Abstract
Introduction
New treatments to inhibit neointimal formation after percutaneous transluminal angioplasty (PTA) are needed for patients undergoing chronic hemodialysis (HD). We compared the efficacy and safety of AMG0102, a balloon catheter containing nuclear factor κB (NF-κB) decoy oligodeoxynucleotide (ODN) with the PTA balloon catheter (control group) for arteriovenous fistula (AVF) stenosis.
Methods
In total, 175 patients (age ≥20 years, undergoing HD, with venous stenosis at the anastomotic region) were registered in this prospective open-label, randomized study. Patients were followed postoperatively for 36 weeks. The duration of primary patency on the targeted venous stenosis site (primary endpoint) was estimated by the Kaplan–Meier method.
Results
A lower restenosis risk was observed for the AMG0102 group, but it was not statistically significant (stratified log-rank test P = 0.250, hazard ratio [HR] 0.774; 95% confidence interval [CI]: 0.500–1.198). The median duration of primary patency was 245 days and 172 days in the AMG0102 and control groups, respectively. After stratification based on the status of diabetes complications, the HR was 0.666 (95% CI: 0.366–1.212; P = 0.183) and the median duration of primary patency was prolonged by 108 days in the AMG0102 group with diabetes complications (245 days) compared with the control group (137 days). Adverse event (AE) incidence up to 36 postoperative weeks did not differ between groups. Four device failures occurred in 3 patients (AMG0102 group), but none resulted in AEs.
Conclusion
Further modifications to enhance NF-κB decoy ODN uptake and efficacy are necessary to show its clinical utility for AVF stenosis in chronic HD.
Keywords: arteriovenous fistula stenosis, balloon catheter, diabetes, hemodialysis, nuclear factor κB, percutaneous transluminal angioplasty
Based on the findings of the 2015 nationwide survey by the Japanese Society for Dialysis Therapy, 324,986 patients with end-stage renal disease required HD/renal transplantation in Japan by the end of 2015.1 With the increasing prevalence of diabetes in the past decade, diabetic nephropathy has surpassed chronic glomerulonephritis as the most common underlying disease among individuals undergoing HD. Approximately, 43.7% of patients who started HD in Japan for the first time in 2015 had diabetic nephropathy.1 In Japan, blood purification (i.e., HD and peritoneal dialysis) remains the mainstay treatment option for end-stage renal disease, given the limited availability of renal transplant donors.
Although the native AVF is the preferred and most frequently used vascular access,2, 3 AVF dysfunction caused by complications such as stenosis or occlusion is a common issue.2 The development of AVF stenosis is known to be multifactorial4; however, several studies have concluded that neointimal proliferation is the main culprit of stenosis.5
The fact that there are limited HD access sites in a patient’s lifetime,6 together with the increase in the number of long-term HD patients, elderly patients, and diabetic patients in whom AVF reconstruction is not an option, has led to a growing focus on prolonging the life of an existing AVF rather than creating a new one.
In Japan, PTA is the first-line treatment for AVF stenosis/occlusion.7 However, PTA is associated with high rates of reinterventions secondary to restenosis.8 In previous reports, up to 60% of patients presented restenosis within the first 12 months after PTA.8 Moreover, Japanese national health insurance often does not cover the use of PTA balloon treatments for HD because of the high cost. New means to inhibit continuous neointimal proliferation (potentially leading to restenosis) after PTA are needed for patients undergoing chronic HD.
AMG0102 is a nylon balloon doubly coated catheter with anionic polymer and cationic poly (lactic-co-glycolic acid) particles containing NF-κB decoy ODNs. Once the balloon is inflated inside the target blood vessel, NF-κB decoy ODN can be applied to the vessel wall.
NF-κB is a transcription factor that regulates the transcription of cytokines and adhesion molecules controlling immune/inflammatory response.9 NF-κB decoy ODN, a double-stranded phosphorothioate ODN of 20 base pairs, includes an NF-κB binding sequence. When NF-κB decoy ODN is taken up by a target cell, ODN binds specifically to the NF-κB transcription factor acting as a decoy to the NF-κB DNA binding sequence. Thus, ODN exerts anti-inflammatory/immunosuppressive effects by inhibiting NF-κB transcription activity and reducing the expression of genes involved in the inflammatory response.10, 11 In a rabbit model, the therapeutic effect at the site of the neointima was verified using fluorescence-labeled, NF-κB decoy ODN-loaded nanospheres. These nanospheres were successfully transferred into the neointima in short-term contact with target vessels, and fluorescence could be detected ≥1 week after angioplasty. Local application of NF-κB decoy ODN-loaded nanospheres inhibited neointimal formation and lumen loss after balloon angioplasty, and it also accelerated restoration of the endothelial cell monolayer associated with enhanced expression of phosphorylated Bcl-2 in endothelial cells.12 Thus, the expected pharmacological action of NF-κB decoy ODN is to inhibit the development of restenosis of the intervened vessel.12 Regarding the technical feasibility of the drug delivery system, the drug-coated balloon catheter is considered simple to use while allowing placement of the drug on the target vessel wall during PTA, with a minimal vascular injury.12 We aimed to compare the efficacy and safety of AMG0102 with those of a PTA balloon catheter for the treatment of AVF stenosis.
Methods
Ethics
This study adhered to the Declaration of Helsinki, Pharmaceutical and Medical Device Act, Ordinance of Good Clinical Practice for Medical Device, associated Japanese regulations, and the approved study protocol. All patients provided written informed consent for study enrollment and procedures. The institutional review board at each participating center reviewed and approved the study protocol and associated documents. This trial was registered at the UMIN Clinical Trial Registry under the registration number UMIN000008096.
Patients
Inclusion criteria were as follows: outpatients and inpatients aged ≥20 years who had undergone HD via AVF in the arm at least 3 times and provided informed consent at least 4 weeks before the shunts were created; had untreated venous stenosis at or around the anastomotic region demonstrated by ultrasound or angiography; a stenosis rate ≥50% at the dialysis shunt venous stenotic lesion site; and if PTA was found to be the most appropriate treatment for the identified treatment site as determined by the investigator, based on the following conditions: reduction in blood flow (defined as blood removal of ≤180 ml/min multiple times when performing puncture toward the anastomotic site); increase in venous pressure (defined as increased pressure of ≥50 mm Hg), or if pressure remained stable at 150 mm Hg; reduction in dialysis dose (defined as a decrease of ≥10% in the efficiency in dialysis by recirculation); a lesion with a length of ≤60 mm at the venous treatment site; and patients who were willing to comply with study visits and were registered within 30 days from providing informed consent. Detailed exclusion criteria are listed in Appendix S1.
Study Design and Treatments
This was a prospective, multicenter, open-label randomized study with 2 groups, the AMG0102 treatment group (AMG0102 group) and PTA balloon catheter treatment group (PTA group), and a 36-week postoperative follow-up period. The study was conducted between September 2012 and November 2015 in 25 institutions (listed in Appendix S2) in Japan.
Patients were randomized to each group via dynamic allocation. Assignment to each treatment group was conducted using the central registration method. The randomization process included minimization by the following allocation adjustment factors, which may have influenced the duration of primary patency (primary endpoint): facility and presence of diabetes complications (i.e., patients receiving treatment with insulin or oral hypoglycemic agents).
Patients in the AMG0102 group underwent a single use of AMG0102 and were evaluated until 36 weeks postoperatively. Treatment with cutting balloon, Rotablator (Boston Scientific Japan K.K., Tokyo, Japan), ultra-high-pressure balloon, or laser, and atheroma excision was prohibited. Blood samples were collected before and after the procedures and every 4 weeks thereafter to perform blood biochemistry, hematology, lipid profile, and liver function tests.
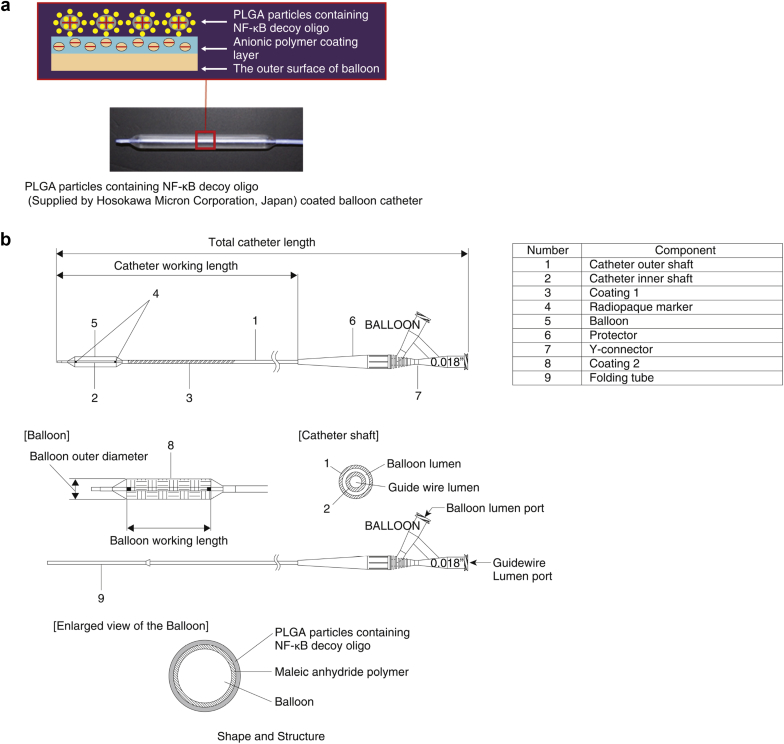
Figure 1a and b show the study device (AMG0102, supplied by Medikit Co., Ltd., Tokyo, Japan) and Table 1 shows its specifications by device size and NF-κB decoy ODN amount. The control device was a commercially available PTA balloon catheter (e.g., HYUGA [Medikit Co., Ltd., Approval number 21500BZZ00120000]). For device insertion, the operator had to expand the balloon at a pressure lower than the rated burst pressure, and the duration of the expansion was ≥60 seconds. When the investigator considered that there was no clinical issue during the first postoperative dialysis, patient discharge was evaluated. The need for a bailout procedure and the specific procedure was evaluated and chosen by each investigator.
Figure 1.
(a) Schematic representation of the layers of AMG0102 and image of AMG0102. (b) Schema of the structural components of AMG0102. PLGA, poly (lactic-co-glycolic acid).
Table 1.
Coated area per balloon and amount (theoretical) of NF-κB decoy ODN in AMG0102
| Balloon size, mm | Area coated, mm2 | Amount, μg/mm2, of NF-κB decoy ODN | Amount, μg, of NF-κB decoy ODN |
|---|---|---|---|
| 4 × 40 | 502 | 0.18 | 89 |
| 5 × 40 | 628 | 0.18 | 112 |
| 6 × 40 | 754 | 0.18 | 134 |
NF-κB, nuclear factor κB; ODN, oligodeoxynucleotide
Endpoints
The primary efficacy endpoint was the duration of primary patency (from the date of surgery to the date of loss of patency) at the targeted venous stenosis treatment site until 36 weeks. Loss of primary patency was considered if the restenosis treatment criteria were met (as defined in the inclusion criteria: reduction in blood flow, increase in venous pressure, and reduction in dialysis dose); if there was confirmation of obstruction at the treatment site; if it was decided that a surgical intervention was needed to remove the treatment site from the access circuit (i.e., the flow of the vessel starting at the venous anastomotic site and flowing into the superior vena cava–right atrium junction); or if the dialysis shunts could no longer be used because of restenosis at the treatment site as judged by the investigator. The date of any of these events was recorded to calculate the duration of primary patency.
The secondary efficacy endpoints were the rate of primary patency, technical success, and safety, evaluated in terms of the incidence of AEs, treatment-related AEs, and serious AEs. Technical success was defined as the lack of any serious AEs and defects or malfunctions during the sequential procedure from the insertion of the catheter into the sheath to balloon expansion and removal of the catheter from the sheath.
Statistical Analyses
The rationale and details of the sample size calculation are provided in Appendix S3. The total target sample size was set to be 177 patients: 118 patients in the AMG0102 group and 59 patients in the PTA group.
The data analysis sets (full analysis set [FAS], per protocol set, and safety analysis set) are defined in Appendix S3. Patients in whom loss of primary patency was not observed were removed from the study on the last day on which the patency was confirmed. Additionally, patients who received a bailout procedure during the treatment that may have influenced the duration of primary patency were removed from the study analysis on the date of surgery. Patients with diabetes complications were defined as receiving treatment with insulin or oral hypoglycemic agents according to the Statistical Analysis Plan.
The changes in the duration of primary patency were shown by Kaplan–Meier method, and the primary patency rate at 36 weeks and the 95% CIs were calculated using Greenwood’s formula. The duration of primary patency until 36 weeks in both treatment groups was analyzed using a stratified log-rank test according to the allocation adjustment factors, except for institutions, and both treatment groups were compared. A Cox proportional-hazards model, which included assignment groups as covariates, was used. The HRs between the treatment groups and the 95% CIs were calculated. Between-group comparisons were made using the χ2 test (using Fisher exact test as needed). All tests were 2-sided at a 5% significance level. Statistical analyses were conducted using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC).
Results
Patients
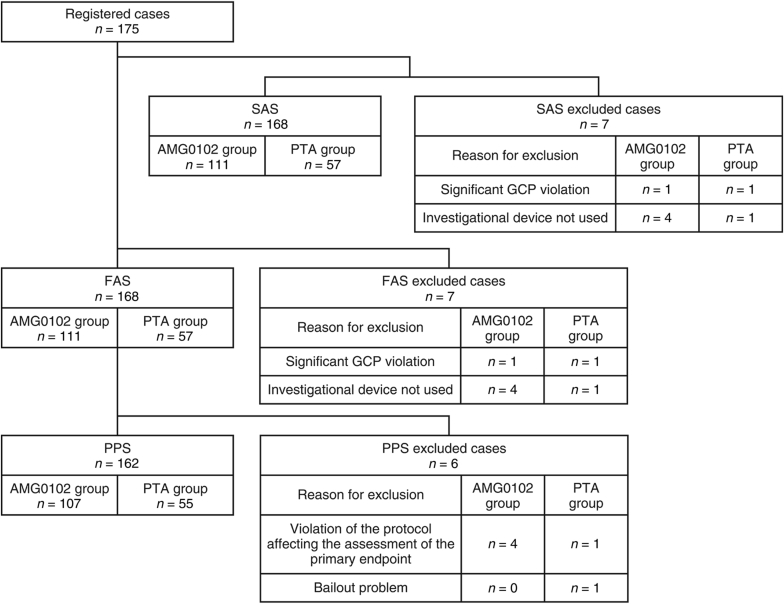
Figure 2 shows the disposition of patients. A total of 175 patients were registered. Of these, 168 patients were in the FAS (AMG0102 group, n = 111; PTA group, n = 57 patients). Nearly all of the patients completed the study. The main reason for exclusion in both the safety analysis set and FAS (AMG0102 group, n = 4; PTA group, n = 1) was “investigational device not used.” In the per protocol set, it was “violation of the protocol affecting the assessment of the primary endpoint” (AMG0102 group, n = 4; PTA group, n = 1). No significant differences were identified between groups at baseline (Table 2 and Supplementary Table S1), except for a larger proportion of patients aged ≥65 years at the time of providing consent in the AMG0102 group; a larger proportion of stenosis in the access circuit (defined as the flow of the vessel that starts from the venous anastomotic site up to the superior vena cava–right atrium junction) in the PTA group; and a larger proportion of patients with other past history in the PTA group.
Figure 2.
Patient disposition. FAS, full analysis set; GCP, Good Clinical Practice Guidelines; PPS, per protocol set; PTA, percutaneous transluminal angioplasty; SAS, safety analysis set.
Table 2.
Baseline demographic and clinical characteristics of patients in the FAS (categorical data)
| Variable | No. of patients | Total | Treatment group |
P | |
|---|---|---|---|---|---|
| AMG0102 n (%) |
PTA n (%) |
||||
| 168 | 111 (66.1) | 57 (33.9) | |||
| Age, yr | <65 | 61 | 46 (75.4) | 15 (24.6) | 0.054a |
| ≥65 | 107 | 65 (60.7) | 42 (39.3) | ||
| Sex | Male | 122 | 82 (67.2) | 40 (32.8) | 0.611a |
| Female | 46 | 29 (63.0) | 17 (37.0) | ||
| Diabetes complications | Absent | 86 | 58 (67.4) | 28 (32.6) | 0.701a |
| Present | 82 | 53 (64.6) | 29 (35.4) | ||
| Target lesion site of interest (treatment site) | Vein near anastomosis | 101 | 66 (65.3) | 35 (34.7) | — |
| Superficial forearm vein (internal shunt main vein) | 82 | 55 (67.1) | 27 (32.9) | ||
| Superficial brachial vein (internal shunt main vein) | 15 | 10 (66.7) | 5 (33.3) | ||
| Deep brachial vein | 0 | 0 | 0 | ||
| Central vein | 1 | 1 (100) | 0 | ||
| Smoking history | No smoking history | 75 | 49 (65.3) | 26 (34.7) | 0.965a |
| Previously smoked | 77 | 51 (66.2) | 26 (33.8) | ||
| Currently smoking | 16 | 11 (68.8) | 5 (31.25) | ||
| Number of shunt constructions | Once | 119 | 80 (67.2) | 39 (32.8) | 0.622a |
| Twice or more | 49 | 31 (63.3) | 18 (36.7) | ||
| Shunt site | Left | 129 | 88 (68.2) | 41 (31.8) | 0.285a |
| Right | 39 | 23 (59.0) | 16 (41.0) | ||
| Shunt anastomosis method | Side-end anastomosis | 119 | 77 (64.7) | 42 (35.3) | — |
| Side-side anastomosis | 31 | 22 (71.0) | 9 (29.0) | ||
| End-end anastomosis | 1 | 0 | 1 (100) | ||
| Other | 17 | 12 (70.6) | 5 (29.4) | ||
| Weekly dialysis frequency | <3 times | 7 | 6 (85.7) | 1 (14.3) | — |
| 3 times | 161 | 105 (65.2) | 56 (34.8) | ||
| 4 times | 0 | 0 | 0 | ||
| ≥5 times | 0 | 0 | 0 | ||
| Target lesion site of interest | Initial onset | 61 | 43 (70.5) | 18 (29.5) | — |
| Recurrence | 107 | 68 (63.6) | 39 (36.4) | ||
| Unknown | 0 | 0 | 0 | ||
| Type of previous treatment in case of recurrence | PTA balloon | 107 | 68 (63.6) | 39 (36.4) | — |
| Other | 0 | 0 | 0 | ||
| Stenosis of access circuit | Absent | 118 | 82 (69.5) | 36 (30.5) | 0.150a |
| Present | 50 | 29 (58.0) | 21 (42.0) | ||
| Stenting at site other than target lesion | Absent | 167 | 110 (65.9) | 57 (34.1) | 1.000b |
| Present | 1 | 1 (100) | 0 | ||
| Etiology of dialysis | Diabetic nephropathy | 89 | 58 (65.2) | 31 (34.8) | — |
| Chronic glomerulonephritis | 23 | 14 (60.9) | 9 (39.1) | ||
| Nephrosclerosis | 32 | 22 (68.8) | 10 (31.3) | ||
| Rapidly progressive glomerulonephritis | 1 | 1 (100) | 0 | ||
| Polycystic kidney disease | 6 | 5 (83.3) | 1 (16.7) | ||
| Chronic pyelonephritis | 1 | 1 (100) | 0 | ||
| Other | 27 | 16 (59.3) | 11 (40.7) | ||
| Diabetes mellitus | Absent | 65 | 43 (66.2) | 22 (33.8) | 0.986a |
| Present | 103 | 68 (66.0) | 35 (34.0) | ||
| Diabetes treatment | Insulin therapy | 45 | 27 (60.0) | 18 (40.0) | — |
| Oral diabetes medication | 54 | 38 (70.4) | 16 (29.6) | ||
| Dietary therapy alone | 9 | 5 (55.6) | 4 (44.4) | ||
| Untreated | 12 | 10 (83.3) | 2 (16.7) | ||
| Other | 12 | 10 (83.3) | 2 (16.7) | ||
| Hyperlipidemia | Absent | 87 | 57 (65.5) | 30 (34.5) | 0.875a |
| Present | 81 | 54 (66.7) | 27 (33.3) | ||
| Hyperlipidemia treatment | Resolved | 2 | 1 (50.0) | 1 (50.0) | 1.000b |
| Ongoing morbidity | 79 | 53 (67.1) | 26 (32.9) | ||
| Drug treatment absent | 28 | 21 (75.0) | 7 (25.0) | ||
| Drug treatment present | 51 | 32 (62.7) | 19 (37.3) | ||
| Hypertension | Absent | 9 | 8 (88.9) | 1 (11.1) | 0.276b |
| Present | 159 | 103 (64.8) | 56 (35.2) | ||
| Hypertension treatment | Resolved | 0 | 0 | 0 | — |
| Ongoing morbidity | 159 | 103 (64.8) | 56 (35.2) | ||
| Drug treatment absent | 15 | 13 (86.7) | 2 (13.3) | ||
| Drug treatment present | 144 | 90 (62.5) | 54 (37.5) | ||
| Other past history | Absent | 96 | 70 (72.9) | 26 (27.1) | 0.030a |
| Present | 72 | 41 (56.9) | 31 (43.1) | ||
| Other concomitant illness | Absent | 0 | 0 | 0 | — |
| Present | 168 | 111 (66.1) | 57 (33.9) | ||
| Allergy to heparin | Absent | 168 | 111 (66.1) | 57 (33.9) | — |
| Present | 0 | 0 | 0 | ||
| Allergy to contrast agent | Absent | 164 | 109 (66.5) | 55 (33.5) | 0.605b |
| Present | 4 | 2 (50.0) | 2 (50.0) | ||
| 12-lead ECG | No abnormality | 98 | 67 (68.4) | 31 (31.6) | 0.417a |
| Abnormality present | 69 | 43 (62.3) | 26 (37.7) | ||
With and without diabetes mellitus complications: a patient who received insulin preparation or oral hypoglycemic agents when obtaining consent was regarded as having diabetes mellitus complications.
Diabetes mellitus: in addition to the described above, the patient who received diet and exercise therapy was regarded as having diabetes mellitus.
ECG, electrocardiogram; FAS, full analysis set; PTA, percutaneous transluminal angioplasty.
χ2 test.
Fisher exact test.
Efficacy Endpoints
The mean (± SD) observation period (FAS) in the AMG0102 and PTA groups was 171.5 ± 82.5 days (minimum–maximum, 0–266 days, median 175.0 days) and 158.5 ± 84.8 days (minimum–maximum, 1–265 days, median 137.0 days), respectively (Table 3). The mean duration of primary patency in the AMG0102 and PTA groups was 220.0 ± 67.3 days (minimum–maximum, 4–265 days, medium 252.0 days) and 207.7 ± 84.7 days (minimum–maximum, 1–265 days, medium 250.5 days), respectively (Table 3).
Table 3.
Summary statistics of the observation period in the FAS
| All patients | Primary patency patients | |
|---|---|---|
| Total | ||
| No. of patients | 168 | 72 |
| Mean (SD) | 167.1 (83.3) | 216.2 (72.6) |
| Median (minimum–maximum) | 161.0 (0–266) | 252.0 (1–265) |
| AMG0102 | ||
| No. of patients | 111 | 50 |
| Mean (SD) | 171.5 (82.5) | 220.0 (67.3) |
| Median (minimum–maximum) | 175.0 (0–266) | 252.0 (4–265) |
| PTA | ||
| No. of patients | 57 | 22 |
| Mean (SD) | 158.5 (84.8) | 207.7 (84.7) |
| Median (minimum–maximum) | 137.0 (1–265) | 250.5 (1–265) |
FAS, full analysis set; PTA, percutaneous transluminal angioplasty.
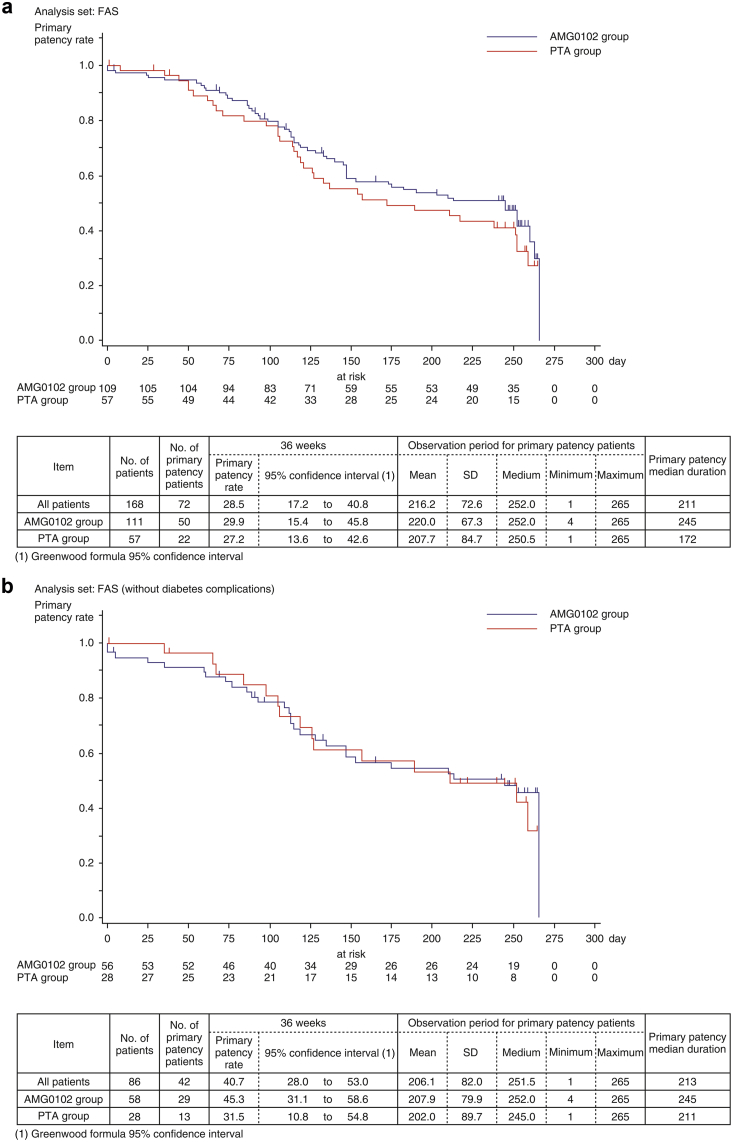
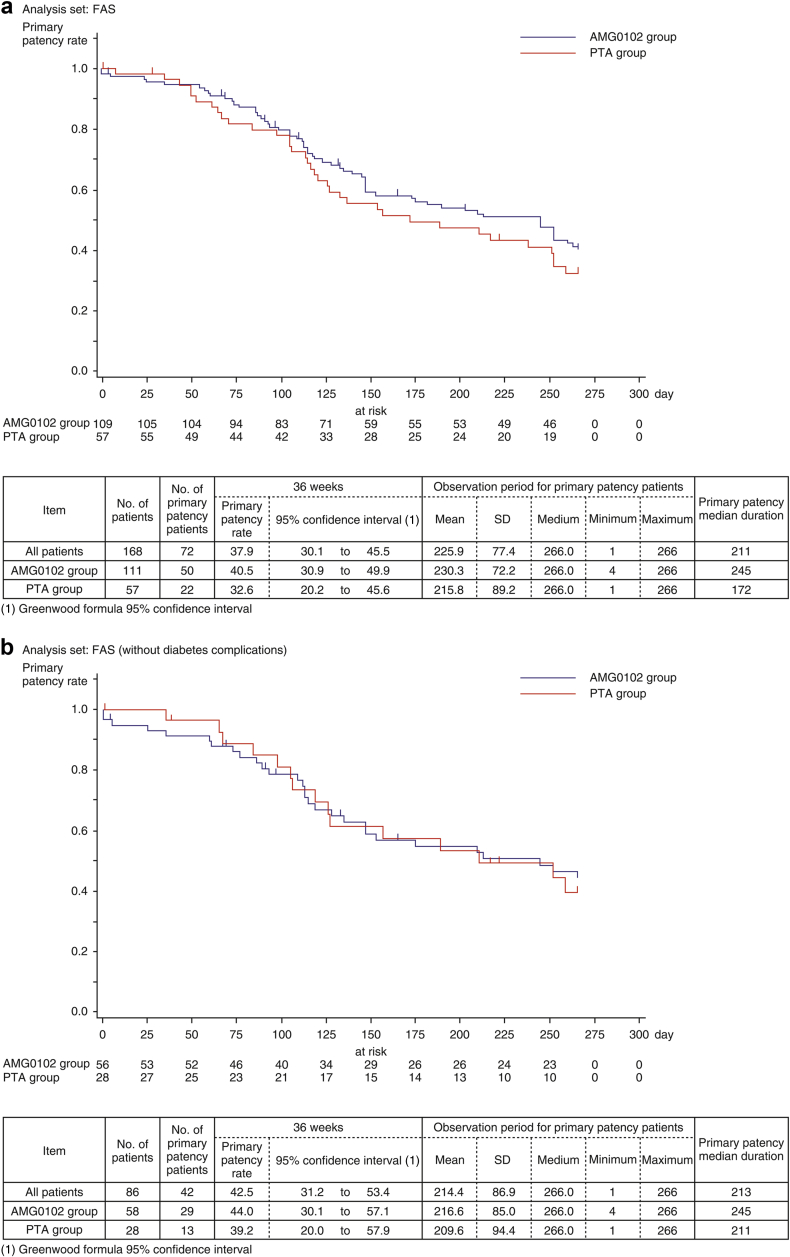
Figure 3a shows the estimated duration of primary patency by Kaplan–Meier method from baseline to week 36. The primary patency rates at week 36 in the AMG0102 and PTA groups were 29.9% (95% CI: 15.4–45.8) and 27.2% (95% CI: 13.6–42.6), respectively; however, the stratified log-rank test found no statistically significant difference between the 2 treatment groups (HR 0.774 [95% CI: 0.500–1.198], P = 0.250) (Table 4). The median duration of primary patency in the AMG0102 and PTA groups was 245 days and 172 days, respectively. Compared with the PTA group, in the AMG0102 group, we observed 73 days (2.4 months) of prolongation of the median duration of primary patency.
Figure 3.
(a) Estimation of the duration of primary patency by Kaplan–Meier method. (b) Estimation of the duration of primary patency by Kaplan–Meier method: Full analysis set (FAS) without diabetes complications.
Table 4.
Stratified log-rank test for the duration of primary patency in the FAS
| Stratum | AMG0102 group, n |
PTA group, n | Score | Square of score | Variance of score | χ2 value | DF | HRa | 95% CIa | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Without diabetes complications | 58 | 28 | −0.801 | 0.642 | 9.445 | 0.068 | 1 | 0.919 | 0.486–1.738 | 0.794 |
| With diabetes complications | 53 | 29 | −4.358 | 18.990 | 10.704 | 1.774 | 1 | 0.666 | 0.366–1.212 | 0.183 |
| Total χ2 value | — | — | — | — | — | 1.842 | — | — | — | — |
| Stratified log-rank test | 111 | 57 | −5.159 | 26.613 | 20.149 | 1.321 | 1 | 0.774 | 0.500–1.198 | 0.250 |
| The test of stratification consistency for treatment efficacy | — | — | — | — | — | 0.521 | 1 | — | — | 0.470 |
CI, confidence interval; DF, degrees of freedom; FAS, full analysis set; HR, hazard ratios; PTA, percutaneous transluminal angioplasty.
Values based on the stratified log-rank test score. Hazard ratio was calculated as AMG0102 group hazard/PTA group hazard.
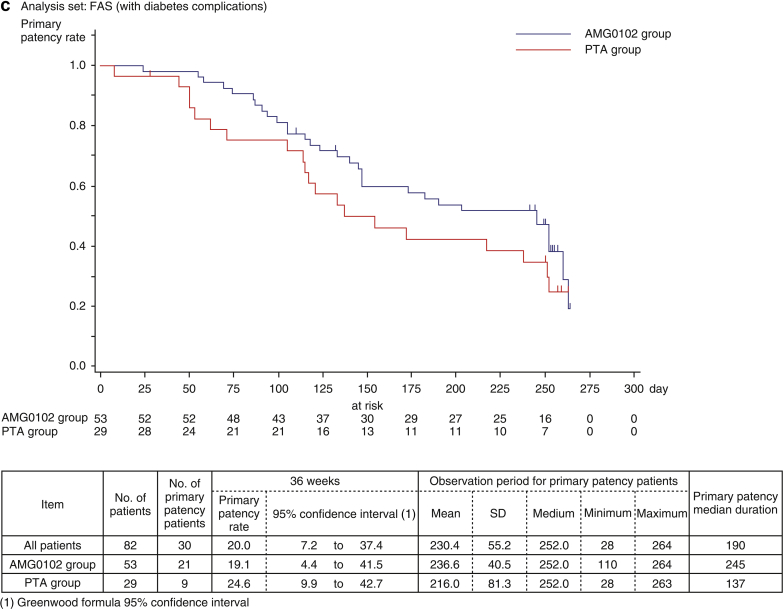
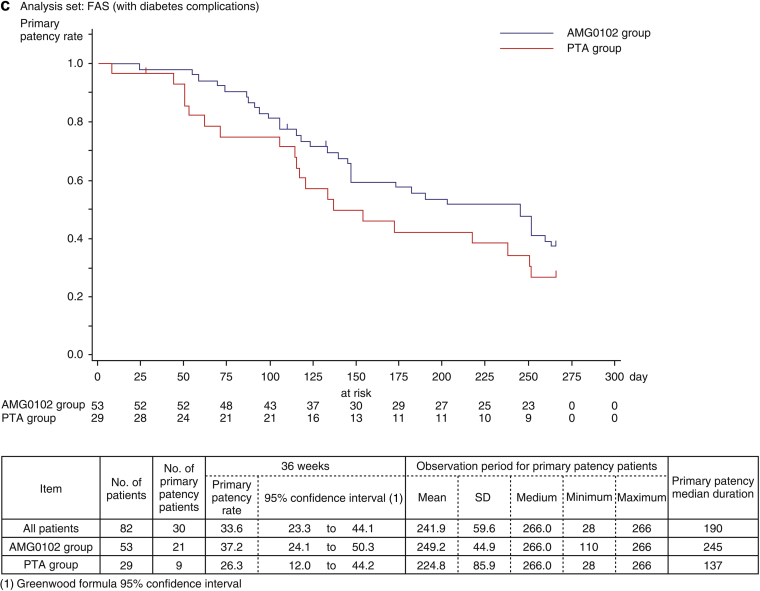
Figure 3b and c show the estimated duration of primary patency by Kaplan–Meier method from baseline to week 36 for patients without and with diabetes complications. In patients without diabetes complications, the primary patency rates at 36 weeks in the AMG0102 and PTA groups were 45.3% (95% CI: 31.1–58.6) and 31.5% (95% CI: 10.8–54.8), respectively. The median duration of primary patency in the AMG0102 and PTA groups was 245 days and 211 days, respectively. Compared with the PTA group, in the AMG0102 group, we observed 34 days (1.1 months) of prolongation of the median duration of primary patency. In patients with diabetes complications, the primary patency rates at 36 weeks in the AMG0102 and PTA groups were 19.1% (95% CI: 4.4–41.5) and 24.6% (95% CI: 9.9–42.7), respectively. The median duration of primary patency in the AMG0102 and PTA groups was 245 days and 137 days, respectively. Compared with the PTA group, in the AMG0102 group, we observed 108 days (3.6 months) of prolongation of the median duration of primary patency. The results in the FAS and per protocol set analysis were similar in patients regardless of the group.
When stratifying patients according to “without diabetes complications,” the HR was 0.919 (95% CI: 0.486–1.738; P = 0.794 [log-rank test]) (Table 4). When stratifying patients according to “with diabetes complications,” the HR was 0.666 (95% CI: 0.366–1.212; P = 0.183 [log-rank test]) (Table 4). The test of stratification consistency for treatment efficacy was not statistically significant (P = 0.470) (Table 4).
We performed the additional analysis after data fixation for the patients whose loss of primary patency was not observed by week 36 (cutoff date on the next day [266 days] as the upper limit). The primary patency rates at 36 weeks were 40.5% (95% CI: 30.9–49.9) in the AMG0102 group and 32.6% (95% CI: 20.2–45.6) in the PTA group. The median duration of primary patency was 245 days and 172 days in the AMG0102 and PTA groups, respectively. Compared with the PTA group, in the AMG0102 group, we observed 73 days (2.4 months) of prolongation of the median duration of primary patency (Figure 4a).
Figure 4.
(a) Estimation of the duration of primary patency by Kaplan–Meier method: Full analysis set (FAS) additional analysis with data cutoff at 36 weeks. For patients in whom primary patency was not lost by 36 weeks, the day (day 266) after the upper limit of the 36-week data range was set as the day of cessation. (b) Estimation of the duration of primary patency by Kaplan–Meier method: FAS without diabetes complications. Additional analysis with data cutoff at 36 weeks.
Figure 4a–c show the estimated duration of primary patency by Kaplan–Meier method from baseline to week 36 for patients in the FAS and stratified according to diabetes status for the revised data with a cutoff at 36 weeks. In the FAS analysis, when patients with and without diabetes complications were stratified and treatment groups were compared, there were no statistically significant differences in primary patency rate (HR 0.785; 95% CI: 0.508–1.211; P = 0.274 [stratified log-rank test]) (Supplementary Table S2).
No statistically significant differences between the treatment groups were found in terms of technical success rates (AMG0102 group: 99.1%, PTA group: 100%; P = 1.00) (Table 5).
Table 5.
Summary of technical success in the FAS
| Treatment group | No. of patients | Successful | Unsuccessful | 95% CI for success ratea | Success rate difference | 95% CI for success rate differenceb | P |
|---|---|---|---|---|---|---|---|
| AMG0102 | 111 | 110 (99.1) | 1 (0.9) | 95.1–100.0 | −0.9 | −2.7 to 0.9 | 1.000c |
| PTA | 57 | 57 (100.0) | 0 (0.0) | 93.7–00.0 |
CI, confidence interval; FAS, full analysis set; PTA, percutaneous transluminal angioplasty.
Exact 95% CI based on binomial probability.
95% CI obtained by normal approximation to binomial probability (Wald test).
Fisher exact test.
Safety
Four device failures occurred with AMG0102 in 3 patients; these failures did not result in any AE. No device failures occurred in the PTA group.
Serious AEs were observed in 37 (33.3%) (95% CI: 24.7%–42.9%) of 111 patients in the AMG0102 group and 17 (29.8%) (95% CI: 18.4%–43.4%) of 57 patients in the PTA group (Supplementary Table S3). No serious AE for which a causal relationship could be established was observed in the AMG0102 group. In the PTA group, 1 patient (1.8%) (95% CI: 0.0–9.4) developed a serious AE (shunt stenosis) for which a causal relation cannot be ruled out (Supplementary Table S3). Five patients died during the study (4 of 111 patients in the AMG0102 group and 1 of 57 patients in the PTA group), but a causal relationship with the study device was ruled out in all of them. The summary of AEs in each group classified by system organ class and preferred term of the Medical Dictionary for Regulatory Activities is shown in Supplementary Table S4. Of the AEs with an incidence ≥5%, those with a difference in the incidence of 5% or more between groups were vasospasm, peripheral arterial occlusive disease, dermatitis contact, skin exfoliation, puncture site pain, and postangioplasty restenosis. The incidence was higher for all of these in the PTA group.
Discussion
Interestingly, among patients without diabetes complications, the median duration of primary patency was prolonged by 34 days in the AMG0102 group; however, among patients with diabetes complications, the median duration of primary patency was prolonged by 108 days (3.6 months) in the AMG0102 group. Thus, a more favorable result was obtained in the patient population complicated with diabetes in the AMG0102 group.
Previous reports indicate that advanced glycation end products (AGEs) increase adhesion of inflammatory cells that then stimulate endothelial proliferation.13, 14 One of the causes of diabetic angiopathy is the accelerated formation and accumulation of AGEs (also known as saccharified products). When AGEs bind to the receptor for AGE (RAGE), NF-κB is activated through the Ras/MAP kinase pathway in vascular endothelial cells. Additionally, NF-κB in endothelial cells increases the expression of inflammatory mediators, such as monocyte chemoattractant protein 1 and vascular cell adhesion protein 1, via RAGE.13, 14 Thus, we consider that AMG0102 prolonged the primary patency period further in patients with diabetes complications than in those without diabetes complications by suppressing inflammation caused by activation of NF-κB.
Although NF-κB is found in other types of cells, it is primarily found in immune/inflammatory cells. NF-κB regulates the transcription of cytokines and adhesion molecules, such as tumor necrosis factor-α and interleukins-1β, -6, and -8, thereby controlling immune/inflammatory responses. NF-κB also activates interleukin-2 receptor, major histocompatibility complex class I/II, costimulatory molecules CD80 and CD86 (involved in antigen presentation), intercellular adhesion molecule-1 and vascular cell adhesion protein 1 (adhesion molecules), cyclooxygenase-2 (prostaglandin synthase), and collagenase I (matrix metalloprotease),9 all of which are associated with vascular restenosis. Theoretically, NF-κB decoy ODN bound with such inflammatory cells, inhibiting NF-κB transcription activity and suppressing the inflammatory response.11, 12
In the present study, no significant difference was noted between the 2 study groups in terms of technical success, indicating that AMG0102 is applicable as a medical device without any clinical issue compared with PTA, which is the recommended standard of care. The incidence of AEs up to 36 postoperative weeks did not differ between the 2 treatment groups. Further, causal relationships with all serious AEs were ruled out in both groups. Thus, an NF-κB decoy ODN-coated PTA balloon catheter would be feasible in chronic HD patients.
Unexpectedly, we were unable to meet the primary endpoint of this study, even though AMG0102 demonstrated better efficacy, especially in patients with diabetes complications in the AMG0102 group. One of the reasons for this failure might be that the device did not transfect the NF-κB decoy ODN efficiently. Further modification of the potency of inhibition of NF-κB activity or the transfection efficiency of the PTA balloon catheter could enhance its clinical utility.
This study had several limitations. To be used for dialysis, the AVF must mature appropriately. That is, the vein must undergo dilation and remodeling to accommodate the marked increased blood flow. Vein trauma from hemodynamic stress (i.e., altered shear stress and venous hypertension), in particular, may affect the application of AMG0102 to the venous vessel wall. As mentioned previously, AMG0102 is a balloon catheter coated first with an anionic polymer and then with poly (lactic-co-glycolic acid) particles (cationic, biocompatible, and bioerodible polymer) containing NF-κB decoy ODN. The poly (lactic-co-glycolic acid) particle is coated with an electric charge but it is not lipid-soluble. Thus, when AMG0102 is inserted into the blood vessel, the marked increase in blood flow at the AVF may wash the poly (lactic-co-glycolic acid) particle away. Possibly, the amount of NF-κB decoy ODN is decreased and insufficient to produce the expected effect. Several factors are involved in the pathology and pathogenesis of repeated stenosis and occlusion, particularly in patients with HD5; thus, such patients are typically treated with a PTA balloon. In such cases, the vein wall may be hardened by remodeling, and it is possible that AMG0102 may not be as effective in the venous vessel walls.
Many laboratory animal studies have addressed the topic of prevention of neointimal formation after angioplasty using NF-κB decoy ODN-coated balloon catheters in rat artery,11 rabbit,12 and porcine15 models. However, few studies have been conducted using venous models, especially an HD venous model. Further, there may be differences between such models using arteries and veins. Particularly, more experimental trials of AVF stenosis in animal models are needed. In this study, the primary patency rate may have varied in each facility and affected the present results. This study was powered to detect a 25% primary patency rate. This effect size may be overly large to allow the detection of a clinically meaningful effect. Finally, our sample included only Japanese patients; thus, the present results may not be generalizable to other populations and countries with different practice patterns.
In conclusion, AMG0102 was safe for clinical use and effective for the prolongation of the primary patency period; no significant differences between AMG0102 and PTA were noted. The clinical application of AMG0102 for maintenance of HD AVF patency is expected to decrease the onset of restenosis and benefit patients in terms of a decreased burden from early stenosis or occlusion of the HD shunt. In the AMG0102 group with diabetes complications (i.e., patients receiving treatment with insulin or oral hypoglycemic agents), we observed a trend toward prolongation of the duration of primary patency.
Disclosure
HH declares the receipt of lecture fees and travel support from FujiFilm, Medicon, Boston, Toray Industries, Gore, Toshiba, and Kyowa Kirin. RM discloses having received consulting fees or payments for advisory boards from AnGes, MSD, Bayer, Boehringer Ingelheim, Takeda, and Daiichi-Sankyo; has equity ownership/stock options and has received lecture fees from AnGes; has received grant support from AMED, AnGes, Boehringer Ingelheim, and Bayer; and holds the patent for the NF-κB decoy ODN. All the other authors declared no competing interests.
Acknowledgments
The authors thank Keyra Martinez Dunn, MD (Edanz Medical Writing), for providing medical writing support, which was funded by AnGes, Inc. The authors also thank Mr. Kazuki Ide, Director of the Medical Device Clinical Development Department, AnGes, Inc., and the principal investigators at all the study sites.
This study was funded by AnGes, Inc. and Medikit Co., Ltd.
This work was partially supported by a Grant-in-Aid from the Japan Innovation Promotion Program and the Translational Research Promotion Project of the New Energy and Industrial Technology Development Organization.
Author Contributions
MF, MI, SN, MN, and HH conceived and designed the study. TM and RM contributed to the interpretation of results. MF wrote the manuscript. All authors approved the final manuscript.
Footnotes
Appendix S1. Exclusion criteria.
Appendix S2. List of participating principal investigators and institutions.
Appendix S3. Rationale for sample size and corresponding calculations and definitions of analysis sets.
Table S1. Baseline demographic and clinical characteristics of patients in the FAS (continuous data).
Table S2. Stratified log-rank test for duration of primary patency in the FAS: additional analysis with data cutoff at 36 weeks.
Table S3. Summary of adverse events and serious adverse events in the safety analysis set.
Table S4. Adverse events for which a causal relationship with the study drug cannot be ruled out by SOC and PT in the safety analysis set.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Exclusion criteria.
List of participating principal investigators and institutions.
Rationale for sample size and corresponding calculations and definitions of analysis sets.
Baseline demographic and clinical characteristics of patients in the FAS (continuous data).
Stratified log-rank test for duration of primary patency in the FAS: additional analysis with data cutoff at 36 weeks.
Summary of adverse events and serious adverse events in the safety analysis set.
Adverse events for which a causal relationship with the study drug cannot be ruled out by SOC and PT in the safety analysis set.
References
- 1.Japanese Society for Dialysis Therapy Current status of chronic dialysis therapy in Japan (as of December 31, 2015), JSDT Registry. Journal of the Japanese Society of Dialysis Medicine. 2017;50:1–62. [Google Scholar]
- 2.Roy-Chaudhury P., Sukhatme V.P., Cheung A.K. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 3.Ethier J., Mendelssohn D.C., Elder S.J. Vascular access use and outcomes: an international perspective from the dialysis outcomes and practice patterns study. Nephrol Dial Transplant. 2008;23:3219–3226. doi: 10.1093/ndt/gfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmbhatt A., Remuzzi A., Franzoni M. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016;89:303–316. doi: 10.1016/j.kint.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P., Arend L., Zhang J. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–790. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Trinh K.N., Wilson S.E., Gordon I.L. Postintervention patency: a comparison of stenting versus patch angioplasty for dysfunctional hemodialysis access sites. Ann Vasc Surg. 2016;33:120–125. doi: 10.1016/j.avsg.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Kukita K., Ohira S., Amano I. 2011 update Japanese Society for Dialysis Therapy Guidelines of Vascular Access Construction and Repair for Chronic Hemodialysis. Ther Apher Dial. 2015;19(Suppl 1):S1–S39. doi: 10.1111/1744-9987.12296. [DOI] [PubMed] [Google Scholar]
- 8.Schillinger M., Minar E. Restenosis after percutaneous angioplasty: the role of vascular inflammation. Vasc Health Risk Manag. 2005;1:73–78. doi: 10.2147/vhrm.1.1.73.58932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pahl H. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 10.Morishita R., Sugimoto T., Aoki M. In vivo transfection of cis element “decoy” against nuclear factor-κB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura S., Morishita R., Hayashi K. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element ‘decoy’ of nuclear factor-κB binding site as a novel molecular strategy. Gene Ther. 2001;8:1635–1642. doi: 10.1038/sj.gt.3301566. [DOI] [PubMed] [Google Scholar]
- 12.Miyake T., Ihara S., Miyake T. Prevention of neointimal formation after angioplasty using nuclear factor-κB decoy oligodeoxynucleotide-coated balloon catheter in rabbit model. Circ Cardiovasc Interv. 2014;7:787–796. doi: 10.1161/CIRCINTERVENTIONS.114.001522. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt A.M., Hori O., Chen J.X. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basta G., Lazzerini G., Massaro M. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki K., Asai T., Shimizu M. Inhibition of NFkappaB activation using cis-element 'decoy' of NFkappaB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2003;10:356–364. doi: 10.1038/sj.gt.3301875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exclusion criteria.
List of participating principal investigators and institutions.
Rationale for sample size and corresponding calculations and definitions of analysis sets.
Baseline demographic and clinical characteristics of patients in the FAS (continuous data).
Stratified log-rank test for duration of primary patency in the FAS: additional analysis with data cutoff at 36 weeks.
Summary of adverse events and serious adverse events in the safety analysis set.
Adverse events for which a causal relationship with the study drug cannot be ruled out by SOC and PT in the safety analysis set.