Abstract
Introduction
Vancomycin pharmacokinetic data in patients with acute kidney injury (AKI) on high-volume peritoneal dialysis (HVPD) are lacking. The aims were to study the pharmacokinetics of i.v. vancomycin in patients with AKI treated by HVPD who received an i.v. dose of vancomycin (15–20 mg/kg), to determine the vancomycin removal, and to establish vancomycin dosing and evaluation pharmacokinetics target attainment achievement for the empirical treatment of patients with AKI treated by HVPD.
Methods
Vancomycin was administered 1 hour before dialysis start. Samples of all dialysate were collected for a 24-hour period. Blood samples were collected after 1, 2, 4, and 24 hours of therapy. Vancomycin concentrations were determined using a liquid chromatographic (high-performance liquid chromatography)–fluorescence method. Pharmacokinetic calculations were completed assuming a 1-compartment model.
Results
Ten patients completed the study. The mean vancomycin dose administered was 18.0 ± 2.95 mg/kg (14.7–21.8 mg/kg) on the day of study (first day) and the mean percentage of vancomycin removal by HVPD was 21.7% ± 2.2% (16%–29%). Peritoneal clearance was 8.1 ± 2.2 ml/min (5.3–12 ml/min). The serum vancomycin half-life was 71.2 ± 24.7 hours (42–110 hours) during HVPD session, the maximum serum concentration was 26.2 ± 3.5 mg/l, which occurred 1 hour after vancomycin administration and HVPD start. Area under the curve (AUC)0–24/minimum inhibitory concentration (MIC) ratio ≥400 was achieved in all patients when MIC = 1 mg/l was considered.
Conclusion
HVPD removes considerable amounts of vancomycin in septic patients with AKI. Administration of 18 mg/kg vancomycin each 48 to 72 hours in patients with AKI undergoing HVPD was required to reach and maintain therapeutic concentrations.
Keywords: acute kidney injury, peritoneal dialysis, sepsis, vancomycin
Sepsis is the main etiology of AKI in critically ill patients and can reach the surprising mortality rate of 70%.1, 2, 3, 4 Current renal replacement therapy modalities include peritoneal dialysis (PD), intermittent hemodialysis (conventional hemodialysis or prolonged hemodialysis), and continuous renal replacement therapy. Continuous methods are preferred in hemodynamically unstable patients; however, previously published studies have shown no difference in patient survival among continuous renal replacement therapy, intermittent hemodialysis, or PD.5
Recently, the interest in PD to manage patients with AKI has been increased, mainly in developing countries, due to its lower cost and minimal infrastructural requirements. Brazilian studies have shown that, with careful thought and planning, patients with AKI can be successfully treated using PD.6, 7
Our previous studies have demonstrated that HVPD can offer adequate small solute clearances and ultrafiltration using a flexible catheter, 2-l exchanges (total dialysate volume ranged from 32 to 44 l per day) and 35- to 60-minute dwell times. HVPD was rapidly effective in the correction of blood urea nitrogen, creatinine, bicarbonate, and fluid overload. The achieved weekly Kt/V (K, dialyzer clearance of urea; t, dialysis time; V, volume of distribution of urea) was 3.8 ± 0.6 and the mortality was 57%.6, 7, 8
Considering the high mortality rate of septic AKI and its related costs of hospitalization and treatment, the early administration of antimicrobials should be adopted.
Vancomycin is an antibiotic used in the parenteral treatment of infections caused by multidrug-resistant gram-positive bacteria, especially methicillin-resistant Staphylococcus aureus. Its antibacterial action is time-dependent and concentration-independent.9, 10 Based on the pharmacodynamics of vancomycin, an AUC (ratio) is recommended by the MIC (AUC/MIC) of 400 or greater. Due to the logistic challenges of such determination in clinical practice, it was demonstrated that such value could be obtained with trough serum levels of vancomycin between 10 and 20 mg/l.9, 11 A serum level of less than 10 mg/l does not guarantee an AUC/MIC ratio greater than 400 and may develop bacterial resistance. However, the dosage above 20 mg/l can be nephrotoxic.9, 10
Vancomycin has almost exclusive renal elimination, making dosage adjustment during kidney failure obligatory. The half-life of vancomycin is 5 to 8 hours in patients with normal kidney function and higher than 180 hours in patients with advanced chronic kidney disease.12, 13, 14, 15, 16 Volume of distribution of vancomycin is 0.4 to 1.0 l/kg, molecular weight is approximately 1450 Da, and protein binding from 10% to 50%.16
Vancomycin is not removed to a significant extent during PD and should be administered each 72 to 120 hours. However, there is no study that evaluated the vancomycin removal during HVPD sessions in patients with AKI and no strategies have been proposed to appropriately dose vancomycin in HVPD.14, 15, 16
Our aim was to study the pharmacokinetics of i.v. vancomycin in patients with AKI treated by HVPD who received a single i.v. dose of vancomycin (15 mg/kg), to determine the vancomycin removal, to compare vancomycin levels obtained in blood with MICs of typical gram-positive bacterial pathogens, to evaluate pharmacokinetic target attainment achievement, and to establish vancomycin dosing guidelines for the empirical treatment of patients with AKI receiving HVPD.
Methodology
This was a prospective cohort clinical study performed at the University Hospital Sao Paulo State in Botucatu Medical School, Brazil. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.The study protocol was approved by local ethics committees. Written informed consent was obtained from patients or families. It was not registered because it is not a clinical trial study. It is a prospective cohort clinical study.
Ten critically ill adult patients with sepsis and AKI treated by HVPD on vancomycin therapy were included. Classification of AKI was made according to Kidney Disease: Improving Global Outcomes criteria.17 All patients were oliguric (urine output lower than 0.5 ml/kg per hour). They were followed for 2 days after introduction to the study.
PD was performed using a flexible catheter, an automated cycler (HomeChoice; Baxter, Deerfield, IL), and a high volume of dialysis fluid, as described in our previous studies.6, 7, 8 Each session of HVPD lasted 24 hours, and sessions were repeated daily, 7 times per week. The total dialysate volume per session ranged from 32 to 38 l and the prescribed Kt/V was 0.50 per session.
Vancomycin was administered 1 hour before dialysis start. The protocol required that vancomycin 15 to 20 mg/kg was reconstituted with 100 ml 0.9% sodium chloride and was administered for 1 hour. Blood samples were collected after 1, 2, and 4 hours of dialysis start and end of dialysis (after 24 hours). Dialysate samples were collected at the end of each dwell using a sample port directly on the automatic cycler machine for a 24-hour period. Vancomycin concentrations were determined using a liquid chromatographic (high-performance liquid chromatography)–fluorescence method. Blood samples were placed in an ice bath and centrifuged within 3 hours of collection. Serum was transferred to a labeled polypropylene tube and stored frozen at –80°C until assayed. Volumes of dialysate and of ultrafiltration were recorded for each patient.
Throughout, the analysis was carried out using a high-performance liquid chromatography system (Agilent Series 1100; Agilent Technologies, Palo Alto, CA), according to the protocol described by Hu et al.18 The following pharmacokinetic parameters were calculated for each patient assuming a 1-compartment model: volume of distribution (Vd), dialysis total drug clearance (Cl), and elimination half-life. The AUC was determined using the trapezoidal rule to the last nonzero time point. The AUC from the last nonzero time point was determined by the pharmacokinetic method, concentration/k, where k is the elimination rate constant determined from log-linear regression of the terminal phase of the concentration-time profile. A monoexponential model was used for all pharmacokinetic calculations. The following equations were used: the serum elimination rate constant (k el) for dwells was obtained by regression of the serum concentrations over the time between the start of the dwell and the end of the dwell. The formula used to determine the approximate serum concentration was C = C 0 * e–k t, where C = concentration, C 0 = original concentration, –k = elimination rate constant, and t = time between sampling points. The serum elimination half-life for patients on the cycler was calculated as (0.693)/(mean k el dwells). The serum elimination half-life for patients off the cycler was calculated as (0.693)/(mean k el dwells). The serum AUC for the first 24 hours was calculated by summation of the AUC of each dwell, using the trapezoid rule, and from 24 hours to infinity by extrapolation of the serum concentration at 24 hours/k el last dwell.
Data are expressed as median and range. The vancomycin removal from blood during the HVPD session was calculated from the following formula and expressed as % vancomycin removed:
where CpreHVPD = the concentration of vancomycin before HVPD, and C24hHVPD = the concentration of vancomycin at end of HVPD.
Sample Size Calculation
This study was performed to estimate the mean amount of vancomycin removed by HVPD and to establish an 80% confidence interval about that value such that the width was less than ±20%, using α = 0.05, β = 0.2, and standard sample size equations for parametric data; it was estimated that 10 patients were required.19
Results
Table 1 shows the patients and clinical characteristics. All patients had sepsis or septic shock. The main bacterial agents were gram-positive Staphylococcus haemolyticus, methicilin-resistant S aureus, and Enterococcus faecalis. All patients were in stage 3 according to Kidney Disease: Improving Global Outcomes criteria of AKI.17 Mean glomerular filtration rate according to MDRD was 0.3 ml/min (0 to 0.74 ml/min). The mean dwell times for cycles ranged from 35 to 50 minutes and 7 patients were anuric.
Table 1.
Patients’ demographic and clinical characteristics
| Patient | Sex | Age, yr | Weight, kg | SOFA | Diagnosis | Pathogen/MIC | Urine output, ml/kg per h |
|---|---|---|---|---|---|---|---|
| 1 | F | 58 | 65 | 11 | Pneumonia | MRSA | 0 |
| 2 | F | 62 | 55 | 12 | Endocarditis | Enterococcus | 0.32 |
| 3 | F | 59 | 58 | 14 | Meningitis | MRSA | 0.38 |
| 4 | M | 76 | 71 | 13 | Pneumonia | Staphylococcus haemolyticus | 0.40 |
| 5 | M | 72 | 62 | 12 | Peritonitis | Enterococcus | 0 |
| 6 | M | 59 | 58 | 14 | Meningitis | MRSA | 0 |
| 7 | F | 44 | 75 | 13 | Pneumonia | MRSA | 0 |
| 8 | M | 67 | 68 | 12 | Blood Stream | Enterococcus | 0.44 |
| 9 | M | 63 | 72 | 13 | Blood Stream | MRSA | 0 |
| 10 | M | 72 | 62 | 14 | Peritonitis | Enterococcus | 0.42 |
F, female; M, male; MRSA, methicillin-resistant Staphylococcus aureus; SOFA, Sequential Organ Failure Assessment.
Vancomycin removal by HVPD was 21.7% ± 2.2% (16%–29%). Vancomycin serum concentrations higher than 15 mg/l at the day of study dialysis were obtained in all patients at the end of dialysis. The median vancomycin dose was 18 ± 2.95 mg/kg (14.7–21.8 mg/kg) on the day of study (first day). In all patients, it was the first vancomycin dose administered.
AUC0–24/MIC ratio ≥ 400 was achieved in all patients on the study days (Table 2), when MIC = 1 mg/l was considered. Table 2 shows the vancomycin pharmacokinetic parameters.
Table 2.
PK/PD vancomycin parameters of individual patients
| Patient | Doses, mg/kg | C Van T1h, mg/l | C Van T2h, mg/l | C Van T4h, mg/l | C Van T24h, mg/l | C Van dialysate, mg/l | Half-life, h | AUC/MIC | Removal, % | Peritoneal Cl, ml/min |
Distribution volume, l/kg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Van | Ur | |||||||||||
| 1 | 15.3 | 22.5 | 19.4 | 17.3 | 16.8 | 9.5 | 42.2 | 424.4 | 25 | 12 | 12.5 | 0.68 |
| 2 | 21.8 | 28.6 | 27.7 | 25.1 | 23.8 | 5.6 | 64 | 568 | 16 | 8.8 | 10.8 | 0.85 |
| 3 | 17.2 | 24.5 | 21.3 | 19.3 | 17.9 | 8.2 | 52,4 | 424.5 | 25 | 9.2 | 11.5 | 0.7 |
| 4 | 21.3 | 28.2 | 27.6 | 25 | 23.1 | 6.1 | 105 | 557.4 | 18 | 5.7 | 8.2 | 0.74 |
| 5 | 16.1 | 22.2 | 19.7 | 19 | 17.8 | 8.1 | 52.6 | 422.5 | 23 | 8.3 | 9.7 | 0.72 |
| 6 | 17 | 24.5 | 21.2 | 17.3 | 16.9 | 9.3 | 65 | 490 | 24 | 6.8 | 9.1 | 0.73 |
| 7 | 13.5 | 32.7 | 28.6 | 27.1 | 25.9 | 8.1 | 77.5 | 605 | 2 | 9.7 | 13.4 | 0.61 |
| 8 | 19 | 26.6 | 22.4 | 19.6 | 18.8 | 9 | 53.4 | 448 | 29 | 5.3 | 9.8 | 0.65 |
| 9 | 14 | 24.6 | 22.3 | 21 | 20.5 | 5.2 | 110 | 477.5 | 16 | 8.1 | 11.4 | 0.7 |
| 10 | 22.3 | 22.1 | 19.6 | 19.2 | 17.7 | 8.2 | 77.6 | 401 | 22 | 8.6 | 10.5 | 0.62 |
AUC/MIC, area under the curve/minimum inhibitory concentration; C Van, serum concentration of vancomycin; Cl, clearance; Ur, urea.
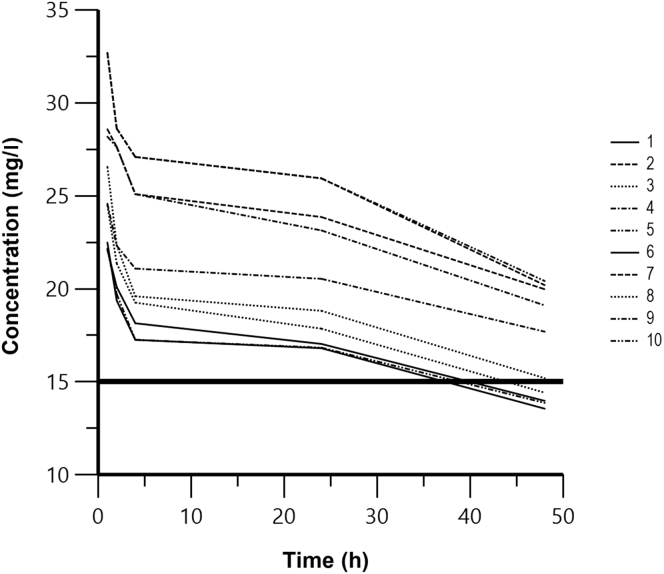
The mean serum and dialysate concentrations remained in excess of MICs for sensitive organisms (approximately 1 mg/l) in all patients during the treatment. The mean vancomycin peak serum concentration was 26.2 ± 3.5 mg/l, which occurred 1 hour after vancomycin administration and HVPD start. The mean dwell dialysate concentration was 7.3 ± 1.77 mg/l. Figure 1 shows serum vancomycin concentrations during HVPD therapy.
Figure 1.
Serum vancomycin concentrations during high-volume peritoneal dialysis therapy.
Peritoneal clearance was 8.1 ± 2.2 ml/min (5.3–12 ml/min) and vancomycin half-life during dialysis was 71.2 ± 24.7 hours (42–110 hours).
Discussion
The purpose of this study was to characterize the pharmacokinetics of vancomycin and provide dosing recommendations based on a single i.v. 15-mg/kg dose of vancomycin in patients with AKI treated by HVPD (prescribed Kt/V of 0.5 per session) using 18 to 24 cycler exchanges over 24 hours.
Although the pharmacokinetics of vancomycin have been studied in chronic patients on continuous ambulatory PD, the data are limited for automated PD and there were no data in HVPD. Vancomycin clearance is minimal during continuous ambulatory PD and should be administered approximately every 5 to 7 days,14 whereas during an automatic PD treatment, the vancomycin clearance is significant, and should be administered every 3 to 5 days. In a small study of 4 pediatric patients with peritonitis while on automated PD, vancomycin serum concentrations decreased 17% after the automated dialysis session.20, 21 It was recommended that vancomycin should be readministered to maintain therapeutic serum concentrations. Dialysate concentrations were not provided.
In this study, vancomycin removal mean was 21% during HVPD. Previous study reported that it was 10% in continuous ambulatory PD, 17% in automated PD, and ranged from 17% to 31% in intermittent hemodialysis therapy, according to flux membrane dialysis used.14, 22 Variability in vancomycin removal in HVPD was modest (16% to 29%), and it is different from that found in studies that described hemodialysis removal using low- and high-flux dialysis. Peritoneal clearance was 8.1 ± 2.2 ml/min (5.3–12 ml/min), whereas high-flux membranes have estimated vancomycin clearance between 43.3 and 120.0 mL/min. Clearance of vancomycin during PD may be affected by inflammation that affects peritoneal permeability and dialysate flow. These conditions may lead to variability in vancomycin clearance during PD dosage regimens in a critical care setting.
Due to high pharmacokinetic variability in septic patients with AKI, we suggest daily monitoring of vancomycin concentrations to reach optimal trough vancomycin concentrations and its dose may be readministered every 48 to 72 hours to maintain therapeutic serum concentrations in patients with AKI undergoing HVPD. In hybrid and conventional hemodialysis therapies, vancomycin dose should be administered over the last hour of dialysis and vancomycin intradialytic clearance was approximately 2-fold higher with high-flux membrane compared with low-flux membrane.22
The vancomycin peak serum concentration occurred 1 hour after vancomycin administration and HVPD start, and vancomycin serum concentrations higher than 15 mg/l were obtained in all patients at the end of 1 session of HVPD. Vancomycin half-life during HVPD session was 71.2 ± 24.7 hours (42–110 hours). Serum and dialysate concentrations suggest that i.v. vancomycin 18 mg/kg each 72 hours would provide adequate concentrations over a 24-hour period.
An AUC/MIC ratio ≥400 is necessary to achieve clinical effectiveness with vancomycin therapy.7 However, because it can be difficult in the clinical setting to obtain multiple serum vancomycin concentrations to determine AUC and subsequently calculate AUC/MIC, trough serum concentration monitoring, which can be used as a surrogate marker for AUC, is recommended as the most accurate and practical method for vancomycin monitoring. Trough vancomycin serum concentrations maintained above 10 mg/l are recommended.9, 11, 12, 13 All our patients having vancomycin after 1 hour of dialysis start serum concentrations above 10 mg/l, and AUC/MIC of at least 400 was achieved in all patients on the study days. This desired AUC/MIC ratio was achieved almost only in patients with vancomycin MIC < 1.0 mg/l. In patients with MIC ≥ 1.0 mg/l, higher dosing will be required. A target AUC/MIC of ≥ 400 is not achievable with conventional dosing methods if the pathogen vancomycin MIC is ≥ 2 mg/l. Achievement of this ratio would lead to undesirable vancomycin toxicity.9, 11, 12, 13, 16
Vancomycin-induced nephrotoxicity has been related to drug plasma concentrations.9 Its incidence varies greatly among various studies, with rates as low as zero in the absence of other concomitant nephrotoxins, up to 40%23, 24, 25, 26 in combination with other potentially nephrotoxic drugs. Among the patient-related factors,27, 28, 29, 30 the most important are advanced age, reduced kidney function, dehydration, reduced renal mass, sex (women have lower muscle mass and body water quantity), obesity, hypoalbuminemia, and sepsis, whereas drug-related risk factors include administration concomitant with other nephrotoxic drugs, such as aminoglycosides, loop diuretics, amphotericin B, piperacillin-tazobactam, acyclovir, vasopressors, and i.v. contrast media; as well as long treatment duration and high serum dosage of this antimicrobial.24, 25, 26, 27, 28, 29 Data suggesting a causal relationship between doses and therapeutic targets of vancomycin and nephrotoxicity are conflicting and marked by confounding factors.30 Although there is minimal evidence supporting efficacy in maintaining therapeutic levels between 15 and 20 mg/l, several studies have evaluated the safety of this recommendation, comparing nephrotoxicity rates above and below 15 mg/l levels.31 A systematic review and a meta-analysis conducted by van Hal et al.31 included 15 studies in which trough serum levels of vancomycin ≥15 mg/l were associated with a higher risk of nephrotoxicity when compared with levels <15 mg/l (odds ratio 2.67, P < 0.01).
The pharmacokinetic parameters should not change. However, in critically ill patients, drug pharmacokinetic parameters may be expected to change, especially regarding absorption, distribution, and metabolism, resulting in variations in serum levels. Thus, there is an increased risk of overdosing and drug toxicity, or a subtherapeutic dose and an increased risk of bacterial resistance, infection by opportunistic germs, and mortality.16, 19, 21
The removal of antimicrobials by different dialysis therapies in critically ill patients is a complex issue. This depends on the modality and intensity of dialysis, as well as drug characteristics, such as water solubility, molecular weight, and the extent of protein binding. There are no validated guidelines to assist in antibiotic dose adjustment in septic patients on acute renal supportive therapy, and the extrapolated recommendations were obtained from studies on noncritical patients with end-stage chronic kidney disease receiving substitutive renal therapy. Thus, because of the importance of maintaining therapeutic levels of antimicrobial drugs, more studies on this very complex subject are needed to reduce microbial resistance and mortality.22, 32, 33
Our study has some limitations, as the small number of patients studied and the dialysis elimination have been considered the only route of vancomycin elimination. We did not consider the role of renal and biliary elimination of vancomycin as possible confounders in the vancomycin plasma reduction. We also evaluated the pharmacokinetics of i.v. vancomycin in patients with AKI treated by HVPD (prescribed Kt/V of 0.5 per session). However, recent studies have presented similar outcomes of patients with AKI treated with lower PD doses.34, 35 Parapiboon et al.35 presented a study using PD to treat 80 critically ill patients with AKI. This was a randomized controlled trial comparing the 2 regimens recommended in the International Society for Peritoneal Dialysis guidelines, aimed at achieving target weekly Kt/V of 3.5 and 2.1, respectively.33 Patients were randomized 1:1 to receive 1.5 l of PD fluid using manual PD and a single-bag open system delivered either hourly (36 l/24 hours) or every 2 hours (18 l/24 hours) for the first 48 hours. Following this, they could perform exchanges less often, based on metabolic parameters and fluid balance. Seventy-five patients were included in the analysis. The achieved weekly Kt/V was 2.26 in the low-intensity group and 3.3 in the high-intensity group. There was no significant difference in metabolic control, although ultrafiltration was higher in the high-intensity group. The mortality was 72% in the high-intensity and 63% in the low-intensity groups (P = 0.18), suggesting no advantage to the higher-intensity treatment. Certainly, when lower PD doses are used, the clearance of vancomycicn and its pharmacokinetics can be different from our results.
Conclusion
HVPD removes considerable amounts of vancomycin in septic patients with AKI, and antibiotic underdosing is undesirable in these patients. Application of 18 mg/kg vancomycin each 48 to 72 hours in patients with AKI undergoing HVPD was required to maintain therapeutic concentrations. Daily vancomycin serum concentration monitoring is recommended to maintain therapeutic concentrations, and vancomycin pharmacokinetics in patients with AKI undergoing HVPD warrants further investigation.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank all of the doctors and nurses of nephrology and intensive care units who contributed to this study. We also thank the participants of the study.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All articles published in this journal must include this declaration within the acknowledgments. The ICMJE authorship criteria can be found at http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html.
Acknowledgments
Funding
No funding or sponsorship was received for this study or publication of this article. The article processing charges were funded by the authors. All authors had full access to all of the data in this study and take complete esponsibility for the integrity of the data and accuracy of the data analysis.
Compliance With Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the ethics committee of Brazil Plataform, under number: 17086413.3.0000.5411. Informed consent was obtained from all individual participants included in the study.
References
- 1.Zarjou A., Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 2.Davenport A. Dialytic treatment for septic patients with acute kidney injury. Kidney Blood Press Res. 2011;34:218–224. doi: 10.1159/000326898. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S., Bellomo R., Morimatsu H. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33:1563–1570. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 4.Ostermann M., Joannidis M., Pani A. Patient selection and timing of continuous renal replacement therapy. Blood Purif. 2016;42:224–237. doi: 10.1159/000448506. [DOI] [PubMed] [Google Scholar]
- 5.Pannu N., Klarenbach S., Wiebe N. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 6.Ponce D., Berbel M.N., Regina de Goes C. High-volume peritoneal dialysis in acute kidney injury: indications and limitations. Clin J Am Soc Nephrol. 2012;7:887–894. doi: 10.2215/CJN.11131111. [DOI] [PubMed] [Google Scholar]
- 7.Ponce D., Caramori J.T., Barretti P., Balbi A.L. Peritoneal dialysis in acute kidney injury: Brazilian experience. Perit Dial Int. 2012;32:242–246. doi: 10.3747/pdi.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponce D., Buffarah M.B., Goes C., Balbi A. Peritoneal dialysis in acute kidney injury: trends in the outcome across time periods. PLoS One. 2015;10:e0126436. doi: 10.1371/journal.pone.0126436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandecasteele S.J., De Vriese A.S. Recent changes in vancomycin use in renal failure. Kidney Int. 2010;77:760–764. doi: 10.1038/ki.2010.35. [DOI] [PubMed] [Google Scholar]
- 10.Blot S., Lipman J., Roberts D.M., Roberts J.A. The influence of acute kidney injury on antimicrobial dosing in critically ill patients: are dose reductions always necessary? Diagn Microbiol Infect Dis. 2014;79:77–84. doi: 10.1016/j.diagmicrobio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Singer M., Deutschman C.S., Seymour C.W. The third international consensus definitions for sepsis and septic shock. JAMA. 2016;23;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother. 2011;66:S25–S31. doi: 10.1093/jac/dkq516. [DOI] [PubMed] [Google Scholar]
- 13.Rybak M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42:S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 14.Manley H.J., Bailie G.R., Frye R.F., McGoldrick M.D. Intravenous vancomycin pharmacokinetics in automated peritoneal dialysis patients. Perit Dial Int. 2001;21:378–385. [PubMed] [Google Scholar]
- 15.Lewis S., Ba M. Antibiotic dosing in patients with acute kidney injury: “enough but not too much.”. J Intensive Care Med. 2014;31:164–176. doi: 10.1177/0885066614555490. [DOI] [PubMed] [Google Scholar]
- 16.Zamoner W., Freitas F.M., Garms D.S.S. Pharmacokinetics and pharmacodynamics of antibiotics in critically ill acute kidney injury patients. Pharmacol Res Perspect. 2016;4:e00280. doi: 10.1002/prp2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 18.Hu L.Q., Yin C.L., Du Y.A., Zeng Z.P. Simultaneous and direct determination of vancomycin and cephalexin in human plasma by using HPLC-DAD coupled with second-order calibration algorithms. J Anal Methods Chem. 2012;2012:256963. doi: 10.1155/2012/256963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva J.M., Oliveira A.M., Campos E.V. Vancomycin dose adjustment in severe burn patients based on trough level for drug effectiveness against pathogens at 1 mg/l minimum inhibitory concentration. Crit Care. 2013;17(Suppl 3):29. [Google Scholar]
- 20.Awdishu L., Bouchard J. How to optimize drug delivery in renal replacement therapy. Semin Dial. 2011;24:176–182. doi: 10.1111/j.1525-139X.2011.00826.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogge M.C., Johnson C.A., Zimmerman S.W., Welling P.G. Vancomycin disposition during continuous ambulatory peritoneal dialysis: a pharmacokinetic analysis of peritoneal drug transport. Antimicrob Agents Chemother. 1985;27:578–582. doi: 10.1128/aac.27.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petejova N., Martinek A., Zahalkova J. Vancomycin removal during low flux and high-flux extended daily hemodialysis in critically ill septic patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156:342–347. doi: 10.5507/bp.2012.002. [DOI] [PubMed] [Google Scholar]
- 23.Prybylski J.P. Vancomycin trough concentration as a predictor of clinical outcomes in patients with Staphylococcus aureus bacteremia: a meta-analysis of observational studies. Pharmacotherapy. 2015;35:889–898. doi: 10.1002/phar.1638. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A., Biyani M., Khaira A. Vancomycin nephrotoxicity; myths and facts. Neth J Med. 2011;69:379–383. [PubMed] [Google Scholar]
- 25.King D.W., Smith M.A. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro. 2004;18:797–803. doi: 10.1016/j.tiv.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Strokes M.B. Vancomycin in the kidney—a novel cast nephropathy. J Am Soc Nephrol. 2017;28:1669–1670. doi: 10.1681/ASN.2017010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mergenhagen K.A., Borton A.R. Vancomycin nephrotoxicity: a review. J Pharm Pract. 2014;27:545–553. doi: 10.1177/0897190014546114. [DOI] [PubMed] [Google Scholar]
- 28.Pazhayattil G.S., Shirali A.C. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457–468. doi: 10.2147/IJNRD.S39747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi Y.C., Saw S., Soliman D. Intravenous vancomycin is associated with the development of nephrotoxicity in patients with class iii obesity. Ann Pharmacother. 2017;51:937–944. doi: 10.1177/1060028017720946. [DOI] [PubMed] [Google Scholar]
- 30.Su Y.-C., Lin P.-C., Wu C.-C. Risk of nephrotoxicity among patients who received vancomycin. Infect Dis. 2018;50:152–155. doi: 10.1080/23744235.2017.1366046. [DOI] [PubMed] [Google Scholar]
- 31.van Hal S.J., Paterson D.L., Lodise T.P. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kielstein J.T., Czock D., Schöpke T. Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis. Crit Care Med. 2006;34:51–56. doi: 10.1097/01.ccm.0000190243.88133.3f. [DOI] [PubMed] [Google Scholar]
- 33.Launay-Vacher V., Izzedine H., Mercadal L., Deray G. Clinical review: use of vancomycin in haemodialysis patients. Crit Care. 2002;6:313–316. doi: 10.1186/cc1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho S., Lee Y.-J., Kim S.-R. Acute peritoneal dialysis in patients with acute kidney injury. Perit Dial Int. 2017;37:529–534. doi: 10.3747/pdi.2016.00264. [DOI] [PubMed] [Google Scholar]
- 35.Parapiboon W., Jamratpan T. Intensive versus minimal standard dosage for peritoneal dialysis in acute kidney injury: a randomized pilot study. Perit Dial Int. 2017;37:523–528. doi: 10.3747/pdi.2016.00260. [DOI] [PubMed] [Google Scholar]