Introduction
Anti−glomerular basement membrane (anti-GBM) disease presents with rapidly progressive glomerulonephritis, often associated with alveolar hemorrhage and characterized histologically by crescentic glomerulonephritis. Typically, there is linear deposition of Ig along the glomerular basement membrane (GBM), which, in the majority of cases, is due to IgG autoantibodies directed against the noncollagenous domain of the α3 chain of type IV collagen (α3[IV]NC1).1
Early disease recognition relies on detecting circulating IgG anti-GBM antibodies in serum samples. However, conventional assays do not detect IgA antibodies or those directed against other target antigens, including α5(IV) found in some Alport disease patients following renal transplantation.2 The presence and specificity of the antibody can be confirmed by Western blotting, usually at a reference center, although this is not routinely performed.
We describe a case of anti-GBM disease mediated by IgA anti-GBM antibodies not detected by standard serological tests, and suggest a method of detection and monitoring that can be used in the right clinical context.
Case Presentation
A 37-year-old Turkish man presented with a 2-month history of breathlessness, chest pain, and weight loss. He worked as a hairdresser and had no past medical history. Investigation revealed bilateral pleural effusions and a pericardial effusion. The pleural effusion was tapped at the referring hospital, revealing an exudate. Pending the AFB culture of his pleural aspirate, he was started on empirical treatment for presumed tuberculous pleuro-pericarditis. Three weeks later, he was admitted to hospital with new-onset acute kidney injury requiring hemodialysis, and 1 week later was transferred to our renal unit for ongoing management. His serum creatinine was 1108 μmol/l, Hb 72 g/l, phosphate 3.73 mmol/l, and serum bicarbonate 16 mmol/l. His pleural aspirate was negative for AFB. He was still passing urine with nephrotic-range proteinuria (urine protein-to-creatinine ratio: 1110 mg/mmol, normal range <30) and microscopic hematuria. Immunological test results were negative for anti-GBM antibodies by enzyme-linked immunosorbent assay (EliA, Phadia using recombinant α3 chain of collagen IV as target). Antinuclear antibodies (ANAs) and antineutrophil cytoplasm antibodies (ANCAs) were negative; complement C3 and C4 levels were normal; IgG, IgM, and IgA were normal; and no paraprotein was found in serum or urine. Ultrasound examination revealed 11-cm kidneys with normal corticomedullary differentiation. Computed tomography ruled out significant alveolar hemorrhage.
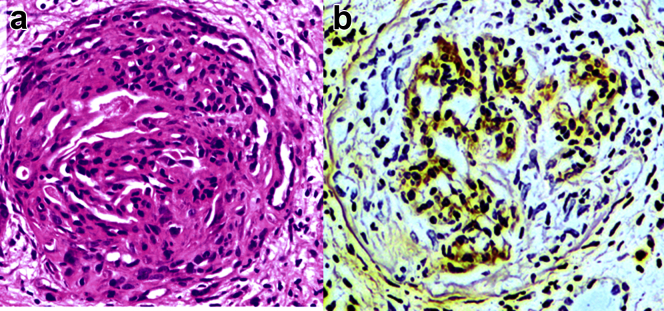
A kidney biopsy was performed. Histopathological examination revealed acute diffuse and global crescentic glomerulonephritis on the background of moderate chronic damage (Figure 1a). A total of 19 glomeruli were present, 3 of which were obsolete. All patent tufts were severely shrunken and some obliterated by large cellular crescents. The vessels showed fibrinoid necrosis adjacent to some glomerular vascular poles. There was no deposition of IgG in glomeruli, but there was linear deposition of complement C3 and IgA along the GBM as well as the Bowman’s capsule, the glomerular mesangium, and the tubular basement membranes (Figure 1b).
Figure 1.
Patient renal biopsy tissue stained with (a) hematoxylin and eosin, showing a large circumferential cellular crescent associated with compression of the glomerular tuft, and (b) by immunohistochemistry for IgA, showing linear IgA deposition along the glomerular basement membrane, Bowman’s capsule, and mesangial matrix.
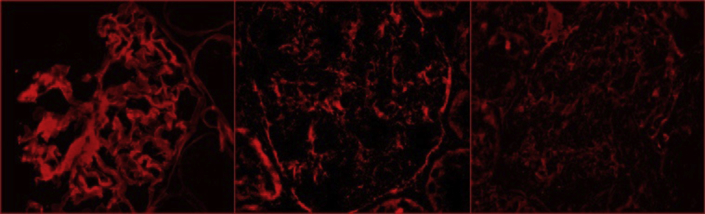
The patient received 3 pulses of methylprednisolone and daily plasma exchange. In view of his prolonged dialysis dependence and the extensive chronic damage on the biopsy sample, he was not treated with cyclophosphamide, but started mycophenolate mofetil in an attempt to accelerate autoantibody removal to facilitate transplantation. As there are no standard means for following the levels of circulating IgA anti-GBM antibodies, we modified an indirect immunofluorescence method, using patient serum to stain primate kidney sections, which confirmed prominent linear IgA binding to the glomerular and tubular basement membranes and to the Bowman’s capsule, accompanied by weaker IgA binding to mesangial areas with the patient’s acute serum (Figure 2). It was reassuring that there was no such staining with convalescent serum obtained 9 months after treatment initiation (Figure 2), allowing him to be placed on the transplant waiting list. The patient received a cadaveric renal transplant 12 months later. At the 9-month follow-up, his serum creatinine was 104 μmol/l, with no evidence of urinary abnormalities suggesting disease recurrence.
Figure 2.
Indirect immunofluorescence staining of primate kidney sections (Euroimmun, product number FB 1250-1010) incubated overnight at 4 °C with (left) control patient serum with IgG antibodies against the noncollagenous domain of the α3 chain of type IV collagen, (center) patient acute, and (right) patient convalescent serum. Patient’s and control sera (diluted 1:10) antibody binding was visualized with Alexa Fluor 647−labeled secondary antibodies (diluted 1:1000) to human IgA (diluted 1:250; center, right) or IgG (diluted 1:500, left).
Discussion
Commonly, IgG anti-GBM antibodies bind the α3 subunit of the noncollagenous (NC1) domain of type IV collagen α3(IV)NC1, distributed along the glomerular, alveolar, and cochlear basement membranes as well as the distal tubular basement membranes, resulting in linear GBM staining (Figure 2) on renal immunohistochemistry. However, the IgA staining pattern that we noted in this case appeared different, with additional prominent Bowman’s capsule and mesangial and diffuse tubular basement membrane staining, suggesting a potentially different target epitope.
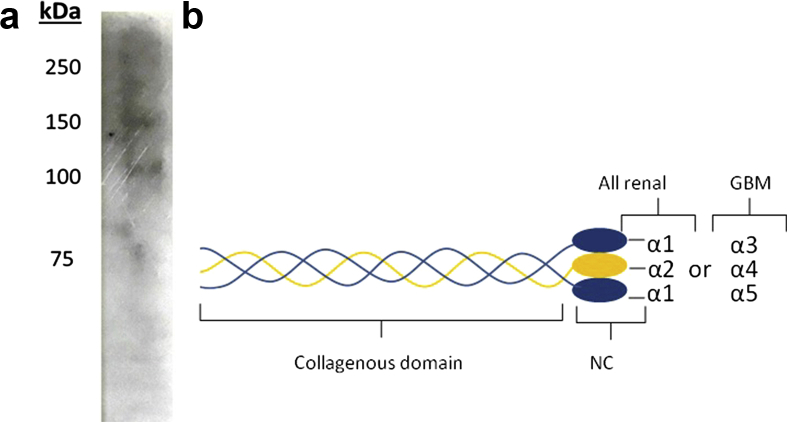
We used western blot analysis of the acute serum to characterize the IgA anti-GBM epitope. Using type IV collagen derived from collagenase-digested human GBM, no binding was found. However, using intact type IV collagen derived from human fibroblasts, we found IgA binding to proteins of molecular weights 100 and 150 kDa (Figure 3a), confirming that the IgA antibodies reacted with a collagenous portion of the molecule. As type IV collagen derived from human fibroblasts contains only α1(IV) and α2(IV) chains but no α3(IV) to α6(IV) chains, our data imply that the IgA anti-GBM antibodies bound the collagenous domains of α1(IV) or α2(IV) collagen chains (Figure 3b).
Figure 3.
Western blot analysis of the circulating IgA antibody reactivity with type IV collagen. (a) Type IV collagen antigens derived from human fibroblasts and epithelial cells (Collagen Type IV from human cell culture, Bornstein and Traub Type IV, Sigma) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (6% Tris−glycine gels) under nonreducing conditions and transferred to Immobilon P membranes for immunoblotting with patient acute serum (diluted 1:10), which was subsequently stained with a biotinylated antibody to human IgA (diluted 1:250) and visualized with horseradish peroxidase−streptavidin (diluted 1:1000). (b) Schematic representation of type IV collagen protomers (triple helical molecules). GBM, glomerular basement membrane; NC, noncollagenous domain.
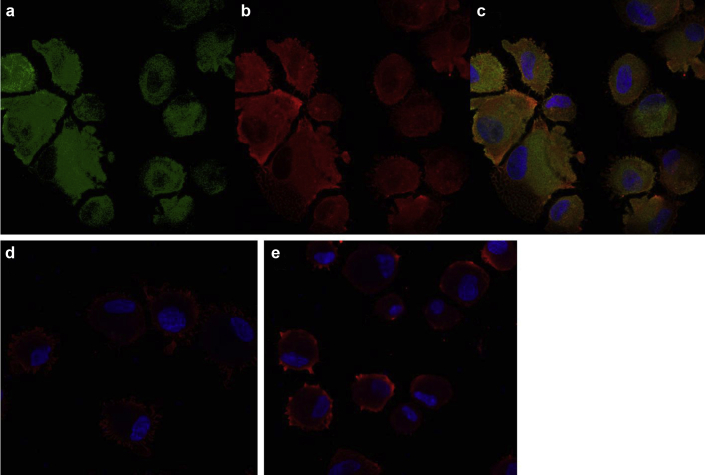
To specify whether the IgA antibody is targeted against the α1(IV) or α2(IV) collagen chains, we performed immunofluorescence on human skin fibroblasts from healthy controls. In skin fibroblasts, collagen IV is a heterotrimeric molecule containing two α1 and one α2 chains. Cells were stained with acute patient serum visualized by Alexa Fluor 647−labeled antibody to human IgA and a mouse monoclonal antibody against the α1(IV). We found extensive expression of IgA on fibroblasts with the patient’s acute serum, which colocalized with the monoclonal antibody staining to the α1 chain of type IV collagen. There was no such IgA binding found with the patient’s convalescent serum or serum from a healthy control (Figure 4). The colocalization of IgA antibody staining with the monoclonal anti-α1(IV) antibody, as well as the Western blotting with fibroblast collagen IV, and the pattern of IgA deposition along the GBM, Bowman’s capsule, mesangial matrix, and tubular basement membranes localize the IgA antibody epitope to the collagenous domain of α1(IV).
Figure 4.
Immunofluorescence of human skin fibroblasts. Fibroblasts were centrifuged using a Cytospin centrifuge on Superfrost slides (Thermofisher) (5 × 104 cells/slide), fixed, blocked with 5% goat serum, and incubated with (a) mouse antihuman α1(IV) monoclonal antibody (catalog number MAB6308) at 10 μg/ml for 3 hours at room temperature and visualized with Alexa 488 and (b) acute and (d) patient convalescent serum and (e) serum from a healthy control (all at dilution 1:10) incubated overnight at 4 °C and visualized with Alexa Fluor 647−labeled secondary antibodies (diluted 1:1000) to human IgA (diluted 1:250). (c) Merged image of (a) and (b) demonstrating colocalization of IgA antibodies in the patient’s acute serum and α1 chains of type IV collagen.
The occurrence of IgA anti-GBM disease in very uncommon. Among 53 patients who presented with anti-GBM disease in 2 local renal units over the past 3 decades, this has been the only case of IgA-mediated disease that we have encountered. So far, only 13 cases have been reported in the literature.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 In all cases, the histology was consistent with crescentic glomerulonephritis and demonstrated linear GBM staining with IgA. The percentage of crescentic glomeruli ranged from 6% to 75%. Pulmonary hemorrhage was present in 6 of 12 cases (50%). At last follow-up, 6 of 12 patients (50%) had died. Only 2 patients received a kidney transplant, in 1 of whom there was recurrence of disease 2 years later in the absence of a standard serological tests to monitor IgA anti-GBM antibody titers.5, 6, 16 These outcomes emphasize the importance of prompt diagnosis and having the means to monitor the IgA anti-GBM antibodies.
The specificity of the IgA anti-GBM antibodies has been considered only twice. Similar to the epitope that we identified, Borza et al. described the case of recurrent anti-GBM disease mediated by a monoclonal IgA antibody that recognized epitopes located in the collagenous domain of the α1/2(IV) collagen network.6 Interestingly, their patient’s IgA staining pattern also demonstrated an additional prominent Bowman’s capsule and mesangial staining, similar to our case. The Bowman’s capsule, mesangial matrix, and tubular basement membranes are rich in other α(IV) chains, supporting the location of the IgA antibody epitope on the α1/2(IV) chains.17 The second case was that of a patient with crescentic glomerulonephritis and subepidermal blisters, with IgA anti-GBM autoantibodies directed against the noncollagenous domain of the α5 and α6 chains of type IV collagen.14
In 1988, Cederholm et al. were first to describe circulating IgA−fibronectin complexes in IgA nephropathy patients.18 The IgA−fibronectin complexes have an affinity for the collagen backbone of all collagen IV chains (α1–α6). To investigate an effect of IgA−fibronectin immune complexes mediating the GBM binding, we performed western blot analysis using the patient’s acute serum binding to fibroblast-derived collagen IV and developed with an antifibronectin secondary antibody. No binding was found, suggesting that the antibody targeting the GBM was not due to formation of IgA−fibronectin complexes (data not shown).
Interestingly, in 5 of 14 case reports, the patients had concurrent autoimmune or alternative renal diseases: Henoch–Schonlein purpura/IgA nephropathy,8, 19 Crohn's disease,9 systemic lupus erythematosus,13 autoimmune bullous dermatitis,14 and thin basement membrane disease.12
Whether the coexistence of the conditions could facilitate the development of anti-GBM disease is an interesting hypothesis that remains to be proved. Ongoing glomerular inflammation may alter GBM components, triggering an autoimmune reaction to modified antigens and subsequent production of nephritogenic antibodies, as proposed for the association between anti-GBM disease and ANCA glomerulonephritis.20
Our patient received a cadaveric kidney transplant, with no evidence of recurrence at 9-month follow-up. In the past, recurrence of anti-GBM disease has occurred in patients who underwent transplantation in the presence of circulating anti-GBM antibodies. In the absence of a validated IgA-GBM detection assay, our modified indirect immunofluorescence test demonstrating a lack of IgA binding to the GBM with the patient’s convalescent serum provided reassurance for us to proceed with transplantation with minimal risk of disease recurrence (Table 1).
Table 1.
Teaching points
| • Conventional anti−glomerular basement membrane (anti-GBM) assays used in clinical practice would not detect IgA antibodies or those directed against other target antigens such α5(IV) found in some Alport disease patients following renal transplantation. |
| • Delayed establishment of the correct diagnosis and treatment of anti-GBM disease can result in need for permanent renal replacement therapy. |
| • If the clinical presentation and histopathological findings are suggestive of anti-GMB disease in the absence of positive serology results, alternative laboratory tests such as immunoblotting and indirect immunofluorescence can be used to confirm the diagnosis. |
Disclosure
All the authors declared no competing interests.
Acknowledgments
MA is a Medical Research Council Clinical Research Fellow.
References
- 1.Turner N., Mason P.J., Brown R. Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest. 1992;89:592–601. doi: 10.1172/JCI115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browne G., Brown P.A.J., Tomson C.R.V. Retransplantation in Alport post-transplant anti-GBM disease. Kidney Int. 2004;65:675–681. doi: 10.1111/j.1523-1755.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakano H., Suzuki A., Tojima H. A case of Goodpasture’s syndrome with IgA antibasement membrane antibody [in Japanese] Nihon Kyobu Shikkan Gakkai Zasshi. 1990;28:634–638. [PubMed] [Google Scholar]
- 4.Savage C.O., Pusey C.D., Bowman C. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. BMJ. 1986;292:301–304. doi: 10.1136/bmj.292.6516.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fervenza F.C., Terreros D., Boutaud A. Recurrent Goodpasture’s disease due to a monoclonal IgA-kappa circulating antibody. Am J Kidney Dis. 1999;34:549–555. doi: 10.1016/s0272-6386(99)70084-3. [DOI] [PubMed] [Google Scholar]
- 6.Borza D.-B., Chedid M.F., Colon S. Recurrent Goodpasture’s disease secondary to a monoclonal IgA1-κ antibody autoreactive with the α1/α2 chains of type IV collagen. Am J Kidney Dis. 2005;45:397–406. doi: 10.1053/j.ajkd.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 7.Maes B., Vanwalleghem J., Kuypers D. IgA antiglomerular basement membrane disease associated with bronchial carcinoma and monoclonal gammopathy. Am J Kidney Dis. 1999;33:E3. doi: 10.1016/s0272-6386(99)70324-0. [DOI] [PubMed] [Google Scholar]
- 8.Carreras L., Poveda R., Bas J. Goodpasture syndrome during the course of a Schönlein-Henoch purpura. Am J Kidney Dis. 2002;39:E21. doi: 10.1053/ajkd.2002.32799. [DOI] [PubMed] [Google Scholar]
- 9.Shaer A.J., Stewart L.R., Cheek D.E. IgA antiglomerular basement membrane nephritis associated with Crohn’s disease: a case report and review of glomerulonephritis in inflammatory bowel disease. Am J Kidney Dis. 2003;41:1097–1109. doi: 10.1016/s0272-6386(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 10.Gris P., Pirson Y., Hamels J. Antiglomerular basement membrane nephritis induced by IgA1 antibodies. Nephron. 1991;58:418–424. doi: 10.1159/000186473. [DOI] [PubMed] [Google Scholar]
- 11.Border W.A., Baehler R.W., Bhathena D., Glassock R.J. IgA antibasement membrane nephritis with pulmonary hemorrhage. Ann Intern Med. 1979;91:21–25. doi: 10.7326/0003-4819-91-1-21. [DOI] [PubMed] [Google Scholar]
- 12.de Caestecker M.P., Hall C.L., MacIver A.G. Atypical antiglomerular basement membrane disease associated with thin membrane nephropathy. Nephrol Dial Transplant. 1990;5:909–913. doi: 10.1093/ndt/5.11.909. [DOI] [PubMed] [Google Scholar]
- 13.Ho J., Gibson I.W., Zacharias J. Antigenic heterogeneity of IgA anti-GBM disease: new renal targets of IgA autoantibodies. Am J Kidney Dis. 2008;52:761–765. doi: 10.1053/j.ajkd.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghohestani R.F., Rotunda S.L., Hudson B. Crescentic glomerulonephritis and subepidermal blisters with autoantibodies to alpha5 and alpha6 chains of type IV collagen. Lab Investig J Tech Methods Pathol. 2003;83:605–611. doi: 10.1097/01.lab.0000067497.86646.4d. [DOI] [PubMed] [Google Scholar]
- 15.Ke C.-L., Wen Y.-K., Chen M.-L. IgA variant of anti-glomerular basement membrane glomerulonephritis associated with pulmonary hemorrhage and microangiopathic hemolytic anemia. Ren Fail. 2012;34:657–660. doi: 10.3109/0886022X.2012.664807. [DOI] [PubMed] [Google Scholar]
- 16.Moulis G., Huart A., Guitard J. IgA-mediated anti-glomerular basement membrane disease: an uncommon mechanism of Goodpasture’s syndrome. Clin Kidney J. 2012;5:545–548. doi: 10.1093/ckj/sfs087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidet L., Cai Y., Guicharnaud L. Glomerular expression of type IV collagen chains in normal and X-linked Alport syndrome kidneys. Am J Pathol. 2000;156:1901–1910. doi: 10.1016/S0002-9440(10)65063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cederholm B., Wieslander J., Bygren P., Heinegård D. Circulating complexes containing IgA and fibronectin in patients with primary IgA nephropathy. Proc Natl Acad Sci U S A. 1988;85:4865–4868. doi: 10.1073/pnas.85.13.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang A., Wang Y., Wang G. Mesangial IgA deposits indicate pathogenesis of anti-glomerular basement membrane disease. Mol Med Rep. 2012;5:1212–1214. doi: 10.3892/mmr.2012.809. [DOI] [PubMed] [Google Scholar]
- 20.Pedchenko V., Bondar O., Fogo A.B. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]