Abstract
Introduction
Abnormalities in lipid metabolism may contribute to the development and progression of chronic kidney disease (CKD) in patients with type 2 diabetes. Fenofibrate induces early and reversible reduction in estimated glomerular filtration rate (eGFR), but it may have protective effects on microvascular complications of diabetes. We hypothesized that randomization to fenofibrate versus placebo would be associated with beneficial long-term effects on kidney outcomes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial participants.
Methods
We conducted a post hoc analysis in the ACCORD Lipid Trial to examine the association of randomization to fenofibrate versus placebo with change in eGFR and with time-to-development of microalbuminuria, macroalbuminuria, CKD, and kidney failure.
Results
We analyzed 2636 participants in the fenofibrate arm and 2632 in the placebo arm. During a median follow-up of 4 years, treatment with fenofibrate was associated with lower rate of eGFR decline (−0.28 ml/min per 1.73 m2 per year in the fenofibrate group vs. −1.25 ml/min per 1.73 m2 per year in the placebo group, P < 0.01) and with lower incidence of microalbuminuria (hazard ratio [HR] 0.56, 95% confidence interval [CI] 0.43–0.72, P < 0.001) and macroalbuminuria (HR 0.72, 95% CI 0.57–0.91, P < 0.001). There was no difference in incidence of CKD (HR 0.92, 95% CI 0.74–1.15, P = 0.46) and/or kidney failure (HR 0.95, 95% CI 0.68–1.33, P = 0.76).
Conclusion
Compared with placebo, randomization to fenofibrate was associated with lower rates of incident albuminuria and a slower eGFR decline, but no difference in incidence of CKD or kidney failure in ACCORD participants.
Keywords: albuminuria, chronic kidney disease, diabetic nephropathy, fenofibrate, kidney failure
Diabetes is the leading cause of CKD, which affects more than 30 million Americans and is a major public health problem.1 Individuals with diabetes and CKD have approximately 4 times the rate of mortality than unaffected peers.2, 3 Glycemic control and blockade of the renin-angiotensin system form the mainstay of treatment. Emerging data from clinical trials of glucagon-like peptide 1 agonists and sodium–glucose cotransporter 2 inhibitors suggest that use of these new classes of hypoglycemic medications may improve kidney outcomes in patients with type 2 diabetes.4, 5, 6 Despite exciting advances, the prevalence of diabetic kidney disease remains stable, and large numbers of diabetic patients continue to progress to end-stage renal disease (ESRD).7, 8
Dyslipidemia and abnormalities in renal lipid metabolism may predispose diabetic individuals to lipid accumulation in glomeruli and tubules, which may lead to fibrosis and accelerated CKD progression.9, 10 In support of this hypothesis are data suggesting that use of statins is associated with modest preservation of eGFR,11 and the finding of beneficial effects of fenofibrate on albuminuria and eGFR in the diabetic population.12 Data from the ACCORD trial previously demonstrated that fenofibrate induced early and reversible reduction in kidney function without significant differences in onset of ESRD.13, 14 The long-term effects of fenofibrate on development and progression of CKD in ACCORD participants have not been examined in detail in dedicated studies. Therefore, we conducted a post hoc analysis to test whether, compared with placebo, fenofibrate treatment would be associated with reduced eGFR decline over time and with lower risks of development of microalbuminuria, macroalbuminuria, incident CKD, and kidney failure.
Methods
The ACCORD Trial
The ACCORD glycemia trial was a multicenter randomized trial that tested intensive blood glucose control compared with standard therapy in 10,251 patients with type 2 diabetes.15, 16 The main outcomes examined were cardiovascular endpoints. Within the glycemia trial, in a double 2 × 2 factorial design, participants were divided into 2 subgroups, one investigating intensive versus standard blood pressure control and the other examining lipid therapies.14, 17 Eligibility criteria included patients with type 2 diabetes for >3 months and hemoglobin A1c of 7.5% or higher. Participants were age 40 to 79 with known cardiovascular disease; or age 55 to 79 with 2 risk factors for cardiovascular disease, microalbuminuria (≥ 30 mg/g creatinine), significant atherosclerosis, or left ventricular hypertrophy. Patients with a creatinine >1.5 mg/dl were excluded.16 Participants were randomized from 2001 to 2005. In 2008, the glycemia trial was stopped early due to an increase in mortality in the intensive blood glucose arm.16 Follow-up for the blood pressure and lipid arms ended in 2009.14, 17 The ACCORD protocol was approved by institutional review boards at all sites, and written informed consent was obtained from all participants.
The ACCORD Lipid Trial randomized 5518 participants to either fenofibrate and simvastatin, or placebo and simvastatin.14 Simvastatin was started at the randomization visit and fenofibrate or placebo was started 1 month later. Participants with eGFR >50 ml/min per 1.73 m2 were initially started on fenofibrate 160 mg daily. In 2004, individuals with eGFR between 30 and 50 ml/min per 1.73 m2 received fenofibrate 54 mg daily. If the eGFR dropped below 30 ml/min per 1.73 m2, the fenofibrate was stopped.
Study Population
We used ACCORD Research Materials obtained from the National Heart, Lung, and Blood Institute to conduct a post hoc analysis. We examined 5268 participants in the ACCORD Lipid Trial (n for fenofibrate arm = 2636, n for placebo arm = 2632), who had an eGFR from the month 4 study visit. We excluded 250 participants of the ACCORD Lipid Trial who did not have a month 4 eGFR. We used the month 4 eGFR for our baseline eGFR given the increase in creatinine seen with fibrates after initiating therapy.13
Exposure and Outcomes
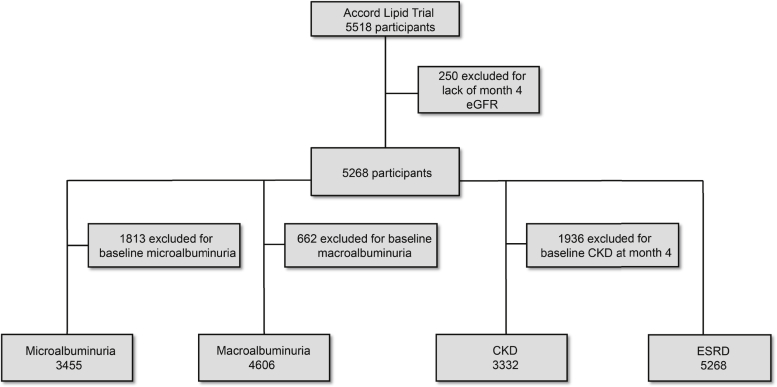
The primary exposure was allocation to the fenofibrate arm, which we analyzed in an intention-to-treat manner. We examined the association of fenofibrate with longitudinal change in eGFR from the month 4 study visit to the end of follow-up. We also examined the time-to-development of microalbuminuria, macroalbuminuria, incident CKD, and kidney failure. Because of baseline prevalence of microalbuminuria, macroalbuminuria, and CKD, the analytic sample size for each incident time-to-event analysis varied (Figure 1). Serum creatinine was measured every 4 months and albuminuria was assessed yearly.18 Microalbuminuria and macroalbuminuria were defined as urinary albumin-to-creatinine ratio ≥ 30 mg/g and ≥ 300 mg/g in a random urine sample, respectively. We defined incident CKD as new onset of eGFR < 60 ml/min per 1.73 m2, ≥25% decrease from month 4 eGFR, or decrease in eGFR slope greater than 1 ml/min per 1.73 m2 per year, which was the median yearly change in eGFR in our study population. Kidney failure was defined as initiation of dialysis, kidney transplantation, or rise in serum creatinine >3.3 mg/dl without a reversible cause.
Figure 1.
Sample size of participants for the time-to-event analyses. The flow chart demonstrates the available sample size for analyses of incident kidney outcomes after exclusion of baseline presence of the relevant kidney outcome. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease.
Measurements and Assessment of Baseline Covariates
Covariate information was obtained from questionnaires, which included questions about sociodemographic characteristics, medical history, and concomitant medications. All participants had baseline measurements that included height, weight, and blood pressure. eGFR was estimated from serum creatinine, measured by the Roche Creatinine Plus enzymatic method (Roche Diagnostics, Basel, Switzerland), using the Chronic Kidney Disease Epidemiology Collaboration equation.19 Urine creatinine was measured enzymatically on a Roche Double Modular P Analytics automated analyzer. Urinary albumin was measured by immunonephelometry on a Siemens (Munich, Germany) BN II nephelometer. HbA1C was determined by an automated high-performance liquid chromatography.
Statistical Analysis
We examined baseline participant characteristics by fenofibrate treatment assignment.
We used t-test for continuous variables with normal distribution, Wilcoxon-Mann-Whitney tests for the skewed continuous variables, and χ2 tests for categorical variables. We compared self-reported adherence to the randomized interventions among participants in the fenofibrate arm and those in the placebo arm throughout the duration of follow-up.
We applied linear mixed models to estimate the effect of randomization to fenofibrate on the longitudinal change in eGFR from the month 4 visit to the end of follow-up. All models included a random intercept for each participant and a random slope for time as a continuous variable to account for within-subject correlation. In model 1, we adjusted for glycemia trial and network. In model 2, we adjusted for factors in model 1 and for age, gender, and race. In model 3, we adjusted for factors in model 2 and for kidney-specific factors, including month 4 eGFR, and presence of microalbuminuria and macroalbuminuria at baseline. In model 4, we adjusted for factors in model 3 and baseline comorbidities and concomitant medications.
In the analysis of development of microalbuminuria or macroalbuminuria, participants were excluded if they had microalbuminuria or macroalbuminuria at the baseline visits, respectively. Participants with baseline visit eGFR <60 ml/min per 1.73 m2 or baseline visit urinary albumin-to-creatinine ratio >30 mg/g were excluded from the incident CKD analysis. We adjusted the Cox models for the same covariates we included in the linear mixed models with exceptions for model 3. In the microalbuminuria and CKD analyses, the data were adjusted for month 4 eGFR. In the macroalbuminuria analyses, we adjusted for month 4 eGFR and microalbuminuria. In the kidney failure analyses, we adjusted for month 4 eGFR, microalbuminuria, and macroalbuminuria. HRs were reported with 95% CIs with nonuse of fenofibrate as the reference category. We tested proportional hazards assumption using the Schoenfeld residual for the effect of fenofibrate use. The proportional hazards assumption was met for kidney failure, and development of macroalbuminuria, but it was violated for development of microalbuminuria. To accommodate non–proportional hazards of development of microalbuminuria, we included time by fenofibrate interaction term in the microalbuminuria models.
In additional exploratory analyses, to test whether the effects of fenofibrate were mediated by changes in lipid levels during follow-up, we further adjusted our final models for time-varying levels of total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein. To determine whether the eGFR levels rebounded after discontinuation of fenofibrate at the end of the ACCORD Lipid Trial, in 2736 follow-on study participants we examined the mean eGFR levels throughout the trial and at 2 time points during the ACCORD Follow-on (ACCORDION) study,20 an observation period during which no active therapies were provided by the study and measurements were collected and analyzed according to the group to which participants were originally allocated.
All the statistical analyses were performed by using SAS version 9.4 (SAS Institute Inc, Cary, NC) and R version 3.4.0 (2017-04-21; http://cran.r-project.org). All statistical tests were 2-sided, and P < 0.05 was considered statistically significant. Because the objective of our post hoc analysis study was to produce hypothesis-generating results, we did not adjust for multiple testing. Reported P values are of nominal significance and serve as guides for possible associations.
Results
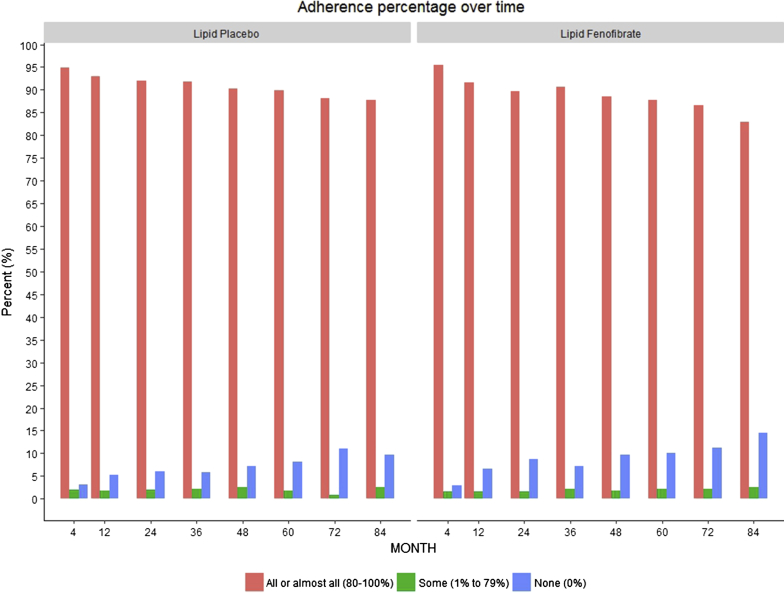
The baseline characteristics of our study population are listed in Table 1. Overall, participants had diabetes for more than 10 years, 12% had retinopathy, and 36% had CKD at baseline, defined as eGFR <60 ml/min per 1.73 m2 or urinary albumin-to-creatinine ratio >30 mg/g. Baseline characteristics, including eGFR values, were matched between the study groups. Due to the acute effect of fenofibrate on eGFR,13 the fenofibrate arm had a significantly lower month 4 eGFR than the placebo arm (71.9 vs. 84.0 ml/min per 1.73 m2, P <0.001). Self-reported adherence was comparable between the 2 groups throughout the duration of follow-up (Figure 2).
Table 1.
Baseline characteristics of study population by fenofibrate randomization arm
| Baseline characteristics | All patients, n = 5268 | Fenofibrate arm, n = 2636 | Placebo arm, n = 2632 | P |
|---|---|---|---|---|
| Age, y | 62.8 ± 6.6 | 62.8 ± 6.5 | 62.8 ± 6.7 | 0.91 |
| Female, n (%) | 1614 (30.6) | 810 (30.7) | 804 (30.6) | 0.89 |
| SBP, mm Hg | 133.6 ± 17.0 | 133.6 ± 17.0 | 133.7 ± 17.1 | 0.71 |
| BMI, kg/m2 | 32.3 ± 5.3 | 32.2 ± 5.3 | 32.4 ± 5.3 | 0.32 |
| Total cholesterol, mg/dl | 175.2 ± 36.9 | 174.9 ± 36.5 | 175.5 ± 37.2 | 0.56 |
| HbA1C, % | 8.3 ± 1.0 | 8.3 ± 1.0 | 8.2 ± 1.0 | 0.45 |
| Current smoking, n (%) | 2485 (54.5) | 1262 (55.4) | 1223 (53.5) | 0.20 |
| Duration of diabetes, y | 10.7 ± 7.4 | 10.7 ± 7.3 | 10.6 ± 7.4 | 0.75 |
| Heart failure, n (%) | 274 (5.2) | 141 (5.4) | 133 (5.1) | 0.63 |
| CVD, n (%) | 1905 (36.2) | 948 (36.0) | 957 (36.4) | 0.76 |
| Baseline retinopathy, n (%) | 525 (11.5) | 265 (11.7) | 260 (11.4) | 0.70 |
| Trial baseline eGFR, ml/min per 1.73 m2 | 83.5 ± 16.9 | 83.5 ± 16.9 | 83.6 ± 16.9 | 0.73 |
| Month 4 eGFR, ml/min per 1.73 m2 | 77.9 ± 18.8 | 71.9 ± 18.7 | 84.0 ± 16.9 | <0.001 |
| Microalbuminuria, n (%) | 1235 (24.5) | 629 (24.9) | 606 (24.1) | 0.54 |
| Macroalbuminuria, n (%) | 359 (7.1) | 182 (7.2) | 177 (7.1) | 0.84 |
| UACR, mg/g | 14.0 (7.0–45.0) | 14.0 (7.0–48.0) | 14.0 (7.0–42.0) | 0.49 |
| Prevalent CKD, n (%) | 1875 (35.6) | 948 (36.0) | 927 (35.3) | 0.57 |
| ACE/ARB inhibitors, n (%) | 3545 (67.6) | 1746 (66.6) | 1799 (68.7) | 0.10 |
| Insulin use, n (%) | 940 (17.8) | 481 (18.3) | 459 (17.4) | 0.44 |
| TZD use, n (%) | 1069 (20.3) | 526 (20.0) | 543 (20.6) | 0.55 |
| Randomization to intensive glycemic control arm, n (%) | 2627 (49.9) | 1299 (49.3) | 1328 (50.5) | 0.39 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1C, hemoglobin A1C; SBP, systolic blood pressure; TZD, thiazolidinedione; UACR, urine albumin-to-creatinine ratio.
Figure 2.
Self-reported adherence to fenofibrate and placebo in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid Trial Participants throughout the duration of follow-up. Bars represent percentages of ACCORD Lipid Trial participants reporting good, intermediate, and poor adherence to fenofibrate and placebo throughout the duration of follow-up.
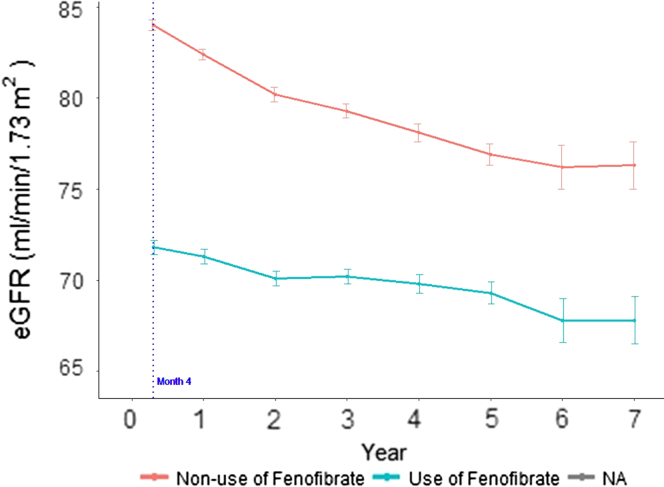
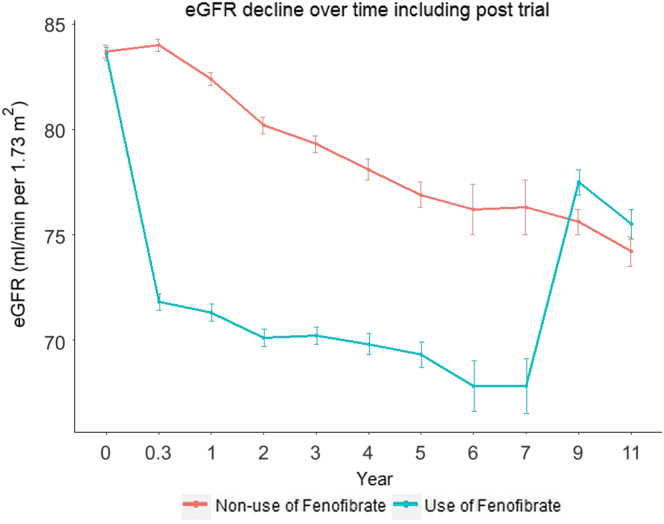
The mean values of eGFR during follow-up are shown in Figure 3. Despite having a lower month 4 eGFR, participants randomized to fenofibrate experienced a slower decline in eGFR than individuals randomized to placebo (Table 2 and Figure 3). In the unadjusted analysis, those randomized to fenofibrate had an eGFR slope of −0.27 ml/min per 1.73 m2 per year (95% CI −0.56 to 0.01) compared with −1.26 ml/min per 1.73 m2 per year (95% CI −1.38 to −1.14, P < 0.001) in the placebo group. Following adjustment for glycemia trial, network, comorbidities, kidney-specific factors, and medications, the eGFR slopes for the 2 study groups remained similar to unadjusted values, consistent with well-matched distribution of baseline characteristics between the 2 groups.
Figure 3.
Estimated GFR (eGFR) over time in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid Trial participants according to randomization. Mean absolute follow-up values are shown. Error bars indicate SEM.
Table 2.
Mean annualized change of eGFR by fenofibrate randomization arm
| Models Total, n = 5268 | Mean annualized change of eGFR (95% CI) |
P | |
|---|---|---|---|
| Fenofibrate arm, n = 2636 | Placebo arm, n = 2632 | ||
| Unadjusted | –0.27 (–0.56 to 0.01) | –1.26 (–1.38 to –1.14) | <0.001 |
| Model 1 | –0.27 (–0.56 to 0.01) | –1.26 (–1.38 to –1.14) | <0.001 |
| Model 2 | –0.27 (–0.56 to 0.01) | –1.26 (–1.38 to –1.14) | <0.001 |
| Model 3 | –0.28 (–0.57 to 0.01) | –1.26 (–1.38 to –1.14) | <0.001 |
| Model 4 | –0.28 (–0.57 to 0.01) | –1.25 (–1.38 to –1.13) | <0.001 |
Model 1: Adjusts for glycemia trial, and network.
Model 2: Adjusts for factors in model 1 and for demographics: age, gender, race.
Model 3: Adjusts for factors in model 2 and for kidney-specific factors: month 4 eGFR, microalbuminuria, macroalbuminuria.
Model 4: Adjusts for factors in model 3 and for presence of comorbidities at baseline: systolic blood pressure, body mass index, HbA1c, smoking status, cholesterol, T2DM duration, history of heart failure, history of CVD (myocardial infarction, stroke, revascularization, or angina), history of retinopathy, and for baseline use of medications: ACE/ARB, insulin, TZD.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; TZD, thiazolidinedione; T2DM, type 2 diabetes mellitus.
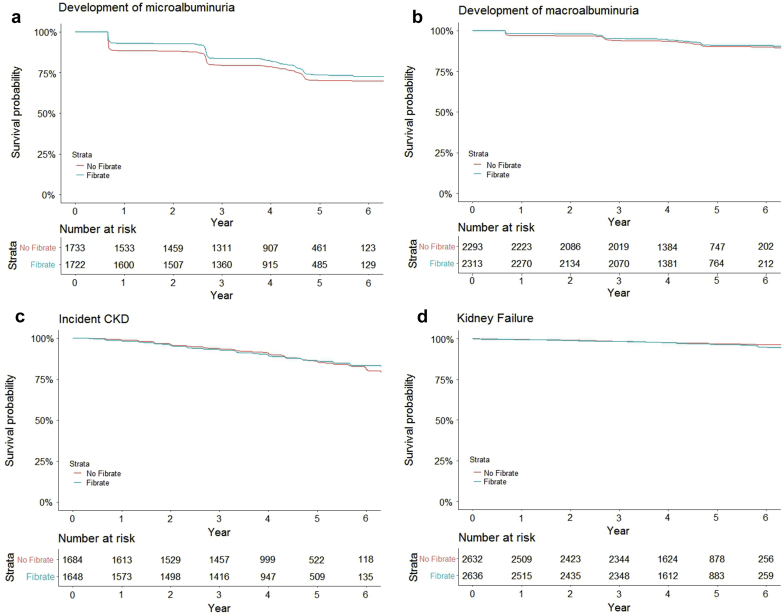
The associations of randomization to fenofibrate versus placebo on risks of development of albuminuria, incident CKD, and kidney failure are shown in Table 3 and Figure 4. During a median follow-up time of 4 years, randomization to the fenofibrate arm resulted in a decreased risk of incident microalbuminuria, both in the unadjusted (HR 0.62, 95% CI 0.49–0.79, P < 0.001) and adjusted analyses (model 4, HR 0.56, 95% CI 0.43–0.72, P < 0.001). The risk of development of macroalbuminuria did not differ between the 2 groups in the unadjusted analysis, but after adjusting for kidney-specific factors in model 3, participants randomized to the fenofibrate arm had a lower risk of developing macroalbuminuria compared with those assigned to the placebo arm (model 3, HR 0.66, 95% CI 0.53–0.83, P < 0.001). There were no statistical differences in risks of developing incident CKD or kidney failure between the 2 groups.
Table 3.
Risks of kidney outcomes by fenofibrate randomization arm
| Outcomes | Development of microalbuminuria (UAlb≥30 mg/g)a,b | Development of macroalbuminuria (UAlb≥300 mg/g)c | Incident CKDb | Kidney failured | ||||
|---|---|---|---|---|---|---|---|---|
| Total n | 3455 | 4606 | 3332 | 5268 | ||||
| n of events | 837 | 355 | 400 | 160 | ||||
| Median follow-up time, y | 4.0 | 4.3 | 4.3 | 4.3 | ||||
| Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | Hazard ratio | P | |
| Unadjusted | 0.62 (0.49–0.79) | <0.001 | 0.87 (0.70–1.07) | 0.18 | 0.99 (0.82–1.21) | 0.95 | 1.08 (0.79–1.47) | 0.64 |
| Model 1 | 0.61 (0.48–0.78) | <0.001 | 0.86 (0.70–1.06) | 0.14 | 0.99 (0.81–1.20) | 0.90 | 1.07 (0.78–1.45) | 0.69 |
| Model 2 | 0.61 (0.48–0.78) | <0.001 | 0.86 (0.70–1.06) | 0.14 | 0.98 (0.81–1.19) | 0.84 | 1.07 (0.78–1.46) | 0.68 |
| Model 3 | 0.56 (0.44–0.72) | <0.001 | 0.66 (0.53–0.83) | <.001 | 0.89 (0.72–1.11) | 0.30 | 0.92 (0.66–1.28) | 0.60 |
| Model 4 | 0.56 (0.43–0.72) | <0.001 | 0.72 (0.57–0.91) | 0.006 | 0.92 (0.74–1.15) | 0.46 | 0.95 (0.68–1.33) | 0.76 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c; SCr, serum creatinine; TZD, thiazolidinedione; T2DM, type 2 diabetes mellitus; UAlb, urinary albumin.
Adding fenofibrate and time interaction term.
Model 1: Stratified by network and adjusts for glycemia trial.
Model 2: Stratified by network and adjusts for factors in model 1 and for demographics: age, gender, race.
Model 3: (Development of microalbuminuria/Incident CKD) Stratified by network and glycemia trial, and adjusts for factors in model 2 and for kidney-specific factors: month 4 eGFR.
Model 3: (Development of macroalbuminuria) Stratified by network and glycemia trial, and adjusts for factors in model 2 and for kidney-specific factors: month 4 eGFR, microalbuminuria.
Model 3: (Kidney failure OR ESRD (dialysis) OR SCr >3.3) Stratified by network and glycemia trial, and adjusts for factors in model 2 and for kidney-specific factors: month 4 eGFR, microalbuminuria, macroalbuminuria.
Model 4: Stratified by network and glycemia trial, and adjusts for factors in model 3 and for presence of comorbidities at baseline: systolic blood pressure, body mass index, HbA1c, smoking status, cholesterol, T2DM duration, history of heart failure, history of CVD (myocardial infarction, stroke, revascularization, or angina), history of retinopathy, and for baseline use of medications: ACE/ARB, insulin, TZD.
Figure 4.
Proportion of Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid Trial participants free from development or progression of chronic kidney disease (CKD). Proportion ACCORD Lipid Trial participants free from microalbuminuria (a); macroalbuminuria (b); incident CKD (c); kidney failure (d).
Overall, time-varying lipid levels had little effect on kidney outcomes (Supplementary Table S1). Adjustments for follow-up levels of total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein did not attenuate the relationships between fenofibrate use and risks of development of microalbuminuria and macroalbuminuria, and the associations with incident CKD and kidney failure remained nonsignificant.
The evolution of eGFR values throughout the trial and during the observational ACCORDION study period in 2736 follow-on study participants is shown in Figure 5. Consistent with the findings in the ACCORD Lipid Trial, among the follow-on participants, we observed an early steep decline in eGFR in the fenofibrate group, which was followed by more gradual decline in eGFR in both groups. Discontinuation of fenofibrate resulted in reversal of eGFR decline, such that mean eGFR at the first posttrial time point was modestly higher in the fenofibrate arm compared with the placebo arm (77.4 ± 21.4 ml/min per 1.73 m2 vs. 75.6 ± 21.8 ml/min per 1.73 m2, P = 0.04). At the second posttrial endpoint, mean eGFR values were similar in both groups (75.4 ± 22.6 ml/min per 1.73 m2 vs. 74.3 ± 22.0 ml/min per 1.73 m2, P = 0.28).
Figure 5.
Estimated glomerular filtration rate over time in 2736 follow-on study participants according to randomization during the Action to Control Cardiovascular Risk in Diabetes Lipid Trial. Mean absolute follow-up values are shown during the trial and the follow-up observational period. Error bars indicate SEM.
Discussion
In this post hoc analysis of the ACCORD Lipid Trial, randomization to fenofibrate led to a less rapid decline in glomerular filtration rate and reduced the risks of incident microalbuminuria and macroalbuminuria, but had no effect on risks of incident CKD or kidney failure. These findings suggest that fenofibrate may have renoprotective effects, despite causing early decline in eGFR. Our findings corroborate previous studies that show an initial rise in serum creatinine when starting fibrates that is reversible on cessation of the medication.12, 13, 21, 22 In addition, we confirm prior reports that demonstrated the association of fenofibrate use with preservation of kidney function in patients with diabetes.12, 13 These findings are promising given the burden of diabetic nephropathy in the United States and the urgent need for effective therapies to slow progression of disease.
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study previously showed beneficial effects of fenofibrate on kidney function.12 This study was a randomized controlled trial with 9795 patients aged 50 to 75 with type 2 diabetes who were randomized to fenofibrate or placebo for 5 years. Similar to our analysis, the FIELD study showed that compared with placebo, use of fenofibrate led to a rise in serum creatinine in the short-term, but in the long-term it reduced eGFR decline and lowered albuminuria without impacting the rate of ESRD onset. After a washout phase of 1 year in the FIELD study, participants who previously took fenofibrate had significantly lower serum creatinine levels compared with individuals assigned to placebo.
Our findings of improved albuminuria and reduced eGFR decline associated with fenofibrate use in the ACCORD study confirm the findings of the FIELD study. For the first time, we now demonstrate that fenofibrate use did not increase the risk of incident CKD in the ACCORD population. In addition, we were able to examine changes in eGFR after stopping fenofibrate over a 4-year follow-up period, which was longer than the 1-year follow-up after fenofibrate washout in the FIELD study. Finally, the FIELD study took place in Finland, New Zealand, and Australia, whereas the ACCORD study recruited participants from 77 North American centers. Therefore, our data may be more relevant to a North American population.
Fenofibrate may have renoprotective effects by affecting circulating lipid levels. Previous studies have suggested that high lipid levels and altered lipid metabolism in diabetic kidney disease may contribute to kidney function decline.9, 10 Individuals with diabetes and CKD have high levels of circulating lipids that are filtered by the kidney and accumulate in tubular epithelial cells, which may lead to fibrosis and accelerated CKD progression.23, 24 Although we did not detect any evidence of effect mediation by lipid levels in the ACCORD Lipid Trial, the FIELD investigators found that participants with higher baseline lipid levels had greater preservation in their eGFR with fenofibrate compared with those with more normal lipid levels.12
Effects of fenofibrate on metabolism have also been proposed as a possible mechanism for nephroprotection. Patients with diabetic kidney disease have altered activity of lipid-metabolizing enzymes, which may lead to a reduction in fatty acid metabolism.25 The abnormal fatty acid levels create toxic byproducts that cause cell apoptosis, contributing to kidney failure. Because fenofibrate stimulates the enzymes that metabolize lipids within the renal cells, it has been suggested that fenofibrate may mitigate kidney damage caused by reduced fatty acid metabolism. Experimental studies support this hypothesis by demonstrating that fenofibrate increases fatty acid oxidation and protects against renal fibrosis in animals.10
Fibrates may also protect the kidney through another mechanism suggested by an experimental study of hyperfiltrating diabetic rats in whom renal cyclooxygenase-2 expression is upregulated.26 Fenofibrate treatment lowered GFR, reduced renal cyclooxygenase-2 expression, and prevented the rise in renal prostaglandin production in response to vasoconstriction in this animal model. The investigators concluded that fenofibrate may attenuate diabetes-induced hyperfiltration by preventing the upregulation of renal cyclooxygenase-2 expression that accompanies diabetes.26
Other studies suggest that fenofibrate has anti-inflammatory effects with reduction of inflammatory cytokines, which may also have beneficial effects on the kidney.27 Given this and other evidence for pleotropy of fenofibrate, which includes beneficial effects on endothelium, coagulation and fibrinolytic pathways, oxidative stress, and hyperuricemia,28 additional research is needed to further elucidate the exact mechanisms behind the renoprotection we and others observed with long-term use of fenofibrate in diabetic patients.
Strengths of our study include use of data from a large randomized controlled trial that assessed predefined microvascular endpoints, including microalbuminuria, macroalbuminuria, and ESRD events. In addition, we were able to assess incident CKD, which we defined based on rigid criteria. Finally, we were able to use data from the follow-on observational period in ACCORDION, which allowed us to evaluate changes in eGFR after discontinuation of randomized interventions in the ACCORD Lipid Trial.
Our study had several limitations. It is a post hoc analysis of the ACCORD Lipid Trial, and thus our results are hypothesis-generating. Given the short study duration and that patients with creatinine greater than 1.5 were excluded from this study, there were few incident ESRD events. Thus, it is unclear what effect, if any, fenofibrate has on this outcome. In addition, most patients in the ACCORD study were middle-aged white males with mostly normal kidney function. These findings may not apply to minorities, younger populations, or those with advanced kidney dysfunction.
In our study, we found that patients with diabetes had slower kidney function decline when on fenofibrate than those not taking the medication. Currently, even with treatment, approximately 35% of patients with diabetes develop diabetic nephropathy. Despite medical advances, this rate has been stable for approximately 20 years.7 Given the high morbidity and mortality associated with kidney disease, new medications are needed for the treatment of diabetic nephropathy. Fenofibrate may be a tool to help reduce diabetic complications in a vulnerable population. However, additional studies should be done to further elucidate the effects of fenofibrate on kidney disease.
Disclosure
TI received funding from Gilead, GSK, Boehringer Ingelheim, and Regeneron. She has received grant support from Shire and honorarium from Bayer. RM has interest in Abbot Laboratories, AbbVie, Inc., and Teva Pharmaceuticals Industries Ltd. KS has received funding from Gilead, GSK, Boehringer Ingelheim, Regeneron, Merk and Ono Pharmacuetical. All the other authors declared no competing interests.
Acknowledgments
This manuscript was prepared using ACCORD Research Materials obtained from the National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ACCORD or the NHLBI.
This work was supported by grants R01DK102438 (TI), R01DK110087 (TI), and T32DK108738 (RF and AS) from the National Institutes of Health, United States. The authors were responsible for the design, analysis, and interpretation of results, in addition to reviewing and approving the manuscript for publication. The funders had no role in the design, analysis, or interpretation of data, preparation and review of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Table S1. Risks of kidney outcomes by fenofibrate randomization arm.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Risks of kidney outcomes by fenofibrate randomization arm.
References
- 1.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2016. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 2.Barkoudah E., Skali H., Uno H. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc. 2012;1:8–15. doi: 10.1161/JAHA.111.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkarian M., Sachs M.C., Kestenbaum B. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann J.F.E., Orsted D.D., Brown-Frandsen K. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 5.Wanner C., Inzucchi S.E., Lachin J.M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 6.Neal B., Perkovic V., Mahaffey K.W. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 7.de Boer I.H., Rue T.C., Hall Y.N. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregg E.W., Li Y., Wang J. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 9.Chahil T.J., Ginsberg H.N. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35:491–510. doi: 10.1016/j.ecl.2006.06.002. vii–viii. [DOI] [PubMed] [Google Scholar]
- 10.Stadler K., Goldberg I.J., Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15:40. doi: 10.1007/s11892-015-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler D.C. Does lipid-lowering therapy slow progression of chronic kidney disease? Am J Kidney Dis. 2004;44:917–920. [PubMed] [Google Scholar]
- 12.Davis T.M., Ting R., Best J.D. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011;54:280–290. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 13.Mychaleckyj J.C., Craven T., Nayak U. Reversibility of fenofibrate therapy-induced renal function impairment in ACCORD type 2 diabetic participants. Diabetes Care. 2012;35:1008–1014. doi: 10.2337/dc11-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg H.N., Elam M.B., Lovato L.C. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buse J.B., Bigger J.T., Byington R.P. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Gerstein H.C., Miller M.E., Byington R.P. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cushman W.C., Evans G.W., Byington R.P. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ismail-Beigi F., Craven T., Banerji M.A. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Group A.S. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39:701–708. doi: 10.2337/dc15-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hottelart C., El Esper N., Rose F. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002;92:536–541. doi: 10.1159/000064083. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y.Y., Weir M.A., Manno M. New fibrate use and acute renal outcomes in elderly adults: a population-based study. Ann Intern Med. 2012;156:560–569. doi: 10.7326/0003-4819-156-8-201204170-00003. [DOI] [PubMed] [Google Scholar]
- 23.Susztak K., Ciccone E., McCue P. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggiero C., Elks C.M., Kruger C. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am J Physiol Renal Physiol. 2014;306:F896–F906. doi: 10.1152/ajprenal.00484.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son N.H., Yu S., Tuinei J. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y.J., Quilley J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal cyclooxygenase-2 expression, and enhanced renal prostaglandin release. J Pharmacol Exp Ther. 2008;324:658–663. doi: 10.1124/jpet.107.129197. [DOI] [PubMed] [Google Scholar]
- 27.Han S.H., Quon M.J., Koh K.K. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptor-alpha activators. Hypertension. 2005;46:1086–1092. doi: 10.1161/01.HYP.0000187900.36455.4c. [DOI] [PubMed] [Google Scholar]
- 28.Tsimihodimos V., Liberopoulos E., Elisaf M. Pleiotropic effects of fenofibrate. Curr Pharm Des. 2009;15:517–528. doi: 10.2174/138161209787315675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risks of kidney outcomes by fenofibrate randomization arm.