Abstract
Aim: The Japan Diet nutritional education program effects on serum fatty acid compositions for prevention of atherosclerotic cardiovascular disease in middle-aged men brought up in the westernized dietary environment of modern Japan were examined.
Methods: Thirty-three men, 30–49 years of age, attended a nutrition education class and were recommended to consume Japan Diet volumes (more fish, soybeans and soy products, vegetables, seaweed, konjak, mushrooms, and unrefined cereals and less animal fat, meat and poultry with fat, sweets, desserts and snacks, and alcoholic drinks) for 6 weeks. Three-day weighted dietary records were kept, and fatty acid intakes were calculated. Serum phospholipid fatty acid compositions were examined.
Results: During the 6 weeks, fish, soy, and seaweed and/or mushrooms and/or konjak were consumed 1.0, 1.1, and 1.0 times daily on average, whereas daily fatty meat and poultry and sweet consumptions were 0.3 and 0.3, respectively. These changes were attributed to increased intake of n-3 polyunsaturated fatty acids (PUFAs) such as C20:5(n-3), C22:6(n-3), C18:4(n-3), and C20:4(n-3) and decreased intakes of all saturated fatty acids and unsaturated fatty acids such as C18:1 and C18:2(n-6). As to the phospholipid fatty acid composition, C18:0 decreased, whereas C15:0, C17:0, and C20:0 increased. Marked increases in C20:5(n-3) and C22:6(n-3) raised total n-3 PUFA from 10.30% to 13.20% along with n-6 PUFA decreasing from 33.92% to 31.16%. Despite decreases in C20:4(n-6) and C20:3(n-6), the C20:4(n-6)/C20:3(n-6) ratio used as an estimate of delta-5 desaturase activities increased and correlated positively with fish intake at completion of the intervention.
Conclusions: The Japan Diet is effective for changing the fatty acids to an anti-atherosclerotic profile. The clinical trial registration number: UMIN000020639.
Keywords: Japan Diet, Fish, Delta-5 desaturase activities, Icosapentaenoic acid, Docosahexaenoic acid
See editorial vol. 26: 1–2
Introduction
There has been major research and clinical interest in elucidating the effects of dietary fatty acids on cardiovascular disease risk reduction. Over the years, numerous studies have corroborated the blood cholesterol predictive equations showing saturated fatty acids (SFAs) to be hypercholesterolemic and that polyunsaturated fatty acids (PUFAs) reduce cholesterol1).
In the Seven Countries Study, a high serum cholesterol level was found to be a risk factor for coronary artery disease, and high SFA intake was recognized as leading to a high serum cholesterol level2). From the end of World War Ⅱ, problems of malnutrition remained in Japan up to 1960. The traditional Japanese diet in the 1960s to mid-1970s, which was rich in fish, with relatively low consumptions of meat, poultry, and animal fats, was described as a cardioprotective dietary style. From the 1960s, the Japanese dietary style has changed markedly, with Westernization and diversification, and the percentage of energy derived from lipid increasing from 10.6% in 1960 to 18.9% in 1970 and finally to 22.3% in 1975. From 1975 to 1990, the changes were moderate, and the percentage of energy derived from lipid was rather stable. Ever since, lipid intake has been remarkably high, especially in the younger generation. In 2016, people with energy intake derived from lipid of over 30% were 40% in the all three age groups (20s, 30s, and 40s). Lack of fish intake and excessive oils and meat and poultry intakes have been recognized as major problems in younger generations3). Along with these trends, fatty acid intakes have also changed, and this is contributing to increased obesity, dyslipidemia, and type 2 diabetes. There is now a need to verify the effects of the traditional Japanese diet on dietary fatty acid intakes and circulating fatty acid levels.
We previously conducted a pilot study showing the anti-atherosclerotic effects of the Japan Diet (higher consumptions of fish, soybeans and soy products, vegetables, seaweed/mushrooms/konjak, and unrefined cereals with reduced consumptions of animal fat, meat and poultry with fat, sweets including desserts and snacks, and alcoholic drinks). At baseline, their food consumption was rich in meat and poultry, but poor in fish and soybeans. The percentage of energy derived from lipid was 31%, twice as high as that of the 1960–1970s. After the 6 week education program, fish; soy; and the sum of seaweed, mushroom, and konjak intakes doubled, whereas intakes of meat and poultry, sweets including desserts and snacks, and margarine and shortening all decreased. These changes contributed to significant decreases in serum low-density lipoprotein (LDL) cholesterol, malondialdehyde-modified LDL, and triglyceride concentrations and to the maintenance of high levels of high-density lipoprotein (HDL) cholesterol4).
The Japan Diet has a characteristic fatty acid content derived from seafood and plant foods. Serum fatty acid compositions would be expected to evolve together with the anti-atherogenic pattern in response to Japan diet consumption. The effects of purified fatty acid supplementation as part of a prescribed dietary intervention that increased circulating n-3 PUFA have been reported5). However, there have been no studies examining changes in serum fatty acid profiles achieved by the educational approach of recommending intake of the Japan Diet.
Aim
This study aimed to examine how the Japan Diet affects fatty acid intakes and serum fatty acid compositions.
Methods
Study Design and Subjects
The study design, methods, and subjects were described in detail in our previous report4). The 34 participants were male, between 30 and 49 years of age, with a body mass index (BMI) over 22 kg/m2, and all were non-smokers. Users of dietary supplements or health food products were excluded. After being given a detailed explanation of the program, all 34 subjects consented to participate. The present study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures were approved by the ethics committee of Japan Women's University (No. 209). Written informed consent was obtained from all subjects prior to participation. The clinical trial registration number is UMIN000020639.
Intervention Program
The 6 week intervention program was explained in our previous report4). Briefly, a lecture and group work were conducted focusing on easy ways to consume the foods classified as constituting the Japan Diet (consumption of more fish, soybeans and soy products, vegetables, seaweed, mushrooms, konjak, and unrefined cereals while reducing animal fat, meat and poultry with fat, sweets including desserts and snacks, and alcoholic drink intakes). Each participant was required to keep a daily performance record in terms of the intakes of foods comprising the Japan Diet for 6 weeks. The subjects were instructed to maintain their habitual physical activity levels during the study period.
Food Intake
Subjects were instructed to record their daily food intake, counting the number of dishes (fish, soybeans and soy products, vegetables, seaweed/mushrooms/konjak and unrefined cereals, fatty meat and poultry, and sweets/desserts/snacks) during the intervention period.
Dietary Fatty Acid Intake
Three-day weighted dietary records were kept at baseline and at the end of the intervention as previously reported4). Fatty acid intakes were calculated employing Excel-Eiyokun Ver.6, (Kenpaku-sha Co., Ltd., Tokyo, Japan) software.
Measurement of Metabolic Parameters and Serum Phospholipid Fatty Acid Compositions
Fasting blood collections were conducted on day 1 and after 6 weeks, in the morning following a 12 h overnight fast. Plasma and serum samples were obtained and stored at −80°C until analysis. Serum biochemical parameters were measured as described in our previous report4). For fatty acid measurement, serum lipids were extracted by the method of Folch et al.6). The phospholipid fraction was obtained with thin-layer chromatography and methylated as described in our previous report with minor modifications7). The fatty acid methyl esters were separated by gas–liquid chromatography (Shimadzu GC14B, Tokyo, Japan) using a 0.25 mm × 30 m capillary column containing DB-FFAP (J&W Scientific, CA, USA). The injector temperature was 230°C, and the column temperature rose at 2°C/min from 160°C to 200°C. Nitrogen was employed as the carrier gas, and the split ratio was 22.0.
Statistical Analysis
The data of the 33 Japanese men who completed this program were analyzed. One participant was hospitalized for an acute illness and had to drop out.
Statistical analyses were carried out using SPSS for Windows (version 16.0J; SPSS Japan, Inc.). All values are presented as means with standard deviations. The statistical significance of differences in values obtained at baseline and at the end of the intervention was assessed using the non-parametric Wilcoxon matched pair's signed-rank test. Spearman correlation analyses were performed to identify correlations between metabolic parameters and the fatty acid ratio in the serum phospholipids. P < 0.05 was considered to indicate a statistically significant difference.
Results
Subjects
The mean age of the subjects was 39 ± 5 years. Other clinical parameters were shown in our previous report4). In short, after the intervention, body weight, BMI, and umbilical circumference decreased with a coincident decrease in the serum leptin concentration. Serum LDL-cholesterol, malondialdehyde-LDL, and triglyceride concentrations decreased significantly, whereas HDL cholesterol was unchanged4).
Food Intake
Food intake volumes per day at baseline and at the end of this intervention were reported previously; that is, mean fish and soy intakes increased from 49.0 and 39.8 g to 98.0 and 86.5 g, respectively, and seaweed intake from 9.6 to 33.4 g. The sum volume of meat and poultry intake, which totaled 134.3 g at baseline, had decreased to 95.4 g at the end of the intervention4). The number of dishes consumed daily, which provided each type of target food during the 6 weeks, is shown in Table 1. Fish, soy, and seaweed and/or mushrooms and/or konjak were consumed 1.0, 1.1, and 1.0 times per day on average, respectively, whereas fatty meat and poultry, and sweets were consumed 0.3 and 0.3 times daily, respectively.
Table 1. Number of dishes which provide each of target foods in a day during the 6 week intervention.
| number in a day | (minimum, maximum) | |
|---|---|---|
| Fish | 1.0 ± 0.3 | (0.4, 2.1) |
| Soybeans and other soy products | 1.1 ± 0.5 | (0.1, 2.4) |
| Vegetables | 2.3 ± 0.9 | (0.7, 5.0) |
| Seaweed, mushrooms and konjak | 1.0 ± 0.5 | (0.2, 2.0) |
| Fatty meat and poultry | 0.3 ± 0.2 | (0.0, 0.9) |
| Sweets, desserts and snacks | 0.3 ± 0.2 | (0.0, 0.9) |
n = 33, Values are expressed as means ± SD.
Dietary Fatty Acid Intakes
Energy intakes derived from lipids and SFAs were 31.4 (23.7, 42.9) (mean (minimum, maximum)) % and 8.8 (5.61, 15.0) % at baseline, and had decreased to 30.6 (18.4, 44.9) % and to 7.8 (3.6, 14.2) %, respectively, at the end of the intervention4).
Almost all SFAs intake including C12:0, C16:0, and C18:0 significantly decreased (p = 0.002, 0.002, 0.005), and C14:0 tended to decrease (p = 0.053). The sum of C12:0, C14:0, and C16:0, which are known to increase the LDL-C concentration, was decreased by 3882 mg corresponding to 1.4% of total energy intake.
Monounsaturated fatty acid (MUFA) intake decreased (p = 0.008), principally that of C18:1, which is the major MUFA (p = 0.003).
C18:2 intake, which accounts for the majority of n-6 PUFA, tended to decrease (p = 0.055), though C20:3 (n-6) and C20:4(n-6) did not change (p = 0.122, 0.922).
Mean n-3 PUFA intake was 3103 mg at baseline and had increased to 3723 mg (p = 0.036), mainly due to C20:5(n-3) and C22:6(n-3), by the end of the intervention (p = 0.005, 0.016).
The SFA/MUFA/PUFA ratio changed from 1/1.3/0.7 to 1/1.4/0.9, and the n-6/n-3 ratio decreased from 4.9 to 3.7 (Table 2).
Table 2. Fatty acid intakes at baseline and the end of the 6 week intervention.
| Baseline (mg) | 6 weeks (mg) | Change (mg) | p | |
|---|---|---|---|---|
| C4:0 | 328 ± 241 | 223 ± 284 | −105 ± 301 | 0.003 |
| C6:0 | 214 ± 156 | 144 ± 177 | −70 ± 187 | 0.002 |
| C7:0 | 1 ± 2 | 1 ± 2 | −0 ± 2 | 0.129 |
| C8:0 | 198 ± 171 | 119 ± 134 | −79 ± 172 | 0.002 |
| C10:0 | 328 ± 232 | 215 ± 233 | −113 ± 267 | 0.004 |
| C12:0 | 820 ± 840 | 454 ± 573 | −366 ± 895 | 0.002 |
| C13:0 | 4 ± 4 | 3 ± 5 | −1 ± 6 | 0.127 |
| C14:0 | 1733 ± 867 | 1426 ± 969 | −307 ± 1072 | 0.053 |
| C15:0 | 165 ± 85 | 135 ± 104 | −30 ± 112 | 0.024 |
| C15:0ant | 47 ± 34 | 32 ± 40 | −15 ± 46 | 0.002 |
| C16:0 | 14504 ± 3626 | 11296 ± 5105 | −3209 ± 5222 | 0.002 |
| C16:0iso | 23 ± 17 | 16 ± 20 | −7 ± 23 | 0.004 |
| C17:0 | 202 ± 76 | 173 ± 103 | −30 ± 115 | 0.075 |
| C17:0ant | 44 ± 32 | 30 ± 38 | −14 ± 43 | 0.003 |
| C18:0 | 5533 ± 1616 | 4236 ± 1970 | −1297 ± 2361 | 0.005 |
| C20:0 | 259 ± 59 | 218 ± 58 | −42 ± 82 | 0.009 |
| C22:0 | 129 ± 56 | 110 ± 37 | −19 ± 68 | 0.140 |
| C24:0 | 62 ± 28 | 47 ± 17 | −16 ± 33 | 0.014 |
| SFA subtotal | 24593 ± 7168 | 18870 ± 8946 | −5723 ± 9572 | 0.003 |
| C10:1 | 26 ± 19 | 18 ± 22 | −8 ± 26 | 0.004 |
| C14:1 | 146 ± 80 | 114 ± 159 | −32 ± 160 | 0.006 |
| C15:1 | 0 ± 0 | 0 ± 0 | −0 ± 0 | 0.144 |
| C16:1 | 1240 ± 424 | 1242 ± 758 | 2 ± 836 | 0.448 |
| C17:1 | 134 ± 52 | 121 ± 95 | −13 ± 106 | 0.161 |
| C18:1 | 29991 ± 6651 | 24137 ± 8618 | −5855 ± 10664 | 0.003 |
| C20:1 | 628 ± 321 | 802 ± 486 | 174 ± 582 | 0.106 |
| C22:1 | 329 ± 472 | 511 ± 608 | 182 ± 781 | 0.102 |
| C24:1 | 49 ± 43 | 74 ± 56 | 25 ± 74 | 0.031 |
| MUFA subtotal | 32583 ± 6931 | 27013 ± 9518 | −5570 ± 11440 | 0.008 |
| C18:2(n-6) | 14937 ± 3197 | 13384 ± 3468 | −1552 ± 4710 | 0.055 |
| C18:3(n-6) | 4 ± 5 | 8 ± 7 | 4 ± 8 | 0.021 |
| C20:2(n-6) | 79 ± 28 | 76 ± 44 | −4 ± 47 | 0.280 |
| C20:3(n-6) DGLA | 43 ± 15 | 38 ± 24 | −5 ± 22 | 0.122 |
| C20:4(n-6) AA | 207 ± 67 | 220 ± 128 | 13 ± 115 | 0.922 |
| C22:2(n-6) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.213 |
| C22:4(n-6) | 17 ± 7 | 16 ± 11 | −2 ± 10 | 0.249 |
| C22:5(n-6) | 9 ± 8 | 19 ± 17 | 10 ± 16 | 0.001 |
| n-6 PUFA subtotal | 15143 ± 3150 | 13760 ± 3594 | −1534 ± 4786 | 0.067 |
| C18:3(n-3) | 2195 ± 627 | 2141 ± 701 | −54 ± 912 | 0.741 |
| C18:4(n-3) | 71 ± 105 | 125 ± 148 | 54 ± 185 | 0.039 |
| C20:4(n-3) | 28 ± 30 | 54 ± 43 | 26 ± 56 | 0.020 |
| C20:5(n-3) EPA | 237 ± 235 | 454 ± 313 | 217 ± 398 | 0.005 |
| C21:5(n-3) | 6 ± 10 | 14 ± 15 | 8 ± 16 | 0.010 |
| C22:5(n-3) | 84 ± 55 | 144 ± 93 | 61 ± 111 | 0.007 |
| C22:6(n-3) DHA | 490 ± 440 | 797 ± 547 | 306 ± 730 | 0.016 |
| n-3 PUFA subtotal | 3103 ± 1022 | 3723 ± 1476 | 620 ± 1637 | 0.036 |
| PUFA subtotal | 18427 ± 3768 | 17541 ± 4639 | −886 ± 5589 | 0.249 |
n = 33, Values are expressed as means ± SD.
SFA: saturated fatty acid, MUFA: monounsaturated fatty acid, PUFA: polyunsaturated fatty acid
DGLA: di-homo gamma linolenic acid, AA: arachidonic acid, EPA: icosapentaenoic acid, DHA: docosahexaenoic acid
At the end of the intervention, a positive correlation was observed between n-3 PUFA intake and the number of fish dishes consumed (p < 0.001) (Fig. 1).
Fig. 1.
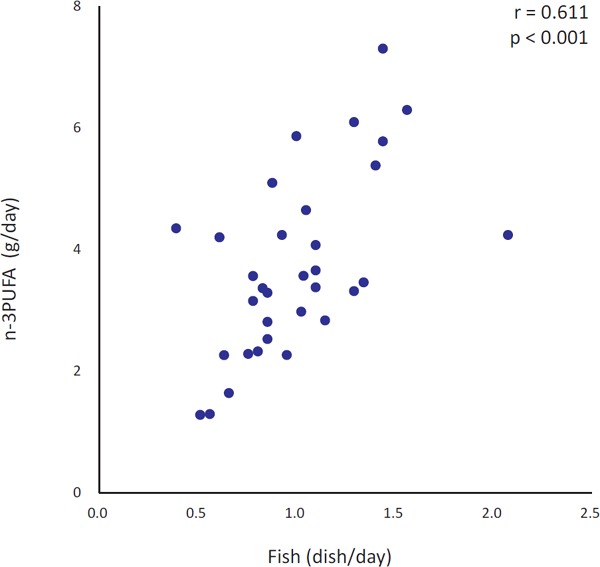
Simple correlation between fish dish intake during the 6 week intervention and n-3 PUFA intake at the end of the 6 week intervention
n = 33, PUFA: polyunsaturated fatty acid
Serum Phospholipid Fatty Acid Compositions
Changes in the fatty acid composition in serum phospholipids are presented in Table 3.
Table 3. Fatty acid compositions in serum phospholipids at baseline and the end of the 6 week intervention.
| Baseline | 6 weeks | Change | p | |
|---|---|---|---|---|
| C14:0 | 0.14 ± 0.04 | 0.13 ± 0.04 | −0.01 ± 0.05 | 0.406 |
| C15:0 | 0.09 ± 0.02 | 0.10 ± 0.05 | 0.01 ± 0.05 | 0.044 |
| C16:0 | 23.04 ± 1.32 | 22.96 ± 1.12 | −0.08 ± 1.13 | 0.809 |
| C17:0 | 0.32 ± 0.05 | 0.34 ± 0.04 | 0.02 ± 0.04 | 0.020 |
| C18:0 | 14.64 ± 0.93 | 14.24 ± 1.19 | −0.39 ± 1.12 | 0.046 |
| C20:0 | 0.62 ± 0.11 | 0.66 ± 0.12 | 0.04 ± 0.08 | 0.003 |
| C22:0 | 1.84 ± 0.38 | 1.85 ± 0.34 | 0.02 ± 0.30 | 0.586 |
| C24:0 | 1.60 ± 0.29 | 1.56 ± 0.26 | −0.04 ± 0.28 | 0.623 |
| SFA subtotal | 42.28 ± 1.58 | 41.85 ± 1.55 | −0.43 ± 1.82 | 0.140 |
| C14:1 | 0.04 ± 0.02 | 0.03 ± 0.03 | −0.01 ± 0.02 | 0.092 |
| C16:1 | 0.29 ± 0.07 | 0.27 ± 0.07 | −0.02 ± 0.08 | 0.118 |
| C17:1 | 0.14 ± 0.08 | 0.15 ± 0.09 | 0.01 ± 0.09 | 0.561 |
| C18:1(n-9) | 8.10 ± 0.94 | 7.47 ± 0.88 | −0.63 ± 1.18 | 0.009 |
| C20:1 | 0.21 ± 0.04 | 0.29 ± 0.15 | 0.08 ± 0.15 | 0.001 |
| C22:1 | 0.01 ± 0.02 | 0.09 ± 0.18 | 0.09 ± 0.18 | 0.002 |
| C24:1 | 3.19 ± 0.66 | 3.88 ± 0.95 | 0.69 ± 0.81 | < 0.001 |
| MUFA subtotal | 11.98 ± 0.93 | 12.18 ± 1.01 | 0.21 ± 0.99 | 0.257 |
| C18:2(n-6) | 19.83 ± 2.34 | 18.26 ± 3.08 | −1.57 ± 2.81 | 0.004 |
| C20:2(n-6) | 0.32 ± 0.04 | 0.29 ± 0.07 | −0.03 ± 0.07 | 0.010 |
| C20:3(n-6) DGLA | 2.33 ± 0.81 | 1.79 ± 0.69 | −0.54 ± 0.57 | < 0.001 |
| C20:4(n-6) AA | 10.74 ± 1.71 | 10.14 ± 1.87 | −0.60 ± 1.26 | 0.003 |
| C22:2(n-6) | 0.69 ± 0.14 | 0.68 ± 0.17 | −0.01 ± 0.19 | 0.823 |
| n-6 PUFA subtotal | 33.92 ± 2.64 | 31.16 ± 2.74 | −2.75 ± 2.99 | < 0.001 |
| C18:3(n-3) | 0.28 ± 0.10 | 0.28 ± 0.13 | 0.00 ± 0.11 | 0.741 |
| C18:4(n-3) | 0.17 ± 0.03 | 0.17 ± 0.03 | 0.00 ± 0.03 | 0.908 |
| C20:4(n-3) | 0.12 ± 0.06 | 0.13 ± 0.07 | 0.01 ± 0.09 | 0.782 |
| C20:5(n-3) EPA | 1.91 ± 1.40 | 3.21 ± 1.83 | 1.30 ± 2.01 | < 0.001 |
| C22:5(n-3) | 0.98 ± 0.17 | 1.03 ± 0.14 | 0.05 ± 0.15 | 0.201 |
| C22:6(n-3) DHA | 6.84 ± 1.61 | 8.37 ± 1.67 | 1.53 ± 1.83 | < 0.001 |
| n-3 PUFA subtotal | 10.30 ± 2.95 | 13.20 ± 3.33 | 2.89 ± 3.58 | < 0.001 |
| PUFA subtotal | 44.22 ± 1.85 | 44.36 ± 1.92 | 0.14 ± 1.54 | 0.574 |
n = 33, Values are expressed as means ± SD. Results are presented as the percentage of total fatty acids.
SFA: saturated fatty acid, MUFA: monounsaturated fatty acid, PUFA: polyunsaturated fatty acid
DGLA: di-homo gamma linolenic acid, AA: arachidonic acid, EPA: icosapentaenoic acid, DHA: docosahexaenoic acid
As to the SFAs, the C18:0 percentage had decreased by the end of the intervention as compared with baseline (p = 0.046), whereas C15:0 (p = 0.044), C17:0 (p = 0.020), and C20:0 (p = 0.003) were increased.
The total MUFA percentage did not change significantly, though cisC18:1(n-9) decreased (p = 0.009), whereas C20:1, C22:1, and C24:1 increased (p > 0.001, p = 0.002, p < 0.001).
As to n-3 PUFA, marked increases in C20:5(n-3) and C22:6(n-3) (p < 0.001, both) resulted in a significant increase in total n-3 PUFA from 10.30% to 13.20% (p < 0.001) along with a total n-6 PUFA decrease from 33.92% to 31.16% (p < 0.001). The n-3 PUFA percentage increased as much as the n-6 PUFA percentage decreased, suggesting n-6 PUFAs to have been replaced by n-3 PUFAs. Almost all n-6 PUFAs, including C18:2(n-6), 20:4(n-6), C20:3(n-6), and C20:2 (n-6) decreased (p = 0.004, p = 0.003, p < 0.001, p = 0.010).
Fatty acid ratios such as C20:5(n-3)/C20:4(n-6), C22:6(n-3)/C20:4(n-6), and (C20:5(n-3)+C22:6(n-3))/C20:4(n-6) increased (p < 0.001). Furthermore, the C20:4(n-6)/C20:3(n-6) ratio, which serves as an estimate of delta-5 desaturase (D5D) activities also increased (p = 0.002) (Table 4).
Table 4. Fatty acid ratios of serum phospholipids at baseline and at the end of the 6 week intervention.
| Baseline | 6 weeks | p | |
|---|---|---|---|
| C20:5(n-3)/C20:4(n-6) | 0.188 ± 0.174 | 0.329 ± 0.210 | < 0.001 |
| C22:6(n-3)/C20:4(n-6) | 0.656 ± 0.211 | 0.854 ± 0.242 | < 0.001 |
| (C20:5(n-3)+C22:6(n-3))/C20:4(n-6) | 0.844 ± 0.372 | 1.183 ± 0.418 | < 0.001 |
| C20:4(n-6)/C20:3(n-6) | 5.149 ± 1.928 | 6.365 ± 2.252 | 0.002 |
n = 33, Values are expressed as means ± SD.
Correlations between Changes in Metabolic Parameters and Fatty Acid Ratios in Serum Phospholipids
Changes in the C20:4(n-6)/C20:3(n-6) ratio correlated negatively with changes in body weight (p < 0.001), BMI (p < 0.001), total cholesterol (p = 0.013), LDL cholesterol (p = 0.012), non-HDL cholesterol (p = 0.002), triglycerides (p = 0.040), and the insulin concentration (p = 0.036). However, other fatty acid ratios showed no significant correlations with metabolic parameters (Table 5).
Table 5. Correlations between changes in metabolic parameters and fatty acid ratios in serum phospholipids.
|
ΔC20:5(n-3)/C20:4(n-6) |
ΔC22:6(n-3)/C20:4(n-6) |
Δ(C20:5(n-3)+C22:6(n-3))/C20:4(n-6) |
ΔC20:4(n-6)/C20:3(n-6) |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| ΔBody weight (kg) | −0.147 | 0.415 | −0.293 | 0.099 | −0.179 | 0.320 | −0.618 | < 0.001 |
| ΔBody Mass Index (kg/m2) | −0.146 | 0.418 | −0.296 | 0.094 | −0.179 | 0.318 | −0.600 | < 0.001 |
| ΔSBP (mmHg) | −0.086 | 0.636 | −0.061 | 0.736 | −0.062 | 0.732 | −0.305 | 0.084 |
| ΔDBP (mmHg) | −0.055 | 0.762 | 0.005 | 0.978 | −0.025 | 0.890 | −0.264 | 0.138 |
| ΔTC (mg/dL) | 0.111 | 0.537 | 0.108 | 0.551 | 0.154 | 0.392 | −0.426 | 0.013 |
| ΔLDL-C (mg/dL) | −0.020 | 0.914 | 0.060 | 0.742 | 0.065 | 0.721 | −0.434 | 0.012 |
| Δnon-HDL-C (mg/dL) | 0.181 | 0.314 | 0.202 | 0.259 | 0.250 | 0.160 | −0.517 | 0.002 |
| ΔHDL-C (mg/dL) | 0.025 | 0.888 | −0.120 | 0.504 | −0.068 | 0.706 | 0.058 | 0.749 |
| ΔTriglyceride (mg/dL) | −0.055 | 0.761 | 0.119 | 0.510 | 0.028 | 0.876 | −0.360 | 0.040 |
| ΔGlucose (mg/dL) | 0.003 | 0.989 | −0.068 | 0.707 | −0.022 | 0.904 | −0.098 | 0.589 |
| ΔInsulin (µU/mL) | 0.037 | 0.836 | 0.037 | 0.836 | 0.044 | 0.807 | −0.367 | 0.036 |
n = 33
r: Spearman correlation coefficients, p: P value with two-sided tests
SBP: systolic blood pressure, DBP: diastolic blood pressure
TC: total cholesterol, LDL-C: LDL-cholesterol, non-HDL-C: non-HDL-cholesterol, HDL-C: HDL-cholesterol
Correlations between Food Intake Volume and Fatty Acid Ratios in Serum Phospholipids
There was a tendency toward a positive association between the number of fish dishes consumed and the C20:4(n-6)/C20:3(n-6) ratio. Fish intake volume showed a positive association with the C20:4(n-6)/C20:3(n-6) ratio in serum phospholipids at the end of the intervention (p < 0.001) (Fig. 2). No associations were observed between intake volumes of other foods and the C20:4(n-6)/C20:3(n-6) ratio.
Fig. 2.
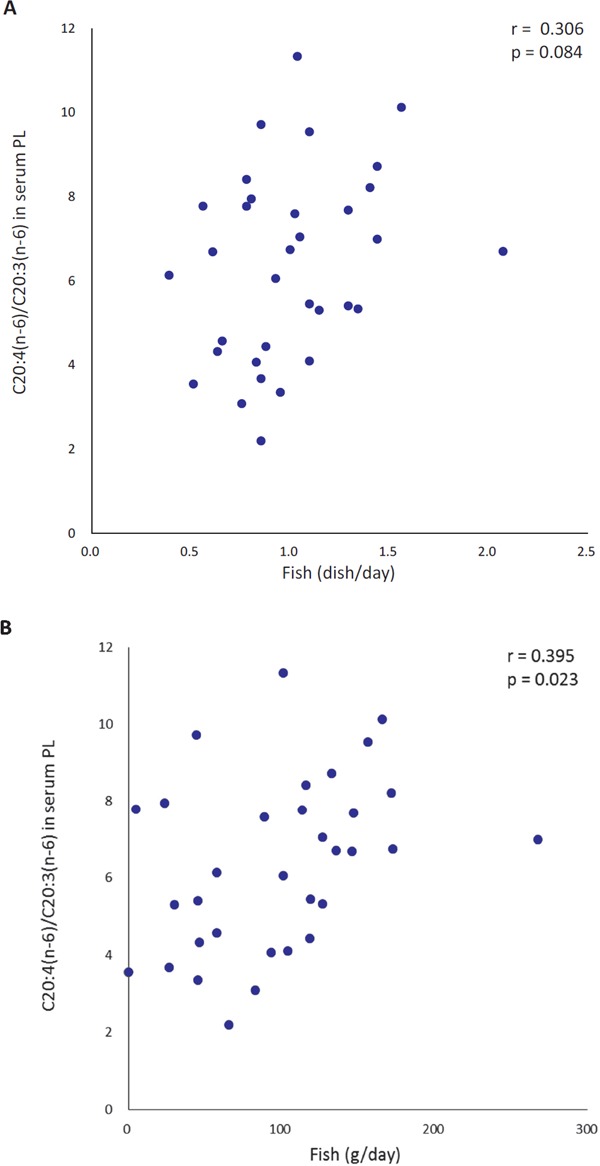
Simple correlations between fish dish intake (A) and fish intake (B) during the 6 week intervention and C20:4(n-6)/C20:3 (n-6) fatty acid ratios in serum phospholipids (PL) at the end of the 6 week intervention
n = 33, PL: phospholipid, PUFA: polyunsaturated fatty acid
Discussion
Our educational intervention recommending the Japan Diet resulted in daily consumption of one dish each on average of fish, soy, and seaweed, as well as increased vegetable intake, and decreased consumptions of meat and poultry, sweets including desserts and snacks, and margarine and shortening4). These changes were attributed to increased intakes of n-3 PUFAs such as C20:5(n-3), C22:6(n-3), C18:4(n-3), and C20:4(n-3) and decreased intakes of all SFAs, as well as unsaturated fatty acids such as C18:1 and C18:2(n-6).
For dietary treatment of dyslipidemia, the fatty acid intake ratio of SFAs (S)/MUFAs (M)/PUFAs (P) was to be 1/1.3/1 according to dietary guidelines8). The Japanese S/M/P ratio in the 1970s was equivalent to this recommended ratio on average9). In this study, the baseline average was 1/1.3/0.7, clearly moving in a worsening direction, approaching the ratio in the UK, which is 1/1/0.510) while showing an improvement to 1/1.4/0.9 with SFA decreasing from 8.8% to 7.8% after the educational intervention. Most notably, the sum of dietary C12:0, C14:0, and C16:0 fatty acids, which are known as hyper-LDL-cholesterolemic fatty acid concentrations1, 11, 12), were reduced by 3882 mg, corresponding to 1.4% of total energy intake, and the LDL cholesterol concentration was significantly decreased as mentioned in our previous report4). Reduced consumptions of meat and poultry, sweets including desserts and snacks, as well as margarine and shortening, were confirmed in our study to effectively limit SFA consumption.
Dietary n-3 PUFA has attracted attention from the perspective of maintaining the physiological balance of active substances13). The dietary n-6/n-3 ratio was 4.9, thus being much higher than the recommended level14) at baseline. The subjects achieved improvement to 3.7 by increasing seafood consumption, as one dish each of fish and seaweed per day. Consuming one fish dish daily could increase n-3 PUFA intake by at least 2.1 g per day, according to the results of this study (Fig. 1), being consistent with the results of a Japanese cohort study, in which subjects who ate fish eight times per week consumed 2.1 g of n-3 PUFA per day. Such high n-3 PUFA consumers were reported to have significantly lower coronary heart disease risk than those with lower fish intakes15).
Dramatic changes in the fatty acid compositions of serum phospholipids were observed after the 6 week intervention. Although C20:5(n-3) and C22:6(n-3) compositions were much higher than those of subjects in other countries at baseline16), these n-3 PUFA compositions were additionally increased. The ratios of C20:5(n-3)/C20:4(n-6) and C22:6(n-3)/C20:4(n-6) were increased as C20:4(n-6) decreased.
Decreased serum C20:5(n-3)/C20:4(n-6) and C22:6(n-3)/C20:4(n-6) ratios have been demonstrated to be risk factors for early onset of acute coronary syndrome17, 18). It is noteworthy that despite decreases in both C20:4(n-6) and C20:3(n-6) in phospholipids, the C20:4(n-6)/C20:3(n-6) ratio, which serves as an estimate of D5D activities, was increased in this study. There was a positive correlation between fish intake and the C20:4(n-6)/C20:3(n-6) ratio. As D5D converts not only C20:3(n-6) to C20:4(n-6) but also C20:4(n-3) to C20:5(n-3), increased C20:5(n-3) from fish may reduce the competitive inhibitory effect on C20:3(n-6) desaturation. Furthermore, changes in the C20:4(n-6)/C20:3(n-6) ratio showed significant correlations with changes in body weight, serum insulin, and lipid concentrations, though no correlations were observed between the change in the C20:5(n-3)/C20:4(n-6) ratio and any of the metabolic markers in this study. Our results suggest the C20:4(n-6)/C20:3(n-6) ratio in phospholipids to be a somewhat more effective index than the C20:5(n-3)/C20:4(n-6) or the C22:6(n-3)/C20:4 (n-6) ratios in a dynamic nutritional assessment of atherosclerotic risk.
Maruyama et al. reported that estimated D5D in patients with metabolic syndrome was low as compared with that in healthy Japanese subjects19). A defect in D5D is regarded as being a factor in the initiation and progression of insulin resistance, that is, the metabolic syndrome19–21). In Japanese people, a higher serum concentration of C20:3(n-6) and lower estimated D5D activity are reportedly associated with an elevated risk of type 2 diabetes independently of BMI according to a nested case–control study22). In a population-based prospective study, low estimated phospholipid D5D activity was found to be associated with the increased mortality of cardiovascular disease23, 24).
PUFAs exhibit a range of biological effects, many of which are mediated through the formation and actions of lipid mediators such as prostaglandins, leukotrienes, lipoxins, resolvins, and protectins25, 26). Supplementation with fish oil results in decreased production of PGE2, TXB2, LTB4, 5-HETE, and LTE4 by inflammatory cells27) and marked increase in the abundance of the E-series resolvin precursor, 18-HEPE28). Further study is needed on the production of lipid mediators in response to Japan Diet intake.
In SFAs, the C18:0 percentage was significantly decreased, whereas those of C15:0 and C17:0 were increased. Total SFA did not change in phospholipids, because glycerophospholipids are the major components of serum phospholipids and SFAs attach to carbon number 1, whereas MUFA and PUFA can attach to carbon number 229). For C18:0 in phospholipids, consistent results have not been obtained. One study showed higher concentrations of C18:0 to be associated with a lower risk of total mortality30), whereas another study reported the proportions of C18:0 to not be associated with any mortality risk23). Thus, further studies are needed.
The odd-chain fatty acids C15:0 and C17:0, which account for only a small proportion of total SFA in milk fat and ruminant meat, are accepted biomarkers of dairy fat intake31, 32). On the other hand, some studies report that they can also be synthesized endogenously, for example, from gut-derived C3:032). To date, it has been reported that increases in C15:0 and C17:0 in serum phospholipids are anti-atherosclerotic; for example, C15:0 and C17:0 concentrations were low in patients with metabolic syndrome19, 33, 34). In this intervention study, although intake volumes of dairy products were unchanged and meat and poultry consumptions were decreased4), C15:0 and C17:0 were increased. These results might be caused by the significant decrease in BMI and improvement of insulin resistance. However, the mechanisms underlying these results have not been elucidated, so further studies are needed.
Therefore, this 6 week nutrition education intervention, recommending the Japan Diet, improved fatty acid intakes and fatty acid profiles in phospholipids.
This study has limitations. First, as the study design was single arm, the actual effects on changes in the parameters assessed could not be demonstrated. Second, our subjects were all men in their 30s to 40s not receiving any form of disease treatment. It is necessary to examine effects according to age and gender, as well as to investigate patients with risk factors for atherosclerosis including dyslipidemia. Most other studies used whole blood containing triglycerides to measure circulating fatty acid compositions, but such data reflect the most recent fatty acid intake. Because this study examined fatty acid composition changes in phospholipids reflecting habitual dietary intakes, we cannot simply compare our results with those of studies that examined fatty acid composition changes in whole blood. We did not directly examine vascular function or perform blood vessel imaging studies that depict atherosclerotic change. A potential weakness is the use of fatty acid product: precursor ratio to estimate desaturase activities indirectly, instead of directly measuring the desaturase activity. The use of a product: precursor ratio as a substitute measure is, however, well established, and desaturase activities are commonly estimated from phospholipid fatty acids. The utility of D5D as a comprehensive indicator of the effects of arteriosclerosis prevention and treatment requires detailed evaluation in future studies.
Conclusion
With the nutrition education recommendation to consume the Japan Diet, intakes of meat and poultry and sweets including desserts and snacks decreased, whereas fish, soybean, and seaweed intakes increased. Dietary n-3 PUFA intake increased, but SFA and MUFA decreased and blood phospholipid fatty acid compositions changed to a more anti-atherosclerotic profile. The Japan Diet is effective for rapidly improving fatty acid compositions.
Acknowledgment
We are grateful to Dr. Hiromasa Shijo and Dr. Ayako Maruyama for their help with blood collection.
COI
Chizuko Maruyama received a grant from PPD Japan.
References
- 1). Kris-Etherton PM, Yu S: Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr 1997; 65: 1628-1644 [DOI] [PubMed] [Google Scholar]
- 2). Keys A, Mientotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, Kromhout D, Nedeljkovic S, Punsar S, Seccareccia F, Toshima H: The diet and 15-year death rate in the Seven Countries Study. Am J Epidemiol 1986; 124: 903-915 [DOI] [PubMed] [Google Scholar]
- 3). Ministry of Health, Labor and Welfare: The national Health and Nutrition Survey in Japan, 2015. http://www.mhlw.go.jp/bunya/kenkou/eiyou/h27-houkoku.html
- 4). Maruyama C, Nakano R, Shima M, Mae A, Shijo Y, Nakamura E, Okabe Y, Park S, Kameyama N, Hirai S, Nakanishi M, Uchida K, Nishiyama H: Effects of a Japan diet intake program on metabolic parameters in middle-aged men. J Atheroscler Thromb 2017; 24: 393-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K: Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke 2008; 39: 2052-2058 [DOI] [PubMed] [Google Scholar]
- 6). Folch J, Lees M, Stanley GHS: A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497-509 [PubMed] [Google Scholar]
- 7). Maruyama C, Kijimoto R, Ito K, Doi K, Imamura M, Nakamori T, Fukushima T, Maruyama T, Tsushima M: Serum fatty acid composition in hyperlipidemic females. J Clin Biochem Nutr 1993; 15: 143-153 [Google Scholar]
- 8). Krauss RM, Deckelbaum RJ, Ernst N, Fisher E, Howard BV, Knopp RH, Kotchen T, Lichtenstein AH, McGill HC, Pearson TA, Prewitt TE, Stone NJ, Horn LV, Weinberg R: Dietary Guidelines for Healthy American Adults. A Statement for Health Professionals From the Nutrition Committee, American Heart Association. Circulation 1996; 94: 1795-1800 [DOI] [PubMed] [Google Scholar]
- 9). Wen CP, Gershoff SN: Changes in serum cholesterol and coronary heart disease mortality associated with changes in the postwar Japanese diet. Am Journal Clin Nutr 1973; 26: 616-619 [DOI] [PubMed] [Google Scholar]
- 10). Food Standards Agency: The National Diet and Nutrition Survey in the UK, 2008–2009. http://webarchive.nationalarchives.gov.uk/20101209182500/http://www.food.gov.uk/science/dietarysurveys/ndnsdocuments/ndns0809year1
- 11). Mensink RP, Zock PL, Kester ADM, Katan MB: Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003; 7777: 1146-1155 [DOI] [PubMed] [Google Scholar]
- 12). Hegsted DM, McGandy RB, Myers ML, Stare FJ: Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr 1965; 17: 281-295 [DOI] [PubMed] [Google Scholar]
- 13). Endo J, Arita M: Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J Cardiol 2016; 67: 22-27 [DOI] [PubMed] [Google Scholar]
- 14). Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM: N-3 Fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr 2006; 83: 1526-1535 [DOI] [PubMed] [Google Scholar]
- 15). Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane Y: Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) study cohort?. Circulation 2006; 113: 195-202 [DOI] [PubMed] [Google Scholar]
- 16). Laidlaw M, Holub BJ: Effects of supplementation with fish oil-derived n-3 fatty acids and γ-linolenic acid on circulating plasma lipids and fatty acid profiles in women. Am J Clin Nutr 2003; 77: 37-42 [DOI] [PubMed] [Google Scholar]
- 17). Takahashi M, Ando J, Shimada K, Nishizaki Y, Tani S, Ogawa T, Yamamoto M, Nagao K, Hirayama A, Yoshimura M, Daida H, Nagai R, Komuro I: The ratio of serum n-3 to n-6 polyunsaturated fatty acids is associated with diabetes mellitus in patients with prior myocardial infarction: a multicenter cross-sectional study. BMC Cardiovasc Disord 2017; 17: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Yagi S, Aihara K, Fukuda D, Takashima A, Bando M, Hara T, Nishimoto S, Ise T, Kusunose T, Yamaguchi K, Tobiume T, Iwase T, Yamada H, Soeki T, Wakatsuki T, Shimabukuro M, Akaike M, Sata M: Reduced ratio of eicosapentaenoic acid and docosahexaenoic acid to arachidonic acid is associated with early onset of acute coronary syndrome. Nutr J 2015; 14: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Maruyama C, Yoneyama M, Suyama N, Kasuya Y, Teramoto A, Sakaki Y, Suto Y, Takahashi K, Araki R, Ishizaka Y, Yamakado M, Teramoto T: Differences in serum phospholipid fatty acid compositions and estimated desaturase activities between Japanese men with and without metabolic syndrome. J Atheroscler Thromb 2008; 15: 306-313 [DOI] [PubMed] [Google Scholar]
- 20). Lee S, Do HJ, Kang SM, Chung JH, Park E, Shin MJ: Plasma phospholipid fatty acid composition and estimated desaturase activity in heart failure patients with metabolic syndrome. J Clin Biochem Nutr 2012; 51: 150-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Das UN: A defect in Δ6 and Δ5 desaturases may be a factor in the initiation and progression of insulin resistance, the metabolic syndrome and ischemic heart disease in South Asians. Lipids Health Dis 2010; 9: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Akter S, Kurotani K, Sato M, Hayashi T, Kuwahara K, Matsushita Y, Nakagawa T, Konishi M, Honda T, Yamamoto S, Hayashi T, Noda M, Mizoue T: High serum phospholipid dihomo-γ-linoleic acid concentration and low Δ5-desaturase activity are associated with increased risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. J Nutr 2017; 147: 1558-1566 [DOI] [PubMed] [Google Scholar]
- 23). Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U: Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. Am J Clin Nutr 2008; 88: 203-209 [DOI] [PubMed] [Google Scholar]
- 24). Wang L, Folsom AR, Eckfeldt JH: Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr Metab Cardiovasc Dis 2003; 13: 256-266 [DOI] [PubMed] [Google Scholar]
- 25). Stables MJ, Gilroy DW: Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res 2011; 50: 35-51 [DOI] [PubMed] [Google Scholar]
- 26). Arita M: Mediator lipidomics in acute inflammation and resolution. J Biochem 2012; 152: 313-319 [DOI] [PubMed] [Google Scholar]
- 27). Baukje de R, Yiannis M, Ingeborg AB: Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol 2009; 158: 413-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Markworth JF, Kaur G, Miller EG, Larsen AE, Sinclair AJ, Maddipati KR, Cameron-Smith D: Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J 2016; 30: 3714-3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Martin DW, Mayes PA, Rodwell VW, Grodsky GM, Nestle M: Lipids. In: Harper's review of biochemistry 18th Ed, ed by Maruzen Asia (Pte), Ltd., pp 189-190, Lange Medical Publications, Singapore, 1981 [Google Scholar]
- 30). Fretts AM, Mozaffarian D, Siscovick DS, King IB, McKnight B, Psaty BM, Rimm EB, Sitlani C, Sacks FM, Song X, Sotoodehnia N, Spiegelman D, Lemaitre RN: Associations of plasma phospholipid SFAs with total and cause-specific mortality in older adults differ according to SFA chain length. J Nutr 2016; 146: 298-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Smedman AEM, Gustafsson I, Berglund LGT, Vessby BOH: Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am J Clin Nutr 1999; 69: 22-29 [DOI] [PubMed] [Google Scholar]
- 32). Pfeuffer M, Jaudszus A: Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv Nutr 2016; 7: 730-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Warensjö E, Jansson JH, Cederholm T, Boman K, Eliasson M, Hallmans G, Johansson I, Sjögren P: Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr 2010; 92: 194-202 [DOI] [PubMed] [Google Scholar]
- 34). Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW, van Woudenbergh GJ, Wang L, Summerhill K, Griffin JL, Feskens EJ, Amiano P, Boeing H, Clavel-Chapelon F, Dartois L, Fagherazzi G, Franks PW, Gonzalez C, Jakobsen MU, Kaaks R, Key TJ, Khaw KT, Kühn T, Mattiello A, Nilsson PM, Overvad K, Pala V, Palli D, Quirós JR, Rolandsson O, Roswall N, Sacerdote C, Sánchez MJ, Slimani N, Spijkerman AM, Tjonneland A, Tormo MJ, Tumino R, van der A DL, van der Schouw YT: Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014; 2: 810-818 [DOI] [PMC free article] [PubMed] [Google Scholar]


