Abstract
Introduction
Cardiac biomarkers soluble ST2 (sST2) and galectin-3 may reflect cardiac inflammation and fibrosis. It is plausible that these mechanisms may also contribute to the progression of kidney disease. We examined associations of sST2 and galectin-3 with kidney function decline in participants with chronic kidney disease (CKD).
Methods
This was a pooled analysis of 2 longitudinal cohorts of participants with CKD: the Clinical Phenotyping and Resource Biobank (C-PROBE) study and the Seattle Kidney Study (SKS). We measured circulating concentrations of sST2 and galectin-3 at baseline. Our primary outcome was progression to estimated glomerular filtration rate (eGFR) <15 ml/min per 1.73 m2 or end-stage renal disease (ESRD). We used competing risk Cox regression models to study the association of sST2 and galectin-3 with CKD progression, adjusting for demographics, kidney function, and comorbidity.
Results
Among the 841 participants in the pooled cohort, baseline eGFR was 51 ± 27 ml/min per 1.73 m2 and median urine albumin-to-creatinine ratio (UACR) was 141 (interquartile range = 15−736) mg/g. Participants with higher sST2 and galectin-3 were more likely to be older, to have heart failure and diabetes, and to have lower eGFR. Adjusting for demographics, kidney function, and comorbidity, every doubling of sST2 was not associated with progression to eGFR <15 ml/min per 1.73 m2 or ESRD (adjusted hazard ratio 1.02, 95% confidence interval = 0.76−1.38). Every doubling of galectin-3 was significantly associated with a 38% (adjusted hazard ratio = 1.35, 95% confidence interval = 1.01−1.80) increased risk of progression to eGFR <15 ml/min per 1.73 m2 or ESRD.
Conclusion
Higher concentrations of the cardiac biomarker galectin-3 may be associated with progression of CKD, highlighting potential novel mechanisms that may contribute to the progression of kidney disease.
Key words: CKD, galectin-2, sST2
Heart failure is common among patients with chronic kidney disease (CKD) and end-stage-renal disease (ESRD),1, 2 and is associated with significant morbidity and mortality in these populations.3, 4, 5, 6 Heart failure is also associated with kidney function decline in patients with5, 7, 8 and without9 CKD. Recent studies have suggested that circulating heart failure biomarkers are also associated with kidney function decline.10, 11, 12 Novel heart failure biomarkers that are thought to signal cardiac inflammation and fibrosis have been recently identified, and may also link cardiovascular disease (CVD) with kidney disease.
Soluble ST2 (sST2) is a member of the interleukin (IL)−1 receptor family that is thought to induce inflammation, myocardial hypertrophy, and fibrosis by neutralizing the effects of IL-33.13 Galectin-3 is a β-galactoside−binding lectin expressed ubiquitously and thought to be involved in inflammation and fibrosis in both the heart and kidney.14, 15, 16 Several studies have established a link between these biomarkers and heart failure, as well as with major cardiovascular events and death,11, 13, 16, 17, 18 although less is known about their relationship with kidney disease. It is possible that elevations in these biomarkers represent parallel processes affecting both the kidneys and heart, or, alternatively, heart disease leading to kidney disease.
Cross-sectional studies have shown associations of sST2 and galectin-3 with lower estimated glomerular filtration rate (eGFR)19, 20, 21, 22, 23 and albuminuria.24 However, the few studies that have evaluated the associations of sST2 and galectin-3 with longitudinal kidney function decline have produced somewhat conflicting results. An earlier study found that neither sST2 nor galectin-3 was associated with rapid kidney function decline or development of ESRD in older adults without CKD.25 However, sST2 has suggestive associations with incident albuminuria in patients without CKD11 and has also been found to predict acute kidney injury.26 Similarly, galectin-3 has been associated with rapid decline in kidney function, incident CKD, and incident albuminuria in patients without CKD.27 Few studies have evaluated these biomarkers in the CKD population, who are at greatest risk for progressive loss of kidney function. Therefore, in this study, we examined the association of circulating sST2 and galectin-3 with kidney function decline in patients with CKD in a pooled study of 2 longitudinal cohort studies, the Clinical Phenotyping and Resource Biobank (C-PROBE) study and the Seattle Kidney Study (SKS).
Methods
Study Populations
This was a pooled study of 2 longitudinal CKD cohorts: the C-PROBE study and the SKS.
Clinical Phenotyping and Resource Biobank Study
The C-PROBE study, supported by the George M. O’Brien Michigan Kidney Translational Core Center at the University of Michigan, is a multicenter ongoing, prospective observational cohort study that began enrollment in March 2009. Patients with CKD stage 1 to 4 are recruited from 6 sites in the United States: John H. Stroger Hospital, Chicago; Renaissance Renal Research Institute, Detroit; University of Michigan Nephrology Program, Ann Arbor; Wayne State University Nephrology Program, Detroit; Temple University Nephrology Program, Philadelphia; and Carolinas Medical Center, Levine Children’s Hospital, Charlotte (http://kidneycenter.med.umich.edu/C-PROBE).28, 29, 30 Patient demographics, socioeconomic variables, clinical information and biosamples were collected at enrollment and annually thereafter. Patients with polycystic kidney disease and those who underwent dialysis or transplantation were excluded. At the time of this study, 741 participants were enrolled in C-PROBE. Inclusion criteria for this study were age >18 years and baseline eGFR >15 and ≤90 ml/min per 1.73 m2. With these exclusions, 561 participants were included in our analysis (Supplementary Figure S1).
Seattle Kidney Study
The SKS is a nephrology clinic−based, ongoing cohort study of persons with CKD, which began recruitment in June 2004.31, 32, 33, 34 Participants were recruited from outpatient nephrology clinics at the University of Washington Medical Center, Harborview Medical Center, and the Veterans Affairs Puget Sound Health Care Center in Seattle, Washington. Inclusion criteria were age ≥18 years and either eGFR ≤90 ml/min per 1.73 m2 or a urinary protein-to-creatinine ratio of >30 mg/g. Exclusion criteria included current dialysis, current or previous kidney transplantation, inability to provide informed consent, or expectation of dialysis initiation within 3 months. The study was approved by the University of Washington Institutional Review Board. The protocol includes annual in-person study visits with blood collections that are separate from the clinical events and therefore reflective of ambulatory health status. A total of 530 participants met inclusion criteria and were enrolled in SKS. Inclusion criteria for our study were: available follow-up beyond baseline study visit, available biospecimens, and baseline eGFR >15 ml/min per 1.73 m2. With these exclusions, 280 participants were included in this analysis (Supplementary Figure S1).
Circulating sST2 and Galectin-3 Measurement in C-PROBE and SKS
Soluble ST2 and galectin-3 concentrations were measured in plasma (C-PROBE) or serum (SKS) using enzyme-linked immunosorbent assay (for sST2: Critical Diagnostics, San Diego, CA; for galectin-3: R&D Systems, Minneapolis, MN). All assays were performed at the University of Washington. The absorbances for each analyte were measured by spectrophotometry (450 nm) and unknown concentrations determined through a 4-parameter logistic curve fit. The intraassay coefficients of variation were 9.58% for sST2 and 9.80% for galectin-3. We performed duplicate measures of each biomarker in 44 participants in our study, which showed good repeatability (Pearson correlation coefficients, 0.9588 for sST2 and 0.9407 for galectin-3).
Progression of Kidney Disease
Progression of kidney disease was defined as progression to eGFR <15 ml/min per 1.73 m2 or ESRD (defined as need for dialysis or transplantation). End-stage renal disease was determined by review of the medical records and participant self-report. End of the follow-up was July 2016 for C-PROBE and February 2013 for SKS. Serum creatinine was measured by the modified rate Jaffe method with an assay traceable to isotope dilution mass spectrometry. We calculated eGFR annually using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.35
Covariates
Covariates were obtained from clinical records for participants in C-PROBE and from research study visits in SKS. Prevalent conditions were determined on the basis of physician diagnosis in the medical record (C-PROBE) or by self-report (SKS). In C-PROBE, medications were ascertained from the patients’ medical records. In SKS, medications were assessed by inventory assessment, and missing medication data were completed by chart review.36 In SKS, diabetes was defined by any of the following: use of an oral hypoglycemic medication or insulin, fasting blood sugar ≥126 mg/dl, nonfasting blood sugar ≥200 mg/dl, or hemoglobin A1c ≥6.5%. In SKS, 3 seated blood pressure (BP) measurements were recorded using an automated sphygmomanometer; the average of the last 2 readings was retained for analysis. Hypertension was defined by the use of any antihypertensive medication, systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg. Body mass index (BMI) was measured at study visits. General chemistries were measured on Beckman-Coulter DxC autoanalyzer (Beckman-Coulter, Brea, CA). Urine albumin and creatinine were measured in spot morning or overnight urine collections, by a timed end point method and with the modified Jaffe method.
Statistical Analyses
This was a pooled analysis of C-PROBE and SKS. Characteristics of the pooled study populations were described across quartiles of each biomarker. Spearman correlation coefficients were calculated for each biomarker of interest and kidney function measures, with statistical significance at P values <0.05.
Unadjusted incidence rates for progression to eGFR <15 ml/min per 1.73 m2 or ESRD (per doubling of each biomarker) were calculated as the number of events divided by person-years at risk in both study cohorts. Kaplan−Meier curves were generated to examine the rates of CKD progression across quartiles of sST2 and galectin-3. We used a Cox proportional hazards regression in a competing risks context using the method proposed by Lunn and McNeil37 to test the association of each biomarker (per doubling and in quartiles) at baseline and risk of progression to eGFR <15 ml/min to 1.73 m2 or ESRD. This approach stratifies on event type and allows for estimation of the separate associations of each risk factor with the relative hazard of each outcome under a proportional hazards assumption. Covariates for adjustment were selected a priori based on previous literature regarding potential confounders. Nested models were used to explore the confounding effects of demographics, known cardiovascular risk factors, and kidney function. The model adjusted for age, race, gender, log(eGFR), log(urine albumin-to-creatinine ratio [UACR]), education, body mass index (BMI), smoking, prevalent heart failure, prevalent coronary artery disease, prevalent diabetes, systolic BP, and antihypertensive medication use. Interaction of each biomarker with baseline eGFR was assessed in each model. Censoring events included loss to follow-up (1% in SKS and 0% in C-PROBE) or end of the study period (23 February 2013 for SKS and 29 July 2016 for C-PROBE). Patients were followed forward from study enrollment to the first occurrence of a study outcome, censoring event, or competing event (death).
All analyses were performed using Stata version 13.1 (StataCorp, College Station, TX) and IBM SPSS Statistics for Windows version 23.0 (IBM Corp., Armonk, NY).
Results
Characteristics of Study Populations
Mean ages of participants from C-PROBE and SKS were 55 and 62 years, respectively. In comparison to C-PROBE, SKS had more male and white participants. In comparison to C-PROBE, SKS participants were more likely to have prevalent cardiovascular disease, diabetes, and hypertension, and to use statins at baseline. Mean eGFR was 55 and 42 ml/min per 1.73 m2, and median UACR was 217 and 118 mg/g, in C-PROBE and SKS participants, respectively (Supplementary Table S1).
In the pooled study population of 841 participants, those in the higher quartiles of sST2 were more likely to be older and male and to have prevalent CVD and heart failure, higher blood pressure, lower eGFR, and higher UACR (Table 1). Participants in the highest quartile of galectin-3 were more likely to be older and female and to have prevalent CVD and diabetes, higher blood pressure, higher BMI, lower eGFR, and higher UACR (Table 2).
Table 1.
Baseline characteristics of pooled cohort across quartiles of soluble ST2 (sST2)
| Participant characteristics | Overall, N (%) | sST2 quartiles (ng/ml), N (%) |
|||
|---|---|---|---|---|---|
| <20.52 | 20.52−26.00 | 26.01−34.14 | >34.14 | ||
| n | 841 | 211 | 210 | 210 | 210 |
| Cohort | |||||
| SKS | 280 | 30 (14) | 52 (25) | 79 (38) | 119 (57) |
| C-PROBE | 561 | 181 (86) | 158 (75) | 131 (62) | 91 (43) |
| Age, yr | 57 (16) | 57 (16) | 56 (16) | 57 (15) | 59 (15) |
| Male | 465 (55) | 78 (37) | 111 (53) | 131 (62) | 145 (69) |
| Race | |||||
| White | 464 (55) | 102 (49) | 123 (59) | 114 (54) | 125 (60) |
| Black | 289 (34) | 86 (41) | 72 (34) | 67 (32) | 64 (31) |
| Other | 86 (10) | 22 (11) | 15 (7) | 29 (14) | 20 (10) |
| Education | |||||
| <High school | 83 (10) | 22 (11) | 14 (7) | 29 (14) | 18 (9) |
| Completed high school | 444 (55) | 105 (51) | 116 (58) | 111 (54) | 112 (57) |
| Completed college or more | 285 (35) | 81 (39) | 69 (35) | 67 (32) | 68 (34) |
| Current smoker | 106 (13) | 25 (12) | 29 (14) | 26 (12) | 26 (12) |
| Prevalent CVD | 303 (36) | 60 (28) | 75 (36) | 76 (36) | 92 (44) |
| Prevalent heart failure | 118 (14) | 14 (7) | 32 (15) | 29 (14) | 43 (21) |
| Statin use | 353 (42) | 74 (35) | 73 (35) | 101 (48) | 105 (50) |
| Diabetes | 354 (42) | 66 (31) | 86 (41) | 88 (42) | 114 (54) |
| Hypertension | 724 (86) | 168 (80) | 182 (87) | 179 (85) | 195 (93) |
| Antihypertensive use | 686 (82) | 161 (76) | 172 (82) | 170 (81) | 183 (88) |
| SBP, mm Hg | 132 (20) | 128 (17) | 134 (22) | 132 (19) | 135 (20) |
| DBP, mm Hg | 75 (12) | 74 (11) | 75 (12) | 74 (12) | 75 (12) |
| BMI, kg/m2 | 31.7 (7.9) | 32.0 (8.3) | 31.0 (6.9) | 32.4 (8.3) | 31.4 (8.0) |
| eGFR, ml/min per 1.73 m2 | 51 (27) | 54 (29) | 51 (27) | 51 (28) | 46 (26) |
| UACR, mg/g | 141 [15−736] | 47 [8−396] | 110 [11−668] | 172 [17−754] | 290 [34−1186] |
BMI, body mass index; C-PROBE, Clinical Phenotyping and Resource Biobank; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SKS, Seattle Kidney Study; UACR, urine albumin-to-creatinine ratio.
Data are N (%) or [interquartile range].
Table 2.
Baseline characteristics of pooled cohort across quartiles of galectin-3
| Participant characteristics | Galectin-3 quartiles (ng/ml) |
|||
|---|---|---|---|---|
| <9.04 | 9.04−12.65 | 12.66− 16.76 | >16.76 | |
| n | 211 | 210 | 210 | 210 |
| Cohort | ||||
| SKS | 54 (26) | 78 (37) | 85 (41) | 63 (30) |
| C-PROBE | 157 (74) | 132 (63) | 125 (60) | 147 (70) |
| Age, yr | 52 (16) | 58 (15) | 59 (15) | 60 (14) |
| Male | 126 (60) | 131 (62) | 108 (51) | 100 (48) |
| Race | ||||
| White | 124 (59) | 109 (52) | 125 (60) | 106 (51) |
| Black | 65 (31) | 79 (38) | 65 (31) | 80 (38) |
| Other | 22 (10) | 22 (11) | 19 (9) | 23 (11) |
| Education | ||||
| <High school | 17 (9) | 16 (8) | 22 (11) | 28 (14) |
| Completed high school | 100 (50) | 117 (57) | 115 (56) | 112 (55) |
| Completed college or more | 84 (42) | 72 (35) | 67 (33) | 62 (31) |
| Current smoker | 23 (11) | 25 (12) | 33 (16) | 25 (12) |
| Prevalent CVD | 63 (30) | 74 (35) | 71 (34) | 95 (45) |
| Prevalent heart failure | 23 (11) | 33 (16) | 24 (11) | 38 (18) |
| Prevalent CAD | 43 (20) | 50 (24) | 53 (25) | 61 (29) |
| Statin use | 65 (31) | 85 (41) | 99 (47) | 104 (50) |
| Diabetes | 65 (31) | 83 (40) | 92 (44) | 114 (54) |
| Hypertension | 165 (78) | 183 (87) | 184 (88) | 192 (91) |
| Antihypertensive use | 157 (74) | 164 (78) | 177 (84) | 188 (90) |
| SBP, mm Hg | 129 (17) | 132 (20) | 132 (19) | 135 (22) |
| DBP, mm Hg | 74 (11) | 76 (12) | 75 (12) | 74 (12) |
| BMI, kg/m2 | 30.1 (7.3) | 31.7 (7.7) | 31.1 (7.2) | 33.9 (8.8) |
| eGFR, ml/min per 1.73 m2 | 65 (30) | 53 (24) | 45 (24) | 39 (25) |
| UACR, mg/g | 93 [11−601] | 93 [13−366] | 230 [15−934] | 329 [42−1186] |
BMI, body mass index; C-PROBE, Clinical Phenotyping and Resource Biobank; CAD, coronary artery disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; SKS, Seattle Kidney Study; UACR, urine albumin-to-creatinine ratio.
Data are N (%) or [interquartile range].
Correlations of sST2 and Galectin-3 With Measures of Kidney Function
In the pooled cohort, eGFR and UACR were significantly correlated with both sST2 and galectin-3 (Table 3). The strongest correlations were seen for eGFR with galectin-3 and UACR with sST2.
Table 3.
Correlations of galectin-3, soluble ST2, and measures of kidney function in the pooled cohort
| Measure | Creatinine | eGFR | UACR | Galectin-3 | sST2 |
|---|---|---|---|---|---|
| Creatinine | 1 | −0.919a | 0.108b | 0.353a | 0.148a |
| eGFR | 1 | −0.119a | −0.426a | −0.098a | |
| UACR | 1 | 0.164a | 0.203a | ||
| Galectin-3 | 1 | 0.148a | |||
| sST2 | 1 |
eGFR, estimated glomerular filtration rate; sST2, soluble ST2; UACR, urine albumin-to-creatinine ratio.
Correlation significant at the 0.01 level.
Correlation significant at the 0.05 level.
Association of sST2 and Progression of CKD
Median follow-up time was 2.81 (interquartile range [IQR] = 1.78−3.98) and 3.41 (IQR = 1.84−) years in C-PROBE and SKS, respectively. The mean numbers of eGFR measurements were 3.05 ± 1.74 and 2.99 ± 1.45 measurements in C-PROBE and SKS, respectively.
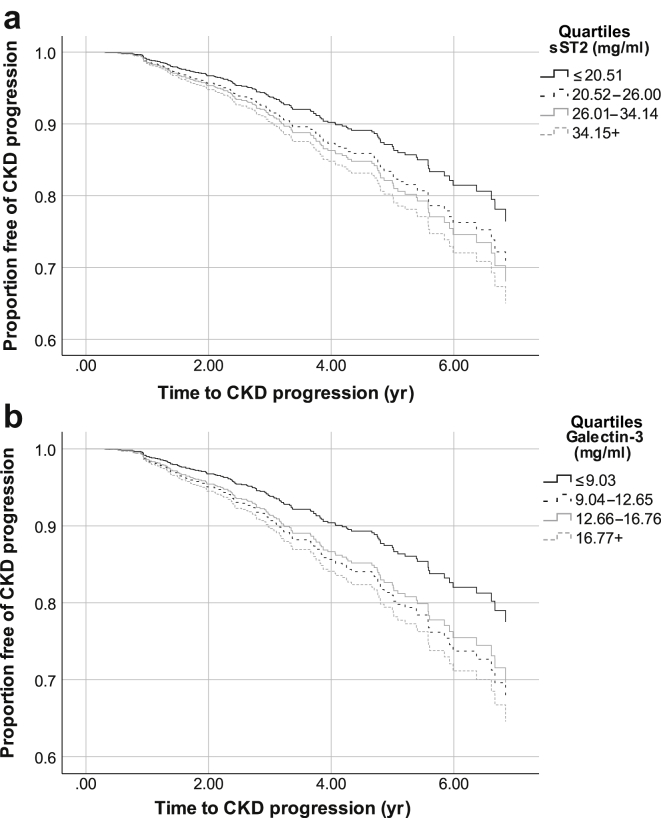
In the pooled cohort, median follow-up time was 3.10 (IQR = 1.83−4.83) years. Incidence rates of ESRD or eGFR <15 ml/min per 1.73 m2 were higher among participants in the top 3 quartiles compared with the lowest quartile of sST2. The proportion of patients with CKD progression over time was greatest among those with the highest sST2 (Figure 1a). When examining sST2 continuously or in categories, there was no association of higher sST2 with progression to ESRD or eGFR <15 ml/min per 1.73 m2 in unadjusted or adjusted models (Table 4). The interaction between sST2 and baseline eGFR was not statistically significant (P = 0.3).
Figure 1.
(a) Kaplan−Meier curve of chronic kidney disease (CKD) progression across quartiles of sST2. (b) Kaplan−Meier curve risk of CKD progression across quartiles of galectin-3.
Table 4.
Association of sST2 and galectin-3 with progression of chronic kidney disease (CKD)
| Cardiac biomarker | Incidence rate (per 1000 person- yr) | No. of events | Unadjusted |
ESRD or eGFR <15 |
||||
|---|---|---|---|---|---|---|---|---|
| Adjusted for age, sex, race, and study cohort |
Fully adjusted modela |
|||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||
| sST2 (ng/ml) | ||||||||
| Quartile 1 (<20.52) | 39.4 | 34 | Ref | Ref | Ref | |||
| Quartile 2 (20.52−26.00) | 51.2 | 37 | 1.29 (0.80−2.08) | 1.35 (0.84−2.18) | 1.38 (0.84−2.27) | |||
| Quartile 3 (26.01−34.14) | 49.4 | 34 | 1.26 (0.77−2.05) | 1.49 (0.91−2.45) | 1.36 (0.81−2.29) | |||
| Quartile 4 >34.14 | 59.4 | 38 | 1.37 (0.85−2.20) | 1.49 (0.91−2.45) | 1.54 (0.92−2.58) | |||
| Continuous (per doubling) | 1.03 (0.79−1.36) | 0.824 | 1.06 (0.81−1.40) | 0.676 | 1.02 (0.76−1.38) | 0.873 | ||
| Galectin-3 (ng/ml) | ||||||||
| Quartile 1 (<9.04) | 25.6 | 20 | Ref | Ref | Ref | |||
| Quartile 2 (9.04–12.65) | 41.1 | 28 | 1.62 (0.91−2.87) | 1.59 (0.89−2.84) | 1.49 (0.82−2.71) | |||
| Quartile 3 (12.66–16.76) | 54.5 | 37 | 1.98 (1.15−3.41) | 2.09 (1.20−3.64) | 1.33 (0.73−2.39) | |||
| Quartile 4 (>16.76) | 79.1 | 55 | 2.92 (1.75−4.87) | 3.03 (1.78−5.13) | 1.58 (0.88−2.83) | |||
| Continuous (per doubling) | 1.77 (1.41−2.22) | <0.001 | 1.76 (1.40−2.20) | <0.001 | 1.35 (1.01−1.80) | 0.044 | ||
CI, confidence interval; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HR, hazard ratio; Ref, reference.
Adjusted for age, race, gender, study cohort, log(eGFR), log(urine albumin-to-creatinine ratio), education, body mass index, smoking, prevalent coronary artery disease, prevalent heart failure, prevalent diabetes, systolic blood pressure, and antihypertensive medication use.
Association of Galectin-3 and Progression of CKD
The incident rates for CKD progression increased with higher quartiles of galectin-3 (Table 4). The proportion of patients with CKD progression over time was greatest among those with the highest galectin-3 (Figure 1b). In quartile analyses, there was a graded association between higher galectin-3 with CKD progression in unadjusted models. However, these associations were attenuated and no longer statistically significant with adjustment for potential confounders in the final model (with the greatest attenuation with adjustment for eGFR). In continuous analyses, higher concentrations of galectin-3 were associated with increased risk of ESRD or eGFR <15 ml/min per 1.73 m2 in unadjusted models (hazard ratio = 1.77, 95% CI = 1.41−2.22 for every doubling of galectin-3). Each doubling of galectin-3 was associated with a 35% (adjusted hazard ratio 1.38, 95% CI = 1.01−1.80) increased the risk of progression to ESRD or eGFR <15 ml/min per 1.73 m2 with adjustment for demographic characteristics, kidney function, clinical characteristics, and medication use (Table 4). There was no interaction between galectin-3 and baseline eGFR (P = 0.6).
Discussion
In this pooled study of 2 CKD cohorts, we found that the novel cardiac biomarker galectin-3 may be associated with progression of CKD, independent of demographic characteristics, kidney function, comorbid conditions, or medication use. The association of sST2 with progression of CKD was not statistically significant. These findings highlight possible shared cardiac and renal mechanisms that contribute to the progression of kidney disease.
Higher levels of circulating galectin-3 were associated with increased risk of progression to ESRD or eGFR <15 ml/min per 1.73 m2. In the heart, galectin-3 is linked to cardiac fibrosis, remodeling, and ventricular dysfunction.16, 38 In support of these mechanisms, clinical studies have reported an association of higher galectin-3 with all-cause mortality and cardiovascular mortality in patients with impaired kidney function19 and those on hemodialysis.39, 40 Galectin-3 has been linked to the pathogenesis of several kidney diseases as well, including diabetic nephropathy,41 infections, acute tubular necrosis, autosomal recessive polycystic kidney disease, renal cell carcinoma, lupus nephritis, and chronic allograft rejection.15 Galectin-3 is thought to mediate kidney injury by modulating the systemic and local inflammatory response42, 43 and promoting renal fibrosis.44, 45 Some, but not all,25 clinical studies have shown an association between higher galectin-3 and kidney function decline. Among 2450 participants without CKD in the Framingham Offspring Study, higher galectin-3 was associated with a 50% increased risk of incident CKD and rapid decline in kidney function, but not with incident albuminuria.27 In contrast, our previous study of older adults found no association of higher galectin-3 with rapid decline of kidney function.25 Our findings augment this prior literature by reporting a possible association between higher galectin-3 with CKD progression in 2 well-defined CKD cohorts who are at high risk for adverse kidney outcomes.
We did not find a significant association between higher sST2 with CKD progression in this pooled analysis of 2 CKD cohorts. Experimental models have shown that sST2, an IL-1−like cytokine receptor for IL-33, is expressed by cardiac myocytes in response to myocardial infarction46 and mediates injury by neutralizing the cardioprotective effects of IL-33, which reduces cardiac hypertrophy, fibrosis, and apoptosis and improves myocardial function.47 Higher sST2 has also been linked to cardiac matrix remodeling.38 Clinically, sST2 has been shown to predict heart failure, major cardiovascular events, and mortality independently48, 49, 50 and in association with other biomarkers and clinical characteristics in the general population17, 51 and among patients with kidney disease.39, 52 Fewer studies have examined the association of sST2 with kidney outcomes. Previous studies have shown an association of sST2 with acute kidney injury in patients with ST-elevation myocardial infarction26 and with early recurrence of idiopathic nephrotic syndrome in kidney transplant recipients.53 One study based on the Framingham Heart Study showed a trend toward an association between higher sST2 with incident microalbuminuria in participants without CKD, but this did not reach statistical significance.11 Furthermore, our previous study of a cohort of older adults did not find a statistically significant association of higher sST2 with kidney function decline25; however, that study was limited largely to participants without CKD, which differs from our study population.
The exact mechanism linking elevation of galectin-3 with CKD progression is not clear, but may represent parallel processes affecting both the heart and kidneys or, alternatively, heart disease leading to kidney disease. “Cardiorenal syndrome” is a complex process mediated by intersecting physiologic, neurohormonal, and biochemical derangements and may explain these associations. For example, volume overload is a key mechanism that likely contributes to both cardiac and kidney disease. Galectin-3 has been associated with increased pulmonary artery and biventricular pressures54, 55, 56 and decreased left ventricular function57; thus, elevations in galectin-3 may reflect pathways that affect kidney function via renal venous congestion.58 Inflammation is also likely an important mechanism in cardiorenal pathophysiology. Galectin-3 has been found to be associated with inflammatory pathways.59
There are several important next directions for our study findings. Further studies are needed to confirm and extend the findings in our study. It is plausible that novel cardiac biomarkers may help risk stratify patients with CKD in relation to adverse kidney outcomes; further studies are warranted. Furthermore, the pathways represented by these biomarkers may represent novel therapeutic targets. For example, galectin-3 inhibitors have been shown to prevent cardiac and renal fibrosis60 and to improve hypertensive nephropathy,61 acute kidney injury,62 and glomerulopathy63 in animal models. Further preclinical and clinical research is needed to determine whether blockade of galectin-3 or sST2 ameliorates the risk of CKD progression.
Our study had several strengths. We studied 2 independent, well-characterized cohorts composed of participants with geographic and clinical diversity. We had several years of follow-up time to evaluate longitudinal outcomes. All biomarker measures were performed at the same laboratory at the University of Washington. We also recognize some limitations. The analyses may have been underpowered, which may have affected our ability to detect statistically significant differences. All eGFR measures to determine the outcomes of interest were taken in the ambulatory setting. It is possible that participants had fluctuations in eGFR or acute kidney injury, which may have affected our results. We did not have longitudinal cystatin C measures in C-PROBE, so eGFR was defined using serum creatinine; however, serum creatinine is the kidney biomarker most widely used clinically. The etiology of CKD was not available in SKS or C-PROBE. Finally, we cannot determine causality or mechanisms from this observational study.
In conclusion, elevated galectin-3 may be associated with greater risk of CKD progression among participants with CKD. Novel cardiac biomarkers may inform mechanisms underlying cardiac and kidney injury, and may be promising tools for disease monitoring and targeted interventions to slow the progression of CKD. Further larger studies should confirm and extend these findings.
Disclosure
All the authors declared no competing interests.
Acknowledgments
NB received funding from R03 DK102452 and R01 DK103612.
Footnotes
List of Abbreviations and Declarations.
Table S1. Characteristics of the study participants in C-PROBE and SKS.
Figure S1. CONSORT diagram.
Supplementary material is linked to the online version of the article at www.kireports.org.
Supplementary Material
Characteristics of the study participants in C-PROBE and SKS.
CONSORT diagram.
References
- 1.Bansal N., Katz R., Robinson-Cohen C. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2017;2:314–318. doi: 10.1001/jamacardio.2016.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K., Chertow G.M., Fried L.F. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166:1396–1402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 3.USRDS. 2016 USRDS annual data report: epidemiology of kidney disease in the United States. Available at: https://www.usrds.org/. Accessed August 18, 2018.
- 4.Muntner P., He J., Hamm L. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ahmad A., Rand W.M., Manjunath G. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee D., Ma J.Z., Collins A.J. Long-term survival of incident hemodialysis patients who are hospitalized for congestive heart failure, pulmonary edema, or fluid overload. Clin J Am Soc Nephrol. 2007;2:1186–1190. doi: 10.2215/CJN.01110307. [DOI] [PubMed] [Google Scholar]
- 7.McAlister F.A., Ezekowitz J., Tonelli M. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 8.Smith G.L., Lichtman J.H., Bracken M.B. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 9.Khan N.A., Ma I., Thompson C.R. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244–253. doi: 10.1681/ASN.2005030270. [DOI] [PubMed] [Google Scholar]
- 10.Bansal N., Katz R., Dalrymple L. NT-ProBNP and troponin T and risk of rapid kidney function decline and incident CKD in elderly adults. Clin J Am Soc Nephrol. 2015;10:205–214. doi: 10.2215/CJN.04910514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho J.E., Hwang S.J., Wollert K.C. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin Chem. 2013;59:1613–1620. doi: 10.1373/clinchem.2013.205716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal N., Hyre Anderson A., Yang W. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. J Am Soc Nephrol. 2015;26:946–956. doi: 10.1681/ASN.2014010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberg E.O., Shimpo M., Hurwitz S. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 14.Bichara M., Attmane-Elakeb A., Brown D. Exploring the role of galectin 3 in kidney function: a genetic approach. Glycobiology. 2006;16:36–45. doi: 10.1093/glycob/cwj035. [DOI] [PubMed] [Google Scholar]
- 15.Desmedt V., Desmedt S., Delanghe J.R. Galectin-3 in renal pathology: more than just an innocent bystander. Am J Nephrol. 2016;43:305–317. doi: 10.1159/000446376. [DOI] [PubMed] [Google Scholar]
- 16.Sharma U.C., Pokharel S., van Brakel T.J. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 17.Wang T.J., Wollert K.C., Larson M.G. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R., Zhang Y., An T. Prognostic value of sST2 and galectin-3 for death relative to renal function in patients hospitalized for heart failure. Biomark Med. 2015;9:433–441. doi: 10.2217/bmm.15.12. [DOI] [PubMed] [Google Scholar]
- 19.Drechsler C., Delgado G., Wanner C. Galectin-3, renal function, and clinical outcomes: results from the LURIC and 4D studies. J Am Soc Nephrol. 2015;26:2213–2221. doi: 10.1681/ASN.2014010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang W.H., Shrestha K., Shao Z. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011;108:385–390. doi: 10.1016/j.amjcard.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok D.J., Van Der Meer P., de la Porte P.W. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal D.M., Kommineni M., Ayalon N. Relationship of plasma galectin-3 to renal function in patients with heart failure: effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc. 2012;1:e000760. doi: 10.1161/JAHA.112.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao Y.S., Na S.P., Zhang P. Characterization of interleukin-33 and soluble ST2 in serum and their association with disease severity in patients with chronic kidney disease. J Clin Immunol. 2012;32:587–594. doi: 10.1007/s10875-011-9622-7. [DOI] [PubMed] [Google Scholar]
- 24.Iacoviello M., Aspromonte N., Leone M. Galectin-3 serum levels are independently associated with microalbuminuria in chronic heart failure outpatients. Res Cardiovasc Med. 2016;5:e28952. doi: 10.5812/cardiovascmed.28952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal N., Katz R., Seliger S. Galectin-3 and soluble ST2 and kidney function decline in older adults: the Cardiovascular Health Study (CHS) Am J Kidney Dis. 2016;67:994–996. doi: 10.1053/j.ajkd.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tung Y.C., Chang C.H., Chen Y.C. Combined biomarker analysis for risk of acute kidney injury in patients with ST-segment elevation myocardial infarction. PLoS One. 2015;10:e0125282. doi: 10.1371/journal.pone.0125282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Seaghdha C.M., Hwang S.J., Ho J.E. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol. 2013;24:1470–1477. doi: 10.1681/ASN.2012090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martini S., Nair V., Keller B.J. Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol. 2014;25:2559–2572. doi: 10.1681/ASN.2013080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju W., Nair V., Smith S. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7:316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair V., Robinson-Cohen C., Smith M.R. Growth differentiation factor-15 and risk of CKD progression. J Am Soc Nephrol. 2017;28:2233–2240. doi: 10.1681/ASN.2016080919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roshanravan B., Khatri M., Robinson-Cohen C. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roshanravan B., Robinson-Cohen C., Patel K.V. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24:822–830. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosworth C.R., Levin G., Robinson-Cohen C. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012;82:693–700. doi: 10.1038/ki.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson-Cohen C., Littman A.J., Duncan G.E. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25:399–406. doi: 10.1681/ASN.2013040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith N.L., Psaty B.M., Heckbert S.R. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52:143–146. doi: 10.1016/s0895-4356(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 37.Lunn M., McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 38.Savic-Radojevic A., Pljesa-Ercegovac M., Matic M. Novel biomarkers of heart failure. Adv Clin Chem. 2017;79:93–152. doi: 10.1016/bs.acc.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z., Shen B., Cao X. Increased soluble suppression of tumorigenicity 2 level predicts all-cause and cardiovascular mortality in maintenance hemodialysis patients: a prospective cohort study. Blood Purif. 2017;43:37–45. doi: 10.1159/000452924. [DOI] [PubMed] [Google Scholar]
- 40.Hogas S., Schiller A., Voroneanu L. Predictive calue for galectin 3 and cardiotrophin 1 in hemodialysis patients. Angiology. 2016;67:854–859. doi: 10.1177/0003319715623397. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi Y., Kobayashi S., Hemmi N. Galectin-3-positive cell infiltration in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19:602–607. doi: 10.1093/ndt/gfg603. [DOI] [PubMed] [Google Scholar]
- 42.Vasta G.R. Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol. 2012;946:21–36. doi: 10.1007/978-1-4614-0106-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeon S.B., Yoon H.J., Chang C.Y. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol. 2010;185:7037–7046. doi: 10.4049/jimmunol.1000154. [DOI] [PubMed] [Google Scholar]
- 44.Henderson N.C., Mackinnon A.C., Farnworth S.L. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang Z., MacKinnon A., Marson L.P. Tubular atrophy and interstitial fibrosis after renal transplantation is dependent on galectin-3. Transplantation. 2012;93:477–484. doi: 10.1097/TP.0b013e318242f40a. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg E.O., Shimpo M., De Keulenaer G.W. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitz J., Owyang A., Oldham E. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Mueller T., Dieplinger B., Gegenhuber A. Increased plasma concentrations of soluble ST2 are predictive for 1-year mortality in patients with acute destabilized heart failure. Clin Chem. 2008;54:752–756. doi: 10.1373/clinchem.2007.096560. [DOI] [PubMed] [Google Scholar]
- 49.Piper S.E., Sherwood R.A., Amin-Youssef G.F. Serial soluble ST2 for the monitoring of pharmacologically optimised chronic stable heart failure. Int J Cardiol. 2015;178:284–291. doi: 10.1016/j.ijcard.2014.11.097. [DOI] [PubMed] [Google Scholar]
- 50.Broch K., Ueland T., Nymo S.H. Soluble ST2 is associated with adverse outcome in patients with heart failure of ischaemic aetiology. Eur J Heart Fail. 2012;14:268–277. doi: 10.1093/eurjhf/hfs006. [DOI] [PubMed] [Google Scholar]
- 51.Shah R.V., Chen-Tournoux A.A., Picard M.H. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2:311–319. doi: 10.1161/CIRCHEARTFAILURE.108.833707. [DOI] [PubMed] [Google Scholar]
- 52.Bayes-Genis A., Zamora E., de Antonio M. Soluble ST2 serum concentration and renal function in heart failure. J Card Fail. 2013;19:768–775. doi: 10.1016/j.cardfail.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Bruneau S., Le Berre L., Herve C. Potential role of soluble ST2 protein in idiopathic nephrotic syndrome recurrence following kidney transplantation. Am J Kidney Dis. 2009;54:522–532. doi: 10.1053/j.ajkd.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 54.Lala R.I., Darabantiu D., Pilat L. Galectin-3: a link between myocardial and arterial stiffening in patients with acute decompensated heart failure? Arq Bras Cardiol. 2016;106:121–129. doi: 10.5935/abc.20150149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lala R.I., Lungeanu D., Darabantiu D. Galectin-3 as a marker for clinical prognosis and cardiac remodeling in acute heart failure. Herz. 2018;43:146–155. doi: 10.1007/s00059-017-4538-5. [DOI] [PubMed] [Google Scholar]
- 56.Shah R.V., Chen-Tournoux A.A., Picard M.H. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim S.H., Behnes M., Natale M. Galectin-3 reflects mitral annular plane systolic excursion being assessed by cardiovascular magnetic resonance imaging. Dis Markers. 2016;2016:7402784. doi: 10.1155/2016/7402784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullens W., Abrahams Z., Francis G.S. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henderson N.C., Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 60.Calvier L., Martinez-Martinez E., Miana M. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59–67. doi: 10.1016/j.jchf.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Hart P.D., Bakris G.L. Hypertensive nephropathy: prevention and treatment recommendations. Expert Opin Pharmacother. 2010;11:2675–2686. doi: 10.1517/14656566.2010.485612. [DOI] [PubMed] [Google Scholar]
- 62.Kolatsi-Joannou M., Price K.L., Winyard P.J. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6:e18683. doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Traber P.G., Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the study participants in C-PROBE and SKS.
CONSORT diagram.