Thailand | 40 | NR | 60– | Aqua-aerobic exercise and 70% HRmax or a Borg scale of 10–16 | 30 | 3 | 12 | 34–36 |
| Shibata et al36), 2012, Japan | 22 | 81 | 68 | Walking (side-walking, back-walking, twist-walking, hurdler-walking, and cross-walking) and a Borg scale of 11–13 | 30 | 1 | 10 | 32 |
| Arca et al37), 2013, Brazil | 33 | 100 | 64 | Walking and running, and 50–60% HRR deducted 17 heartbeat | 50 | 3 | 12 | 33–33.5 |
| Rahimi et al38), 2013, Iran | 24 | 100 | 45–55 | Walking and 50–70% HRmax | 35 | 3 | 6 | NR |
| Chung et al39), 2014, China | 23 | 100 | 72 | Water tai chi (24-Form) | 50 | 3 | 8 | 32 |
| Mohr et al40), 2014a, UK and Faroe Islands | 41 | 100 | 45 | Intermittent swimming by free-style (front crawl) and 6–10 (1–6 week: 6 interval; 7–12 week: 8 interval; 13–15: 10 interval) × 30 s, and all-out (interspersed by 2 min recovery) | 15–25 | 2.9 | 15 | NR |
| Mohr et al40), 2014b, UK and Faroe Islands | 41 | 100 | 46 | Continuous swimming by free-style (front crawl) and encour-aged to swim as far as possible | 60 | 2.9 | 15 | NR |
| Kantyka et al41), 2015, Poland | 21 | 100 | 56 | Aqua–aerobics comprised running and arm exercise and maintained at approximately 128–137 bpm of target HR | 25–30 | 3 | 14 | 26–28 |
| Kim et al42), 2016, Korea | 50 | 100 | 72 | Aquarobics and maintained at about 129–138 bpm of tar-get HR | 50 | 3 | 12 | NR |
Borg scale: Borg rating of perceived exertion scale; HR: heart rate; HRmax: maximal heart rate; HRR: heart rate reserve; NR: not reported; LT: lactate threshold; V̇O2: oxygen uptake; V̇O2max: maximal oxygen uptake; WT: water temperature.
Supplementary Table 1. Study quality based on PEDro.
| Study name | Items | Total Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Takeshima et al32), 2002 | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Colado et al33), 2009 | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Nualnim et al34), 2012 | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Nuttamonwarakul et al35), 2012 | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Shibata et al36), 2012 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
| Arca et al37), 2013 | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Rahimi et al38), 2013 | Yes | No | No | Yes | No | No | No | Yes | Yes | 3 |
| Chung et al39), 2014 | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Mohr et al40), 2014a | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Mohr et al40), 2014b | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Kantyka et al41), 2015 | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | 5 |
| Kim et al42), 2016 | Yes | No | No | Yes | No | No | Yes | Yes | Yes | 4 |
1: Random allocated to groups, 2: Concealed allocation, 3: Groups that were similar at baseline, 4: The blinding of assessors, 5: Outcome measures that were obtained from more than 85% of subjects, 6: Intention to treat analysis, 7: Between-group statistical comparisons, 8: Provided both point measures and measures of variability, 9: Eligibility criteria (note: eligibility criteria does not contribute to total score).
Net Changes and Subgroup Analysis
Figs.2–5 show the results of baseline and forest plots of net changes in lipids and lipoproteins from each trial and the pooled net changes in lipids and lipoproteins. The pooled net changes in HDL-C, LDL-C, and TC in the trials of aquatic endurance exercise decreased significantly. The figures include the baseline HDL-C, LDL-C, TC, and TG levels and net changes in these levels according to trials not involving aquatic endurance exercise, and the pooled net changes did not improve significantly. The pooled net changes in LDL-C and TC decreased significantly in all the trials. In addition, the pooled net changes in HDL-C, LDL-C, TC, and TG did not improve significantly in trials involving swimming34, 40), and the pooled net changes in LDL-C and TC improved significantly in trials not involving swimming (Supplementary Table 2)32, 33, 35–39, 41, 42).
Fig. 2.
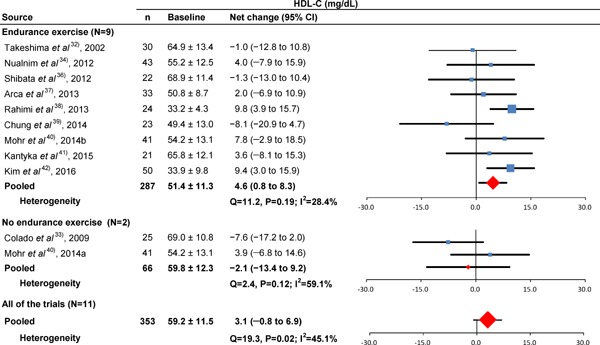
Baseline HDL-C and forest plot for the net changes in HDL-C. Each trial is represented with black squares (net change) and widths (95% CI); the size of the black squares is in proportion to the weighting by inverse variance in each trial. The pooled net changes are represented by black rhombuses (net change) and widths (95% CI).
Fig. 5.
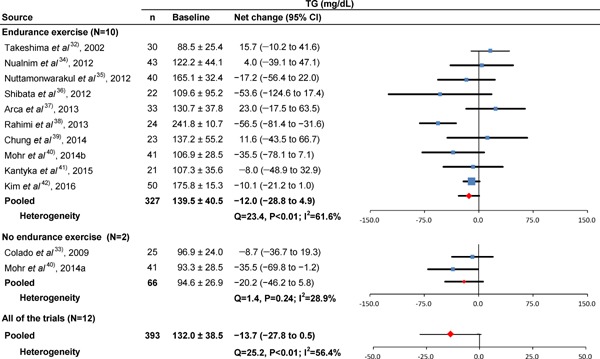
Baseline TG and forest plot for the net changes in TG. Each trial is represented with black squares (net change) and widths (95% CI); the size of the black squares is in proportion to the weighting by inverse variance in each trial. The pooled net changes are represented by black rhombuses (net change) and widths (95% CI).
Supplementary Table 2. Baseline, net changes, and subgroup analyses when RCTs limited involving swimming or no swimming.
| Variable (category) | Trials | Baseline, mg/dL | Net change, mg/dL | Q | I2% |
|---|---|---|---|---|---|
| HDL-C | |||||
| Swimming | 3 (125) | 54.6 ± 12.9 | 5.3 (−1.1 to 11.7) | 0.3 | 0.0 |
| No swimming | 8 (228) | 52.1 ± 10.7 | 2.0 (−3.2 to 7.1) | 17.1* | 59.0 |
| LDL-C | |||||
| Swimming | 3 (125) | 138.0 ± 27.1 | −0.6 (−14.4 to 13.2) | 0.01 | 0.0 |
| No swimming | 8 (228) | 147.8 ± 22.4 | −13.1 (−23.7 to −2.6) | 12.7 | 45.0 |
| TC | |||||
| Swimming | 3 (125) | 211.9 ± 28.2 | −4.0 (−17.7 to 9.8) | 1.5 | 0.0 |
| No swimming | 7 (223) | 227.3 ± 24.0 | −11.3 (−21.8 to −0.8) | 10.3 | 41.9 |
| TG | |||||
| Swimming | 3 (125) | 107.7 ± 34.7 | −24.2 (−48.8 to 0.4) | 2.3 | 14.1 |
| No swimming | 9 (268) | 143.3 ± 40.2 | −10.8 (−27.8 to 6.1) | 21.8* | 63.3 |
CI: confidence intervals; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride.
Trials are expressed number of trials (number of subjects)
Baseline lipids and lipoproteins are expressed as mean ± SD
Net changes in lipids and lipoproteins are expressed as pooled net change (95% CI)
Significant heterogeneity (P < 0.05)
Fig. 3.
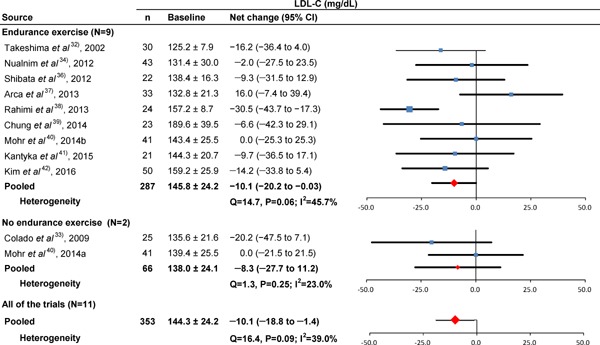
Baseline LDL-C and forest plot for the net changes in LDL-C. Each trial is represented with black squares (net change) and widths (95% CI); the size of the black squares is in proportion to the weighting by inverse variance in each trial. The pooled net changes are represented by black rhombuses (net change) and widths (95% CI).
Fig. 4.
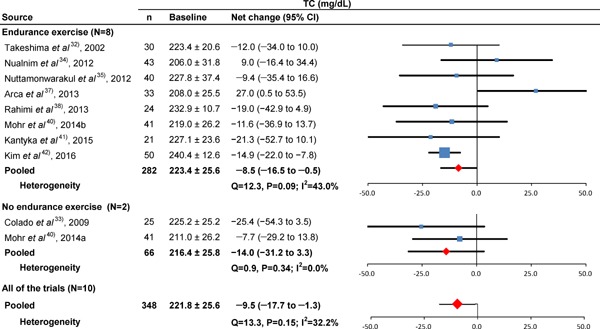
Baseline TC and forest plot for the net changes in TC. Each trial is represented with black squares (net change) and widths (95% CI); the size of the black squares is in proportion to the weighting by inverse variance in each trial. The pooled net changes are represented by black rhombuses (net change) and widths (95% CI).
Table 2 shows the results of the subgroup analyses when trials were limited to those involving subjects who performed endurance exercise32, 34–39, 41, 42). When trials were limited to those involving only women32, 33, 37–42), the pooled net changes in HDL-C, LDL-C, and TC improved significantly, but LDL-C contained significant heterogeneity. When trials were limited to those involving subjects with a mean age < 60 years, the pooled net changes in HDL-C, TC, and TG improved significantly. When trials were limited to those involving subjects with dyslipidemia35, 38–42), the pooled net changes in HDLC, LDL-C, TC, and TG improved significantly, but TG contained significant heterogeneity. When trials were limited to those involving subjects without dyslipidemia32–34, 36, 37), the pooled net changes in HDL-C improved significantly.
Table 2. Baseline, net changes, and subgroup analyses when trials are limited to aquatic endurance exercise.
| Variable (category) | Trials | Baseline, mg/dL | Net change, mg/dL | Q | I2 % |
|---|---|---|---|---|---|
| HDL-C | |||||
| Women only | 7 (222) | 48.9 ± 11.1 | 5.0 (0.6 to 9.4) | 9.7 | 38.2 |
| Women and men | 2 (65) | 59.8 ± 12.2 | 1.3 (−7.0 to 9.6) | 0.4 | 0.0 |
| Mean age < 60 years | 3 (86) | 51.2 ± 11.1 | 8.4 (3.7 to 13.1) | 0.9 | 0.0 |
| Mean age ≥ 60 years | 6 (201) | 51.5 ± 11.4 | 2.2 (−2.9 to 7.4) | 7.7 | 35.3 |
| Intervention duration ≥ 8 weeks | 8 (263) | 53.0 ± 11.8 | 3.4 (−0.5 to 7.4) | 8.3 | 15.8 |
| Intervention duration ≥ 12 weeks | 6 (218) | 51.8 ± 11.6 | 5.5 (1.6 to 9.4) | 3.5 | 0.0 |
| Dyslipidemia | 4 (118) | 50.8 ± 11.5 | 6.4 (2.0 to 10.8) | 6.4 | 53.0 |
| No dyslipidemia | 5 (169) | 51.7 ± 11.2 | 4.4 (0.01 to 8.8) | 4.3 | 8.2 |
| LDL-C | |||||
| Women only | 7 (222) | 149.3 ± 23.6 | −12.3 (−23.6 to −1.0) | 13.7* | 56.3 |
| Women and men | 2 (65) | 133.8 ± 26.2 | −6.1 (−22.9 to 10.6) | 0.2 | 0.0 |
| Mean age < 60 years | 3 (86) | 147.5 ± 20.9 | −16.0 (−36.0 to 4.1) | 5.3 | 62.2 |
| Mean age ≥ 60 years | 6 (201) | 145.0 ± 25.5 | −6.6 (−16.2 to 3.0) | 5.2 | 3.9 |
| Intervention duration ≥ 8 weeks | 8 (263) | 144.7 ± 25.2 | −6.2 (−14.6 to 2.2) | 5.5 | 0.0 |
| Intervention duration ≥ 12 weeks | 6 (218) | 140.6 ± 23.9 | −5.5 (−15.3 to 4.3) | 5.4 | 7.7 |
| Dyslipidemia | 4 (118) | 156.3 ± 26.0 | −17.3 (−31.7 to −2.9) | 5.9 | 49.3 |
| No dyslipidemia | 5 (169) | 139.3 ± 23.1 | −6.3 (−17.5 to 4.9) | 5.2 | 23.1 |
| TC | |||||
| Women only | 6 (199) | 225.7 ± 20.6 | −9.9 (−17.8 to −2.0) | 9.5 | 47.8 |
| Women and men | 1 (43) | 206.0 ± 31.8 | 9.0 (−16.4 to 34.4) | 0.0 | 0.0 |
| Mean age < 60 years | 3 (86) | 224.8 ± 22.2 | −16.9 (−32.1 to −1.7) | 0.2 | 0.0 |
| Mean age ≥ 60 years | 5 (196) | 222.2 ± 26.9 | −2.6 (−17.5 to 12.4) | 11.5* | 65.1 |
| Intervention duration ≥ 8 weeks (or ≥ 12 weeks) | 7 (258) | 222.1 ± 26.5 | −6.2 (−17.5 to 5.0) | 11.9 | 49.0 |
| Dyslipidemia | 4 (135) | 225.8 ± 28.0 | −15.0 (−28.1 to −1.8) | 0.5 | 0.0 |
| No dyslipidemia | 4 (147) | 220.8 ± 23.4 | −0.4 (−19.0 to 18.2) | 11.5* | 73.8 |
| TG | |||||
| Women only | 7 (222) | 141.2 ± 30.8 | −10.4 (−31.5 to 10.6) | 21.5* | 72.1 |
| Women and men | 2 (65) | 118.0 ± 66.0 | −17.6 (−72.3 to 37.1) | 1.8 | 45.9 |
| Mean age < 60 years | 3 (84) | 144.6 ± 27.0 | −36.9 (−66.0 to −7.8) | 4.0 | 50.6 |
| Mean age ≥ 60 years | 7 (241) | 137.7 ± 44.3 | −1.9 (−15.4 to 11.6) | 7.8 | 22.9 |
| Intervention duration ≥ 8 weeks | 9 (303) | 131.4 ± 41.9 | −5.0 (−16.8 to 6.8) | 9.7 | 17.2 |
| Intervention duration ≥ 12 weeks | 7 (258) | 132.8 ± 32.0 | −4.4 (−16.7 to 7.9) | 7.6 | 20.6 |
| Dyslipidemia | 5 (158) | 149.0 ± 34.2 | −26.3 (−50.9 to −1.7) | 8.0 | 50.0 |
| No dyslipidemia | 5 (169) | 131.6 ± 45.0 | 0.4 (−17.8 to 18.5) | 7.1 | 43.4 |
CI: confidence intervals; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride.
Trials are expressed as the number of trials (number of subjects).
Baseline lipids and lipoproteins are expressed as the mean ± SD.
Trials with a mean HDL-C < 40 mg/dL, mean LDL-C ≥ 140 mg/dL, or mean TG ≥ 150 mg/dL of subjects were designated as having dyslipidemic subjects.
Net changes in lipids and lipoproteins are expressed as pooled net change (95% CI).
Significant heterogeneity (P < 0.05).
BMI was examined as a secondary outcome. The baseline BMI was 26.5 ± 4.4 kg/m2 (404 subjects in 11 trials32–35, 37–42)). The pooled net change in BMI was −0.2 kg/m2 (95% CI, −1.1 to 0.7 for 297 subjects in 9 trials32–35, 37, 39–41)), and no significant heterogeneity in the BMI was noted (P = 0.99 and I2 = 0.0%).
Supplementary Fig.1–4 show the results of funnel plots in lipids and lipoproteins. When the publication bias was evaluated using the trim and fill method, there were no missing trials for TG. However, there were five missing trials for HDL-C, five missing trials for LDL-C, and two missing trials for TC. After adjusting for the missing trials, the pooled net changes in HDL-C improved to 7.4 mg/dL (95% CI, 3.1 to 11.8), those in LDL-C improved to −18.9 mg/dL (95% CI, −28.3 to −9.5), and those in TC improved to −8.5 mg/dL (95% CI, −15.5 to −1.5). Egger's regression test revealed significant asymmetry of the funnel plots of HDL-C and LDL-C (P = 0.01 and P = 0.03, respectively). In a subgroup analysis where trials were limited to those involving subjects who performed swimming alone, subjects with dyslipidemia, or subjects without dyslipidemia, the funnel plots for HDL-C, LDL-C, TC, and TG were not significantly asymmetrical and the trim and fill method of Duval and Tweedie suggested that there were no missing trials (Supplementary Table 3).
Supplementary Fig. 1.
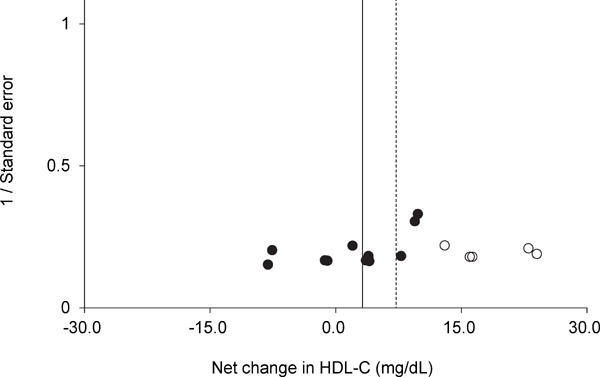
Funnel plot of HDL-C for each trial. Egger's regression test indicated that the funnel plot had significant asymmetry. A vertical line indicates the pooled net change in HDL-C (3.1 mg/dL). The trim and fill method of Duval and Tweedie suggested that there were five missing trials (white circles). A dotted vertical line indicates the pooled net change in HDL-C after adjusting for the missing trials (7.4 mg/dL).
Supplementary Fig. 4.
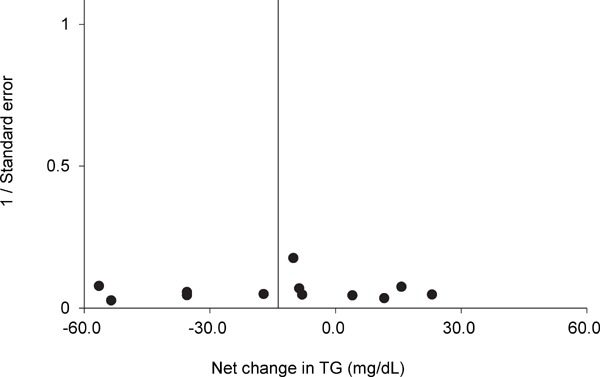
Funnel plot of TG for each trial. Egger's regression test indicated that the funnel plot did not have significant asymmetry. A vertical line indicated the pooled net change in TG (−13.7 mg/dL). The trim and fill method of Duval and Tweedie did not suggest that there were any missing trials.
Supplementary Table 3. Publication bias.
| Variable (category) | Trials | Net change, mg/dL | Trim and fill method |
Regression test |
||
|---|---|---|---|---|---|---|
| Missing trials | Adjusted net change, mg/dL | Interception | P | |||
| HDL-C | ||||||
| All trials | 11 | 3.1 (−0.8 to 6.9) | 5 | 7.4 (3.1 to 11.8) | −3.3 | 0.01 |
| Endurance exercise | 9 | 4.6 (0.8 to 8.3) | 4 | 8.0 (3.7 to 12.2) | −3.2 | 0.01 |
| No endurance exercise | 2 | −2.1 (−13.4 to 9.2) | N/A | N/A | ||
| Swimming | 3 | 5.3 (−1.1 to 11.7) | 0 | −3.1 | 0.70 | |
| No swimming | 8 | 2.0 (−3.2 to 7.1) | 4 | 7.3 (1.7 to 12.8) | −4.3 | 0.01 |
| Women only | 7 | 5.0 (0.6 to 9.4) | 2 | 7.2 (2.3 to 12.2) | −3.4 | 0.02 |
| Women and men | 2 | 1.3 (-7.0 to 9.6) | N/A | N/A | ||
| Mean age < 60 years | 3 | 8.4 (3.7 to 13.1) | 2 | 9.8 (5.7 to 13.9) | −1.6 | 0.30 |
| Mean age ≥ 60 years | 6 | 2.2 (−2.9 to 7.4) | 3 | 6.1 (0.7 to 11.5) | −3.9 | 0.02 |
| Intervention duration ≥ 8 weeks | 8 | 3.4 (−0.5 to 7.4) | 3 | 6.0 (1.6 to 10.4) | −3.3 | 0.03 |
| Intervention duration ≥ 12 weeks | 6 | 5.5 (1.6 to 9.4) | 3 | 8.0 (4.3 to 11.7) | −2.3 | 0.11 |
| Dyslipidemia | 4 | 6.4 (2.0 to 10.8) | 0 | −3.1 | 0.20 | |
| No dyslipidemia | 5 | 4.4 (0.01 to 8.8) | 2 | 6.2 (1.6 to 10.7) | −3.2 | 0.04 |
| LDL-C | ||||||
| All trials | 11 | −10.1 (−18.8 to −1.4) | 5 | −18.9 (−28.3 to −9.5) | 3.2 | 0.03 |
| Endurance exercise | 9 | −10.1 (−20.2 to −0.03) | 4 | −18.4 (−28.9 to −7.9) | 3.6 | 0.02 |
| No endurance exercise | 2 | −8.3 (−27.7 to 11.2) | N/A | N/A | ||
| Swimming | 3 | −0.6 (−14.4 to 13.2) | 0 | −3.3 | 0.62 | |
| No swimming | 8 | −13.1 (−23.7 to −2.6) | 4 | −22.1 (−33.3 to −11.0) | 2.9 | 0.09 |
| Women only | 7 | −12.3 (−23.6 to −1.0) | 3 | −20.0 (−33.3 to −6.7) | 3.6 | 0.06 |
| Women and men | 2 | −6.1 (−22.9 to 10.6) | N/A | N/A | ||
| Mean age < 60 years | 3 | −16.0 (−36.0 to 4.1) | 2 | −30.5 (−50.6 to −10.5) | 3.9 | 0.19 |
| Mean age ≥ 60 years | 6 | −6.6 (−16.2 to 3.0) | 1 | −3.9 (−13.1 to 5.4) | 1.8 | 0.50 |
| Intervention duration ≥ 8 weeks | 8 | −6.2 (−14.6 to 2.2) | 0 | 1.6 | 0.43 | |
| Intervention duration ≥ 12 weeks | 6 | −5.5 (−15.3 to 4.3) | 0 | 4.0 | 0.30 | |
| Dyslipidemia | 4 | −17.3 (−31.7 to −2.9) | 2 | −23.2 (−37.9 to −8.4) | 2.9 | 0.10 |
| No dyslipidemia | 5 | −6.3 (−17.5 to 4.9) | 0 | 7.5 | 0.19 | |
| TC | ||||||
| All trials | 10 | −9.5 (−17.7 to −1.3) | 2 | −8.5 (−15.5 to −1.5) | 0.7 | 0.33 |
| Endurance exercise | 8 | −8.5 (−16.5 to −0.5) | 1 | −6.8 (−13.1 to −0.5) | 1.0 | 0.27 |
| No endurance exercise | 2 | −14.0 (−31.2 to 3.3) | N/A | N/A | ||
| Swimming | 3 | −4.0 (−17.7 to 9.8) | 0 | 3.3 | 0.75 | |
| No swimming | 7 | −11.3 (−21.8 to −0.8) | 2 | −8.3 (−16.0 to −0.6) | 0.5 | 0.61 |
| Women only | 6 | −9.9 (−17.8 to −2.0) | 2 | −5.9 (−16.8 to 5.0) | 0.8 | 0.49 |
| Women and men | 1 | 9.0 (−16.4 to 34.4) | N/A | N/A | ||
| Mean age < 60 years | 3 | −16.9 (−32.1 to −1.7) | 0 | −1.1 | 0.72 | |
| Mean age ≥ 60 years | 5 | −2.6 (−17.5 to 12.4) | 0 | 2.0 | 0.15 | |
| Intervention duration ≥ 8 weeks (or ≥ 12 weeks) | 7 | −6.2 (−17.5 to 5.0) | 0 | 1.2 | 0.22 | |
| Dyslipidemia | 4 | −15.0 (−28.1 to −1.8) | 1 | −13.6 (−25.7 to −1.5) | −1.2 | 0.67 |
| No dyslipidemia | 4 | −0.4 (−19.0 to 18.2) | 0 | 2.5 | 0.18 | |
| TG | ||||||
| All trials | 12 | −13.7 (−27.8 to 0.5) | 0 | −0.2 | 0.85 | |
| Endurance exercise | 10 | −12.0 (−28.8 to 4.9) | 0 | 0.0 | 0.97 | |
| No endurance exercise | 2 | −20.2 (−46.2 to 5.8) | N/A | N/A | ||
| Swimming | 3 | −24.2 (−48.8 to 0.4) | 0 | 4.8 | 0.60 | |
| No swimming | 9 | −10.8 (−27.8 to 6.1) | 0 | 0.0 | 0.97 | |
| Women only | 7 | −10.4 (−31.5 to 10.6) | 1 | −15.1 (−35.4 to 5.2) | 0.2 | 0.88 |
| Women and men | 2 | −17.6 (−72.3 to 37.1) | N/A | N/A | ||
| Mean age < 60 years | 3 | −36.9 (−66.0 to −7.8) | 2 | −56.5 (−86.3 to −26.7) | 4.0 | 0.34 |
| Mean age ≥ 60 years | 7 | −1.9 (−15.4 to 11.6) | 0 | 0.5 | 0.58 | |
| Intervention duration ≥ 8 weeks | 9 | −5.0 (−16.8 to 6.8) | 0 | 0.1 | 0.87 | |
| Intervention duration ≥ 12 weeks | 7 | −4.4 (−16.7 to 7.9) | 0 | 0.4 | 0.63 | |
| Dyslipidemia | 5 | −26.3 (−50.9 to −1.7) | 2 | −39.5 (−63.7 to −15.3) | −4.3 | 0.03 |
| No dyslipidemia | 5 | 0.4 (−17.8 to 18.5) | 0 | 0.6 | 0.63 | |
CI: confidence intervals; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; N/A: not available; TC: total cholesterol; TG: triglyceride.
Net changes in lipids and lipoproteins are expressed as pooled net change (95% CI).
Trials with a mean HDL-C < 40 mg/dL, mean LDL-C ≥ 140 mg/dL, or mean TG ≥ 150 mg/dL of subjects were designated as having dyslipidemia of subjects.
Supplementary Fig. 2.
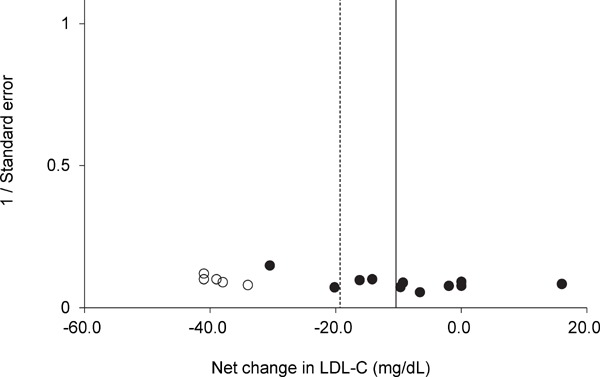
Funnel plot for LDL-C for each trial. Egger's regression test indicated that the funnel plot had significant asymmetry. A vertical line indicates the pooled net change in LDL-C (−10.1 mg/dL). The trim and fill method of Duval and Tweedie suggested that there were five missing trials (white circles). A dotted vertical line indicates the pooled net change in LDL-C after adjusting for the missing trials (−18.9 mg/dL).
Supplementary Fig. 3.
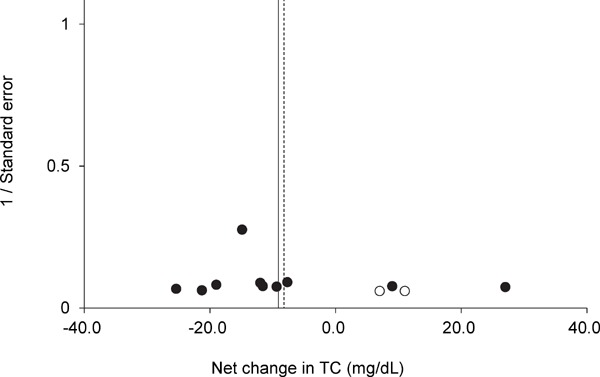
Funnel plot for TC for each trial. Egger's regression test indicated that the funnel plot had significant asymmetry. A vertical line indicates the pooled net change in TC (−9.5 mg/dL). The trim and fill method of Duval and Tweedie suggested that there were two missing trials (white circles). A dotted vertical line indicates the pooled net change in TC after adjusting for the missing trials (−8.5 mg/dL).
Discussion
According to previous meta-analyses, regular endurance exercise resulted in pooled net changes in HDL-C of approximately 2 mg/dL, pooled net changes in LDL-C of approximately −4 mg/dL, pooled net changes in TC of approximately −4 mg/dL, and pooled net changes in TG of approximately −2 mg/dL10–13). In addition, resistance training resulted in pooled net changes in HDL-C of 0.7 mg/dL, pooled net changes in LDL-C of −6.1 mg/dL, pooled net changes in TC of −5.5 mg/dL, and pooled net changes in TG of −8.1 mg/dL, but HDL-C did not improve significantly14). The current meta-analysis evaluated the effects of aquatic exercise on the lipid and lipoprotein levels. The results indicated that exercise resulted in pooled net changes in HDL-C of 3.1 mg/dL, pooled net changes in LDL-C of −10.1 mg/dL, pooled net changes in TC of −9.5 mg/dL, and pooled net changes in TG of −13.7 mg/dL, but the pooled net changes in HDL-C and TG did not improve significantly and contained significant heterogeneity.
When trials were limited to those involving subjects who performed endurance exercise, the HDL-C, LDL-C, and TC levels improved significantly, and subgroup analyses revealed beneficial effects on the lipid and lipoprotein levels in women, subjects with a mean age < 60 years, and patients with dyslipidemia. In addition, the current meta-analysis revealed greater improvement in lipid and lipoprotein levels than previous meta-analyses10–14), likely because of the influence of baseline dyslipidemia. The baseline LDL-C, TC, and TG levels in the current study were higher than those in previous meta-analyses8–11, 14). Several meta-analyses have indicated that changes in the lipid and lipoprotein levels are related to the baseline lipid and lipoprotein levels7, 8). However, the current study showed that HDL-C improved significantly in trials involving endurance exercise and subjects without dyslipidemia. Thus, aquatic endurance exercise is expected to increase the HDL-C levels, regardless of whether a subject has dyslipidemia or not. In addition, the current study showed that the HDL-C levels improved significantly when trials were limited to those involving an intervention duration ≥ 12 weeks. Previous meta-analyses have similarly suggested that the exercise time or volume might be associated with the effects of exercise on the lipid and lipoprotein levels7, 8, 13). For example, a meta-analysis reported that the HDL-C levels increased by approximately 1.4 mg/dL for every 10 min an exercise session was prolonged13). The current meta-analysis involved trials lasting a minimum of 30 min per session, but previous meta-analyses included trials of < 30 min per session11–13).
The mechanisms by which exercise improved the lipid and lipoprotein levels are still unclear. However, given that adipocytokines are considered to cause dyslipidemia43), improvements in lipid and lipoprotein metabolism are presumably related to changes in the adipocytokine levels. A large randomized controlled trial reported that both HDL-C and adiponectin levels increased as a result of lifestyle management and that changes in HDL-C levels were associated with changes in adiponectin levels44). A recent meta-analysis also indicated that endurance exercise reduced the serum leptin levels and increased the adiponectin levels45), so endurance exercise may alleviate dyslipidemia via changes in adipocytokine levels.
A meta-analysis suggested that exercise therapy was as effective at preventing cardiovascular disease as drug therapy46). Because dyslipidemia is a risk factor for cardiovascular disease1–3) and regular exercise improves the lipid and lipoprotein levels10–16), improved lipid and lipoprotein levels may prevent cardiovascular disease. The mechanism by which exercise prevents cardiovascular disease is thought to be by improving the vascular function. A meta-analysis reported that endurance exercise on land significantly improved the pulse wave velocity47). A cross-sectional study indicated that pulse wave velocity was associated with the HDL-C, TC, and TG levels48). In addition, a controlled clinical trial involving regular aquatic exercise indicated that the percentage change in LDL-C or TC during intervention was related to the percentage change in pulse wave velocity during intervention49). A randomized controlled trial included in the current meta-analysis examined the related vascular function and indicated that swimming training significantly increased the carotid artery compliance34). However, that study found that the lipid and lipoprotein levels did not change significantly from the baseline to after intervention. Because there is little evidence indicating the mechanism by which regular aquatic exercise affects the relationship between the vascular function and lipid or lipoprotein levels, this mechanism is a topic for future study.
The current meta-analysis had several limitations. First, this meta-analysis has a small sample of only 393 subjects, and most were older and/or female. In addition, publication bias was suspected of affecting the net changes in HDL-C, LDL-C, and TC. Once these influences were taken into account and adjusted for, the pooled net changes in HDL-C and LDL-C improved drastically. When trials were limited to those involving women or subjects ≥ 60 years of age, there were no signs of publication bias with regard to LDL-C, TC, and TG. Therefore, new randomized controlled trials — particularly those involving men and subjects < 60 years of age—should be included in a future meta-analysis. Second, this meta-analysis did not perform a subgroup analysis of the effects of baseline levels of lipids and lipoproteins based on the guidelines of the American College of Cardiology and the American Heart Association2). According to these guidelines, statin therapy is indicated when a patient's LDL-C level is ≥ 190 mg/dL (70–189 mg/dL if a patient has diabetes), a patient has atherosclerotic cardiovascular disease, or a patient has a 10-year risk of developing atherosclerotic cardiovascular disease ≥ 7.5%2). The current meta-analysis suggested that not all the trial subjects met these criteria based on their mean LDL-C levels at the baseline, and patients with cardiovascular disease and those undergoing cardiac rehabilitation were excluded as subjects. Therefore, the current meta-analysis did not analyze individuals who needed to take statins. A cohort study reported that statin therapy and increased fitness were independently associated with mortality50). Thus, the combination of statin therapy and increased physical activity is a crucial way of improving the health in patients with dyslipidemia. However, there is little evidence regarding the efficacy of the combination of exercise and statin therapy, so future studies should evaluate the effects of exercise on the lipid and lipoprotein levels (in individuals at risk of developing dyslipidemia).
Conclusion
The results of a meta-analysis indicated that aquatic endurance exercise improved the lipid and lipoprotein levels. This form of exercise particularly benefited women, the middle-aged, and patients with dyslipidemia. However, the current meta-analysis had a small sample size, so additional trials must be included in the future in order to confirm the effects of regular aquatic exercise on lipid and lipoprotein levels.
Acknowledgment
A special thanks to the staff of Osaka University of Health and Sports Sciences Library for collecting literature for our analysis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
List of Abbreviations
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- TC
total cholesterol
- TG
triglyceride
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- SD
standard deviation
- BMI
body mass index
- PEDro
Physiotherapy Evidence Database
- CI
confidence intervals
Conflicts of Interests
None.
References
- 1). Madsen CM, Varbo A, Nordestgaard BG: Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J, 2017; 38: 2478-2486 [DOI] [PubMed] [Google Scholar]
- 2). Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014; 129: S1-45 [DOI] [PubMed] [Google Scholar]
- 3). Scandinavian Simvastatin Survival Study Group: Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet, 1994; 344: 1383-1389 [PubMed] [Google Scholar]
- 4). Yu-Poth S, Zhao G, Etherton T, Naglak M, Jonnalagadda S, Kris-Etherton PM: Effects of the National Cholesterol Education Program's Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta-analysis. Am J Clin Nutr, 1999; 69: 632-646 [DOI] [PubMed] [Google Scholar]
- 5). Mensink RP, Zock PL, Kester AD, Katan MB: Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr, 2003; 77: 1146-1155 [DOI] [PubMed] [Google Scholar]
- 6). Uauy R, Aro A, Clarke R, Ghafoorunissa R, L'Abbé MR, Mozaffarian D, Skeaff CM, Stender S, Tavella M: WHO Scientific Update on trans fatty acids: summary and conclusions. Eur J Clin Nutr, 2009; 63: S68-S75 [Google Scholar]
- 7). Leon AS, Sanchez OA: Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc, 2001; 33: S502-515 [DOI] [PubMed] [Google Scholar]
- 8). Kelley GA, Kelley KS, Roberts S, Haskell W: Combined effects of aerobic exercise and diet on lipids and lipoproteins in overweight and obese adults: a meta-analysis. J Obes, 2012; 2012: 985902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Kelley GA, Kelley KS, Roberts S, Haskell W: Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Clin Nutr, 2012; 31: 156-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Kelley GA, Kelley KS, Tran ZV: Walking, lipids, and lipoproteins: a meta-analysis of randomized controlled trials. Prev Med, 2004; 38: 651-661 [DOI] [PubMed] [Google Scholar]
- 11). Kelley GA, Kelley KS, Tran ZV: Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Womens Health (Larchmt), 2004; 13: 1148-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Kelley GA, Kelley KS, Tran ZV: Exercise, lipids, and lipoproteins in older adults: a meta-analysis. Prev Cardiol, 2005; 8: 206-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H: Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med, 2007; 167: 999-1008 [DOI] [PubMed] [Google Scholar]
- 14). Kelley GA, Kelley KS: Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med, 2009; 48: 9-19 [DOI] [PubMed] [Google Scholar]
- 15). Pan XH, Mahemuti A, Zhang XH, Wang YP, Hu P, Jiang JB, Xiang MX, Liu G, Wang JA: Effect of Tai Chi exercise on blood lipid profiles: a meta-analysis of randomized controlled trials. J Zhejiang Univ Sci B, 2016; 17: 640-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Chu P, Gotink RA, Yeh GY, Goldie SJ, Hunink MG: The effectiveness of yoga in modifying risk factors for cardiovascular disease and metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol, 2016; 23: 291-307 [DOI] [PubMed] [Google Scholar]
- 17). Denning WM, Bressel E, Dolny D, Bressel M, Seeley MK: A review of biophysical differences between aquatic and land-based exercise. Int J Aquatic Res Educ, 2012; 6: 46-67 [Google Scholar]
- 18). Torres-Ronda L, Del Alcázar XS: The properties of water and their applications for training. J Hum Kinet, 2014; 44: 237-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Igarashi Y, Nogami Y: The effect of regular aquatic exercise on blood pressure: A meta-analysis of randomized controlled trials. Eur J Prev Cardiol, 2018; 25: 190-199 [DOI] [PubMed] [Google Scholar]
- 20). Uthman OA, van der Windt DA, Jordan JL, Dziedzic KS, Healey EL, Peat GM, Foster NE: Exercise for lower limb osteoarthritis: systematic review incorporating trial sequential analysis and network meta-analysis. BMJ, 2013; 347: f5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS: 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc, 2011; 43: 1575-1581 [DOI] [PubMed] [Google Scholar]
- 22). Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group : Preferred reporting items for systematic review and metaanalysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ, 2015; 349: g7647. [DOI] [PubMed] [Google Scholar]
- 23). Cornelissen VA, Smart NA: Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc, 2013; 2: e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Moseley AM, Herbert RD, Sherrington C, Maher CG: Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother, 2002; 48: 43-49 [DOI] [PubMed] [Google Scholar]
- 25). DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials, 1986; 7: 177-188 [DOI] [PubMed] [Google Scholar]
- 26). Follmann D, Elliott P, Suh I, Cutler J: Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol, 1992; 45: 769-773 [DOI] [PubMed] [Google Scholar]
- 27). Higgins JP, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med, 2002; 21: 1539-1558 [DOI] [PubMed] [Google Scholar]
- 28). Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A, American College of Sports Medicine; American Heart Association : Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation, 2007; 116: 1081-1093 [DOI] [PubMed] [Google Scholar]
- 29). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for Epidemiology and Clinical Management of Atherosclerosis : Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Duval S, Tweedie R: Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 2000; 56: 455-463 [DOI] [PubMed] [Google Scholar]
- 31). Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ, 1997; 315: 629-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Takeshima N, Rogers ME, Watanabe E, Brechue WF, Okada A, Yamada T, Islam MM, Hayano J: Waterbased exercise improves health-related aspects of fitness in older women. Med Sci Sports Exerc, 2002; 34: 544-551 [DOI] [PubMed] [Google Scholar]
- 33). Colado JC, Triplett NT, Tella V, Saucedo P, Abellán J: Effects of aquatic resistance training on health and fitness in postmenopausal women. Eur J Appl Physiol, 2009; 106: 113-122 [DOI] [PubMed] [Google Scholar]
- 34). Nualnim N, Parkhurst K, Dhindsa M, Tarumi T, Vavrek J, Tanaka H: Effects of swimming training on blood pressure and vascular function in adults > 50 years of age. Am J Cardiol, 2012; 109: 1005-1010 [DOI] [PubMed] [Google Scholar]
- 35). Nuttamonwarakul A, Amatyakul S, Suksom D: Twelve weeks of aqua-aerobic exercise improve health-related physical adaptations and glycemic control in elderly patients with type 2 diabetes. J Exerc Physiol Online, 2012; 15: 64-70 [Google Scholar]
- 36). Shibata Y, Hayasaka S, Ojima T, Goto Y: Effects of water exercise on physiological and psychological health in the Japanese: Kawane Spa Study. Int SportMed J, 2012; 13: 190-202 [Google Scholar]
- 37). Arca EA, Martinelli B, Martin LC, Waisberg CB, Franco RJ: Aquatic exercise is as effective as dry land training to blood pressure reduction in postmenopausal hypertensive women. Physiother Res In, 2014; 19: 93-98 [DOI] [PubMed] [Google Scholar]
- 38). Rahimi A, Shabestari MM, Faryadian K, Safaeinejad V, Moazen JS, Fallah Z: The effect of selecting aerobics exercise program (walking in water and in land) on HDLC, LDL-C, TC and TG in non-athlete menopausal women. Eur J Exp Biol, 2013; 3: 463-468 [Google Scholar]
- 39). Chung PK, Mui R, Zhao YN, Liu J: Training effects of water Tai Chi on health indicators among Chinese older females in Hong Kong. Int J Phys Educ Sports Health, 2014; 1: 20-24 [Google Scholar]
- 40). Mohr M, Nordsborg NB, Lindenskov A, Steinholm H, Nielsen HP, Mortensen J, Weihe P, Krustrup P: High-intensity intermittent swimming improves cardiovascular health status for women with mild hypertension. Biomed Res Int, 2014; 728289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Kantyka J, Herman D, Roczniok R, Kuba L: Effects of aqua aerobics on body composition, body mass, lipid profile, and blood count in middle-aged sedentary women. Hum Movement, 2015; 16: 9-14 [Google Scholar]
- 42). Kim WC, Choi SL, Kim SW, Park HR: The effects of aquarobics on blood pressure, heart rate, and lipid profile in older women with hypertension. Indian J Sci Tech, 2016; 9: 1-7 [Google Scholar]
- 43). Izadi V, Farabad E, Azadbakht L: Epidemiologic evidence on serum adiponectin level and lipid profile. Int J Prev Med, 2013; 4: 133-140 [PMC free article] [PubMed] [Google Scholar]
- 44). Belalcazar LM, Lang W, Haffner SM, Hoogeveen RC, Pi-Sunyer FX, Schwenke DC, Balasubramanyam A, Tracy RP, Kriska AP, Ballantyne CM, Look AHEAD Research Group : Adiponectin and the mediation of HDL-cholesterol change with improved lifestyle: the Look AHEAD Study. J Lipid Res, 2012; 53: 2726-2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Yu N, Ruan Y, Gao X, Sun J: Systematic Review and Meta-Analysis of Randomized, Controlled Trials on the Effect of Exercise on Serum Leptin and Adiponectin in Overweight and Obese Individuals. Horm Metab Res, 2017; 49: 164-173 [DOI] [PubMed] [Google Scholar]
- 46). Naci H, Ioannidis JP: Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ, 2013; 347: f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC: Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One, 2014; 9: e110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S: Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis, 2003; 166: 303-309 [DOI] [PubMed] [Google Scholar]
- 49). Kawasaki T, Sullivan CV, Ozoe N, Higaki H, Kawasaki J: A long-term, comprehensive exercise program that incorporates a variety of physical activities improved the blood pressure, lipid and glucose metabolism, arterial stiffness, and balance of middle-aged and elderly Japanese. Hypertens Res, 2011; 34: 1059-1066 [DOI] [PubMed] [Google Scholar]
- 50). Kokkinos PF, Faselis C, Myers J, Panagiotakos D, Doumas M: Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet, 2013; 381: 394-399 [DOI] [PubMed] [Google Scholar]


