Abstract
Pink urine syndrome is mostly seen in patients treated with propofol anesthesia. The pink color is attributed to the presence of large concentrations of uric acid (and pigment), which is excreted in large amounts when propofol is given. We describe a case of propofol-induced pink urine syndrome and perform a comprehensive, evidence-based review. We discuss prior case studies already published in the literature as we speculate on the pathophysiology and how it translates to a clinically relevant entity.
Keywords: acid-base equilibrium, biological, insulin resistance, oxidative stress, pigments, propofol, uric acid
In 1491 a German physician, Dr. Johannes de Ketham, published one of the first medical textbooks Fasciculus medicinae, where he describes using the urine to help diagnose disease.1 Although our technology has improved, from the matula to the urine dipstick, discolored urine continues to intrigue us. Pink urine is most commonly from free heme pigment, which can be seen in hematuria, hemolytic anemia, myoglobinuria (rhabdomyolysis), and porphyria. Another major cause of pink urine is from food, most commonly from beets, rhubarb, and blackberries. A non-heme/non-food pink urine and/or sediment is referred to as pink urine syndrome and has generated interest and discussion for decades. We describe a case of pink urine syndrome in a patient treated with propofol (2,6-diispropylphenol) anesthesia; and provide a comprehensive evidence-based review as we speculate on the many pathophysiological processes occurring.
Case Presentation
A 21-year-old, white male (body mass index 28.2; 91.1kg) with no previous medical history, was admitted to the intensive care unit with acute hypoxic/hypercapnic respiratory failure, acute kidney injury (AKI), and septic shock in the setting of heroin use. The patient was diagnosed with methicillin-susceptible Staphylococcus aureus pneumonia. Due to worsening hypoxia and respiratory acidosis, he was intubated, using propofol for induction. The following morning his creatinine increased to 1.8 mg/dl (from a baseline of 1.1 mg/dl). Urine sediment evaluation revealed a bright pink (Figure 1) pellet upon gross inspection, and light microscopy showed many amorphous crystals (Figure 2). To identify the crystal type, the patient’s urine was acidified and alkalized and reassessed under light microscopy (Figure 3a). On acidification, polychromatic birefringence rhomboid uric acid crystallization became prominent (Figure 3b), whereas when we alkalinized the urine, amorphous urate crystals were present (Figure 3c). The patient was given i.v. fluids and the serum creatinine returned to baseline within 2 days. He was extubated 3 days later and discharged shortly after on oral antibiotics.
Figure 1.

Pink urine sediment after centrifugation in a patient who received propofol.
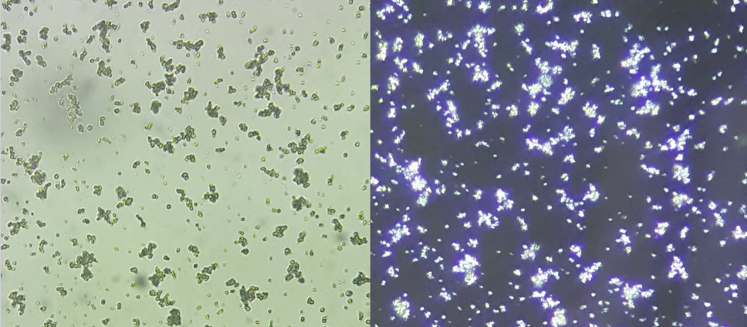
Figure 2.
Amorphous crystals. Urine sediment examination demonstrating amorphous crystals. The left is ×40 light microscopy and the right is the same image but polarized.
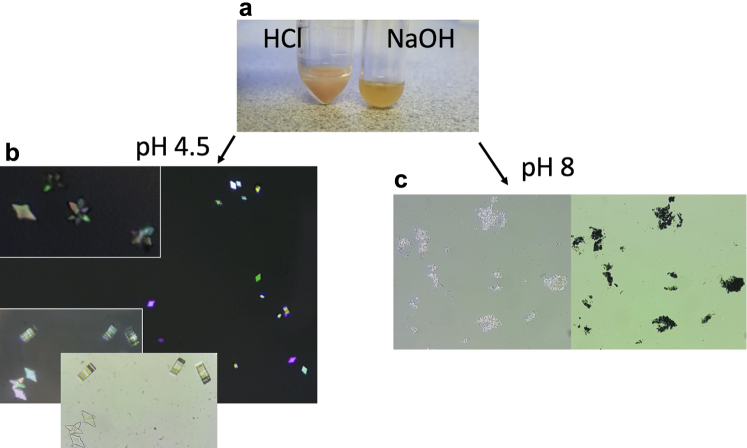
Figure 3.
The effect of pH on uric acid crystallization. One drop of 1 N HCl solution (1 normal = 36.5 g hydrochloric acid/1 liter) was mixed with 2 ml of patient’s urine resulting in a reduction of pH, pinkish hue (a, left test tube) on gross inspection, and polychromatic birefringence uric acid crystallization with light microscopy (b). In a similar fashion, 1 drop of 1 N NaOH solution (1 N = 40 g sodium hydroxide/1 liter) was mixed with 2 ml of urine to serve as a negative-treatment response control. On NaOH addition, urine color became a dirty yellow (a, right test tube), urine pH increased, and amorphous crystals were present when analyzed with light microscopy (c).
A Brief History of Pink Urine Syndrome
Pink urine syndrome refers to either pink urine and/or pink urine sediment that occurs in the absence of a heme-, medication-, or food-based pigment but typically seen in the presence of urinary uric acid and propofol. This entity first appeared in the literature in the 1800s by Louis Proust, where it was described as “substance rosacée” or “acide rosacique.”2 The first clinical evaluation of pink urine syndrome in modern times, was in 1984 in a surgical cohort undergoing gastric partitioning.3 In this obese cohort, one-half of the patients postoperatively had gross pink urine and 100% (n = 187) demonstrated a pink urine sediment on centrifugation. This finding was “rarely” seen in the preoperative period and not seen in the nonobese population. Pink urine syndrome was associated with propofol 10 years later, in 1996, when a milky-pink urine was seen in 9 Japanese surgical patients after propofol anesthesia.4 Before this, propofol was associated with white urine, which has been attributed to its grossly white, oil-in-water emulsion vehicle.5, 6, 7 Propofol has also been associated with green urine and historically blamed on the water-soluble quinol derivatives,8 but this has been challenged recently.9, 10
Patient Characteristics
Case studies with pink urine syndrome have been published throughout the literature (Table 1), and when these cases are evaluated together, similarities emerge. Most patients are obese males, have undergone surgery, were given propofol, have a low urine pH, and present without AKI or prerenal AKI. The association of these factors and their relationship to urinary uric acid are examined.
Table 1.
Pink urine syndrome case studies published in the literature
| Author, year, location [reference] | Age - gender | Body mass index (kg/m2) | Urine pH | Serum uric acid (mg/dl) | Propofol given | AKI etiology | Presenting diagnosis-comorbid conditions |
|---|---|---|---|---|---|---|---|
| Thamcharoen N, et al. 2015 Thailand [92] | 25 - Male | 45 | 6 | 9.3 | Yes | Prerenal, 24-hour correction with IVF |
Mediastinal lymphoma, morbid obesity |
| Potton L, et al. 2013 France [93] | 38 - Male | – | 5 | – | – | – | Liver transplant |
| Saran R, et al. 1998 USA [94] | 42 - Female | 70 | – | 9.8 | – | – | Admitted for gastric bypass. PMHx: Morbid obesity, HTN, non–insulin. dependent diabetes, hypercholesterolemia, OSA, CHF, depression, carpal-tunnel syndrome |
| Okubo K, et al. 2011 Japan [95] | 44 - Male | – | 6 | – | – | No AKI | Liver hepatoma removal |
| Stern AB, et al. 2010 USA [96] | 53 - Female | 5 | 5.5 (postoperative) | Yes | No AKI | Admitted for debulking surgery for ovarian serous adenocarcinoma; HTN, unspecified nephrolithiasis | |
| Schmitt R, et al. 2004 Germany [97] | 69 - Male | – | 5 | 6.44 | No | No AKI | Positional vertigo, HTN |
| Hou JD, et al. 2016 Taiwan [98] | 69 - Male | – | 5.5 | – | Yes | No AKI | Open fracture of the right femoral shaft |
| Verhoeven E, et al. 2014 Belgium [99] | 55 - Male | – | 5 | – | Yes | – | Alcohol abuse with valerian extract overdose |
| Kato N and Ogawa R. 1999 Japan [100] | 32 - Male | – | – | 7 | Yes | No AKI | Admitted for ulnar nerve injury; alcoholic, hypercholesterolemia |
| Tan CK and Lai CC 2012 Taiwan [101] | 67 - Female | 27 | 5.5 | 3.6 | No | – | Admitted with spontaneous bacterial peritonitis and hepatic encephalopathy from upper gastrointestinal bleeding; overweight, diabetes mellitus, liver cirrhosis |
| Sinnollareddy M, et al. 2014 Australia [102] | 29 - Male | – | – | – | Yes | – | Respiratory distress syndrome with acute alcohol toxicity |
| 61 - Male | 95kg (no height given) | Yes | – |
AKI, acute kidney injury; CHF, congestive heart failure; HTN, hypertension; IVF, i.v. fluids; OSA, obstructive sleep apnea; PMHx, past medical history.
This table demonstrates the important characteristics for the development of pink urine syndrome. The dashes represent unpublished/absent data.
Upon closer inspection, specific characteristics (body mass index, male gender, low urinary pH) are also independent risk factors for uric acid crystallization and/or nephrolithiasis.11, 12, 13, 14 A pink urine syndrome cohort confirmed these risk factors for pink urine syndrome.15 These characteristics, commonly seen with insulin resistance, are also more prevalent in males.12, 16, 17, 18 Of the preceding factors, a low urinary pH shows 100% prevalence in our cohort (Table 1), and is an independent risk factor in all the studies discussed, therefore deserves further evaluation.
Urine pH has a large influence on uric acid physiology. Specifically, a low pH will shift uric acid toward its protonated, neutral uric acid form and will increase the likelihood of precipitation. In an alkali solution, the shift is toward the negatively charged, water-soluble, urate, which exists in humans mostly as a salt, monosodium urate.19 Urate has a pKa of approximately 5.4, therefore at a physiological pH of 7.4 (and 37°C), 98% of the urate is in its monovalent form, the urate anion.19 When urinary acidification occurs, less urate is excreted, resulting in higher levels of urate in the blood. If the acidic urine is chronic, the total body urate pool would continue to increase, leading to hyperuricemia. A major determinant of urinary pH is the quantity of ammonia that is present. As a buffer, ammonia will bind free hydrogen ions (H+) forming ammonium (NH4+), thereby decreasing free H+ and increasing urine pH. In the setting of reduced ammoniagenesis, more free H+ will be present because less ammonia is around to bind it, thereby resulting in a more acidic urine. This effect is visualized in Figure 3, where adding acid caused uric acid crystals to form, whereas adding base created amorphous urates.
A low urinary pH, which is a requirement for uric acid crystallization, commonly occurs in the setting of insulin resistance.20 Patients with type 2 diabetes, in whom insulin resistance is present, compared with nondiabetic controls, are observed to have significantly reduced urinary pH in a multivariate analysis controlling for body weight, diet (via urinary sulfate), age, and creatinine clearance.21 This non-diet cause of acidic urine seen in this insulin-resistant population can be attributed to a defect in ammoniagenesis. Specifically, insulin resistance can lead to a reduction in ammonia production, resulting in defective buffering capacity, more free H+ and low urine pH.22, 23 Insulin stimulates the renal tubular sodium-hydrogen exchanger (NHE3) in the proximal tubule via PI3K-SGK1 (phosphatidylinositol 3-kinase--serum and glucocorticoid-dependent kinase), which increases ammoniagenesis.24 Chronic hyperinsulinemia, which is seen during insulin resistance, ineffectively stimulates ammoniagenesis via reduction of the intracellular signal strength.24, 25 The reduced intracellular signal strength in the proximal tubule is attributed to renal tubular epithelial lipid accumulation, or renal steatosis, and is not specific for attenuating insulin signal, but can also delay a glucocorticoid-mediated activation of NHE3 as well.26 Another hypothesis for reduced ammonia excretion in the setting of insulin resistance involves the nuclear factor-erythroid 2 related factor 2 (Nrf2). This regulating transcription factor is upregulated in the setting of oxidative stress, resulting in many more downstream antioxidant enzymes to be active as well. Through Nrf2 activation, we can see upregulation of the pentose phosphate pathway, oxaloacetate to a-ketoglutarate synthesis, and glutamate to glutathione conversion.27 Ogawa et al.27 associates the reduction of urine pH to a relative glutamate deficiency as it is diverted to glutathione synthesis. With a reduction in the available glutamate, ammoniagenesis is reduced and less ammonium is present, resulting in a lower urinary pH.27 This hypothesis is also supported by a significantly lower urinary glutamate seen in patients with pink urine syndrome.27
In general, insulin resistance and hypertriglyceridemia are present before the development of type 2 diabetes mellitus or metabolic syndrome.28 An insulin-resistant state requires a relative increase in endogenous insulin production/release to sustain metabolic activity, and a state of relative hyperinsulinemia occurs. There is a strong independent positive correlation between insulin and insulin resistance with serum urate concentration in a variety of cohorts.29, 30, 31 Insulin resistance reduces renal clearance of urate by increasing its absorption transporter, URAT1, and decreasing urate excretion through downregulation of the ABCG2 transporter.32, 33, 34, 35, 36 Healthy non–insulin-resistant male individuals given a 2- or 4-hour infusion of insulin develop a temporal and graded reduction of urinary uric acid clearance.37, 38 Insulin resistance treated with diet or medications, without body mass index and/or blood pressure improvement, significantly reduces serum uric acid.39
External/Environmental Factors
The external conditions and medications given are of equal importance to the development of pink urine syndrome. Most of these patients have undergone surgery, and even though propofol was given, surgery itself can predispose to urate excretion. Operative patients have more antidiuretic hormone (ADH) release due to stress, hemodynamics, and other factors.40, 41, 42 ADH binds to the V1 receptor in the kidney and enhances urate excretion by downregulating the GLUT9 transporter that reabsorbs urate and upregulating transporters that secrete urate (ABCG2 and NPT1).43, 44 An ADH-dependent urate excretion effect has been demonstrated in hyponatremic patients,45 although no study has evaluated the role of ADH in pink urine syndrome. Patients with pink urine syndrome have a higher postoperative urinary osmolality and reduced urine output as compared with obese, non-pink urine syndrome controls.3 Using urinary osmolality as a surrogate for ADH, one can surmise that increased ADH levels were present. However, surgery is not required for pink urine syndrome to occur, as specific cases in Table 1 did not have surgery and still developed pink urine syndrome. In fact, the prevalence of pink urine syndrome in a nonsurgical Japanese cohort is approximately 4%.15 The data support a role for ADH in the development of pink urine syndrome, but a stronger factor in its development is propofol.
Propofol is a common anesthetic given for induction or maintenance sedation for mechanically ventilated patients in surgery or the intensive care unit. Because of its high lipophilicity, it is given in a lipid emulsion containing soybean oil, egg yolk lecithin, glycerol, sodium hydroxide, and EDTA.46 Propofol has been demonstrated to increase urate excretion in the urine.47 Compared with sevoflurane, propofol cleared significantly more urate (mean 22.9 ml/min vs. 5.9 ml/min) during elective surgery in patients with an American Society of Anesthesiologists physical status I or II (these classifications include healthy patients and mild systemic disease, such as well-controlled diabetes mellitus or hypertension).47 There was no significant difference between urinary pH, creatinine clearance, or urine volume.47 The total urate clearance of propofol is significantly more than other situations that result in increased urate clearance, such as artificially induced hyperuricemia (14.4 ml/min)47, 48 or hypoxanthine-guanine phosphoribosyltransferase deficiency: 11.7 ml/min (range 4.2–20.8 ml/min).49 The only other condition with urate clearance greater, is familial renal hypouricemia, in which urate clearance can be >30 ml/min. This occurs with a genetic mutation in SLC22A12 (encodes URAT1) or SLC2A9 (encodes GLUT9), both are proximal tubule urate reabsorption transporters.50, 51 The total body clearance of propofol is between 20 and 30 ml/min per kg, and when infusing, produces urate excretion at a similar clearance. Clinically, pink urine syndrome presents as a quick onset and abrupt cession of urate clearance in the presence and absence of propofol, respectively. The exact mechanism is unknown, but there are other variables present that influence urate excretion that deserve mention. Diabetic patients can also produce glomerular hyperfiltration (estimated glomerular filtration rate [GFR] >141 ml/min per 1.73 m2), which increases intraglomerular pressure and flow.52 Hyperfiltration, or increased urinary flow, directly increases urinary urate excretion.52, 53, 54 Diabetic patients may also have glycosuria, which also increases urate excretion.55 Propofol may secondarily increase urate efflux transporter in the proximal tubule (ABCG2) through the upregulation of Nrf2.56 Regardless of the etiology, the ability to excrete urate in large amounts is dependent on the GFR.57
Connecting Internal and External Variables
Having an adequate GFR with intact tubular function is one of the requirements for pink urine syndrome. Because acute prerenal AKI maintains tubular function, it is the only type of AKI that is seen with pink urine syndrome. Propofol exhibits many renoprotective benefits, such as improved creatinine clearance, reduced incidence of AKI, prevention of acute tubular necrosis, decreased need for renal replacement therapy, and reduced mortality.58, 59 The mechanism behind these findings is attributed to the antioxidant ability of propofol where it reacts with a free radical turning into a phenoxyl radical and can scavenge harmful radical species.58, 60, 61 The antioxidant ability of propofol is similar to a phenol-based compound: it scavenges reactive species, induces endogenous antioxidant enzymes, and has anti-inflammatory effects.62
Environmental temperature plays a large role in urate physiology, thereby making it important for pink urine syndrome. Propofol administration in the clinical setting typically is seen in the operating room and critical care bed; both locations are considered to be cold. As temperature decreases, the likelihood of uric acid precipitating increases.63 Operating rooms are typically kept below 23 ˚C (73.4 ˚F).64 At a normal physiological temperature (37 ˚C or 98.6 ˚F), precipitation starts to occur at uric acid of 6.8 mg/dl. When the temperature drops to 30 ˚C (86 ˚F), urate precipitation occurs at 4.5 mg/dl. The vehicle propofol is given with, a lipid emulsion, can attenuate this hypothermic effect, which may help maintain urate solubility in the body.65 Once excreted and exposed to the external environment, the significantly reduced temperature would increase the risk of uric acid precipitation. In the presence of both, an acidic urine and reduced external temperatures, the lower the concentration of uric acid needs to be to precipitate.63, 66
The Pink Pigment
The pink hue of the urine in pink urine syndrome is from a pink urinary pigment. Many pink/reddish pigments exist: urobilin, urohematoporphyrin, uroporphyrin, coproporphyrin, urohematin, urocyanin, indirubin, indigotin, uroerythrin, purpurin, skatol red, nephrorosein, urorosein, and uricine (of which some may be even the same compound).67 Uric acid–associated pigments include uricine, urorosein, and biotripyrrin (previously called uroerythrin).67, 68, 69 Of these, uricine is the most likely to be the primary pigment responsible for pink urine syndrome. One of the first descriptions of uricine was described by Dr. Kleeberg in 1974, where he observes: “its pink color in an acid medium and its amber yellow in an alkaline.”70 (See our patient’s urine colors, Figure 3a). Uricine is a pH-dependent, water-soluble, 2-pyrrol compound.71 Like other endogenous pigments, uricine is considered to originate from bilirubin metabolism. Uricine has the strongest attachment to uric acid at an acidic pH, in which the rate-limiting reaction is dependent on uric acid concentration.71 Using the raw data provided in the publication, we performed our own calculations on relationships that were not evaluated in the original article (see Supplementary Table S1, for our statistical methods and full analysis). Excretion of uricine did not correlate with urate excretion, although there was significantly more uricine excretion in uric acid stone-formers (mean 836 mg/24 hours) as compared with non-gouty, non–stone-former controls (242 mg/24 hours) or patients with gout (321 mg/24 hours) (see Supplementary Table S1 for all calculations and data).71 Due to the paucity of clinically oriented data on any of the pink pigments, the exact clinical characterization of uricine excretors cannot be done. The production of color relies on a redox reaction with a metal ion-complex. Therefore, any changes in specific variables that influence this reaction may also influence the color change. Urine pH, being the most important, can directly determine the action of urate as a reducing agent or a metal ion’s structure and optical activity.72, 73, 74
Putting It All Together With a Common Pathway
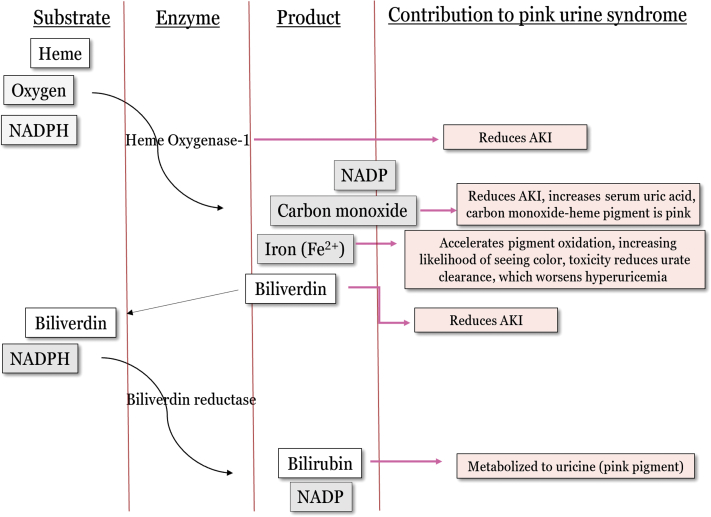
Although no specific studies have evaluated the specific pathway(s) involved in pink urine syndrome, we speculate its overlying mechanism. A specific oxidative stress pathway that includes the enzyme, heme oxygenase-1, is involved with nearly all the requirements to develop pink urine syndrome (Figure 4). Heme oxygenase-1 is found in most cells in the body and is induced by oxidative stress. Its function is to metabolize the toxic, pro-oxidant substrate heme to an antioxidant, bilirubin (this occurs through a biliverdin intermediate), and is a critical cytoprotective factor in the setting of oxidative stress.75, 76 The substrates are heme, oxygen, and reduced NAD phosphate and products are bilirubin, carbon monoxide, and iron (Figure 4). In any inflammatory environment there is an expected degree of heme oxygenase-1 induction to protect against oxidative damage. In the setting of insulin resistance, heme oxygenase-1 induction is overwhelmed, resulting in a suboptimal clinical response to the current oxidative load. A vicious cycle ensues between inflammation and macrophage adipose infiltration contributing to worsening insulin resistance, sustaining an active heme oxygenase-1 pathway.77 This is supported by a strong correlation between heme oxygenase-1 activity and insulin resistance.78
Figure 4.
The heme oxygenase-1 pathway. This enzymatic pathway is activated by oxidative stress and is chronically active during a state of insulin resistance. This pathway becomes overwhelmed and unable to become active enough to completely prevent the increasing amount of oxidative damage. Propofol can further activate this enzyme, which reduces oxidative damage and protects the cell. AKI, acute kidney injury; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen.
The products of this reaction seemingly contribute to some of the clinical variables seen in pink urine syndrome. Figure 4 demonstrates how heme oxygenase interacts with pink urine syndrome. Thus, being chronically active, the products slowly build up, and when propofol is given, a significant increase of heme oxygenase-1 activity is seen acutely. Free iron produced from heme oxygenase-1 is usually quickly sequestered by ferritin. With a chronically active heme oxygenase-1 pathway, more free iron will be present, resulting in more iron seen in the urine.79, 80 Serum urate, at physiological pH, has also been shown to sequester free iron.81 This explains the free iron seen in the urine of some patients with pink urine syndrome, and one should also note that free metal in the urine can accelerate the oxidation of urinary pigments, thereby making it more likely to produce color.82 Bilirubin is also produced and displays strong antioxidant properties, resulting in more of its oxidative metabolites being excreted in the setting of oxidative stress, which include the stress of surgery and intubation.83 Urinary uricine would be expected to increase in the setting of an active heme oxygenase-1 pathway because it is likely a product of bilirubin metabolism.84 Heme oxygenase-1 activity has also been shown to protect against AKI, which is attributed to the removal of a toxins (heme and free iron) and production of cytoprotectants (bilirubin, carbon monoxide, ferritin).85 Specific genetic polymorphisms that make heme oxygenase-1 less active result in significantly higher risk of AKI.86
Propofol reverses many of the effects seen during insulin resistance, which has been attributed to its superior antioxidant and anti-inflammatory activity.62, 87 Specifically, propofol activates the upstream Nrf2, which then activates many different antioxidant enzymes, including heme oxygenase-I.88 The inherent antioxidant activity of propofol and its ability to increase endogenous antioxidant pathways (Nrf2/Heme oxygenase-1) delivers the complete antidiabetic, anti-inflammatory, and antioxidant response to an insulin-resistant, pro-oxidant environment.89, 90
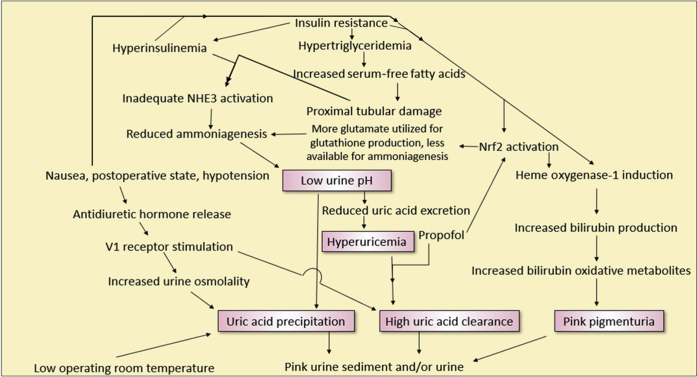
In summary, Figure 5 shows the multiple interactions seen during pink urine syndrome. We created 5 pink boxes in Figure 5, identifying the minimum requirements needed for pink urine syndrome to occur, and they are as follows:
-
(i)
Chronic hyperuricemia: for large amounts of uric acid to appear in the urine, a larger urate pool is required to draw from.
-
(ii)
Clearance of uric acid is acutely increased: seen in the presence of propofol, and to a lesser extent, ADH.
-
(iii)
Acidic urine: required for uric acid precipitation.
-
(iv)
An adequate GFR: required for uric acid clearance.
-
(v)
Chronic oxidative stress: will increase pink pigment production through bilirubin metabolism. Oxidative stress increases bilirubin production, through the upregulation of heme oxygenase-1.
Figure 5.
Flow diagram demonstrating the complex interactions involved in pink urine syndrome. NHE3, renal tubular sodium-hydrogen exchanger; Nrf2, nuclear factor-erythroid 2 related factor 2.
Conclusion
A postulated explanation for the role of insulin resistance and propofol as the most common factors leading to pink urine syndrome is presented in Figure 5. Insulin resistance covers requirements 1, 3, and 5, whereas propofol administration covers requirements 2 and 4. Another scenario would be chronic alcohol use, which may explain some of the cases in Table 1. Alcohol reduces urate excretion and increases serum urate levels.91 The other 4 requirements, a low urinary pH, intact GFR, oxidative stress, and some event/agent to acutely increase urate excretion, would need to be present as well for pink urine syndrome to occur. Using Occam’s razor, or developing a hypothesis with the fewest assumptions, the combination of insulin resistance and propofol would be the quickest pathway to pink urine syndrome.
Disclosure
All the authors declared no competing interests.
Acknowledgments
BMT received funding from Section of Nephrology, Wake Forest School of Medicine, Winston-Salem, NC.
Footnotes
Table S1. Used raw data from Pinto B, Rocha E, Ruiz Marcellan FJ. Urinary excretion of uricine. In: Muller MM, Kaiser E, Seegmiller JE, eds. Purine Metabolism in Man—II. Boston: Springer; 1977:86–89. Performed extra calculations, specifically means of urinary uricine and urinary uric acid excretion, Mann-Whitney U Test for comparison groups, and the Pearson correlation coefficient in the evaluation of whether urinary uricine excretion correlates with urinary uric acid excretion. Significance was defined as 2-tailed P < 0.05.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Used raw data from Pinto B, Rocha E, Ruiz Marcellan FJ. Urinary excretion of uricine. In: Muller MM, Kaiser E, Seegmiller JE, eds. Purine Metabolism in Man—II. Boston: Springer; 1977:86–89. Performed extra calculations, specifically means of urinary uricine and urinary uric acid excretion, Mann-Whitney U Test for comparison groups, and the Pearson correlation coefficient in the evaluation of whether urinary uricine excretion correlates with urinary uric acid excretion. Significance was defined as 2-tailed P < 0.05.
References
- 1.Diskin C.J. de Ketham revisited: a modern-day urine wheel. Med J Aust. 2008;189:658–659. doi: 10.5694/j.1326-5377.2008.tb02232.x. [DOI] [PubMed] [Google Scholar]
- 2.Garrod A.E. A contribution to the study of uroerythrin. J Physiol. 1895;17:432–450. doi: 10.1113/jphysiol.1895.sp000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deitel M., Thompson D.A., Saldanha C.F. “Pink urine“ in morbidly obese patients following gastric partitioning. Can Med Assoc J. 1984;130:1007–1011. [PMC free article] [PubMed] [Google Scholar]
- 4.Masuda A., Hirota K., Satone T., Ito Y. Pink urine during propofol anesthesia. Anesth Analg. 1996;83:666–667. doi: 10.1097/00000539-199609000-00070. [DOI] [PubMed] [Google Scholar]
- 5.Nates J., Avidan A., Gozal Y., Gertel M. Appearance of white urine during propofol anesthesia. Anesth Analg. 1995;81:210. doi: 10.1097/00000539-199507000-00058. [DOI] [PubMed] [Google Scholar]
- 6.Bodenham A., Culank L.S., Park G.R. Propofol infusion and green urine. Lancet. 1987;2:740. doi: 10.1016/s0140-6736(87)91097-x. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen A.B., Kobborg T.K., Larsen J.R. Grass-green urine from propofol infusion. Acta Anaesthesiol Scand. 2015;59:265–267. doi: 10.1111/aas.12437. [DOI] [PubMed] [Google Scholar]
- 8.Shioya N., Ishibe Y., Shibata S. Green urine discoloration due to propofol infusion: a case report. Case Rep Emerg Med. 2011;2011:242514. doi: 10.1155/2011/242514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuiper M. Green urine. Netherlands Journal of Critical Care. 2015;19:30. [Google Scholar]
- 10.Fujii-Abe K., Kawahara H., Fukayama H. An analysis of green discoloration of urine caused by propofol infusion. J Clin Anesth. 2016;35:358–360. doi: 10.1016/j.jclinane.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Daudon M., Lacour B., Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res. 2006;34:193–199. doi: 10.1007/s00240-006-0042-8. [DOI] [PubMed] [Google Scholar]
- 12.Worcester E.M., Bergsland K.J., Gillen D.L., Coe F.L. Mechanism for higher urine pH in normal women compared to men. Am J Physiol Renal Physiol. 2018;314:F623–F629. doi: 10.1152/ajprenal.00494.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor E.N., Curhan G.C. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Maalouf N.M., Sakhaee K., Parks J.H. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S., Takiguchi J., Nako K. Elucidation of the etiology and characteristics of pink urine in young healthy subjects. Clin Exp Nephrol. 2015;19:822–829. doi: 10.1007/s10157-014-1066-y. [DOI] [PubMed] [Google Scholar]
- 16.Carnevale Schianca G.P., Fra G.P., Colli E. Sex differences in lipid profiles in relation to the progression of glucose abnormalities. J Diabetes. 2012;4:95–101. doi: 10.1111/j.1753-0407.2011.00160.x. [DOI] [PubMed] [Google Scholar]
- 17.Marangella M. Uric acid elimination in the urine. Pathophysiological implications. Contrib Nephrol. 2005;147:132–148. doi: 10.1159/000082551. [DOI] [PubMed] [Google Scholar]
- 18.Chen H.W., Chen Y.C., Yang F.M. Mediators of the effects of gender on uric acid nephrolithiasis: a novel application of structural equation modeling. Sci Rep. 2018;8:6077. doi: 10.1038/s41598-018-24485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murea M, Tucker BM. The physiology of uric acid and the impact of end-stage kidney disease and dialysis [e-pub ahead of print]. Semin Dial. doi: 10.1111/sdi.12735. Accessed July 10, 2018. [DOI] [PubMed]
- 20.Maalouf N.M., Cameron M.A., Moe O.W. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 21.Cameron M.A., Maalouf N.M., Adams-Huet B. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 22.Abate N., Chandalia M., Cabo-Chan A.V., Jr. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 23.Maalouf N.M., Cameron M.A., Moe O.W., Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol. 2010;5:1277–1281. doi: 10.2215/CJN.08331109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuster D.G., Bobulescu I.A., Zhang J. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol. 2007;292:F577–F585. doi: 10.1152/ajprenal.00240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocchini A.P., Katch V., Kveselis D. Insulin and renal sodium retention in obese adolescents. Hypertension. 1989;14:367–374. doi: 10.1161/01.hyp.14.4.367. [DOI] [PubMed] [Google Scholar]
- 26.Bobulescu I.A., Dubree M., Zhang J. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315–F1322. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa S., Takiguchi J., Shimizu M. The reduction in urinary glutamate excretion is responsible for lowering urinary ph in pink urine syndrome. Tohoku J Exp Med. 2016;239:103–110. doi: 10.1620/tjem.239.103. [DOI] [PubMed] [Google Scholar]
- 28.Moors C.C., van der Zijl N.J., Diamant M. Impaired insulin sensitivity is accompanied by disturbances in skeletal muscle fatty acid handling in subjects with impaired glucose metabolism. Int J Obes (Lond) 2012;36:709–717. doi: 10.1038/ijo.2011.123. [DOI] [PubMed] [Google Scholar]
- 29.Bo S., Cavallo-Perin P., Gentile L. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest. 2001;31:318–321. doi: 10.1046/j.1365-2362.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 30.Bonora E., Capaldo B., Perin P.C. Hyperinsulinemia and insulin resistance are independently associated with plasma lipids, uric acid and blood pressure in non-diabetic subjects. The GISIR database. Nutr Metab Cardiovasc Dis. 2008;18:624–631. doi: 10.1016/j.numecd.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Facchini F., Chen Y.D., Hollenbeck C.B., Reaven G.M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–3011. [PubMed] [Google Scholar]
- 32.Miao Z., Yan S., Wang J. Insulin resistance acts as an independent risk factor exacerbating high-purine diet induced renal injury and knee joint gouty lesions. Inflamm Res. 2009;58:659–668. doi: 10.1007/s00011-009-0031-9. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Ruiz F., Aniel-Quiroga M.A., Herrero-Beites A.M. Renal clearance of uric acid is linked to insulin resistance and lower excretion of sodium in gout patients. Rheumatol Int. 2015;35:1519–1524. doi: 10.1007/s00296-015-3242-0. [DOI] [PubMed] [Google Scholar]
- 34.Fraile J.M., Puig J.G., Torres R.J. Uric acid metabolism in patients with primary gout and the metabolic syndrome. Nucleosides Nucleotides Nucleic Acids. 2010;29:330–334. doi: 10.1080/15257771003741273. [DOI] [PubMed] [Google Scholar]
- 35.Muscelli E., Natali A., Bianchi S. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9:746–752. doi: 10.1016/0895-7061(96)00098-2. [DOI] [PubMed] [Google Scholar]
- 36.Toyoki D., Shibata S., Kuribayashi-Okuma E. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol. 2017;313:F826–F834. doi: 10.1152/ajprenal.00012.2017. [DOI] [PubMed] [Google Scholar]
- 37.Ter Maaten J.C., Voorburg A., Heine R.J. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 1997;92:51–58. doi: 10.1042/cs0920051. [DOI] [PubMed] [Google Scholar]
- 38.Quinones Galvan A., Natali A., Baldi S. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268:E1–E5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 39.Tsunoda S., Kamide K., Minami J., Kawano Y. Decreases in serum uric acid by amelioration of insulin resistance in overweight hypertensive patients: effect of a low-energy diet and an insulin-sensitizing agent. Am J Hypertens. 2002;15:697–701. doi: 10.1016/s0895-7061(02)02953-9. [DOI] [PubMed] [Google Scholar]
- 40.Hazebroek E.J., de Vos tot Nederveen Cappel R., Gommers D. Antidiuretic hormone release during laparoscopic donor nephrectomy. Arch Surg. 2002;137:600–604. doi: 10.1001/archsurg.137.5.600. discussion 605. [DOI] [PubMed] [Google Scholar]
- 41.Oka Y., Wakayama S., Oyama T. Cortisol and antidiuretic hormone responses to stress in cardiac surgical patients. Can Anaesth Soc J. 1981;28:334–338. doi: 10.1007/BF03007799. [DOI] [PubMed] [Google Scholar]
- 42.Amano J., Suzuki A., Sunamori M. Antidiuretic hormone and cardiovascular responses during and after coronary artery bypass surgery. Thorac Cardiovasc Surg. 1993;41:297–300. doi: 10.1055/s-2007-1013875. [DOI] [PubMed] [Google Scholar]
- 43.Decaux G., Namias B., Gulbis B., Soupart A. Evidence in hyponatremia related to inappropriate secretion of ADH that V1 receptor stimulation contributes to the increase in renal uric acid clearance. J Am Soc Nephrol. 1996;7:805–810. doi: 10.1681/ASN.V75805. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi K., Tamura Y., Kumagai T. Stimulation of V1a receptor increases renal uric acid clearance via urate transporters: insight into pathogenesis of hypouricemia in SIADH. Clin Exp Nephrol. 2016;20:845–852. doi: 10.1007/s10157-016-1248-x. [DOI] [PubMed] [Google Scholar]
- 45.Decaux G., Prospert F., Namias B., Soupart A. Hyperuricemia as a clue for central diabetes insipidus (lack of V1 effect) in the differential diagnosis of polydipsia. Am J Med. 1997;103:376–382. doi: 10.1016/s0002-9343(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 46.Baker M.T., Naguib M. Propofol: the challenges of formulation. Anesthesiology. 2005;103:860–876. doi: 10.1097/00000542-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 47.Masuda A., Asahi T., Sakamaki M. Uric acid excretion increases during propofol anesthesia. Anesth Analg. 1997;85:144–148. doi: 10.1097/00000539-199707000-00026. [DOI] [PubMed] [Google Scholar]
- 48.Puig J.G., Torres R.J., de Miguel E. Uric acid excretion in healthy subjects: a nomogram to assess the mechanisms underlying purine metabolic disorders. Metabolism. 2012;61:512–518. doi: 10.1016/j.metabol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Emmerson B.T., Gordon R.B., Johnson L.A. Urate kinetics in hypoxanthine-guanine phosphoribosyltransferase deficiency: their significance for the understanding of gout. Q J Med. 1976;45:49–61. [PubMed] [Google Scholar]
- 50.Benjamin D., Sperling O., Weinberger A. Familial renal hypouricemia due to isolated tubular defect. Adv Exp Med Biol. 1977;76B:72–76. doi: 10.1007/978-1-4684-3285-5_9. [DOI] [PubMed] [Google Scholar]
- 51.Claverie-Martin F., Trujillo-Suarez J., Gonzalez-Acosta H. URAT1 and GLUT9 mutations in Spanish patients with renal hypouricemia. Clin Chim Acta. 2018;481:83–89. doi: 10.1016/j.cca.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 52.Bjornstad P., Roncal C., Milagres T. Hyperfiltration and uricosuria in adolescents with type 1 diabetes. Pediatr Nephrol. 2016;31:787–793. doi: 10.1007/s00467-015-3299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emmerson B.T., Ravenscroft P.J., Williams G. The effect of urine flow rate on urate clearance. Adv Exp Med Biol. 1977;76B:23–29. doi: 10.1007/978-1-4684-3285-5_2. [DOI] [PubMed] [Google Scholar]
- 54.Diamond H.S., Lazarus R., Kaplan D., Halberstam D. Effect of urine flow rate on uric acid excretion in man. Arthritis Rheum. 1972;15:338–346. doi: 10.1002/art.1780150403. [DOI] [PubMed] [Google Scholar]
- 55.Lytvyn Y., Skrtic M., Yang G.K. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308:F77–F83. doi: 10.1152/ajprenal.00555.2014. [DOI] [PubMed] [Google Scholar]
- 56.Adachi T., Nakagawa H., Chung I. Nrf2-dependent and -independent induction of ABC transporters ABCC1, ABCC2, and ABCG2 in HepG2 cells under oxidative stress. J Exp Ther Oncol. 2007;6:335–348. [PubMed] [Google Scholar]
- 57.Aksenov S., Peck C.C., Eriksson U.G., Stanski D.R. Individualized treatment strategies for hyperuricemia informed by a semi-mechanistic exposure-response model of uric acid dynamics. Physiol Rep. 2018;6(5) doi: 10.14814/phy2.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Zhong D., Lei L. Propofol prevents renal ischemia-reperfusion injury via inhibiting the oxidative stress pathways. Cell Physiol Biochem. 2015;37:14–26. doi: 10.1159/000430329. [DOI] [PubMed] [Google Scholar]
- 59.Chen H., Busse L.W. Novel therapies for acute kidney injury. Kidney Int Rep. 2017;2:785–799. doi: 10.1016/j.ekir.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J.J., Wang Y.L. Propofol attenuation of hydrogen peroxide-mediated oxidative stress and apoptosis in cultured cardiomyocytes involves haeme oxygenase-1. Eur J Anaesthesiol. 2008;25:395–402. doi: 10.1017/S0265021508003542. [DOI] [PubMed] [Google Scholar]
- 61.Murphy P.G., Myers D.S., Davies M.J. The antioxidant potential of propofol (2,6-diisopropylphenol) Br J Anaesth. 1992;68:613–618. doi: 10.1093/bja/68.6.613. [DOI] [PubMed] [Google Scholar]
- 62.Marik P.E. Propofol: therapeutic indications and side-effects. Curr Pharm Des. 2004;10:3639–3649. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- 63.Loeb J.N. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15:189–192. doi: 10.1002/art.1780150209. [DOI] [PubMed] [Google Scholar]
- 64.Hart S.R., Bordes B., Hart J. Unintended perioperative hypothermia. Ochsner J. 2011;11:259–270. [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong C.W., Ju J., Lee D.W. Lipid-emulsion propofol less attenuates the regulation of body temperature than micro-emulsion propofol or sevoflurane in the elderly. Yonsei Med J. 2012;53:198–203. doi: 10.3349/ymj.2012.53.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teotia M., Sutor D.J. Crystallisation of ammonium acid urate and other uric acid derivatives from urine. Br J Urol. 1971;43:381–386. doi: 10.1111/j.1464-410x.1971.tb12056.x. [DOI] [PubMed] [Google Scholar]
- 67.Hall V.M., Cox K.A., Sours R.E., Swift J.A. Urochrome pigment in uric acid crystals. Chemistry of Materials. 2016;28:3862–3869. [Google Scholar]
- 68.Beruter J., Colombo J.P., Schlunegger U.P. Isolation and identification of the urinary pigment uroerythrin. Eur J Biochem. 1975;56:239–244. doi: 10.1111/j.1432-1033.1975.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 69.Pinto B., Rocha E. Isolation and characterization of uricine. In: Muller M.M., Kaiser E., Seegmiller J.E., editors. Purine Metabolism in Man—II. Springer; Boston: 1977. pp. 77–80. [Google Scholar]
- 70.Kleeberg J. A hitherto undescribed pigment found in human urinary uric acid stones. Experientia. 1974;30:455–456. doi: 10.1007/BF01926288. [DOI] [PubMed] [Google Scholar]
- 71.Pinto B., Rocha E. Uricine—uric acid interactions. In: Muller M.M., Kaiser E., Seegmiller J.E., editors. Purine Metabolism in Man—II. Springer; Boston: 1977. pp. 81–85. [Google Scholar]
- 72.de Zawadzki A., Cardoso D.R., Skibsted L.H. Proton-coupled electron transfer promotes the reduction of ferrylmyoglobin by uric acid under physiological conditions. Rsc Advances. 2017;7:17824–17831. [Google Scholar]
- 73.Unnikrishnan AG, Rajaratnam S, John GT, Stephen C Boy with 'rainbow' urine. Nephrol Dial Transplant. 2001;16:2097–2099. doi: 10.1093/ndt/16.10.2097. [DOI] [PubMed] [Google Scholar]
- 74.Cole W.J., Gray C.H., Nicholson D.C., Norman M. The chemistry of the bile pigments. VI. The effect of metal complex formation upon optical activity and spectral absorption of urobilins. J Chem Soc Perkin 1. 1966;15:1321–1326. doi: 10.1039/j39660001321. [DOI] [PubMed] [Google Scholar]
- 75.Tenhunen R., Marver H.S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 76.Takeda T.A., Mu A., Tai T.T. Continuous de novo biosynthesis of haem and its rapid turnover to bilirubin are necessary for cytoprotection against cell damage. Sci Rep. 2015;5:10488. doi: 10.1038/srep10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 78.Bruce C.R., Carey A.L., Hawley J.A., Febbraio M.A. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- 79.Kohgo Y., Ikuta K., Ohtake T. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreno-Navarrete J.M., Ortega F., Rodriguez A. HMOX1 as a marker of iron excess-induced adipose tissue dysfunction, affecting glucose uptake and respiratory capacity in human adipocytes. Diabetologia. 2017;60:915–926. doi: 10.1007/s00125-017-4228-0. [DOI] [PubMed] [Google Scholar]
- 81.Sevanian A., Davies K.J., Hochstein P. Serum urate as an antioxidant for ascorbic acid. Am J Clin Nutr. 1991;54(6 Suppl):1129S–1134S. doi: 10.1093/ajcn/54.6.1129s. [DOI] [PubMed] [Google Scholar]
- 82.Garrod A.E. On the pigmentation of uric acid crystals deposited from urine. The Journal of Pathology and Bacteriology. 1896;3:100–106. [Google Scholar]
- 83.Novio S., Nunez M.J., Ponte C.M., Freire-Garabal M. Urinary biopyrrins: potential biomarker for monitoring of the response to treatment with anxiolytics. Basic Clin Pharmacol Toxicol. 2012;111:206–210. doi: 10.1111/j.1742-7843.2012.00888.x. [DOI] [PubMed] [Google Scholar]
- 84.Pinto B., Rocha E., Ruiz-Marcellan F.J. Isolation and characterization of uricine from uric acid stones. Kidney Int. 1976;10:437–443. doi: 10.1038/ki.1976.130. [DOI] [PubMed] [Google Scholar]
- 85.Nath K.A. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23:17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leaf D.E., Body S.C., Muehlschlegel J.D. Length polymorphisms in heme oxygenase-1 and AKI after cardiac surgery. J Am Soc Nephrol. 2016;27:3291–3297. doi: 10.1681/ASN.2016010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han C., Ding W., Jiang W. A comparison of the effects of midazolam, propofol and dexmedetomidine on the antioxidant system: a randomized trial. Exp Ther Med. 2015;9:2293–2298. doi: 10.3892/etm.2015.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yao W., Luo G., Zhu G. Propofol activation of the Nrf2 pathway is associated with amelioration of acute lung injury in a rat liver transplantation model. Oxid Med Cell Longev. 2014;2014:258567. doi: 10.1155/2014/258567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shravah J., Wang B., Pavlovic M. Propofol mediates signal transducer and activator of transcription 3 activation and crosstalk with phosphoinositide 3-kinase/AKT. JAKSTAT. 2014;3:e29554. doi: 10.4161/jkst.29554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie C.L., Pan Y.B., Hu L.Q., Qian Y.N. Propofol attenuates hydrogenperoxide-induced apoptosis in human umbilical vein endothelial cells via multiple signaling pathways. Korean J Anesthesiol. 2015;68:488–495. doi: 10.4097/kjae.2015.68.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lieber C.S., Jones D.P., Losowsky M.S., Davidson C.S. Interrelation of uric acid and ethanol metabolism in man. J Clin Invest. 1962;41:1863–1870. doi: 10.1172/JCI104643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thamcharoen N., Leeaphorn N., Sanguankeo A. A pretty clue in pink: pink urine in a morbidly obese patient with lymphoma. Clin Nephrol. 2015;84:116–117. doi: 10.5414/CN108585. [DOI] [PubMed] [Google Scholar]
- 93.Potton L., Bonadona A., Minet C., Timsit J.F. Pink urine. Intensive Care Med. 2013;39(3):389–390. doi: 10.1007/s00134-013-2835-x. [DOI] [PubMed] [Google Scholar]
- 94.Saran R., Abdullah S., Goel S. An unusual cause of pink urine. Nephrol Dial Transplant. 1998;13:1579–1580. doi: 10.1093/ndt/13.6.1579. [DOI] [PubMed] [Google Scholar]
- 95.Okubo K., Okubo H., Kamijo Y., Higuchi M. Pink urine syndrome. Intern Med. 2011;50:2057. doi: 10.2169/internalmedicine.50.5972. [DOI] [PubMed] [Google Scholar]
- 96.Stern A.B., Stewart H.D., Singh H.K., Kshirsagar A.V. Pink urine after propofol anesthesia. Kidney Int. 2010;78:1193. doi: 10.1038/ki.2010.363. [DOI] [PubMed] [Google Scholar]
- 97.Schmitt R., Hampich F., Luft F.C. Apricot urine in autumn. Nephrol Dial Transplant. 2004;19:2147–2148. doi: 10.1093/ndt/gfh113. [DOI] [PubMed] [Google Scholar]
- 98.Hou J.-D., Peng C.-Z. Pink urine following propofol anesthesia. Int J Gerontol. 2016;10:245. [Google Scholar]
- 99.Verhoeven E., Capron A., Hantson P. Pink urine. Clin Toxicol (Phila) 2014;52:980–981. doi: 10.3109/15563650.2014.961068. [DOI] [PubMed] [Google Scholar]
- 100.Kato N., Ogawa R. Does use of propofol in heavy alcohol drinkers tend to discolor their urine? Acta Anaesthesiol Scand. 1999;43:868–869. doi: 10.1034/j.1399-6576.1999.430816.x. [DOI] [PubMed] [Google Scholar]
- 101.Tan C.K., Lai C.C. Pink urine in an elderly woman. Geriatr Gerontol Int. 2012;12:358–359. doi: 10.1111/j.1447-0594.2011.00760.x. [DOI] [PubMed] [Google Scholar]
- 102.Sinnollareddy M., Marotti S.B. Propofol-associated urine discolouration in critically ill patients-case reports. Anaesth Intensive Care. 2014;42:268–269. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Used raw data from Pinto B, Rocha E, Ruiz Marcellan FJ. Urinary excretion of uricine. In: Muller MM, Kaiser E, Seegmiller JE, eds. Purine Metabolism in Man—II. Boston: Springer; 1977:86–89. Performed extra calculations, specifically means of urinary uricine and urinary uric acid excretion, Mann-Whitney U Test for comparison groups, and the Pearson correlation coefficient in the evaluation of whether urinary uricine excretion correlates with urinary uric acid excretion. Significance was defined as 2-tailed P < 0.05.