Abstract
Glomerular kidney disorders account for a significant proportion of chronic kidney disease and end-stage renal disease worldwide. Nevertheless, major obstacles make breakthrough progress in diagnosis and cure an ongoing challenge. Here we report the creation of a “grassroots” initiative that aims to provide new opportunities for nephrologists, pathologists, basic and clinical scientists, patients, and industry partners to collaborate in the field of glomerular kidney disease. Members of the medical community, including trainees, nephrologists, and nephropathologists, can participate in the open-access, Web-based, multidisciplinary clinical video case conferences, which provide “peer-to-peer” exchange of clinical and pathological expertise combined with a formal didactic curriculum. Participants can also join other aspects of the broader initiative. These include the participation in a multisite research study to facilitate enrollment of patients into a longitudinal clinical data and biorepository for glomerular kidney disorders. Items included in this prospective registry include the following: an ontology-based patient medical history, which is regularly updated; interval collection and storage of blood and urine samples; DNA collection; and a contact registry for patients who wish to participate in clinical trials. Participating sites and external scientists can leverage access to the database to pursue genetic, biomarker, epidemiological, and observational clinical effectiveness studies. Patients can independently sign up for a supplementary contact registry to participate in clinical trials if eligible. The broad spectrum of activities within this initiative will foster closer collaboration among trainees, practicing nephrologists, pathologists, and researchers, and may help to overcome some of the barriers to progress in the field of glomerular kidney disease.
Keywords: disease registry, glomerular kidney disease, nephrology education, observational clinical studies
Diabetic glomerulopathy together, with other, less common forms of glomerular disorders, account for ∼64% of the prevalent end-stage renal disease (ESRD) patient population in China,1 ∼60% in India,2 ∼59% Australia/New Zealand,3 ∼54% in the United States,4 and ∼38% in Europe.5 Yet, even this majority of glomerulopathies among all causes of ESRD is still likely to be an underestimate. Not all patients are formally diagnosed through a biopsy, leading to a high degree of uncertainty with regard to the underlying cause of ESRD in registry data.4 Furthermore, a positive relationship between the rate of renal biopsy and the number of glomerulonephritis diagnoses was shown by Fiorentino et al,.6 suggesting that prevalence reports are influenced by practice pattern (sampling bias) and that the actual disease prevalence may indeed be higher than widely assumed. In addition, a sizeable portion of patients are likely misclassified as having “hypertensive nephropathy” (or the equivalent tissue diagnosis of “hypertensive nephrosclerosis”), when indeed the primary cause of ESRD is due to a focal segmental glomerulosclerosis lesion related to the presence of high-risk APOL1 alleles, or other yet poorly understood glomerulopathies.4, 7 Within the wider group of glomerulopathies, diabetic nephropathy, although responsible for a sizeable portion of ESRD cases, has repeatedly been shown to be overestimated as the underlying cause of kidney disease, masking the true prevalence of other less common primary and secondary forms of glomerulonephritis.8
These less common forms of glomerulopathies include IgA nephropathy, focal segmental glomerulosclerosis, membranous nephropathy, lupus nephritis, pauci-immune glomerulonephritis, immunotactoid and fibrillary glomerulonephritis, and a plethora of other, newly described inherited or acquired disorders.9 Each individual disease has a reported incidence of <0.1 to 2.5 per 100,000 and a prevalence of approximately 3 to 20 patients per million population.10, 11 Therefore, each represents a rare disorder according to the Orphan Drug Act from 1983 and the Rare Disease Act of 2002.12, 13 This designation highlights the unmet need in understanding and treating glomerulopathies, and makes the research and drug development for these disorders a priority for the medical community.14 These rare and highly heterogeneous presentations of glomerular disorders are not only a challenge during the clinical and pathology training years, but they also pose a significant barrier to the conduct of large observational studies and clinical trials.
Here we report the creation of a platform in which nephrologists, nephropathologists, and basic scientists come together to share experiences, to participate in medical education, and to conduct translational research activities in the field of glomerular kidney disorders. This study aims to create a network of clinicians and pathologists who can share their experience to increase each one’s exposure to rare glomerular disorders, enhance trainees’ education, and combine their patients to establish large patient cohorts to overcome challenges in glomerular disease research. This international initiative, referred to as the “Glomerular Disease Study and Trial Consortium,” is not restricted to any a priori selected center(s), but goes beyond the institutional framework to encourage the active participation of small nonacademic medical centers, community practices, and individual physicians.
Design
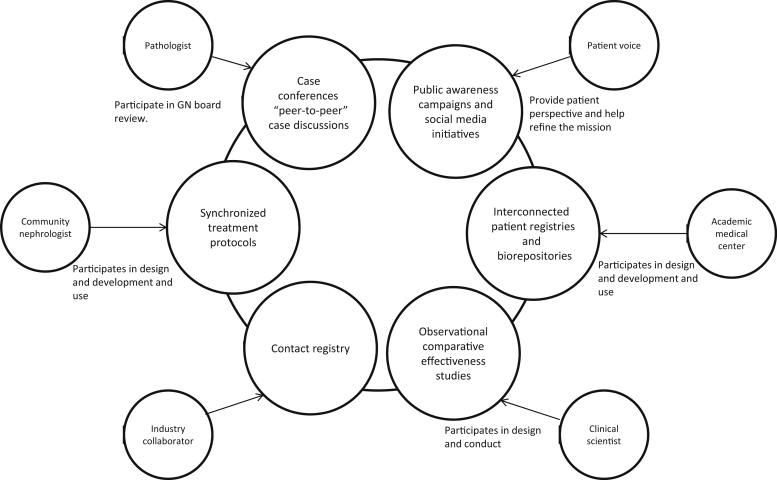
The Glomerular Disease Study and Trial Consortium has 3 focus areas: Clinical practice support, education, and research. Several interconnected platforms aim to create opportunities for members to participate in any of the 3 focus areas. Membership is open to trainees, nephrologists, nephropathologists, scientists, and industry partners. Projects within the consortium are initiated and shaped by the members (Figure 1).
Figure 1.
Overview of the Glomerular Disease Study and Trial Consortium. Six main platforms work synergistically to create new opportunities in field of glomerular kidney disease (inner circles), and one possible example on how the various members and groups may connect through a “Spoke and hub” model (outer circles). Each stakeholder can connect to lead and support any aspect of the larger initiative. GN, glomerulonephritis.
Platforms within this consortium include:
-
•
Tumor board−like case reviews and continuing medical education
-
•
Public awareness campaigns
-
•
Creation of interconnected patient registries and biorepositories
-
•
Supporting design and conduct of long-term observational studies
-
•
Contact registry for enrollment of patients into clinical trials
-
•
Synchronized treatment protocols
Tumor Board−Like Case Reviews and Continuing Medical Education
In their day-to-day practice, clinicians often consult informally with their peers on diagnostic and therapeutic questions. Commonly referred to as “curbside consultation,” this informal yet case-based interaction with colleagues enjoys broad approval among physicians.15 “Curbsiding” colleagues is not only considered helpful in diagnostic and therapeutic decision making, and hence improving patient care, but is also considered an important opportunity for professional development and medical education, and is an enjoyable aspect of a physician’s work activity.15, 16, 17 It is especially necessary for those in lone practice.
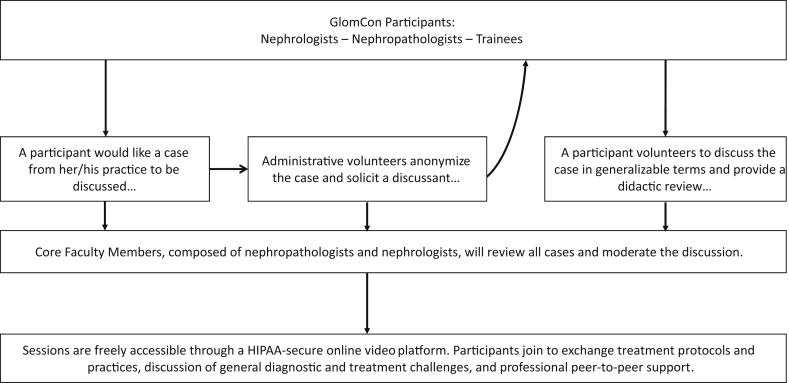
Because of the inherent diagnostic and therapeutic uncertainties, the management of patients with glomerular kidney disorders is especially conducive to curbside consultations. In this field, colleagues with a similar level of experience often seek each other’s opinion when caring for patients either with a challenging presentation, or a rare entity that a given nephrologist may only see a handful of times over the course of a career. Frequent and ongoing communication with nephropathologists also provides vital support for nuanced decision making. Similarly, nephropathologists seek each other’s informal opinion on specific cases. In this initiative, we have created an opportunity for the participation in live Web-based video case conferences, which aim to bring together nephrologists and nephropathologists to review cases from their day-to-day clinical practice (Figure 2). The goal is to promote evidence-based, rather “expert-based” case-based discussions, to access pathology curbside consultation, and to provide an opportunity for all participants to stay up to date with the latest developments in the field of glomerular kidney disorders through case-based, formalized medical education. As of April 2018, nearly 600 nephrologists and nephropathologists from 51 counties have signed up to participate in the twice-monthly virtual online case conferences. Session attendance ranges between 35 and 75 participants. Access to the online conferences are open, and health care professionals wishing to join this initiative are encouraged to sign up through the main Web portal (www.glomcon.org). Cases and discussion topics are proposed by the open community on an informal basis and presented according to the order received.
Figure 2.
Glomerular Disease Clinical Case conferences. Twice monthly web-based video conferences with tumor board−like case review and peer-to-peer exchange among nephrologists and nephropathologists. GlomCon, Glomerular Disease Study and Trial Consortium; HIPAA, Health Insurance Portability and Accountability Act of 1996, is United States legislation that provides data privacy and security provisions for safeguarding medical information, https://www.hhs.gov/hipaa/.
Public Awareness Campaigns
In recent years, several medical research areas have seen an increase in public and private funding, while funding for kidney disease research has steadily declined.18 A decline in funding is considered to be a major contributor to the steady decline in the number of physicians entering a research career.19 Because medical trainees choose their careers based on role models and available opportunities,20 depriving the profession of such may be a contributor to the declining numbers of fellowship applicants, and overall may be eroding a profession in its ability to innovate and rejuvenate.
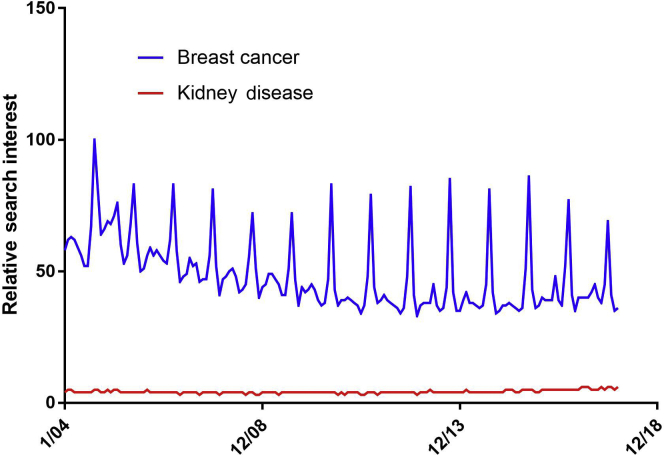
HIV/AIDS and cancer are examples of diseases that have shown that the amount of funding for research does not correlate with disease burden.21, 22 Evidence for why such discrepancies among various medical fields exist is lacking, and one can only speculate whether funding opportunities are influenced by public awareness and disease advocacy.23 Medical research areas that have received outsized public awareness have also historically drawn a disproportionate amount of public and private funding. Using Google trends in search queries for example, one may find that public interest in “kidney disease” is dwarfed by “breast cancer” (Figure 3). This interest is not reflective of their prevalence and socioeconomic burden.4, 21, 24 Although public awareness and perception about disease morbidity and mortality may be an important determinant for public and private funding, it is also likely to be an important aspect in inspiring trainees toward a career in nephrology and a desire to join the force for research and innovation.
Figure 3.
Relative search interest. Numbers represent worldwide search interest relative to the highest point on the chart for the given time. A value of 100 is the peak popularity for the term. In this case, the term “breast cancer” reached peak popularity in October 2014. (Every October “Breast Cancer Awareness Month” is celebrated, leading to annual spikes in search interest.) A value of 50 means that the term is one-half as popular. Likewise, a score of 0 means the term was less than 1% as popular as the peak. Data from Google Trends (https://www.google.com/trends).
This initiative aims to raise awareness about glomerular kidney disease. Through a steady stream of open-access teaching materials and ample opportunity to interact with experts in the field of glomerular kidney disease, we hope to spark curiosity and to inspire medical graduates and trainees to explore nephrology as a career choice.
We have created various online portals (World Wide Web: www.glomcon.org) and social media (https://twitter.com/GlomCon and https://www.facebook.com/glomcon) through which trainees are given the opportunity to connect with senior nephrologists, nephropathologists, and scientists to discuss all matters related to glomerular kidney disease, to build a professional network, and to participate in medical education. Through this, we also hope that this platform will be a positive force for “Creating a Culture of Research”25 and will provide opportunity for nephrology fellows to connect and aspire for a research- and education-oriented career. A dedicated teaching series for trainees, called GlomCon Interactive Fellows' Curriculum, has been created to enable nephrology training programs to connect and to engage in a collective learning experience. Currently, 18 training programs have signed up to provide a didactic seminar each month on a rotating basis. This initiative was awarded the 2017 American Society in Nephrology Innovations in Education Award.
Creation of Interconnected Patient Registries and Biorepositories
The low prevalence of each individual glomerular kidney disorder (except diabetic glomerulopathy), together with their highly variable clinical presentations, has made the systemic study of this disease category extremely challenging. This is not unique to glomerular disease, but applies to all rare disease conditions. Over the past several decades, disease specific human subject biorepositories and genome databases have created tremendous research opportunities for rare diseases.26, 27, 28 Their availability to basic scientists has helped to facilitate the understanding of disease mechanisms and to identify potential therapeutic targets.14 Furthermore, national and international rare disease patient registries and contact registries have made the enrollment of patients into multicenter clinical trials possible.28, 29
In the field of glomerular disease research, the Nephrotic Syndrome Study Network (NEPTUNE),30 and the Cure Glomerulopathy Network (CureGN, https://curegn.org) have been the “trailblazers” in implementing basic and clinical research through the use of prospective patient registries and biorepositories for a select number of glomerular disorders. The British Columbia Glomerulonephritis Network and Registry (BCGN Network) is enrolling a broad category of patients with biopsy-proven glomerulonephritis into a clinical data registry.31 The aims of the BCGN Network include clinical and administrative patient care support, provider and patient education, clinical trial registration, and longitudinal observational studies.
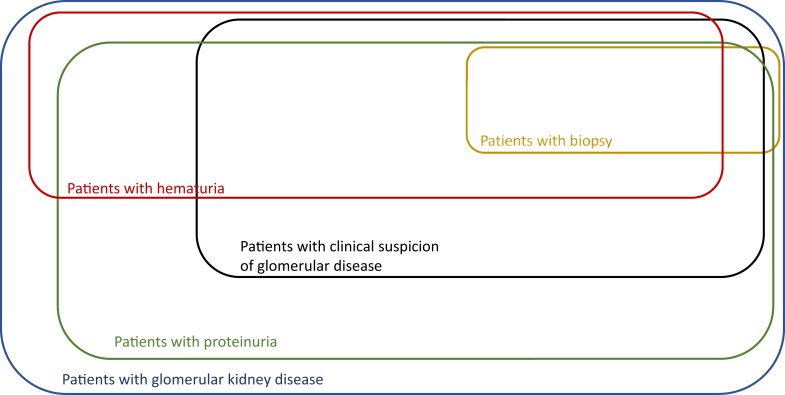
The Glomerular Disease Study and Trial Consortium will build on these efforts, and expand the enrollment into a prospective observational study of all forms of glomerular kidney disease, as defined by clinical parameters, even in the absence of a kidney biopsy (Figure 4). Our broad inclusion criteria will circumvent the inherent selection bias of biopsy registries and allow for the study of patients with glomerular disorders beginning at a much earlier disease stage. Based on level of diagnostic certainty (adjudicated consensus diagnosis among study investigators > single report, biopsy-based diagnosis > clinical diagnosis), the Glomerular Disease Study and Trial Consortium can provide distinct cohorts for “real-world” observations and validation studies in collaboration with other glomerular kidney disease registries. As opposed to a centralized database and biorepository, we are creating a spoke-and-hub pattern of participating sites, whereby centers and large clinical practices can join the initiative in a decentralized fashion. The clinical research database for this initiative is created using the Research Electronic Data Capture (REDCap) application.32 This application is a Health Insurance Portability and Accountability Act (HIPAA)−compliant, Web-based platform specifically developed to support clinical research data entry and maintenance across networks. It provides user-level access control, enabling simultaneous data entry, retrieval, and exchange within multiple investigators and study sites. REDCap also allows for different levels of restricted and privileged access to the data repository, which may contain identifiable information or be removed of all personal identifiers based on level of access, nature of consent, and purpose of the query. More than 1700 data fields are included in 26 data collection forms to allow for the storage of relevant clinical data (see Supplementary Study Protocol). The Unified Medical Language System (UMLS)33 and other biomedical research ontologies34 are used to maintain consistency across sites. This standardized, ontology-driven approach to data storage will further enhance the interoperability with other research registries or observational research initiatives such as the Observational Health Data Sciences and Informatics (OHDSI).35 Larger academic centers and smaller group practices with research infrastructures can adopt and implement this standardized clinical data repository and can rely on a select group of partner sites for collection and maintenance of biospecimens. This will allow for the flexible expansion of the initiative at lower cost. A contact registry will allow for inclusion of patients interested in clinical trial participation. Strong collaboration with private industry partners will not only serve clinical trial collaborations but will facilitate translational studies at an earlier stage. A steering committee composed of site Principal Investigators and sponsors (representatives of prospective funding entities, including industry sponsors or not-for-profit organizations) will guide the direction of this initiative, ensure consensus among investigators and sponsors, and oversee the scientific integrity. Currently 43 patients from a single center (Beth Israel Deaconess Medical Center, Boston, MA) are enrolled in this clinical data and biorepository, and have consented for longitudinal prospective clinical data, biospecimen, and DNA collection. Any individual or center providing care for patients with glomerular disorders who is interested in joining this study is invited to reach out to the authors for protocol solicitation, site feasibility review, and guidance for local protocol implementation. Currently, the Beth Israel Deaconess Medical Center and the University of Wisconsin (Madison, WI) are active study sites where the protocol has been implemented. This dual partnership has created a framework for further interested centers to join this collaborative effort. The overarching requirements within this framework include compliance at an organizational and individual level with all the requirements as stipulated by the regulations for human research protection, and training and compliance with the Good Clinical Practice Guidelines as outlined by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.36 To satisfy the National Institutes of Health requirement of “Single IRB” (single institutional review board) policy for multisite studies,37 this initiative has been implemented through the Streamlined, Multisite, Accelerated Resources for Trials IRB Reliance platform (SMART IRB; https://smartirb.org), which is funded by the National Institutes of Health Clinical and Translational Science Awards (CTSA) Program, and serves to accelerate the implementation of multisite clinical studies (IRB Protocol #: 2016P000414). A steering committee, composed of site Principal Investigator, is in charge of scientific and strategic decisions pertaining to protocol development, ancillary research activities, and the future direction of this study. This study is being conducted in accordance with the Declaration of Helsinki and has been approved by the Institutional Review Board (Protocol Nr. 2016P000414).
Figure 4.
Patient subcategorization. Qualitative schematic representation of various patient subpopulations with glomerular kidney disease.
Supporting Design and Conduct of Long-term Observational Studies
The widespread adoption of electronic health records has provided an opportunity to conduct retrospective clinical studies in a more efficient and cost-effective manner. Nevertheless, retrospective studies conducted using medical records designed for patient care are inherently prone to biases and errors.38, 39 Prospectively collected clinical information, which has been obtained through patients’ medical records, is manually reviewed by study members, and predefined clinical information and patient history is entered into online accessible electronic case report forms. This process of manual review, curation, and standardized categorization can provide well-defined data with higher fidelity and accuracy. The clinical research database within the Glomerular Disease Study and Trial Consortium consists of the following data categories:
-
•
Demographic information
-
•
Medical history
-
•
Family history
-
•
Laboratory data
-
•
Medication history including “over-the-counter” and herbal preparations
-
•
History of therapeutic interventions
-
•
Biopsy reports with individual pathologic parameters and imaging
-
•
Patient-reported quality of life
-
•
Genetic and biomarker information
The design of this prospective clinical data registry is particularly structured to enable large multicenter observational studies in the field of glomerular kidney disorders.
Synchronized Treatment Protocols
Randomized controlled trials (RCTs) are the gold standard in the empiric assessment of efficacy and safety of medical interventions. However, their successful implementation is challenged by difficulties in patient enrollment, high cost, and large administrative and regulatory burden.40, 41 Furthermore, RCTs frequently fail to reproduce their findings in the larger general population.42 This phenomenon has been attributed to, among other factors, the fact that the study population is highly selected, homogenous, and the intervention narrowly defined. Pragmatic trials and observational comparative effectiveness studies (OCES) aim to overcome these challenges by providing a more flexible framework for the design and conduct of clinical studies. “Real-world” interventions and prospective OCES can complement RCTs in the assessment of the safety and efficacy of medical interventions.41, 43, 44
In the field of glomerular kidney disorders, large RCTs and OCES have faced major challenges. The low prevalence of individual disorders and the heterogeneous disease presentation are major barriers to the successful implementation of RCTs. A plethora of treatment protocols that often are supported only by weak evidence are further subject to practice variations and local preferences. This divergent use of treatment approaches among providers, and modifications to management protocols between patients of the same provider, further impede the effective comparison of medical interventions through OCES.
Within the Glomerular Disease Study and Trial Consortium, we aim to create a framework to facilitate a consensus approach in the management of patients with glomerular disorders to enable retrospective and prospective OCES. Without affecting provider autonomy in diagnosis or treatment choice, a core set of standardized protocols will be created to ensure a reproducible, homogeneous, and synchronized practice in the following areas:
-
•
Pathologic evaluation, including the assessment of chronicity
-
•
Disease classification
-
•
Treatment standards
-
•
Supportive care
-
•
Patient follow-up and outcome assessment
This core set of standardized protocols will not be a replacement or alternative to current clinical practice guidelines or individual provider decision but, rather, will ensure that any given approach is pursued in a uniform and standardized fashion. As an example, one may refer to the diagnosis and treatment approaches in lupus nephritis: The tissue pathology reporting, serology testing, disease activity scoring, and patient-reported outcomes will all be recorded and documented in a reproducible and standardized fashion. Although the choice of therapy would be at the discretion of the provider, the implementation would follow a consensus method (e.g., 1000 mg vs. 500 mg for pulse dose methylprednisolone; high-dose cyclophosphamide vs. low-dose cyclophosphamide for “induction”; duration and choice of agent for “maintenance”; which antibiotic prophylaxis regimen to use? How frequently to monitor which clinical and serologic disease markers?). We aim to establish consensus protocols among participating clinicians who enroll patients into the observational study, by first creating a bank of all current routine practice routines, local treatment protocols if available, and protocols as applied in clinical trials. The strengths and limitations of each protocol and its applicability to local practices will be reviewed, and a common acceptable approach will be selected for routine clinical use. We hope that a more uniform application of therapies and clinical response assessments (e.g., currently 1 clinician may check monthly spot urinary albumin-to-creatine ratio, whereas another may obtain biannual 24-hour total urinary protein excretion) will improve our ability to compare and to study patient cohorts across different practices and regions.
Contact Registry for Enrollment of Patients Into Clinical Trials
Interventional clinical trials in patients with glomerular kidney disease have been most successful when large networks and registries supported the efficient identification, screening, and enrollment of patients into a multicenter trial.45 Over the past several years, we have seen steady progress in the identification of therapeutic targets and rapid development of new biologics and small molecules for several glomerular kidney disorders, including antineutrophil cytoplasmic antibody−associated glomerulonephritis, C3 glomerulonephritis, and lupus nephritis.46 This progress suggests new opportunities for patient participation in interventional clinical trials.
Within this initiative, and as part of the study consent process, patients are given the opportunity to enroll into a contact registry for future interventional clinical trials. This will allow for prompt identification of potential study candidates and detailed prescreening (inclusion/exclusion criteria can be extracted from the registry records) without costly outreach and a high number of screening failures. Considering patient identification and screening to be a major time and cost factor in clinical trial operations, this initiative may serve as an additional resource for current investigator-initiated or industry-sponsored clinical trials. Patients with glomerular disorders have a strong interest in clinical trials, a desire that is reflected by the fact that of the 43 patients currently enrolled in this study, all have signed up to participate in the contact registry to be considered for future trials.
Discussion
Glomerular kidney diseases are relatively common; yet, more education and research for clinicians and scientists is required to better understand the intricacies of the pathophysiology and treatment options of these heterogeneous disorders. The variability in clinical presentations, diagnostic criteria, and therapeutic uncertainties pose major challenges in the care of these patients. These and other challenges, which are specific to glomerular disorders, were reviewed in detail by Moxey-Mims et al.47 The authors reviewed and compared several consortia and registries, all of which aim to advance our understanding of autoimmune inflammatory kidney disorders and to help facilitate patient recruitment. Among these initiatives are the national, National Institute of Diabetes and Digestive and Kidney Diseases−funded consortia: The Nephrotic Study Network (NEPTUNE),30 and the Cure Glomerulopathy Network (CureGN). They both are prospective studies that recruit patients with biopsy-proven glomerular disorders. Whereas the NEPTUNE Study focuses on the 3 primary disorders, minimal change disease, focal segmental glomerulosclerosis, and membranous nephropathy, the CureGN study has expanded this list to also include IgA nephropathy. Other glomerulonephritis registries include the British Columbia Glomerulonephritis Network and Registry31 and the Toronto Glomerulonephritis Registry,48 which are single-center initiatives limited to biopsy-proven glomerulonephritis and which do not include biospecimen collections. The UNC Glomerular Disease Collaborative Network49 is enrolling patients with all forms of glomerular diseases into a single-center data- and biorepository. The Glomerular Disease Study and Trial Consortium is a “grassroots” initiative to create multisite interconnected registries and biorepositories for all forms of glomerular disorders without the prerequisite of a biopsy or an established diagnosis. Thus, it will allow large-scale and unbiased collection of clinical data and biospecimens in patients presenting with suspected glomerular disorders, long before a diagnosis is established, or treatment is implemented. This early enrollment of a diverse patient cohort can hopefully complement the aforementioned studies and serve as a reference population for researchers seeking “real-world” clinical information. Furthermore, the Glomerular Disease Study and Trial Consortium is deliberately designed to function without the usual institutional confinements, which has allowed an international group of participants to engage in live, interactive, peer-to-peer discussions while obtaining continuing medical education. This engagement may not only be a welcome opportunity to keep abreast with current practice guidelines and to increase the clinician’s exposure to this category of rare disorders, but may also help to broaden the reach of the registry and, through this, increase research participation and patient enrollment.
There are other disease entities, for example, cancer and autism, with significant exposure to the broader community, particularly through advocacy groups and main stream media. The goal of The Glomerular Disease Study and Trial Consortium is to elevate glomerular disease to a public and scientific community awareness level that approaches that of other disease categories. By providing more exposure to glomerular kidney disorders through various teaching and research activities, we hope to increase awareness among the public, spark interest among trainees, improve avenues for trial funding, and develop study and treatment protocols, all of which will ultimately have an impact on patient outcomes. Furthermore, this initiative has the potential to serve as a platform through which patients and clinicians can come together to share perspectives to advance the care of patients with glomerular kidney diseases. Increasing opportunities for patient−provider interactions through the inclusion of patient representatives into the case discussions will not only increase populationwide awareness and support but may also help clinicians and scientists to better understand patients’ experiences and priorities. The Standardized Outcomes in Nephrology (SONG) Initiative (www.songinitiative.org) is an example of how patients, clinicians, and scientists can come together to define a common path for strengthening clinical research activities for patients with kidney disease.
The creation of a clinical data registry and biorepository, together with consensus-driven “harmonization” of treatment protocols, may enable future large prospective observational effectiveness studies, which currently are challenging to implement. This data/biorepository and contact registry can further serve as a reference population for other large collaborative research initiatives currently underway (e.g., CureGN Project, Kidney Precision Medicine Project) and provides the additional capacity to screen and enroll patients for investigator-initiated or industry-driven clinical trials.
The future direction of this initiative will be dictated by its ability to become sustainable and externally funded. Currently, the “GlomCon” case conferences and public awareness initiatives are supported primarily by uncompensated labor and time commitments of the authors and core participants. The research mission is supported by institutional seed funds. We hope to create a diverse network of partnerships with not-for-profit, private, and public sponsors, who can leverage this Consortium for collaborative research projects. Although there are similar glomerular disease registries and biorepositories available around the world, we believe that the Glomerular Disease Study and Trial Consortium has a unique multifaceted approach that does not apply to only a single objective.
Disclosure
All the authors declared no competing interests.
Acknowledgments
APM is supported by the Chief Academic Officer’s Innovations Grant, Beth Israel Deaconess Medical Center.
Footnotes
Study Protocol.
Supplementary material is linked to the online version of the article at www.kireports.org.
Supplementary Material
References
- 1.Zuo L., Wang M. Chinese Association of Blood Purification Management of Chinese Hospital Association. Current burden and probable increasing incidence of ESRD in China. Clin. Nephrol. 2010;74(suppl 1) S20-22. [PubMed] [Google Scholar]
- 2.Sakhuja V., Jha V., Ghosh A.K. Chronic renal failure in India. Nephrol Dial Transplant. 1994;9:871–872. [PubMed] [Google Scholar]
- 3.Australia and New Zealand Dialysis and Transplant Registry, 37th Annual Report. Adelaide: 2014.
- 4.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2017. 2017 USRDS annual data report: epidemiology of kidney disease in the United States. [Google Scholar]
- 5.Kramer A., Pippias M., Noordzij M. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: a summary. Clin Kidney J. 2018;11:108–122. doi: 10.1093/ckj/sfx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorentino M., Bolignano D., Tesar V. Renal biopsy in 2015—from epidemiology to evidence-based indications. Am J Nephrol. 2016;43:1–19. doi: 10.1159/000444026. [DOI] [PubMed] [Google Scholar]
- 7.Longenkecker J.C., Coresh J., Klag M.J. Validation of comorbid conditions on the End-Stage Renal Disease Medical Evidence Report: the CHOICE Study. J Am Soc Nephrol. 2000;11:520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentino M., Bolignano D., Tesar V. Renal biopsy in patients with diabetes: a pooled meta-analysis of 48 studies. Nephrol Dial Transplant. 2017;32:97–110. doi: 10.1093/ndt/gfw070. [DOI] [PubMed] [Google Scholar]
- 9.Hebert L.A., Parikh S., Prosek J. Differential diagnosis of glomerular disease: a systematic and inclusive approach. Am J Nephrol. 2013;38:253–256. doi: 10.1159/000354390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrogan A., Franssen C.F.M., de Vries C.S. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 11.Braun N., Schweisfurth A., Lohöfener C. Epidemiology of glomerulonephritis in northern Germany. Int Urol Nephrol. 2011;43:1117–1126. doi: 10.1007/s11255-011-9955-4. [DOI] [PubMed] [Google Scholar]
- 12.US Congress. Orphan Drug Act. 96 Stat. 2, Public Law 97-414; 1983.
- 13.US Congress. An act to amend the Public Health Service Act to establish an Office of Rare Diseases at the National Institutes of Health, and for other purposes. H.R. 4013, Public Law 107-280; 2002.
- 14.Griggs R.C., Batshaw M., Dunkle M. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. 2009;96:20–26. doi: 10.1016/j.ymgme.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating N.L., Zaslavsky A.M., Ayanian J.Z. Physicians’ experiences and beliefs regarding informal consultation. JAMA. 1998;280 doi: 10.1001/jama.280.10.900. 900–194. [DOI] [PubMed] [Google Scholar]
- 16.Kuo D., Gifford D.R., Stein M.D. Curbside consultation practices and attitudes among primary care physicians and medical subspecialists. JAMA. 1998;280:905–909. doi: 10.1001/jama.280.10.905. [DOI] [PubMed] [Google Scholar]
- 17.Cook D.A., Sorensen K.J., Wilkinson J.M. Value and process of curbside consultations in clinical practice: a grounded theory study. Mayo Clin Proc. 2014;89:602–614. doi: 10.1016/j.mayocp.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Bryan L., Ibrahim T., Zent R., Fischer M.J. The kidney research predicament. J Am Soc Nephrol. 2014;25:898–903. doi: 10.1681/ASN.2013121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluestone J, Beier D, Glimcher L. The NIH is in danger of losing its edge in creating biomedical innovations. STAT News. Available at: https://www.statnews.com/2018/01/03/nih-biomedical-research-funding/. Accessed January 3, 2018.
- 20.Reynolds E.E. Influencing career choice during residency. J Gen Intern Med. 1999;14:512–513. doi: 10.1046/j.1525-1497.1999.06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moses H., III, Matheson D.H., Cairns-Smith S. The anatomy of medical research: US and international comparisons. JAMA. 2015;313:174–189. doi: 10.1001/jama.2014.15939. [DOI] [PubMed] [Google Scholar]
- 22.Carter A.J.R., Nguyen C.N. A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Public Health. 2007;12:526. doi: 10.1186/1471-2458-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Increasing public awareness. Nat Immunol. 2007;8:109. doi: 10.1038/ni0207-109. [DOI] [PubMed] [Google Scholar]
- 24.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England) 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avins A.L., Goldberg H. Creating a culture of research. Contemp Clin Trials. 2007;28:557–562. doi: 10.1016/j.cct.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 26.De Souza Y.G., Greenspan J.S. Biobanking past, present and future: responsibilities and benefits. AIDS. 2013;27:303–312. doi: 10.1097/QAD.0b013e32835c1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groft S.C., Rubinstein Y.R. New and evolving rare diseases research programs at the National Institutes of Health. Public Health Genomics. 2013;16:259–267. doi: 10.1159/000355929. [DOI] [PubMed] [Google Scholar]
- 28.Aymé S. State of the art of rare disease activities in Europe: a EUCERD perspective. Orphanet J Rare Dis. 2012;7:A1. [Google Scholar]
- 29.Field M.J., Boat T.F., editors. Rare Diseases and Orphan Products. Accelerating Research and Development. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 30.Gadegbeku C.A., Gipson D.S., Holzman L.B. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83:749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbour S., Beaulieu M., Gill J. An overview of the British Columbia Glomerulonephritis Network and Registry: integrating knowledge generation and translation within a single framework. BMC Nephrol. 2013;14:236. doi: 10.1186/1471-2369-14-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindberg D.A., Humphreys B.L., McCray A.T. The Unified Medical Language System. Methods Inf Med. 1993;32:281–291. doi: 10.1055/s-0038-1634945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith B., Ashburner M., Rosse C. The OBO Foundry: coordinated evolution of ontologies to support biomedical data integration. Nat Biotechnol. 2007;25:1251–1255. doi: 10.1038/nbt1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hripcsak G., Duke J.D., Shah N.H. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 36.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Guideline for Good Clinical Practice E6(R1), Step 4 version, 10 June 1996. U.S. Federal Register; 2018:83;41:8882-3.
- 37.Ervin A.M., Taylor H.A., Ehrhardt S. NIH Policy on single-IRB review—a new era in multicenter studies. N Engl J Med. 2016;375:2315–2317. doi: 10.1056/NEJMp1608766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hripcsak G., Knirsch C., Zhou L. Bias associated with mining electronic health records. J Biomed Discov Collab. 2011;6:48–52. doi: 10.5210/disco.v6i0.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan W.R., Wagner M.M. Accuracy of data in computer-based patient records. J Am Med Inform Assoc. 1997;4:342–355. doi: 10.1136/jamia.1997.0040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross S., Grant A., Counsell C. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 41.Mentz R.J., Hernandez A.F., Berdan L.G. Good clinical practice guidance and pragmatic clinical trials: balancing the best of both worlds. Circulation. 2016;133:872–880. doi: 10.1161/CIRCULATIONAHA.115.019902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy-Martin T., Curtis S., Faries D. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:1–14. doi: 10.1186/s13063-015-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohr K.N. Comparative effectiveness research methods symposium overview and summary. Med Care. 2010;48:3–6. doi: 10.1097/MLR.0b013e3181e10434. [DOI] [PubMed] [Google Scholar]
- 44.Bauer Z. Challenges conducting comparative effectiveness research: the Clinical and Health Outcomes Initiative in Comparative Effectiveness (CHOICE) experience. Comparative Effectiveness Research. 2014;4:1–12. [Google Scholar]
- 45.Anders H., Jayne D.R.W., Rovin B.H. Hurdles to the introduction of new therapies for immune-mediated kidney diseases. Nat Rev Nephrol. 2016;12:205–216. doi: 10.1038/nrneph.2015.206. [DOI] [PubMed] [Google Scholar]
- 46.Holdsworth S.R., Gan P., Kitching A.R. Biologics for the treatment of autoimmune renal diseases. Nat Rev Nephrol. 2016;12:217–231. doi: 10.1038/nrneph.2016.18. [DOI] [PubMed] [Google Scholar]
- 47.Moxey-Mims M.M., Flessner M.F., Holzman L. Glomerular diseases: registries and clinical trials. Clin J Am Soc Nephrol. 2016;11:2234–2243. doi: 10.2215/CJN.00540116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regional program for the study of glomerulonephritis. Central Committee of the Toronto Glomerulonephritis Registry. Can Med Assoc J. 1981;124:158–161. [PMC free article] [PubMed] [Google Scholar]
- 49.O’Shaughnessy M.M., Hogan S.L., Poulton C.J. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986–2015. Clin J Am Soc Nephrol. 2017;12:614–623. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.