APOE4 is a genetic risk factor for ageing-related cognitive decline and Alzheimer's disease. Using human and mouse tissue, Peng et al. report that APOE4 reduces brain exosome production and release. Exosome pathway dysfunction may be the initial event in endosomal-lysosomal-exosomal alterations contributing to neuronal vulnerability and neurodegenerative risk in APOE4-carriers.
Keywords: APOE4, Alzheimer's disease, exosomes, endosomes, extracellular vesicles
Abstract
In addition to being the greatest genetic risk factor for Alzheimer's disease, expression of the ɛ4 allele of apolipoprotein E can lead to cognitive decline during ageing that is independent of Alzheimer's amyloid-β and tau pathology. In human post-mortem tissue and mouse models humanized for apolipoprotein E, we examined the impact of apolipoprotein E4 expression on brain exosomes, vesicles that are produced within and secreted from late-endocytic multivesicular bodies. Compared to humans or mice homozygous for the risk-neutral ɛ3 allele we show that the ɛ4 allele, whether homozygous or heterozygous with an ɛ3 allele, drives lower exosome levels in the brain extracellular space. In mice, we show that the apolipoprotein E4-driven change in brain exosome levels is age-dependent: while not present at age 6 months, it is detectable at 12 months of age. Expression levels of the exosome pathway regulators tumor susceptibility gene 101 (TSG101) and Ras-related protein Rab35 (RAB35) were found to be reduced in the brain at the protein and mRNA levels, arguing that apolipoprotein E4 genotype leads to a downregulation of exosome biosynthesis and release. Compromised exosome production is likely to have adverse effects, including diminishing a cell's ability to eliminate materials from the endosomal-lysosomal system. This reduction in brain exosome levels in 12-month-old apolipoprotein E4 mice occurs earlier than our previously reported brain endosomal pathway changes, arguing that an apolipoprotein E4-driven failure in exosome production plays a primary role in endosomal and lysosomal deficits that occur in apolipoprotein E4 mouse and human brains. Disruption of these interdependent endosomal-exosomal-lysosomal systems in apolipoprotein E4-expressing individuals may contribute to amyloidogenic amyloid-β precursor protein processing, compromise trophic signalling and synaptic function, and interfere with a neuron's ability to degrade material, all of which are events that lead to neuronal vulnerability and higher risk of Alzheimer's disease development. Together, these data suggest that exosome pathway dysfunction is a previously unappreciated component of the brain pathologies that occur as a result of apolipoprotein E4 expression.
Introduction
Apolipoprotein E-mediated transport of cholesterol from astrocytes to neurons is crucial for the maintenance of synaptic connections and neuronal membranes (Pfrieger, 2003; Liu et al., 2013). There are three major alleles of the apolipoprotein E (APOE) gene that codes for apolipoprotein E in humans—ɛ2, ɛ3, and ɛ4 (APOE2, APOE3, and APOE4, respectively)—with an approximate worldwide distribution of 7%, 79%, and 14%, respectively (Bertram et al., 2010). Expression of APOE4 has profound consequences within the brain, including greatly increased Alzheimer's disease risk as well as neurological effects that are independent of the hallmark Alzheimer's disease pathologies of amyloid-β plaques or neurofibrillary tangles (Liu et al., 2013; Shi et al., 2014). In the absence of a dementia diagnosis, human APOE4 carriers may still display cognitive decline and olfactory identification impairments compared to age-matched non-carriers (Olofsson et al., 2010; Wisdom et al., 2011). Brain imaging studies also reveal APOE4-driven structural and functional differences within regions of the hippocampus and cortex at ages before APOE4-mediated Alzheimer's disease would be expected (Bookheimer and Burggren, 2009; Filippini et al., 2009; Sheline et al., 2010; Di Battista et al., 2016), with some studies ruling out amyloid-β burden as a contributing factor (Sheline et al., 2010). The idea that APOE4 expression has deleterious effects on the brain outside of its impact on hallmark Alzheimer's disease pathologies is further supported by studies using humanized APOE mouse models that do not develop amyloid-β plaques or neurofibrillary tangles (Sullivan et al., 1997; Tai et al., 2011). APOE4 mice show memory impairments in the Morris water maze (Andrews-Zwilling et al., 2010), which have been suggested to result from regional cell loss (Tong et al., 2016). Moreover, we have recently linked olfactory memory impairments in APOE4 mice to network abnormalities in olfactory brain regions (Peng et al., 2017). While the mechanisms by which APOE4 genotype compromises neuronal viability and function are likely to be multifactorial, recent studies show that a main mechanism may be the APOE4-driven dysfunction of the neuronal endosomal-lysosomal system (Nuriel et al., 2017; Zhao et al., 2017).
Apolipoprotein E-transported cholesterol is made available to neurons upon release into early endosomes after receptor-mediated internalization, where the cholesterol is then delivered to late endosomes/multivesicular bodies (Ikonen, 2008). Within multivesicular bodies, intraluminal vesicles are generated in a process that is mediated by the endosomal sorting complexes required for transport (ESCRT) machinery, which includes the tumor susceptibility 101 (TSG101) gene subunit and its associated protein apoptosis-linked gene 2-interacting protein X (ALIX; encoded by PDCD6IP) (Raiborg and Stenmark, 2009). Intraluminal vesicles are either degraded in lysosomes or released to the extracellular space as exosomes, which requires the function of small Rab-GTPases such as Rab35 for the fusion of intraluminal vesicle-containing multivesicular bodies with the cell plasma membrane (Hsu et al., 2010; Ostrowski et al., 2010). Exosomes play diverse physiological roles in the brain, contributing to both synaptic plasticity and neuronal survival (Rajendran et al., 2014; Levy, 2017). In addition to the beneficial cell-to-cell delivery of RNA, proteins, and lipids (Rajendran et al., 2014; Levy, 2017), exosomes may also contribute to the cell-to-cell and cell-to-extracellular space spread of pathogenic proteins (Levy, 2017). Importantly, exosome secretion offers a mechanism, in addition to lysosomal degradation, for the removal of intracellular materials from the endosomal system (Levy, 2017). Given that APOE4 genotype is linked to neuronal endosome morphological alterations in human Alzheimer's disease brains (Cataldo et al., 2000), that APOE4 genotype in mouse models is sufficient to alter early endosomes and lysosomes (Nuriel et al., 2017; Zhao et al., 2017), and that cholesterol has been implicated in intraluminal vesicle biogenesis (Strauss et al., 2010), we sought to determine whether exosome production from the endosomal pathway is disrupted by APOE4.
We demonstrate APOE4-driven decreases in exosome levels in the post-mortem brains of neuropathologically healthy humans as well as in an age-dependent manner in APOE mice. We further reveal differences in the levels of key exosomal-pathway regulatory proteins as well as of brain-exosome lipids, demonstrating that APOE4 compromises brain exosome production in a manner that may be linked to APOE4-mediated changes in cholesterol levels. Alongside recent reports from our group (Nuriel et al., 2017) and others (Zhao et al., 2017), the present findings demonstrate that APOE4 genotype disrupts exosome production in the context of broad neuronal endosomal-lysosomal pathway alterations. We propose that exosomal pathway dysfunction is an integral component of APOE4-driven endosomal-lysosomal pathway alterations that are likely to contribute to ageing-dependent neuron vulnerability, cognitive impairment, and Alzheimer's disease risk.
Materials and methods
Animals
All experimental procedures involving animals were approved by and complied with the guidelines of the Institutional Animal Care and Use Committee of the Nathan S. Kline Institute. The mice used in this study were from our longstanding colonies. APOE targeted-replacement mice were originally developed on a C57Bl/6 background to express human APOE3 or APOE4 genes, in place of the mouse Apoe gene, under the control of the endogenous murine promoter (Sullivan et al., 1997). Mice homozygous for either the human APOE3 or the human APOE4 genes are referred to in the text as APOE3 and APOE4 mice, respectively. Heterozygous mice expressing one copy of human APOE3 and one copy of human APOE4 are referred to as APOE3/E4 mice. Mice were group housed under controlled temperature and lighting conditions with same-sex littermates and given free access to food and water. Mice were investigated at 6, 12, and 18 months of age as indicated. Approximately equal cohorts of males and females were examined; analysis of data by sex in this study did not reveal significant differences, and the results were pooled. Genotypes were confirmed by restriction fragment length polymorphism (RFLP) analysis following PCR, as previously described (Hixson and Vernier, 1990).
Human brain tissue preparation
Formalin-fixed and corresponding blocks of frozen (−80°C) post-mortem brain tissues from Brodmann area 9/10 of healthy controls with no neuropathological diagnoses were kindly provided by the Emory Alzheimer's Disease Research Center of the Center for Neurodegenerative Disease brain and tissue bank, under the direction of Dr Marla Gearing (see Table 1 for genotype, age at death, post-mortem interval, and diagnosis). Genotypes were confirmed by RFLP analysis following PCR, as described previously (Hixson and Vernier, 1990; Gearing et al., 1995). To confirm that amyloid-β was not present in the cases and region examined, two paraffin sections from each fixed block of brain tissue were stained with 1% thioflavin-S (Millipore Sigma) in parallel with sections from a patient with Alzheimer's disease as a positive control. On average >10 thioflavin-S positive plaques sized at ∼30 μm in diameter were detected in the positive Alzheimer's disease control in a 1 mm field of view. In contrast, no plaques were detected in any brain tissues used in this study.
Table 1.
APOE3, APOE3/E4 and APOE4 human brain samples used in this study
| APOE genotype | Age at death, y | Post-mortem interval (h) | Race/sex | Braak Stage | Neuropathological diagnosis | |
|---|---|---|---|---|---|---|
| Primary | Secondary | |||||
| E3/3 | 74 | 7 | C/f | II | Control | - |
| E3/3 | 46 | 6.5 | C/f | 0 | Control | - |
| E3/3 | 65 | 6 | C/f | II | Control | - |
| E3/3 | 57 | 10 | C/m | II | Control | - |
| E3/3 | 75 | 17 | C/m | I | Control | Coronary artery disease |
| E3/3 | 65 | 19 | C/f | 0 | Control | Metastatic ovarian cancer |
| E3/3 | 98 | 15 | C/m | I | Control | - |
| E3/4 | 90 | 6 | C/m | II | Control | Congestive heart failure |
| E3/4 | 52 | 3 | C/f | 0 | Control | Diffuse plaques |
| E3/4 | 60 | 8 | AA/f | I | Control | - |
| E4/4 | 64 | 17 | C/f | II | Control | Cerebral amyloid angiopathy |
| E4/4 | 53 | 6.5 | AA/m | II | Control | - |
Race: AA = African American; C = Caucasian; sex: m = male; f = female.
Extracellular vesicles isolation from the brain extracellular space
Extracellular vesicles from frozen human tissue as well as from frozen murine right hemibrains (without the cerebellum and olfactory bulbs) were isolated by serial centrifugation and purified on a sucrose gradient, as we have described previously in detail (Perez-Gonzalez et al., 2012, 2017). Human extracellular vesicles were simultaneously isolated from the brain of an APOE4 carrier and an age-matched APOE3 homozygous individual. Mouse extracellular vesicles were isolated simultaneously from APOE3, APOE4 and APOE3/E4 brain tissue in aged-matched cohorts. Briefly, minced tissue was treated with papain (20 units/ml; Worthington) in 3.5 ml Hibernate A (BrainBits) at 37°C for 15 min. The enzymatic papain reaction was stopped with 6.5 ml of cold Hibernate A supplemented with protease inhibitors. Samples were serially spun at 300g at 4°C for 10 min, then 2000g for 10 min, and finally 10 000g for 30 min to pellet and discard cell debris. Extracellular vesicles were pelleted by spinning the resulting supernatants at 100 000g for 1 h. Extracellular vesicle pellets were resuspended in phosphate-buffered saline (PBS) supplemented with protease inhibitors and purified through step-wise sucrose gradients, ranging from 2 M to 0.25 M sucrose, that were spun for 16 h at 4°C at 200 000g. Seven fractions (corresponding to fractions a–g in the text) were collected, starting from the top of the sucrose gradient. Extracellular vesicles were pelleted from these fractions by spinning at 100 000g for 1 h at 4°C after dilution in PBS. Pellets were resuspended in cold PBS supplemented with protease inhibitors and lysed with RIPA buffer supplemented with protease inhibitors prior to protein and western blot analyses (Perez-Gonzalez et al., 2012, 2017). Protein levels of each extracellular vesicle-containing sucrose fraction were quantified using a BCA (bicinchoninic acid) Protein Assay Kit (Thermo Fisher Scientific). Extracellular vesicle protein levels were normalized to the weight of the brain tissue from which the extracellular vesicles were isolated.
Electron microscopy
Isolated extracellular vesicles were diluted 1/10 in 2% paraformaldehyde in 0.1 M sodium cacodylate buffer. Formvar covered carbon-coated copper 200-mesh grids were glow discharged in a Pelco easiGlow™. The extracellular vesicle-containing solution (3 μl) was added to a grid, held by self-clamping tweezers, and left for 20 s. The solution was then wicked off with Whatman™ filter paper and negatively stained using 5 μl of 1% uranyl acetate solution before being immediately wicked off using filter paper. This process was repeated three times before a final incubation of 5 min. The excess solution was wicked off and the grid allowed to air dry. Imaging took place on a Thermo Fisher Talos L120C transmission electron microscope operating at 120 kV.
Lipid analysis
Extracellular vesicle lipid analysis was done using fresh hemibrain tissue, which was otherwise prepared identically to frozen brain tissue. Extracellular vesicle-containing fractions and brain homogenates were lyophilized and resuspended in methyl tert-butyl ether/methanol (1:1). Samples were centrifuged at 500g for 5 min and the lipid-containing supernatant extracted. The resulting delipidated extracellular vesicle pellets were solubilized in RIPA buffer and set aside for western blot analysis. Additional methyl tert-butyl ether and water were added to the supernatant to make a mixture of methanol, methyl tert-butyl ether, and water (1:3:1) that was partitioned into water and organic phases (Matyash et al., 2008). Gangliosides in the water phase were separated by high-performance thin-layer chromatography (HPTLC) using a solvent system, chloroform/methanol/0.2% CaCl2 (60:40:9), stained with an orcinol reagent, and the amount of each ganglioside was determined as described (Saito et al., 2015). The upper organic methyl tert-butyl ether phase was evaporated to dryness and separated into neutral and acidic lipids using DEAE Sephadex® columns (Macala et al., 1983). The neutral lipid fraction containing ceramide and cholesterol were applied on HPTLC plates and developed first with hexane/ethyl acetate/water/acetic acid (3:4:5:2) until the solvent front ascended to 4 cm from origin, and second with hexane/methyl tert-butyl ether/acetic acid (98:2:1) until 9 cm above origin. The plate was then stained with 0.0001% primuline (Millipore Sigma), the fluorescent lipid bands were scanned with the Gel Logic molecular imaging system, and the amounts of cholesterol and ceramide were determined as described previously (Saito et al., 2015).
Western blot analysis
Western blot analysis of mouse tissue was performed on extracellular vesicle fractions from the right hemibrain and parallel homogenates prepared from the left hemibrain. Human tissue western blot analysis was performed on extracellular vesicle fractions and brain homogenates that were prepared from adjacent frozen blocks of tissue. There were no statistical differences in protein band intensities between delipidated samples and untreated samples in any of our analyses, and the data were pooled. Proteins were separated by electrophoresis with Criterion™ 4–20% Tris-HCl gel electrophoresis gels (Bio-Rad) and transferred onto PVDF (polyvinylidene difluoride) membranes (Millipore Sigma). Membranes were incubated with antibodies against the exosomal markers ALIX (1:500, Millipore Sigma #ABC40), TSG101 (1:350, GeneTex Inc #GTX70255), Rab35 (1:1000, Cell Signaling #9690S), or β-actin (1:2500, Abcam #ab8226) overnight, and horseradish peroxidase-coupled secondary antibodies for 1 h. The membranes were incubated in chemiluminescent fluid (Pierce) for 5 min, and chemiluminescence was visualized on reflection autoradiography film. Protein band density was quantified using ImageJ (NIH, Bethesda, MD) and normalized to brain tissue weight.
Quantification of mRNA levels
Quantitative PCR was performed on left hemibrains of APOE mice with RNA purified using the miRNeasy mini kit (Qiagen). Subsequent validation was done for quality and quantity utilizing RNA 6000 Nano (Agilent). RNA was reverse transcribed using random hexamers as described previously (Ginsberg et al., 2012). TaqMan™ qPCR primers (Life Technologies) were used with samples assayed on a real-time qPCR cycler (7900HT, Life Technologies) in 96-well optical plates with coverfilm as described previously (Ginsberg et al., 2012). Standard curves and cycle threshold were generated using standards obtained from total mouse brain RNA. The ddCT method was used to determine relative gene level differences between APOE3 and APOE4 mice (Alldred et al., 2008). Succinate dehydrogenase complex flavoprotein subunit A (Sdha, Mm01352360_m1) qPCR product was used as a control housekeeping gene. Negative controls consisted of the reaction mixture without input RNA and reaction mixture without SuperScript® III enzyme. APOE3 and APOE4 were compared with respect to the PCR product synthesis of Tsg101 (Mm00649956_mH), Pdcd6ip (Mm00478041_m1) and Rab35 (Mm01204416_m1) genes.
Statistical analysis
Western blots were quantified using NIH ImageJ (http://rsb.info.nih.gov). Extracellular vesicle data from mice were analysed using a repeated measures 2 × 3 ANOVA (genotype × age). Two-tailed t-tests assuming equal variance were used to make post hoc pair-wise genotype comparisons. Throughout, results are expressed as the mean ± standard error of the mean (SEM). Statistically significant findings are indicated as shown: *P < 0.05, **P < 0.01, and ***P < 0.001. Dots indicate individual data points. The value of n represents the number of animals and is given in the main text. For qPCR, the product synthesis for each gene was modelled as a function of mouse genotype, using mixed effects models with random mouse effect to account for the correlation between repeated assays on the same mouse. The product synthesis of Tsg101, Pdcd6ip, and Rab35 were normalized to the qPCR product of the housekeeping gene Sdha.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Results
Apolipoprotein E4 expression leads to lower brain exosome levels
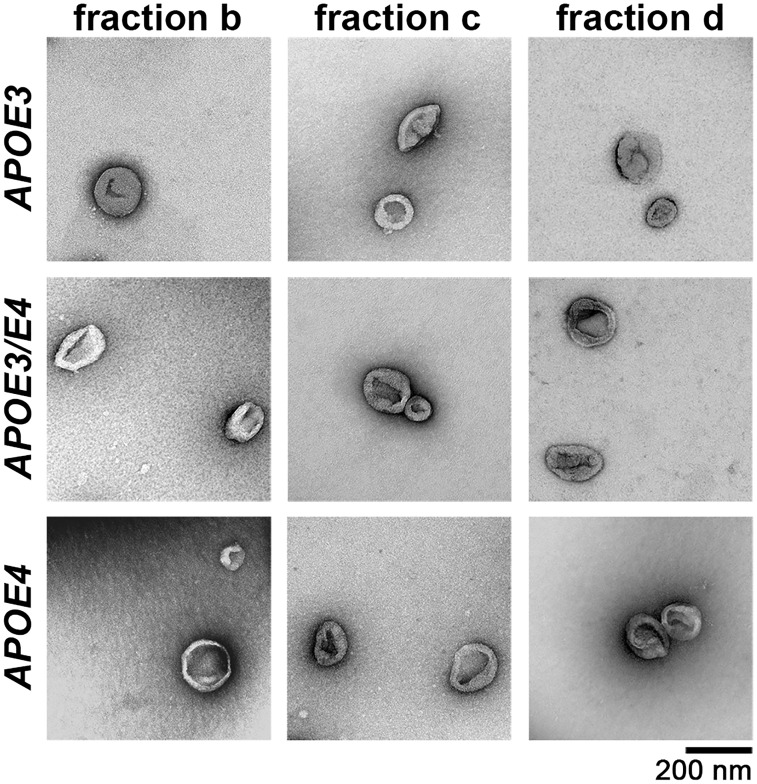
To determine the effect of APOE4 genotype on exosomes, we isolated extracellular vesicles from brains using serial centrifugations and sucrose gradients as described previously (Perez-Gonzalez et al., 2012, 2017). Electron microscopy was used to confirm that extracellular vesicles were only present at sucrose gradient densities ranging from 1.09 to 1.17 g/ml (fractions b–d) and that the isolated extracellular vesicles were consistent in size and morphology with exosomes (Fig. 1) (Perez-Gonzalez et al., 2012). There were no noticeable differences in extracellular vesicle size or morphology by electron microscopy when comparing the extracellular vesicle-containing fractions (fractions b–d) or when comparing extracellular vesicles isolated from APOE3 homozygous, APOE3/E4 heterozygous, and APOE4 homozygous mice (Fig. 1).
Figure 1.
Electron microscopy shows appropriately sized and shaped extracellular vesicles isolated from the brains of APOE mice. Wide field electron microscopy imaging shows extracellular vesicles isolated from the brains of 12-month-old APOE3, APOE3/E4, and APOE4 mice. No obvious differences were observed in size or morphology among the genotypes and among the three extracellular vesicle-containing fractions b–d.
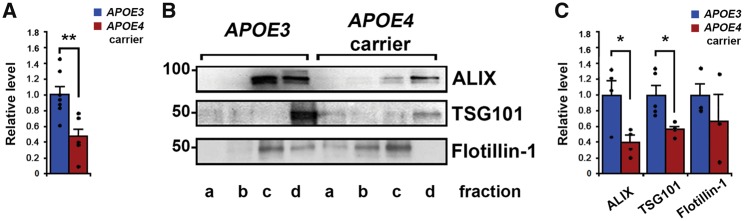
Human brain tissue for extracellular vesicle isolation was from individuals with ages that ranged from 46 to 98 years of age (Table 1), with a mean age of 68.6 ± 6.18 years for APOE3 homozygous individuals and 63.8 ± 6.92 years for APOE4 carriers. Frontal cortices of APOE4 carriers (either APOE4 homozygous or APOE3/E4 heterozygous) contained significantly lower levels of extracellular vesicles than those of non-carriers (APOE3 homozygous) as determined by total extracellular vesicle protein levels pooled from fractions b–d normalized to brain tissue weight [46.9 ± 9.0% of APOE3 individuals; n = 7 APOE3, n = 5 APOE4 carriers, t(10) = 3.26, P = 0.009; Fig. 2A]. Levels of flotillin-1, a lipid-raft protein present in all extracellular vesicle subtypes (Kowal et al., 2016), were not significantly different in extracellular vesicles isolated from APOE4 carriers compared with APOE3 brains [n = 3 for both genotypes, t(4) = 0.85, P = 0.45; Fig. 2B and C]. While brain extracellular vesicles isolated by our protocol are primarily exosomes, other extracellular vesicles such as microvesicles may co-purify (Cocucci and Meldolesi, 2015). We therefore examined levels of the proteins ALIX and TSG101, exosome-specific ESCRT components that are commonly used as exosome markers, in our extracellular vesicle fractions to determine if APOE4 expression specifically affects exosome levels (Fig. 2B and C). Lower levels in fractions b–d normalized to brain tissue weight were revealed for both ALIX [40.02 ± 12.2% of APOE3 individuals; n = 4, t(6) = 2.99, P = 0.02] and TSG101 [52.8 ± 3.6% of APOE3 individuals; n = 4, t(7) = 2.98, P = 0.02] in APOE4 carriers compared with APOE3 by western blot analyses (Fig. 2B and C). Thus, we show that exosome levels are reduced in APOE4-expressing human brains.
Figure 2.
Brain exosome levels are lower in human APOE4-carriers compared with homozygous APOE3 individuals. (A) Extracellular vesicle protein levels from human brains normalized to brain tissue weight. (B) A representative western blot showing ALIX and TSG101 (exosomal proteins), and flotillin-1 (a lipid-raft enriched protein) in the extracellular vesicles. (C) Quantification of band intensities normalized to brain tissue weight is shown for levels of ALIX, TSG101, and flotillin-1. Data are expressed as the mean ± SEM of the ratios of APOE4 carriers to APOE3. *P < 0.05, **P < 0.01.
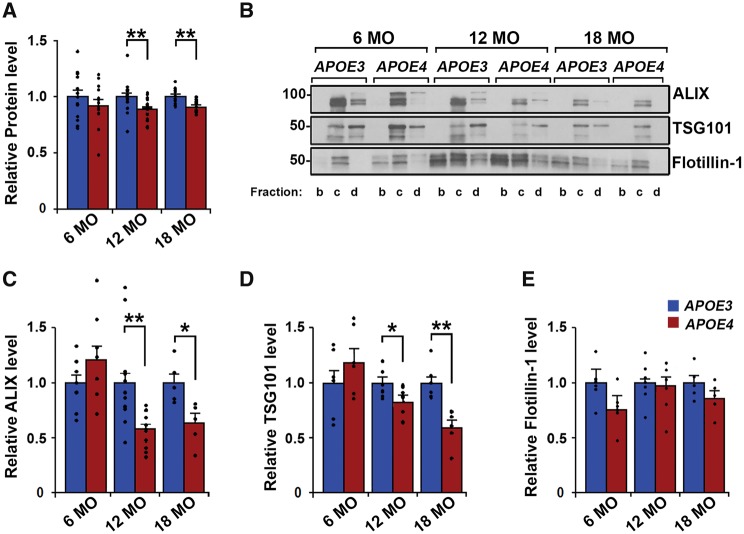
To determine that the observed effects on exosome levels are solely dependent on APOE genotype, we isolated brain extracellular vesicles from humanized APOE homozygous mice that do not develop tau or amyloid-β pathology (Sullivan et al., 1997; Tai et al., 2011). Data from sucrose gradient fractions b–d were pooled for all analyses. Repeated measures 2 × 3 ANOVA (genotype × age) revealed a main effect of genotype [F(1,78) = 9.37, P = 0.003], but not age independent of genotype [F(2,78) = 0.19, P = 0.82], on brain extracellular vesicle total protein levels. In agreement with our human findings, post hoc t-test analysis between groups showed lower extracellular vesicle protein levels in the right hemibrains of 12-month-old [88.2 ± 2.1% of APOE3 levels; n = 18, t(34) = 3.3, P = 0.002; Fig. 3A] and 18-month-old [91.4 ± 1.5% of APOE3 levels; n = 10 APOE3, n = 11 APOE4, t(19) = 3.14, P = 0.005; Fig. 3A] APOE4 compared with age-matched APOE3 mice. Six-month-old APOE4 mice showed no difference in brain extracellular vesicle protein levels when compared with age-matched APOE3 mice [n = 13 APOE3, n = 14 APOE4, t(25) = 0.98, P = 0.33; Fig. 3A], indicating that the APOE4 effect on brain extracellular vesicles is age-dependent. Levels of flotillin-1 were not found to differ by APOE genotype at any age by t-test analyses [6-month-old n = 5, t(8) = 1.67, P = 0.13; 12-month-old n = 5, t(8) = 0.48, P = 0.64; 18-month-old n = 5, t(8) = 2.31, P = 0.18; Fig. 3B and E]. Exosome-specific markers were examined in extracellular vesicle fractions to confirm that brain exosome levels were specifically affected by APOE4 expression (Fig. 3B). Repeated measures 2 × 3 ANOVA (genotype × age) revealed a main effect by genotype with significant interaction with age for both ALIX [genotype F(1,46) = 8.79, P = 0.004; interaction F(2,46) = 8.55, P = 0.0007] and TSG101 [genotype F(1,30) = 5.99, P = 0.02; interaction F(2,30) = 7.05, P = 0.003]. Post hoc t-test analyses between groups revealed that ALIX and TSG101 levels in the brain extracellular vesicles of APOE4 mice were lower at both 12 [ALIX 59.7 ± 4.8% of APOE3; n = 12, t(22) = 3.89, P = 0.0007; TSG101 82.8 ± 4.8% of APOE3; n = 6, t(10) = 2.34, P = 0.04] and 18 months of age [ALIX 62.4 ± 8.6% of APOE3; n = 5, t(8) = 3.10, P = 0.02; TSG101 60.5 ± 6.6% of APOE3; n = 6, t(10) = 4.23, P = 0.002] when normalized to brain tissue weight (Fig. 3C and D). ALIX and TSG101 levels did not differ in the brain extracellular vesicles of 6-month-old APOE4 compared with APOE3 mice [ALIX n = 9, t(16) = 1.58, P = 0.13; TSG101 n = 6, t(10) = 1.13, P = 0.29; Fig. 3C and D].
Figure 3.
Brain exosome levels are lower in APOE4 compared with APOE3 mice in an age-dependent manner. (A) Protein levels in sucrose gradient fractions b–d from each age group are shown for extracellular vesicles isolated from mouse hemibrains normalized to hemibrain weight. (B) ALIX, TSG101, and flotillin-1 were analysed by western blotting. Quantification of band intensities normalized to initial hemibrain weight are shown for levels of ALIX (C), TSG101 (D), and flotillin-1 (E). Data are expressed as the mean ± SEM of the ratios of APOE4 to APOE3. *P < 0.05, **P < 0.01.
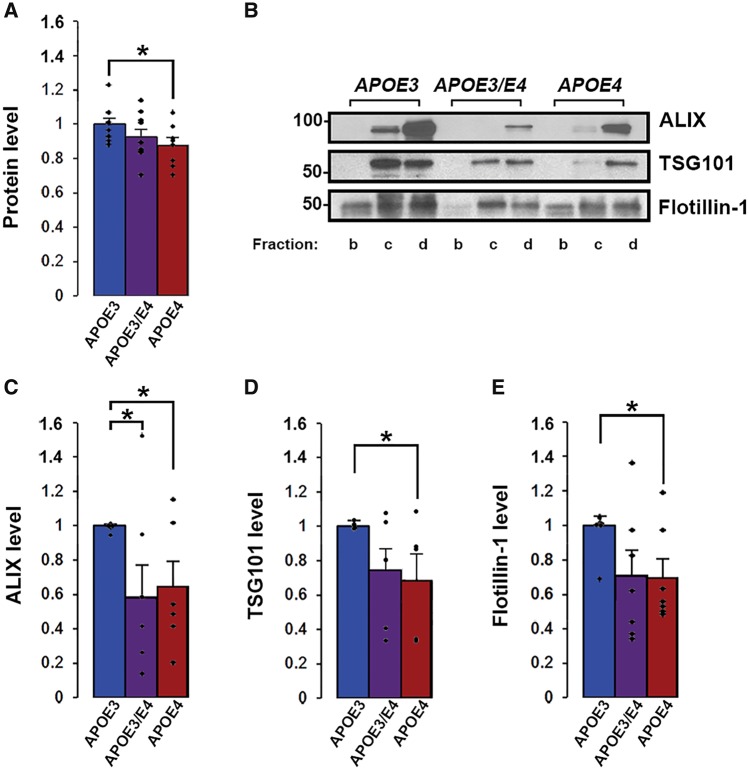
Given that our human APOE4 findings are from both homozygous APOE4 and heterozygous APOE3/E4 individuals (Fig. 2), we repeated a study of 12-month-old mice with the addition of heterozygous APOE3/E4 mice (Fig. 4). These additional data confirmed that 12-month-old APOE4 compared with APOE3 mice showed significantly lower extracellular vesicle levels of total protein [86.6 ± 4.1% of APOE3; n = 10 APOE3, n = 8 APOE4, t(16) = 2.21, P = 0.04], ALIX [64.5 ± 14.9% of APOE3; n = 7 APOE3, n = 6 APOE4, t(11) = 2.59, P = 0.03], and TSG101 [68.1 ± 15.1% of APOE3; n = 6 APOE3, n = 5 APOE4, t(9) = 2.34, P = 0.04] (Fig. 4). In these groups, 12-month-old APOE4 compared with APOE3 mice additionally showed significantly lower extracellular vesicle levels of flotillin-1 [70.0 ± 10.5% of APOE3; n = 7, t(12) = 2.56, P = 0.02] (Fig. 4B and E). Analysis of extracellular vesicles isolated from the brains of 12-month-old APOE3/E4 compared with APOE3 mice revealed significantly lower levels of ALIX [58.1 ± 19.0% of APOE3, n = 7, t(12) = 2.18, P = 0.048] (Fig. 4B and C). Lower but not significant levels were also detected for total extracellular vesicle protein [92.8 ± 4.1% of APOE3; n = 10, t(18) = 1.37, P = 0.19], flotillin-1 [71.0 ± 14.3% of APOE3; n = 7, t(12) = 1.91, P = 0.08], and TSG101 [73.7 ± 12.4% of APOE3; n = 6, t(10) = 2.12, P = 0.06] in the brain extracellular vesicles of 12-month-old APOE3/E4 compared with APOE3 mice (Fig. 4). That these exosome marker levels in 12-month-old APOE3/E4 mice brains were either significantly lower than in age-matched APOE3 mice, or intermediate between the levels found in age-matched APOE3 and APOE4 mice, suggests that 12 months is a critical age when mechanisms of exosomal production and release begin to decline in APOE4 carriers and that this decline may be gene-dose sensitive. Altogether, our findings indicate that APOE4 expression leads to lower exosome levels in the brains of both humans and APOE mice, with the mice data further suggesting that the effect is ageing-dependent.
Figure 4.
APOE4-driven effects on exosome levels are gene dose dependent in 12-month-old mice. (A) Protein levels in sucrose gradient fractions b–d are shown for extracellular vesicles isolated from mouse hemibrains normalized to hemibrain weight. (B) ALIX, TSG101, and flotillin-1 were analysed by western blotting. Quantification of band intensities normalized to initial hemibrain weight are shown for levels of ALIX (c), TSG101 (D), and flotillin-1 (E). Data are expressed as the mean ± SEM of the ratios when compared with APOE3. *P < 0.05.
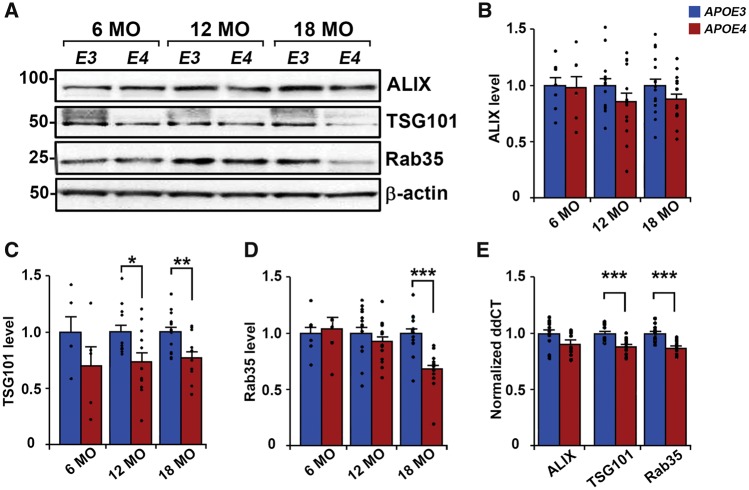
Exosomal formation and secretion are downregulated in aged APOE4 compared with APOE3 mouse brains
To determine whether APOE genotype-mediated differences in brain exosome levels are due to altered exosome biogenesis and/or secretion, we examined the expression of intraluminal vesicle formation regulators (ALIX and TSG101) as well as a regulator of multivesicular body fusion with the plasma membrane (Rab35) (Raiborg and Stenmark, 2009; Hsu et al., 2010) in the brains of APOE mice (Fig. 5). From the same mice in which extracellular vesicles were isolated from the right hemibrain, left hemibrain homogenates were used to measure protein expression levels of ALIX, TSG101, and Rab35. Repeated measures ANOVA (genotype × age) on hemibrain protein levels revealed a main effect of genotype for TSG101 [F(1,50) = 14.14, P = 0.0004], as well as of genotype and age with significant interaction for Rab35 [genotype F(1,64) = 5.32, P = 0.02; age F(2,64) = 4.88, P = 0.01; interaction F(2,64) = 4.86, P = 0.01]. Post hoc t-tests between groups revealed that TSG101 levels were reduced in APOE4 compared with APOE3 mice at 12 [73.4 ± 8.4% of APOE3; n = 11 APOE3, n = 12 APOE4, t(21) = 2.44, P = 0.02] and 18 months of age [76.6 ± 5.8% of APOE3; n = 14 APOE3, n = 13 APOE4, t(25) = 3.16, P = 0.004; Fig 5C]. Rab35 levels in APOE4 mice were significantly lower at 18 months of age [67.4 ± 4.7% of APOE3; n = 14 APOE3, n = 13 APOE4, t(25) = 4.83, P = 0.00006; Fig. 5D]. Consistent with the lack of exosome level differences at 6 months of age, hemibrain levels of TSG101 and Rab35 showed no significant genotype-mediated differences at this age [TSG101 n = 5, t(8) = 1.28, P = 0.24; Rab35 n = 7; t(12) = 0.35, P = 0.74; Fig. 5C and D]. ALIX levels were not significantly different in the hemibrains of APOE4 mice compared with APOE3 mice at any age [6 months old n = 7, t(12) = 0.09, P = 0.93; 12 months old n = 13 APOE3, n = 14 APOE4, t(25) = 1.28, P = 0.21; 18 months old n = 16 APOE3, n = 15 APOE4, t(29) = 1.46, P = 0.16; Fig 5B]. To determine if APOE4-mediated reductions in protein levels were due to downregulated transcription, we conducted qPCR analyses for Pdcd6ip (encodes ALIX), Tsg101, and Rab35 mRNA from the hemibrains of 12-month-old APOE mice. Brain mRNA levels were reduced for Tsg101 [88.8 ± 1.9% of APOE3; n = 6, t(23) = 4.05, P = 0.0005] and Rab35 [87.3 ± 2.2% of APOE3; n = 6, t(25) = 4.24, P = 0.0003] in APOE4 compared with APOE3 mice, whereas Pdcd6ip mRNA levels were not statistically different [91.0 ± 4.1% of APOE3; n = 6, t(24) = 1.60, P = 0.12; Fig. 5E]. Altogether, our findings suggest that a downregulation of molecular events involved in exosomal biogenesis and secretion lead to the reduction of brain exosome levels in APOE4 carriers.
Figure 5.
Expression levels of TSG101 and Rab35 are lower in aged APOE4 mouse brains compared with age-matched APOE3 mice. (A) Representative western blot analyses of mouse hemibrain homogenates are shown for ALIX, TSG101, and Rab35, with β-actin used as a loading control. (B–D) Quantification of bands normalized to levels of β-actin is shown for levels of ALIX (B), TSG101 (C), and Rab35 (D). (E) Quantitative PCR analysis of 12-month-old mouse hemibrains shows Pdcd6ip (encodes ALIX), Tsg101, and Rab35 transcript levels normalized to ddCT levels of the housekeeping gene Sdha. Data are expressed as the mean ± SEM of the ratios of APOE4 to APOE3. *P < 0.05, **P < 0.01, ***P < 0.001.
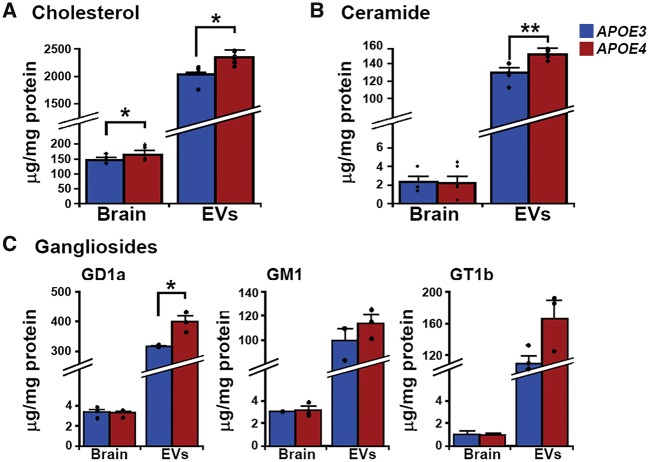
Lipid composition is altered in APOE4 mice exosomes
Lipids such as cholesterol, ceramides, and gangliosides are enriched in exosomal membranes when compared with other cell membranes, reflecting the unique lipid environment of multivesicular bodies (Mobius et al., 2003). We determined the concentrations of cholesterol, ceramide, and gangliosides in 12-month-old APOE mouse brains and brain extracellular vesicles. As previously reported (Mobius et al., 2003), our mouse brain extracellular vesicles were ∼10 times as enriched in cholesterol and ∼50 times as enriched in ceramide and gangliosides compared with total brain lipid concentrations (Fig. 6). At the hemibrain level, cholesterol concentrations were higher in 12-month-old APOE4 compared with age-matched APOE3 mouse brains [112.6 ± 3.9% of APOE3; n = 4 APOE3, n = 5 APOE4, t(7) = 2.59, P = 0.04; Fig. 6A], while concentrations of ceramide and gangliosides did not differ (Fig. 6B and C). Cholesterol concentrations were also higher in the brain extracellular vesicles of APOE4 compared with APOE3 mice [115.0 ± 5.9% of APOE3; n = 5, t(8) = 3.38, P = 0.01; Fig. 6A]. Consistent with previous observations that endosomal cholesterol build-up is commonly associated with ganglioside accumulation (Lloyd-Evans and Platt, 2010), APOE4 mouse brain extracellular vesicles had higher levels of the ganglioside-precursor ceramide [116 ± 4.5% of APOE3; n = 5, t(8) = 3.36, P = 0.01; Fig. 6B] as well as the ganglioside GD1a [125.2 ± 6.3% of APOE3; n = 3; t(4) = 3.97, P = 0.02, Fig. 6C]. These findings demonstrate that APOE4 alters brain and extracellular vesicle cholesterol levels, with associated changes in extracellular vesicle levels of ceramide and ganglioside.
Figure 6.
Differences in brain extracellular vesicle lipids in APOE4 compared with APOE3 mice. Brain and extracellular vesicle lipid concentrations are given in μg/mg of protein for (A) cholesterol, (B) ceramide, and (C) gangliosides. Data are expressed as the mean ± SEM. *P < 0.05, **P < 0.01.
Discussion
Our findings demonstrate an APOE4-mediated decrease in brain exosome levels in both humans and humanized APOE mice, with our mouse findings showing that this is ageing-dependent, consistent with APOE4's contribution to ageing-related disease risk (Mahley, 2016). Our findings further indicate that exosomal decrease follows a downregulation of exosome biogenesis and secretion from the endosomal pathway. We reveal a transcriptional and protein level downregulation of TSG101 in the brains of aged APOE4 mice, consistent with previous findings that depleting specific ESCRT components such as TSG101 is sufficient to decrease exosome secretion in vitro (Colombo et al., 2013). While we did not find a reduction in the brain expression of ALIX, an ESCRT-associated protein that interacts with TSG101 during intraluminal vesical formation, this finding agrees with data from depletion studies suggesting that ALIX's contribution to exosome formation may depend on the cell type (Baietti et al., 2012; Colombo et al., 2013). The observed reduction in exosome secretion is additionally supported by our finding that APOE4 downregulates Rab35, a rab GTPase that functions in the docking of multivesicular bodies to the plasma membrane for exosome release. Inhibition of Rab35 has been shown to lead to impaired exosome secretion in several in vitro models (Hsu et al., 2010; Abrami et al., 2013). Taken together, our data indicate that lower exosome levels in the brains of APOE4 carriers are likely a consequence of downregulated exosome biogenesis and secretion rather than changes in exosome stability or clearance from the brain extracellular space.
The apolipoprotein E protein is likely to exert its effects on endosomal and exosomal pathways through its primary role as a lipid carrier, supported by studies that APOE4 compared with APOE3 expression disrupts neuronal and peripheral cholesterol metabolism (Sing and Davignon, 1985; Michikawa et al., 2000). We found an increase in cholesterol levels in the exosomes of APOE4 compared with APOE3 mice that likely reflects altered lipid metabolism within the endosomal pathway from which they originate. Lipids such as cholesterol accumulate in intracellular vesicles under certain pathological conditions, such as in Alzheimer's disease and select lysosomal storage disorders (Distl et al., 2001; Kuech et al., 2016). Aberrant accumulation of cholesterol, along with the secondary accumulation of sphingolipids, can cause disturbances in the endosomal pathway that affect both the morphology and motility of endosomal compartments (Lebrand et al., 2002; Marquer et al., 2014). For example, endosomal cholesterol accumulation has been shown to immobilize multivesicular bodies through the impaired inactivation of Rab7 (Lebrand et al., 2002; Chen et al., 2008). We therefore suggest that APOE4's disruption of intracellular cholesterol metabolism may be directly associated with compromised exosome production. While several other members of the Rab protein family are known to be regulated by endosomal cholesterol levels (Choudhury et al., 2004; Glodowski et al., 2007), it remains to be determined if Rab35 function, which shows decreased levels in brains of APOE4 mice, may also be affected by changes in cholesterol levels.
An APOE4-mediated alteration in brain exosome pathways introduces an additional component to the endosomal-lysosomal disruption that appears to be a prominent feature of APOE4-driven neuronal changes (Cataldo et al., 2000; Nuriel et al., 2017; Zhao et al., 2017). Alzheimer's disease-related early endosome enlargement in neurons was previously shown by Cataldo et al. (2000) to be more prominent in APOE4 carriers than non-carriers, including in brain regions not affected by amyloid-β pathology. More recently, 22-month-old APOE4 mice were found to have neuronal endosomal pathology that impairs insulin signalling (Zhao et al., 2017). Moreover, we have recently demonstrated with RNA sequencing that carrying an APOE4 allele leads to robust alterations in the expression of multiples genes involved in the endosomal-lysosomal system when comparing the Alzheimer's disease-vulnerable entorhinal cortex to an Alzheimer's disease-resistant brain region (primary visual cortex) of 14 to 15-month-old APOE mice (Nuriel et al., 2017). We additionally showed that the number and size of early endosomes is increased in cingulate cortex pyramidal neurons of APOE4 mice at 18 months of age, but not at 12 months or earlier ages (Nuriel et al., 2017). Further evidence for APOE4-dependent lysosomal changes comes from our findings that lysosome number is increased in the entorhinal pyramidal neurons of 18-month-old APOE4 mice (Nuriel et al., 2017). The current findings show that APOE4-driven brain exosome compromise at 12 months of age occurs earlier than the reported APOE4-driven endosome or lysosome changes, raising the possibility that reduced outward flux through the exosomal pathway might contribute to subsequent endosomal-lysosomal pathway alterations. Down syndrome patients and mouse models of the disease all show early endosome enlargement (Colacurcio et al., 2017), which is also associated with a change in the exosomal pathway, albeit an increase in exosome production (Gauthier et al., 2017). Importantly in this study, reducing exosome generation worsened the endosomal pathology in cultured Down syndrome fibroblasts (Gauthier et al., 2017), suggesting that flux through the exosomal pathway is an important regulator of endosomal compartments. Similarly, knockdown of TSG101 and Rab35 in cells in vitro has been shown to result in morphological endosomal changes in addition to downregulating exosome secretion (Razi and Futter, 2006; Allaire et al., 2010). Thus, reduced exosome release in APOE4 carriers may be a contributing factor to the extensive neuronal endosomal pathway alterations that have been linked to this allele.
Our findings link the APOE4 genotype to a failure in brain exosome production in an in vivo, Alzheimer's disease pathology-free setting, contributing to existing evidence that the neuronal endosomal-lysosomal system is broadly disrupted in individuals expressing this allele. Disturbances of endosomal-lysosomal system function often result in the accumulation of surplus material in neuronal endosomes and lysosomes (Nixon, 2004), and disruptions throughout these pathways are an important factor contributing to neuronal vulnerability in numerous neurodegenerative disorders, including Alzheimer's disease (Nixon, 2005; Schreij et al., 2016). Consistent with our findings here, we propose that endosomal material released into the extracellular space via exosomes is an important mechanism by which neurons remove debris, and that failure of efficient exosome production and release can exacerbate and perhaps lead to endosomal pathway disturbances (Gauthier et al., 2017). This failure to maintain proper functioning of the neuronal endosomal-lysosomal and exosomal pathways in APOE4 carriers during ageing may not only disturb essential homeostatic and catabolic cellular processes, but may also contribute to neuronal vulnerability and the risk of developing neurodegenerative disorders such as Alzheimer's disease (Nixon, 2017).
Supplementary Material
Acknowledgements
We thank Dr. Monika Pawlik for her expert assistance with our mouse colonies, and Sang Han Lee at the Center for Biomedical Imaging and Neuromodulation at the Nathan S. Kline Institute for the statistical analysis of the qPCR data. Human tissue was kindly provided by the Emory ADRC/CND under the direction of Dr. Marla Gearing.
Glossary
Abbreviations
- ALIX
apoptosis-linked gene 2-interacting protein X
- ESCRT
endosomal sorting complexes required for transport
Funding
This work was supported by the NIH (P01 AG017617 and R01 AG057517 to P.M.M. and E.L., and R01 AG043375 and P01 AG014449 to S.D.G.). K.P. was additionally supported by NIH predoctoral and postdoctoral research training grants (T32-GM066704, Dr. Erika Bach; T32-AG052909, Drs Thomas Wisniewski and Helen Scharfman) and the Sackler Institute of Graduate Biomedical Sciences, New York University School of Medicine. The provision of human tissue from the Emory ADRC/CND was supported by the NIH (AG025688 to Dr. Marla Gearing). The funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Competing interests
The authors report no competing interests.
References
- Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, et al. . Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep 2013; 5: 986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire PD, Marat AL, Dall'Armi C, Di Paolo G, McPherson PS, Ritter B.. The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol Cell 2010; 37: 370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Che S, Ginsberg SD.. Terminal Continuation (TC) RNA amplification enables expression profiling using minute RNA input obtained from mouse brain. Int J Mol Sci 2008; 9: 2091–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, et al. . Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci 2010; 30: 13707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. . Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012; 14: 677–85. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE.. The AlzGene Database. Alzheimer Research Forum, January29, 2010. Available from: http://www.alzgene.org (1June2017, date last accessed) [Google Scholar]
- Bookheimer S, Burggren A.. APOE-4 genotype and neurophysiological vulnerability to Alzheimer's and cognitive aging. Annu Rev Clin Psychol 2009; 5: 343–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA.. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol 2000; 157: 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yang J, Low PS, Cheng JX.. Cholesterol level regulates endosome motility via Rab proteins. Biophys J 2008; 94: 1508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A, Sharma DK, Marks DL, Pagano RE.. Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling. Mol Biol Cell 2004; 15: 4500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J.. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 2015; 25: 364–72. [DOI] [PubMed] [Google Scholar]
- Colacurcio DJ, Pensalfini A, Jiang Y, Nixon RA.. Dysfunction of autophagy and endosomal-lysosomal pathways: roles in pathogenesis of down syndrome and Alzheimer's Disease. Free Radic Biol Med 2018; 114: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. . Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 2013; 126 (Pt 24): 5553–65. [DOI] [PubMed] [Google Scholar]
- Di Battista AM, Heinsinger NM, Rebeck GW.. Alzheimer's disease genetic risk factor APOE-ɛ4 also affects normal brain function. Curr Alzheimer Res 2016; 13: 1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distl R, Meske V, Ohm TG.. Tangle-bearing neurons contain more free cholesterol than adjacent tangle-free neurons. Acta Neuropathol 2001; 101: 547–54. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. . Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 2009; 106: 7209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier SA, Perez-Gonzalez R, Sharma A, Huang FK, Alldred MJ, Pawlik M, et al. . Enhanced exosome secretion in Down syndrome brain—a protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol Commun 2017; 5: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Schneider JA, Rebeck GW, Hyman BT, Mirra SS.. Alzheimer's disease with and without coexisting Parkinson's disease changes: apolipoprotein E genotype and neuropathologic correlates. Neurology 1995; 45: 1985–90. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, Che S.. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer's disease. Neurobiol Dis 2012; 45: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C.. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell 2007; 18: 4387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT.. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990; 31: 545–8. [PubMed] [Google Scholar]
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. . Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010; 189: 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E.. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol 2008; 9: 125–38. [DOI] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. . Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 2016; 113: E968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuech EM, Brogden G, Naim HY.. Alterations in membrane trafficking and pathophysiological implications in lysosomal storage disorders. Biochimie 2016; 130: 152–62. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Corti M, Goodson H, Cosson P, Cavalli V, Mayran N, et al. . Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J 2002; 21: 1289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E.. Exosomes in the diseased brain: first insights from in vivo studies. Front Neurosci 2017; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G.. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013; 9: 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM.. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic 2010; 11: 419–28. [DOI] [PubMed] [Google Scholar]
- Macala LJ, Yu RK, Ando S.. Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J Lipid Res 1983; 24: 1243–50. [PubMed] [Google Scholar]
- Mahley RW.. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med 2016; 94: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquer C, Laine J, Dauphinot L, Hanbouch L, Lemercier-Neuillet C, Pierrot N, et al. . Increasing membrane cholesterol of neurons in culture recapitulates Alzheimer's disease early phenotypes. Mol Neurodegener 2014; 9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D.. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res 2008; 49: 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa M, Fan QW, Isobe I, Yanagisawa K.. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem 2000; 74: 1008–16. [DOI] [PubMed] [Google Scholar]
- Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HFG, Slot JW, et al. . Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic 2003; 4: 222–31. [DOI] [PubMed] [Google Scholar]
- Nixon RA.. Niemann-Pick Type C disease and Alzheimer's disease: the APP-endosome connection fattens up. Am J Pathol 2004; 164: 757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA.. Endosome function and dysfunction in Alzheimer's disease and other neurodegenerative diseases. Neurobiol Aging 2005; 26: 373–82. [DOI] [PubMed] [Google Scholar]
- Nixon RA.. Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer's disease: inseparable partners in a multifactorial disease. FASEB J 2017; 31: 2729–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriel T, Peng KY, Ashok A, Dillman AA, Figueroa HY, Apuzzo J, et al. . The endosomal–lysosomal pathway is dysregulated by APOE4 expression in vivo. Front Neurosci 2017; 11: 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M.. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiol Aging 2010; 31: 567–77. [DOI] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. . Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12: 19–30; sup 1–13. [DOI] [PubMed] [Google Scholar]
- Peng KY, Mathews PM, Levy E, Wilson DA.. Apolipoprotein E4 causes early olfactory network abnormalities and short-term olfactory memory impairments. Neuroscience 2017; 343: 364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E.. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 2012; 287: 43108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González R, Gauthier SA, Kumar A, Saito M, Saito M, Levy E.. A method for isolation of extracellular vesicles and characterization of exosomes from brain extracellular space. In: Hill AF, editor. Exosomes and microvesicles: methods and protocols New York, NY: Springer; 2017. p. 139–51. [DOI] [PubMed] [Google Scholar]
- Pfreiger FW.. Role of cholesterol in synapse formation and function. Biochim Biophys Acta 2003; 1610: 271–80. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Stenmark H.. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009; 458: 445–52. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, Picou F, et al. . Emerging roles of extracellular vesicles in the nervous system. J Neurosci 2014; 34: 15482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi M, Futter CE.. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell 2006; 17: 3469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Wu G, Hui M, Masiello K, Dobrenis K, Ledeen RW, et al. . Ganglioside accumulation in activated glia in the developing brain: comparison between WT and GalNAcT KO mice. J Lipid Res 2015; 56: 1434–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreij AM, Fon EA, McPherson PS.. Endocytic membrane trafficking and neurodegenerative disease. Cell Mol Life Sci 2016; 73: 1529–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D'Angelo G, et al. . APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci 2010; 30: 17035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Han P, Kuniyoshi SM.. Cognitive impairment in neurological diseases: lessons from apolipoprotein E. J Alzheimers Dis 2014; 38: 1–9. [DOI] [PubMed] [Google Scholar]
- Sing CF, Davignon J.. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet 1985; 37: 268–85. [PMC free article] [PubMed] [Google Scholar]
- Strauss K, Goebel C, Runz H, Mobius W, Weiss S, Feussner I, et al. . Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem 2010; 285: 26279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, et al. . Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 1997; 272: 17972–80. [DOI] [PubMed] [Google Scholar]
- Tai LM, Youmans KL, Jungbauer L, Yu C, Ladu MJ.. Introducing human APOE into abeta transgenic mouse models. Int J Alzheimers Dis 2011; 2011: 810981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong LM, Yoon SY, Andrews-Zwilling Y, Yang A, Lin V, Lei H, et al. . Enhancing GABA signaling during middle adulthood prevents age-dependent GABAergic interneuron decline and learning and memory deficits in ApoE4 mice. J Neurosci 2016; 36: 2316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA.. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 2011; 32: 63–74. [DOI] [PubMed] [Google Scholar]
- Zhao N, Liu CC, Van Ingelgom AJ, Martens YA, Linares C, Knight JA, et al. . Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 2017; 96: 115–29.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.