Abstract
A fundamental tenet of maternal effects assumes that maternal variance over time should have discordant consequences for offspring traits across litters. Yet, seldom are parents observed across multiple reproductive bouts, with few studies considering anthropogenic disturbances as an ecological driver of maternal effects. We observed captive coyote (Canis latrans) pairs over two successive litters to determine whether among‐litter differences in behavior (i.e., risk‐taking) and hormones (i.e., cortisol and testosterone) corresponded with parental plasticity in habituation. Thus, we explicitly test the hypothesis that accumulating experiences of anthropogenic disturbance reduces parental fear across reproductive bouts, which should have disparate phenotypic consequences for first‐ and second‐litter offspring. To quantify risk‐taking behavior, we used foraging assays from 5–15 weeks of age with a human observer present as a proxy for human disturbance. At 5, 10, and 15 weeks of age, we collected shaved hair to quantify pup hormone levels. We then used a quantitative genetic approach to estimate heritability, repeatability, and between‐trait correlations. We found that parents were riskier (i.e., foraged more frequently) with their second versus first litters, supporting our prediction that parents become increasingly habituated over time. Second‐litter pups were also less risk‐averse than their first‐litter siblings. Heritability for all traits did not differ from zero (0.001–0.018); however, we found moderate support for repeatability in all observed traits (r = 0.085–0.421). Lastly, we found evidence of positive phenotypic and cohort correlations among pup traits, implying that cohort identity (i.e., common environment) contributes to the development of phenotypic syndromes in coyote pups. Our results suggest that parental habituation may be an ecological cue for offspring to reduce their fear response, thus emphasizing the role of parental plasticity in shaping their pups’ behavioral and hormonal responses toward humans.
Keywords: Canis latrans, hormones, human disturbance, parental effects, repeatability, risk‐taking
1. INTRODUCTION
Maternal effects have the potential to drive both the direction and strength of evolutionary change in a population (Bonduriansky & Day, 2009; Marshall & Uller, 2007; Wolf, Brodie, Cheverud, Moore, & Wade, 1998). A fundamental assumption of maternal effects theory is that parents can vary their phenotype as a result of acute changes to environmental conditions, accrued environmental experiences over time, or both (Badyaev & Uller, 2009; Maestripieri & Mateo, 2009; Mousseau, 1998; Mousseau, Uller, Wapstra, & Badyaev, 2009). Despite the centrality of this assumption, most prior work is commonly conducted within a single reproductive bout or season (Marshall & Uller, 2007). These investigations are seldom representative of lifetime maternal fitness (Badyaev & Uller, 2009; Marshall & Uller, 2007; Plaistow, St. Clair, Grant, & Benton, 2007), particularly because maternal effects in one reproductive season may not be predictive of future reproduction due to fluctuations in predation pressures (Marshall & Keough, 2004; Sheriff, Krebs, & Boonstra, 2010), population densities (Dantzer et al., 2013; Plaistow & Benton, 2009), and resources (Forest, Dender, Pitcher, & Semeniuk, 2017; Hafer, Ebil, Uller, & Pike, 2011; Plaistow et al., 2007). While time is an important component of parental effects theory, particularly because such processes are inextricably linked with a mother's ability to translate temporally‐varying environmental conditions into offspring phenotypes (Uller, 2008), few studies have investigated variation in maternal effects over time (Benson, Mills, Loveless, & Patterson, 2013; Marshall & Keough, 2004; Plaistow et al., 2007; Sheriff et al., 2010).
The extent to which parental effects allow parents to mold offspring phenotypic development has received substantial empirical attention over the past decade (Benard & McCauley, 2008; Champagne, 2008; Champagne & Curley, 2009; Crino, Prather, Driscoll, Good, & Breuner, 2014; Duckworth, Belloni, & Anderson, 2015; Hinde et al., 2014; Kemme, Kaiser, & Sachser, 2007; Love, Mcgowan, & Sheriff, 2013; O'Connor, Norris, Crossin, & Cooke, 2014; Uller, 2008; Weaver et al., 2004). Of primary concern in such studies is whether parental phenotype can act as a reliable cue for offspring to use in maximizing their fitness in the current environment (Uller, 2008; Uller, Nakagawa, & English, 2013). This hypothesis necessarily assumes that parental response is sufficiently plastic to adjust to immediate environmental constraints, and offspring are developmentally plastic enough to match parental response changes (Burgess & Marshall, 2014; Uller, 2008; Uller et al., 2013). Evidence supporting this hypothesis has traditionally investigated how predation regimes (Sheriff et al., 2010; Stein & Bell, 2014), density‐dependence (Dantzer et al., 2013), or resource availability (English, Bateman, Mares, Ozgul, & Clutton‐Brock, 2014; Hafer et al., 2011) induce parental and offspring plasticity. Rarely has the contribution of anthropogenic disturbance been considered (Greenberg & Holekamp, 2017; Miranda, Schielzeth, Sonntag, & Partecke, 2013).
Previous empirical work provides evidence to suggest that wildlife perceive humans as predators, and as such, display fear responses that are qualitatively similar to those exhibited in the presence of natural predators (Blumstein, 2006; Carrete & Tella, 2017; Rebolo‐Ifran et al., 2015). This is particularly the case for carnivores, as several recent studies suggest behavioral and ecological patterns of such species are directly modified as a function of anthropogenic disturbance (Clinchy et al., 2016; Moll et al., 2018; Smith et al., 2017; Smith, Thomas, Levi, Wang, & Wilmers, 2018; Wang, Smith, & Wilmers, 2017). Given that wildlife encounters with humans have gradually become more frequent across the globe within recent decades (Barrett, Stanton, & Benson‐Amram, 2018; Ditchkoff, Saalfeld, & Gibson, 2006), it has become necessary for animals to increase their tolerance of human presence to survive in human‐dominated landscapes (Lowry, Lill, & Wong, 2013; Miranda, 2017; Sol, Lapiedra, & González‐Lagos, 2013). Indeed, recent findings emphasize the significance of human disturbance as a source of ecological variance, suggesting that organisms with frequent human encounters (e.g., urban vs. rural individuals) will show reduced fear of, and habituation to, humans over time (Carrete & Tella, 2013; Cook, Weaver, Hutton, & McGraw, 2017; Martin & Réale, 2008; Uchida, Suzuki, Shimamoto, Yanagawa, & Koizumi, 2016; Vincze et al., 2016). Examining the mechanisms that bolster wildlife habituation to human presence both improves our understanding of the functional significance of human‐mediated behavioral plasticity (Love et al., 2013), and informs us on the processes that may contribute to the development of problematic behaviors linked to human‐wildlife conflict (Blackwell et al., 2016; Soulsbury & White, 2015). Thus, prior research on both wildlife habituation and parental effects provide a framework to explore whether human disturbance over time changes parental cues (i.e., behavior) that offspring use to modify their phenotypes.
In this study, we investigated whether changes to parental fear of humans across reproductive episodes differentially affects fear and endocrine responses of offspring born to separate litters (Figure 1). We test this hypothesis in coyotes (Canis latrans), a biparental canid that produces several litters and maintains lifelong monogamous bonds (Hennessy, Dubach, Gehrt, Resources, and Resources (2012)) with near‐equal rates of parental care between mothers and fathers (Schell, Young, Lonsdorf, Mateo, & Santymire, 2018). Our four main questions are as follows: (a) is parental fear of humans reduced over time (i.e., from the first to second reproductive event); (b) does among‐year plasticity in parental fear predict among‐litter plasticity in risk‐taking behavior; (c) do endocrine traits (e.g., cortisol and testosterone) differ between first and second‐litter siblings; and (d) are offspring traits repeatable and heritable? We address these questions in a captive system because the experimental design of among‐litter studies often requires recapture and repeated measures that are difficult to obtain in the wild. Indeed, only two studies prior to this one have observed among‐litter phenotypic plasticity of single mothers (Margulis, Nabong, Alaks, Walsh, & Lacy, 2005; Sheriff et al., 2010), both of which were in captive systems. Moreover, previous evidence suggests behavior in captivity can predict personality variation in the wild (Cole & Quinn, 2014; Herborn et al., 2010), underscoring the ecological significance of such studies.
Figure 1.
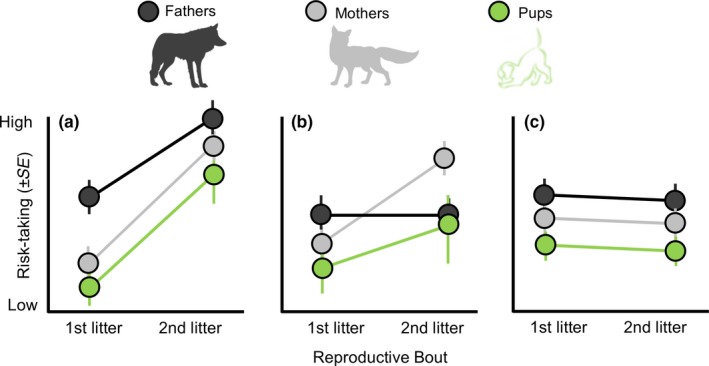
Conceptual diagram of potential scenarios in which parental habituation and offspring risk‐taking behavior are related to predictable cues of anthropogenic environments over time. Dots indicate risk‐taking behavior within fathers (black) and mothers (gray) over successive reproductive events, whereas pups (green) are separate litters. In (a) both mothers and fathers become habituated, and as a result demonstrate riskier behavior across reproductive bouts. If parental cues are a reliable signal of current environmental conditions, then it is predicted that pup risk‐taking will also increase. In (b) only a single parent becomes habituated to anthropogenic disturbance, with the other parent possibly selectively constrained. Second‐litter pups may exhibit slightly greater risk‐taking than their first‐litter siblings, although they may not differ statistically. And in (c), neither parent becomes habituated over time. In all scenarios, it is assumed that parental behavior is a reliable cue of environmental conditions that offspring use to fashion their behavior
From our questions, we make several predictions (Figure 1). First, we predicted that parents would exhibit reduced fear responses with their second versus with their first litters. Parents with their second litters have accumulated more experiences of humans than with their first litters, and thus should be more habituated (Figure 1). We quantified risk‐taking behavior as the willingness to forage with persistent human disturbance (i.e., human observer). This is similar to previous studies that assess individual differences in risk‐taking and boldness in relation to anthropogenic disturbance (Dammhahn & Almeling, 2012; De Meester et al., 2018; Greenberg & Holekamp, 2017; Patrick, Charmantier, & Weimerskirch, 2013; Samia, Nakagawa, Nomura, Rangel, & Blumstein, 2015). Second, we predicted that second‐litter pups would be less risk‐averse than their first‐litter siblings. This prediction necessarily assumes that parental habituation can operate as a cue for offspring to modify their behavior accordingly (Figure 1). Third, we predicted that developmental testosterone, but not cortisol, would be lower in second versus first‐litter siblings. Previously, we demonstrated that parents had reduced gestational testosterone (but not cortisol) as experienced versus naïve parents (Schell, Young, Lonsdorf, Mateo, & Santymire, 2016). Hence, our third prediction assumes that parent‐offspring endocrine responses will positively covary as found in previous work (Meylan, Miles, & Clobert, 2012; Sheriff et al., 2010, 2017). Finally, we predicted that risk‐taking would be consistent within individuals (i.e., demonstrate repeatability) as recent literature suggests that fear of humans is highly repeatable and heritable (Carrete & Tella, 2011, 2013; Carrete et al., 2016). To address this prediction, we used a quantitative genetic approach that allowed us to estimate the contribution of additive genetic, permanent environment, maternal, and cohort effects on all pup traits, calculate repeatability, and estimate correlations among pup traits. This study represents a novel integration of parental effects theory with human‐wildlife interactions to assess how human disturbance may contribute to transgenerational plasticity.
2. METHODS
2.1. Study animals and housing
We observe a captive coyote population, maintained for research purposes, at the United States Department of Agriculture (USDA) – National Wildlife Research Center's (NWRC) Predator Research Facility, in Millville, UT. Eight breeding pairs (male‐female pairs) and their offspring were observed in 2011 and 2013, and all pairs were nulliparous (i.e., had no prior parenting experience) before the study (Figure 2). In 2011, all parents were less than two years of age (1.4 ± 0.1 years [X ± SD]). Pups were born in March and April of both years and observed from 5–15 weeks of age. This age was selected because pup emergence from natal dens becomes more frequent, pups are progressively weaned by their mothers, and pups refine their social skills and conspecific communication (Bekoff & Wells, 1982; Fentress, Ryon, & McLeod, 1987; Messier & Barrette, 1982; Sacks & Neale, 2001; Way, Auger, Ortega, & Strauss, 2001). Parent‐pup family units were housed in 1,000‐m2 outdoor pens from gestation, in early January, until dispersal age in the wild, in late July or early August (i.e., 15 weeks of age; Bekoff & Wells, 1982). Pups were then relocated from their natal pens to outdoor enclosures separate from their parents to reduce parent‐juvenile conflicts (Figure 2). Outdoor enclosures were equipped with artificial den boxes, multi‐tiered wooden structures for cover, and various small objects for environment enrichment. To reduce the influence of environmental familiarity as a covariate with reproductive bout, parents reared second‐litter offspring in different clover pens than those used during 2011.
Figure 2.
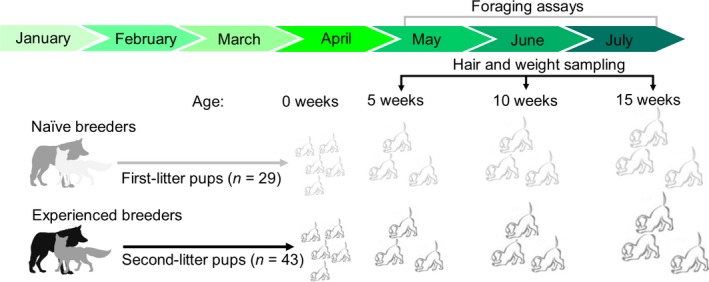
Schematic depicting the general timeline and experimental design used to observe offspring traits of first and second litters. Foraging assays were performed 2–3 times per week from 5–15 weeks of age. At 15 weeks of age, pups were removed from their natal pens to enclosures independent of their parents
2.2. Risk‐taking assays
We use modified foraging assays with anthropogenic disturbance (i.e., human observer present) to assess risk‐taking behavior in coyote parents and pups, as seen in previous work (Dammhahn & Almeling, 2012). Although our study coyotes were fed 6 of 7 days weekly by animal care staff leading up to this experiment, our foraging assays varied in two key ways. First, animal care staff scatter feed coyotes; daily food rations are spread throughout a section of their pens instead of placed in specific piles. Second, animal care staff immediately exits the pen and move on to another pen after scatter feeding, so coyotes typically do not eat with a human present unless they start to forage before the staff has completed exiting the pen. Even then, the human is moving and not static. Our design is fundamentally distinct from routine staff procedures in two ways: (a) a single observer intentionally concentrated food in 3–5 piles at the front half of the pen, and (b) that observer then sat at the pen entrance to visually observe focal individuals. We deemed this process as a proxy for human disturbance.
Foraging assays were performed from 5–15 weeks of age, ~2–3 times over the course of each week. We also randomized the order in which pens were observed during each foraging assay. We recorded whether a pup ate at a food pile independent of their parents (i.e., a parent did not bring or regurgitate food to the focal pup) as a binary response (yes/no) over a 7‐min period. Thus, riskier individuals, by definition, ate at food piles more frequently over development than others. We chose a 7‐min observation period because in preliminary feeding observations, this was the maximum amount of time for coyotes within a pen to consume all food provided, regardless of whether few individuals monopolized food rations or if all animals ate.
2.3. Pup hormones
Hair has quickly become a viable alternative to quantify individual hormone levels, particularly because hair concentrations represent an accumulated hormonal average over a period of months to years, rather than days (Meyer & Novak, 2012; Schell, Young, Lonsdorf, Mateo, & Santymire, 2017; Stalder & Kirschbaum, 2012). Moreover, recent studies have suggested maternal effects can influence hormone levels in neonatal hair (Dettmer, Rosenberg, Suomi, Meyer, & Novak, 2015; Kapoor, Lubach, Ziegler, & Coe, 2016), are useful in examining developmental patterns in endocrine function (Laudenslager, Jorgensen, & Fairbanks, 2012), show heritable variation (Fairbanks et al., 2011), and are responsive to environmental factors (Salaberger et al., 2016), highlighting the functional significance of hair hormone levels. Consequently, we used hair samples as a means of quantifying repeatable variation in pup cortisol and testosterone over development.
We captured pups at 5, 10, and 15 weeks of age and shaved pups using commercially available pet grooming clippers, which were brushed and wiped with 70% alcohol before each shave. We shaved a 4‐cm area of hair for each individual pup and stored the samples in a plastic bag. Bags were then placed in a drawer to reduce prolonged exposure to direct sunlight, as prior study suggests natural sunlight decreases cortisol concentrations in hair (Wester, van der Wulp, Koper, de Rijke, & van Rossum, 2016). Extraction methodology closely followed (Schell et al., 2017). Briefly, hair was pulverized to a fine powder, combined with 5.0 ml of 90% methanol (methanol:distilled water) and sufficiently agitated over a 5‐hr period. Samples were then dried down and reconstituted with 500 μl of phosphate‐buffered saline solution before running on cortisol and testosterone enzyme immunoassays (EIA). Complete description of EIA methods, including validation and differences as a function of body region, can be found in (Schell et al., 2017). Furthermore, in our previous investigation we did not find any differences in pup cortisol or testosterone concentrations as a function of body region (Schell et al., 2017), as seen in other taxa (Acker, Mastromonaco, & Schulte‐Hostedde, 2018; Carlitz, Kirschbaum, Stalder, & van Schaik, 2014). We were therefore able to compare pup hair samples collected from varying body regions in the current study.
2.4. Statistical analyses
We first investigated whether parental risk‐taking behavior changed from the first to the second reproductive bout using univariate generalized Bayesian animal models (i.e., generalized mixed‐models) with Markov chain Monte Carlo (MCMC) estimation (de Villemereuil, 2012; Hadfield, 2010; Wilson et al., 2010). We included litter year (i.e., first vs. second litter), pup developmental age, litter size, and sex as fixed effects in our model. In addition, male‐female pairs in this study were previously exposed to olfactory attractants to simulate high‐density conspecific environments meant to increase glucocorticoid concentrations prepartum (Schell et al., 2016), comparable with prior studies of vertebrate maternal effects (Dantzer et al., 2013; Schweitzer, Schwabl, Baran, & Adkins‐Regan, 2014). Briefly, experimental groups (2011: n = 4; 2013: n = 4) received the odor cues four times over a 20‐day period, whereas control pairs (2011: n = 4; 2013: n = 4) received water as a delivery control (Schell et al., 2016). Our initial study did not have an outgroup odor; however, previous studies indicate that coyote behavioral responses toward other chemical attractants are characteristically similar to the behavioral responses we observed in our previous work (Kimball, Johnston, Mason, Zemlicka, & Blom, 2000; Kimball, Mason, Blom, Johnston, & Zemlicka, 2000; Schell et al., 2016; Shivik, Wilson, & Gilbert‐Norton, 2011). We did not find a statistical effect of our odor manipulation on subsequent parenting behavior (Schell et al., 2018) or prolonged hormonal effects (Schell et al., 2016). Nevertheless, we also included parental odor treatment (i.e., “odor”) as a fixed effect in our statistical analyses. All parental models included animal identity (VA, identity link to the pedigree), individual identity (VPE, identity), maternal identity (VM, mother ID), and cohort identity (VC; common environment or litter) as random effects (Wilson et al., 2010). Because the response variable for risk‐taking behavior was binary, we used a categorical error structure fitted in MCMCglmm with a parameter expanded prior (V = 1, μ = 1,000, α.μ = 0, α.V = 1) for the G priors (random effects) and the residual variance fixed to one (V = 1, fix =1) for the R priors, similar to previous studies (Araya‐Ajoy & Dingemanse, 2017; Patrick & Weimerskirch, 2015; Patrick et al., 2013).
To determine whether offspring traits differed as a function of parental reproductive bout, we used univariate animal models with litter (i.e., first or second) included as a fixed effect, as well as all other fixed effect variables found in parental models (i.e., developmental age, sex, litter size, and parental odor treatment). Pup risk‐taking was fit with a categorical error structure with residual variance fixed to 1, comparable with models for parental risk. Endocrine variables, in contrast, were fit with a Gaussian distribution after confirmation of normality using Levene tests implemented from the “Rcmdr” R package (Fox & Bouchet‐Valat, 2018). Thus, models with a Gaussian error structure were fit with uninformative G and R priors (V = 1, μ = 1.002), and output was robust to slight changes to these priors.
To investigate whether pup traits (and parental risk‐taking) were repeatable, as well as determine the relative weight of our variance components on trait repeatability, we combined parental and pup data (i.e., all age classes) into a single analysis to effectively estimate variance components in our sample population. In addition, we used our previous univariate models from the first two aims to estimate quantitative genetic components within each age class (i.e., within pups and adults). The total phenotypic variance (VP) was partitioned into additive genetic (VA, identity link to the pedigree), permanent environment (VPE, identity), maternal (VM, mother ID), and cohort (VC; common environment or litter) variance parameters by fitting the model with the random terms of “animal”, “ID”, “dam”, and “Litter ID”. Thus, VP = VA + VPE + VM + VC + VR, in which VR accounted for the residual variance (i.e., “units”) in the model (Wilson et al., 2010). We estimated narrow‐sense heritability in risk‐taking behavior as h 2 = VA/(VP + π2/3), which included the distribution‐specific variance term (π2/3) of a binomial model with a logit link (Hadfield, 2010; Nakagawa & Schielzeth, 2010). Permanent environment effects (PE = VPE/VP + π2/3), maternal effects (m 2 = VM/VP + π2/3), and cohort effects (C = VC/VP + π2/3) were similarly estimated (Petelle, Martin, & Blumstein, 2015; Taylor et al., 2012; Wilson et al., 2010). We then estimated repeatability as the among‐individual variance (VI = VA + VPE + VM + VC) divided by the phenotypic variance, r = VI/(VP + π2/3). Again, we included the distribution‐specific variance term (π2/3) of a binomial model with a logit link function. Variance parameters for pup endocrine traits were estimated similarly without the binomial‐specific variance term.
To determine whether offspring traits were correlated, we used a multivariate animal model that contained all fixed and random effects in previous univariate models to estimate genetic, maternal, cohort, and phenotypic correlations among offspring traits. Before analysis, we binned trait data according to the hair hormone survey window, then proceeded to analyze each window separately. In other words, risk‐taking behavioral data were partitioned into a single row with shaved hair samples collected at 10 and 15 weeks of age, respectively. These periods corresponded with ecologically‐relevant developmental periods established in the literature (5–10 weeks: weaning stage; 11–15 weeks: juvenile stage; Bekoff & Wells, 1986; Fentress et al., 1987). Thus, we evaluated each developmental stage separately, with a single pup having a single row of data in the weaning stage, and a single row in the juvenile stage. As a result, we were unable to evaluate permanent environment correlations due to the lack of repeated data (i.e., rows) for each pup within a developmental stage. The remaining random effects were set with an unstructured (“us”) G‐structure, which allowed a fully factorial variance/covariance matrix between pup phenotypic traits and our fixed effects (Boulton et al., 2015; Petelle, McCoy, Alejandro, Martin, & Blumstein, 2013; Sanderson et al., 2015). Litter and the “trait‐1” term were fitted as fixed effects (Wilson et al., 2010). Risk‐taking behavior was fit with the multinomial distribution (“multinomial2”), whereas endocrine variables were fit with a Gaussian distribution. Subsequent phenotypic covariances (COVP) from the multivariate model were partitioned into additive genetic (COVA), maternal (COVM), cohort (COVC), and residual (COVR) covariance components. To calculate correlations, we divided the respective covariance estimate for a pair of traits by the square root of the product of variances. For instance, the genetic correlation among pup risk‐taking and cortisol, where “1” is equal to risk‐taking and “2” is equal to cortisol, was calculated as: rA = COVA(1,2)/√(V A(1)*V A(2)); whereas the maternal correlation was calculated as: rR = COVM(1,2)/√(VM (1) * VM (2))) (Boulton, Grimmer, Rosenthal, Walling, & Wilson, 2014; Brommer, Karell, Ahola, & Karstinen, 2014; Dosmann, Brooks, & Mateo, 2015; Taylor et al., 2012). To calculate phenotypic correlations, we divided the sum of all covariances (COVP (1,2) = COVA (1,2) + COVM (1,2) + COVC (1,2) + COVR (1,2)) by the square root of the sum of all variances (VARP (1,2) = (VA(1) + VM(1) + VC(1) + V R (1))*(VA(2) + VM(2) + VC(2) + V R (2)). Hence, phenotypic correlations among traits were estimated as: rP = COVP(1,2)/√(VARP (1,2)).
All analyses were performed in R version 3.4.4 (R Core Team, 2017). We used the MCMCglmm package to run all Bayesian animal models (Hadfield, 2010) and plots were constructed using ggplot2 (Wickman, 2009). MCMC chains for our animal models were run for 1,000,000 iterations (“nitt”), with the posterior distribution being sampled every 100 iterations (“thin”) after a burn‐in period of 50,000 iterations (“burnin”). In addition, we checked for proper model mixing by examining the levels of autocorrelation (all model runs were <0.04), the variance component plots, and the effective size (all model runs >4,000 per run; (de Villemereuil, 2012; Hadfield, 2010). All models were fit with a pedigree to allow the population variance to be structured among relatives. Sire, dam, grandparental, and great‐grandparental identity were included in the pedigree (Supporting information Appendix S1: Table S1). Estimates for fixed effects (β), repeatability (r), heritability (h 2), and all correlations (rA, rPE, rM, rC, and rP) were derived from animal models as the mode of the posterior distribution with accompanying 95% credibility intervals (low CI, high CI) in parentheses. Bayesian estimates were considered statistically significant when the credibility intervals do not overlap zero (Hadfield & Nakagawa, 2010; Sanderson et al., 2015; Stein & Bell, 2015). Lastly, we found that litters were larger in the second (mean ± SE: 5.4 ± 1.5 pups) versus the first reproductive bout (mean ± SE: 3.6 ± 1.2 pups). Because of the potential confounding relationship between parental parity and litter size, we compared model fit between null animal models containing all previous fixed effects, and alternative models that additionally included the interaction term between litter year and litter size. We then selected the model with the lowest deviance information criterion (DIC) value, in which the optimal model had a ΔDIC = 0 (Hadfield, 2010; Pooley & Marion, 2018; Spiegelhalter, Best, Carlin, & Van Der Linde, 2002). We used the “model.sel” function from the MuMIn package to determine the best‐fit model (Bartoń, 2018), and results are reported from the final (i.e., ΔDIC = 0) animal model.
3. RESULTS
Coyote pairs gave birth to their first litters (n = 29 total pups in 8 L) in 2011 and second litters (n = 43 total pups in 8 L) in 2013 (Figure 2). In 2011, two litters were removed from the study at 10 weeks of age for NWRC‐related research needs. Four additional pups in 2013 (n = 4) died of unknown causes at 6 to 7 weeks of age. Thus, we observed n = 72 pups up to 5 weeks of age, n = 68 pups up to 10 weeks, and n = 60 pups up to 15 weeks for a grand total of n = 1763 observations of pups and n = 790 observations of parents over a 2‐year span. Model selection results can be found in Supporting information Appendix S1: Table S2 and Table S3. For risk‐taking behavior, none of the alternative univariate models containing the interaction between litter year and size performed better than our null models (Supporting information Appendix S1: Table S2). For endocrine traits, however, mixed models with the interaction between litter year and developmental age significantly outperformed null models (Supporting information Appendix S1: Table S3).
3.1. Parental and pup risk‐taking
Both mothers (mean ± SE: 1st year, 0.56 ± 0.12; 2nd year, 0.97 ± 0.02) and fathers (mean ± SE: 1st year, 0.48 ± 0.12; 2nd year, 0.92 ± 0.04) were riskier with their second litters than with their first (Figure 3, Table 1a). We did not find evidence of an effect of developmental age, sex, prepartum odor treatment, or litter size on parental risk‐taking (Table 1a). Second‐litter pups had greater risk‐taking compared to their first‐litter siblings (mean ± SE, first‐litter pups, 0.16 ± 0.06; second‐litter pups, 0.72 ± 0.04; Figure 3, Table 1b). In addition, pup risk‐taking increased over development (Table 1b). There was no effect of sex, prepartum odor treatment, or litter size on pup risk‐taking (Table 1b). Individual reaction norms for each family unit can be found in Supporting information Appendix S1: Figure S1.
Figure 3.
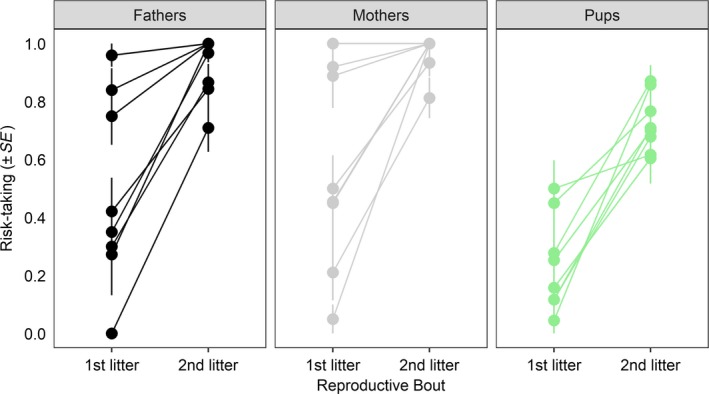
Risk‐taking behavior (i.e., foraging rate) of coyote fathers, mothers, and pups during the first and second reproductive bouts. For mothers and fathers, lines connect the same individuals over time, whereas for pups, lines connect first‐ and second‐litter siblings. Risk‐taking is reported as the average proportion of feeding bouts (±SE) in which the individual fed in the presence of a human
Table 1.
The influence of litter year (i.e., first vs. second), litter age (in weeks), litter size, sex, and prepartum odor treatment on coyote risk‐taking behavior within coyote (a) adults, (b) pups, and (c) both age classes combined
| Fixed effect | (a) β (95% CI) | (b) β (95% CI) | (c) β (95% CI) |
|---|---|---|---|
| Intercept | −2.398 (−5.862, 1.688) | −3.061 (−5.268, −0.950) | −2.413 (−4.705, −0.020) |
| Litter (1st vs. 2nd) | 3.690 (1.738, 6.046) | 2.662 (1.580, 4.076) | 2.705 (1.360, 4.030) |
| Age | 0.0502 (−0.040, 0.155) | 0.103 (0.063, 0.154) | 0.094 (0.055, 0.137) |
| Litter size | 0.632 (−0.107, 1.568) | 0.051 (−0.445, 0.570) | 0.395 (−0.028, 1.054) |
| Sex | −0.451 (−2.623, 0.984) | 0.028 (−0.425, 0.583) | −0.037 (−0.482, 0.487) |
| Odor | −0.753 (−2.623, 1.386) | 0.517 (−0.647, 1.437) | 0.276 (−0.769, 1.395) |
| Age class | – | – | −2.273 (−3.664, −1.222) |
Models (a) and (b) do not examine the fixed effect of age class, as models are partitioned within‐age class. Estimates of fixed effects (β) are given with 95% credible intervals (n = 2,553 observations, 89 individuals and 16 cohorts). Estimates that do not overlap zero (i.e., pMCMC < 0.05) are significant and in bold. Null and alternative model comparisons can be found in the Supporting information Appendix S1: Table S2. Final models were chosen based on ΔDIC = 0.
3.2. Pup hormones
We found that second‐litter pups had higher average cortisol concentrations compared with first‐litter siblings (mean ± SE: first‐litter pups, 9.98 ± 0.48; second‐litter pups, 12.73 ± 0.49; Table 2a, Figure 4). Litter year and developmental age were significant predictors of pup cortisol, with a significant interaction between litter and age, suggesting that second‐litter pups had higher cortisol at 10 and 15 weeks of age (Table 2a, Figure 4). Separate‐year litters also differed in their testosterone over development, with a significant interaction term between litter and age (Table 2b). Compared with their first‐litter siblings, second‐litter pups had lower testosterone at 5 weeks of age, but at 15 weeks of age that trend was reversed (Figure 4).
Table 2.
The influence of litter year, litter age, litter size, sex, prepartum odor treatment, and the interaction term among litter year and age (i.e., litter:age) on coyote pup (a) cortisol and (b) testosterone over development
| Fixed effect | (a) β (95% CI) | (b) β (95% CI) |
|---|---|---|
| Intercept | 15.678 (12.371, 19.407) | 16.771 (11.556, 23.224) |
| Litter (1st vs. 2nd) | −5.452 (−8.130, −2.313) | −13.418 (−18.530, −8.272) |
| Age | −4.557 (−5.368, −3.385) | −1.171 (−3.019, 0.571) |
| Litter size | 0.526 (−0.165, 1.270) | −0.395 (−1.414, 0.901) |
| Sex | 0.844 (−0.352, 1.973) | −0.170 (−2.424, 1.584) |
| Odor | −0.349 (−1.877, 0.876) | 0.416 (−2.282, 2.405) |
| Litter:Age | 3.994 (2.617, 5.130) | 7.460 (4.931, 9.478) |
Estimates of fixed effects (β) are given with 95% credible intervals (n = 200 hair samples, 72 individuals and 16 cohorts). Estimates that do not overlap zero are significant (i.e., pMCMC<0.05) and in bold. Null and alternative model comparisons can be found in the Supporting information Appendix S1: Table S3. Final models were chosen based on ΔDIC = 0.
Figure 4.
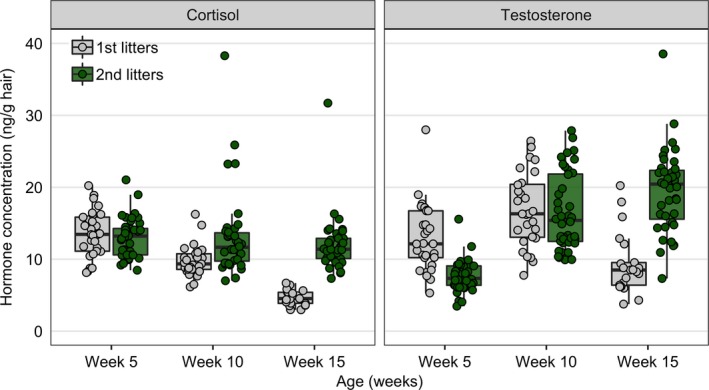
Hormonal differences among first‐ and second‐litter offspring in hair cortisol and testosterone concentrations at 5, 10, and 15 weeks of age. Each dot represents an individual pup
3.3. Variance component estimates and repeatability
We found evidence of repeatability in risk‐taking behavior across age classes (Table 3), and within each age class (Supporting information Appendix S1: Table S4). We additionally found moderate evidence of maternal and cohort effects on risk‐taking behavior (Table 3). However, we did not find statistical support for additive genetic or permanent environment effects on risk‐taking behavior in coyotes. Both cortisol and testosterone were repeatable (Table 3), despite moderate developmental fluctuations in both hormone traits (Figure 4). Moreover, pup cortisol was mildly heritable, with mild permanent environment, maternal, and cohort effects (Table 3). None of the variance components for testosterone differed from zero (Table 3).
Table 3.
Heritability (h2), permanent environmental effects (PE), maternal effects (m2), cohort effects (C), and repeatability (r) of coyote risk‐taking behavior (both age classes), as well as cortisol and testosterone (pups only)
| Trait | h2 | PE | m2 | C | r |
|---|---|---|---|---|---|
| Risk‐takinga | 0.000 (0.000, 0.167) | 0.000 (0.000, 0.127) | 0.171 (0.020, 0.355) | 0.087 (0.026, 0.256) | 0.421 (0.281, 0.573) |
| Cortisol | 0.018 (0.006, 0.102) | 0.015 (0.005, 0.084) | 0.018 (0.005, 0.130) | 0.019 (0.005, 0.097) | 0.143 (0.065, 0.281) |
| Testosterone | 0.007 (0.002, 0.083) | 0.007 (0.002, 0.069) | 0.009 (0.001, 0.097) | 0.008 (0.002, 0.097) | 0.085 (0.032, 0.226) |
All estimates are given with 95% credible intervals (i.e., highest posterior density intervals [HPDI]). Significant estimates are in bold.
Risk‐taking was fit with the "categorical" family distribution; all other variables were fit with a Gaussian distribution. Data from both parents and pups were included in the model (see Supporting information Appendix S1: Table S2 and Table S3 for model specifications).
3.4. Correlation estimates
Within the weaning stage of development (i.e., 5–10 weeks of age), we found evidence of a positive phenotypic correlation among risk‐taking behavior and cortisol (Figure 5a). That correlation was strongly underpinned by substantial cohort correlations (Table 4). We did not find evidence of genetic or maternal correlations among risk‐taking and cortisol. Furthermore, we did not find support for correlations between risk‐taking and testosterone, nor between cortisol and testosterone, during the weaning stage (Table 4). Within the juvenile stage, we found evidence of positive cohort correlations for all trait combinations, and positive phenotypic correlations for two‐thirds of the trait combinations (Table 4, Figure 5). We did not find genetic or maternal correlations among traits within the juvenile stage (Table 4).
Figure 5.
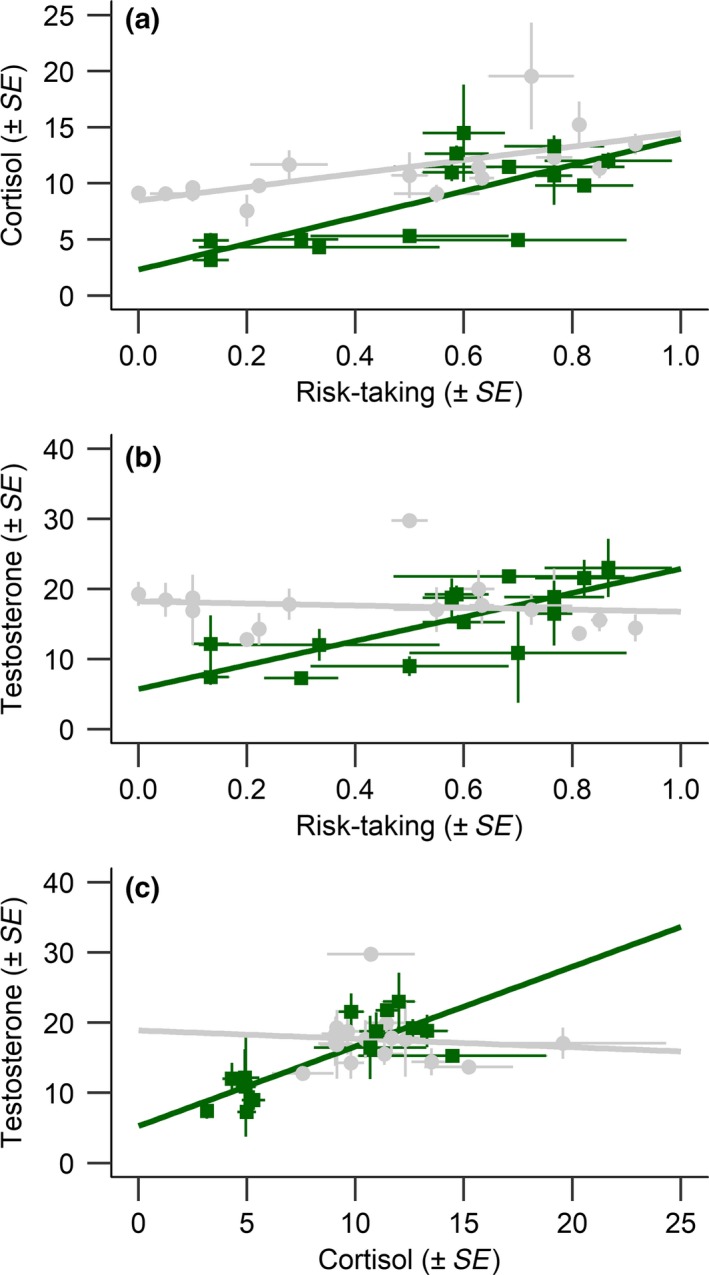
Relationships among risk‐taking and cortisol (a), risk‐taking and testosterone (b) and cortisol and testosterone (c) of offspring during the weaning (5–10 weeks of age; gray circles) and juvenile (10–15 weeks of age; green squares) stages of development. Each point represents the litter average ± SE
Table 4.
Genetic, maternal, cohort, and phenotypic correlations between each pair of pup traits within the weaning (5–10 weeks) and juvenile (10–15 weeks) stages of development
| Development Stage | Trait 1 | Trait 2 | Genetic | Maternal | Cohort | Phenotypic |
|---|---|---|---|---|---|---|
| Weaning (5–10 weeks) | Risk‐taking | Cortisol | 0.507 (−0.321, 0.815) | 0.242 (−0.705, 0.839) | 0.831 (0.285, 0.969) | 0.362 (0.065, 0.593) |
| Risk‐taking | Testosterone | −0.302 (−0.743, 0.569) | −0.298 (−0.802, 0.780) | −0.810 (−0.943, 0.656) | −0.051 (−0.261, 0.204) | |
| Cortisol | Testosterone | −0.954 (−0.984, 0.895) | 0.016 (−0.877, 0.783) | −0.795 (−0.958, 0.680) | 0.197 (−0.105, 0.452) | |
| Juvenile (10–15 weeks) | Risk‐taking | Cortisol | −0.095 (−0.744, 0.787) | 0.020 (−0.738, 0.829) | 0.857 (0.384, 0.972) | 0.304 (−0.032, 0.599) |
| Risk‐taking | Testosterone | 0.493 (−0.573, 0.899) | 0.283 (−0.662, 0.906) | 0.879 (0.368, 0.970) | 0.439 (0.097, 0.651) | |
| Cortisol | Testosterone | 0.941 (−0.899, 0.978) | 0.027 (−0.746, 0.937) | 0.969 (0.784, 0.996) | 0.553 (0.212, 0.749) |
Estimates are given with 95% credible intervals, and significant estimates are in bold.
4. DISCUSSION
The combination of prior experiences paired with current environmental context induce parental plasticity over multiple reproductive bouts (Plaistow et al., 2007; Uller, 2008; Uller et al., 2013), emphasizing the central role of environmental experience in driving transgenerational plasticity (Crean, Dwyer, & Marshall, 2013; Marshall, 2008; Uller, 2008). In anthropogenic contexts, parental habituation of humans may operate as a cue for offspring to modify their fear responses of humans. Our data support this hypothesis: parents were less fearful of human disturbance with their second litters, and pups from second‐litter cohorts were also more tolerant of humans than their first‐litter siblings (Figure 3, Table 1). In addition, we found evidence of parental effects on risk‐taking behavior (Table 3), which provides additional support for the role of parental identity in shaping patterns of offspring fear (Table 3). Finally, pup risk‐taking increased over development (Table 1b), suggesting that individual‐level risk is plastic and can be adjusted over ontogeny. It is well‐known that wildlife with accrued experiences of human disturbance over time become increasingly habituated to, and tolerant of, humans (Carrete & Tella, 2017; Carrete et al., 2016; Greggor, Clayton, Fulford, & Thornton, 2016; Perals, Griffin, Bartomeus, & Sol, 2017; Samia et al., 2015; Sol et al., 2013, 2018; Vincze et al., 2016). Moreover, prior work in coyotes has demonstrated that personality differences in risk can be successfully quantified via response to humans (Darrow & Shivik, 2009; Dawson & Jaeger, 2009; Gilbert‐Norton, Leaver, & Shivik, 2009; Murray, Edwards, Abercrombie, & St. Clair, 2015; Poessel, Gese, & Young, 2017; Schmidt & Timm, 2007; Young, Mahe, & Breck, 2015). The mechanisms that contribute to rapid plasticity in wildlife fear are less well‐understood (Carrete & Tella, 2017). Our results posit that one potential mechanism shaping organismal fear of humans in coyotes may be parental effects.
We provide evidence to suggest that second‐litter pups had lower testosterone at 5 weeks of age (Figure 4, Table 2), supporting our a priori prediction that offspring testosterone levels would match decreased prepartum testosterone of experienced parents found in our previous study (Schell et al., 2016). However, that trend was reversed over time, as second‐litter pups demonstrated higher cortisol and testosterone concentrations at 15 weeks of age compared to their first‐litter siblings (Figure 4, Table 2). One explanation for this trend is that the contribution of parental input to offspring endocrine traits varies over development. Infant coyotes have considerably more contact with their parents early in development (Gese, Roberts, & Knowlton, 2016). By ~6–7 weeks of age, littermates establish relatively stable social hierarchies and are more independent from their parents (Fentress et al., 1987; Kitchen & Knowlton, 2006). Thus, it may be that social interactions amongst littermates contributed more substantially to affecting offspring hormonal development than parental identity. Previous studies on vertebrate sibling competition in relation to maternal influence provide partial insight into this explanation (Carere, Drent, Koolhaas, & Groothuis, 2005; Golla, Hofer, & East Marion, 1999; Hudson & Trillmich, 2007; Wahaj & Holekamp, 2006), although none have explored how the endocrine outcomes of offspring vary over development. Alternatively, increased cortisol and testosterone of second‐litter offspring may be a function of litter size: more siblings may lead to more competition, and thus, higher stress and reproductive physiology. Litter size positively covaried with reproductive bout (Schell et al., 2018); yet, we did not find any evidence that litter size was a significant predictor of endocrine traits over development (Table 2). Another alternative explanation may simply be that individual personalities, and not sheer number of siblings, drive social dynamics that influence individual endocrine function. There is evidence in other taxa suggesting that individual‐level behavioral consistency is more salient to group function than the number of individuals in a social group (Galhardo, Vitorino, & Oliveira, 2012; Laskowski & Bell, 2014; Montiglio, Ferrari, & Reale, 2013). Altogether, these data emphasize the importance of assessing several sources of variance (e.g., maternal, common environment) at different developmental timepoints to fully understand the contribution of parental effects to offspring endocrine development.
Evaluating the genetic and environmental sources of variance in personality and endocrine function is integral to understanding the importance of such effects on evolution (Dingemanse & Araya‐Ajoy, 2015; Dochtermann & Roff, 2010; Petelle et al., 2015). We found evidence of repeatable differences in coyote risk‐taking behavior, with significant maternal and cohort effects contributing to our repeatability estimates (Table 3). These results underscore the importance of common environmental effects and maternal influence in shaping the development of risk sensitivity in coyotes. In contrast with several recent studies (Carrete & Tella, 2017; Carrete et al., 2016; Ducatez, Audet, Rodriguez, Kayello, & Lefebvre, 2017; Sol et al., 2018), we did not find evidence of heritable variation in coyote risk‐taking. This may partially be due to pedigree depth in this study (Supporting information Appendix S1: Table S1), which is an order of magnitude smaller than prior work (Carrete et al., 2016). An alternative explanation may be that personality differences in risk‐taking and fear are both contextually and developmentally plastic in this species. According to the pace of life syndrome (POLS) hypothesis, a slow‐lived species like coyotes should be more risk‐averse with infrequent human disturbance, primarily because their life history strategy largely depends on ensuring a long reproductive lifespan (Careau, Réale, Humphries, & Thomas, 2010; Hall et al., 2015). However, if slow‐lived species manage to survive and reproduce, then such strategies (i.e., risk) can readily be adjusted with accrued experiences (Sol et al., 2018). Indeed, recent work suggests that constant exposure to anthropogenic contexts can lead to divergence in behavioral strategies used to cope with human frequentation (Charmantier, Demeyrier, Lambrechts, Perret, & Grégoire, 2017; Samia et al., 2015). Hence, prior evidence citing reduced fear to people as a necessary component of rapid evolution in anthropogenic contexts infers such processes could also occur in wild and captive coyote populations (Bókony, Kulcsár, Tóth, & Liker, 2012; Carrete & Tella, 2017; Greenberg & Holekamp, 2017; Miranda et al., 2013; Vincze et al., 2016).
Our results provide evidence for the emergence of personality and phenotypic syndromes in early life history, as similarly shown in other mammals (European roe deer, Capreolus capreolus, Debeffe et al., 2015; dwarf hamsters, Phodopus sungorus, Kanda, Louon, & Straley, 2012), fish (convict cichlids, Amatitlania siquia, Mazué, Dechaume‐Moncharmont, & Godin, 2015), and reptiles (red‐eared slider turtles, Trachemys scripta, Carter, Paitz, McGhee, & Bowden, 2016). Phenotypic correlations among traits were substantially influenced by cohort identity, which suggest that the emergence of behavior‐endocrine syndromes in coyote pups is attributed to litter identity (Table 4). Correlated phenotypic suites of behavioral and endocrine traits are important because they often covary with ecological conditions, and thus are sensitive to correlational selection (Bell, 2007; Dingemanse & Réale, 2005; Miles, Sinervo, Hazard, Svensson, & Costa, 2007). Moreover, covariation among personalities and endocrine response underscore the mechanistic and functional links between the two (Boulton et al., 2015; Carere, Caramaschi, & Fawcett, 2010; Miranda, 2017; Taff & Vitousek, 2016). Our study adds to a nascent but growing anthology suggesting that personality differences in fear toward humans is mediated by endocrine function (Bonier, 2012; Rebolo‐Ifran et al., 2015). In addition, our results support evidence from previous studies suggesting that variation in the social environment during development can affect individual phenotype in social organisms (Blumstein, Fuong, & Palmer, 2017; McCowan, Mainwaring, Prior, & Griffith, 2015; Montiglio et al., 2013).
The expression of personality traits can occasionally differ amongst wild and captive individuals (Mason et al., 2013; Niemelä & Dingemanse, 2014), although recent evidence suggests personality in captivity reflects personality in wild settings (Fisher, James, Rodríguez‐Muñoz, & Tregenza, 2015; Herborn, Heidinger, Alexander, & Arnold, 2014). Captivity is generally safer with positive experiences of humans (e.g., via enrichment), whereas even in urban or protected natural settings, the potential for lethal removal still exists (Clinchy et al., 2016; Smith et al., 2017). As a result, the observed levels of risk‐taking in our population may be more exaggerated compared with that of conspecifics in urban or protected natural areas. An individual coyote's perception of risk within the captive environment may also contribute to differences in risk‐taking amongst wild and captive populations. Recent work in other species has shown that urban organisms can identify individual humans (Levey et al., 2009) and exhibit behavioral plasticity when humans diverge from a predictable behavioral pattern (Bateman & Fleming, 2014). Anecdotal evidence suggests coyotes similarly learn to identify individual humans (C. J. Schell pers. obs.), and modify their behavior according to variance in human activity (Schultz & Young, 2018; Séquin, Jaeger, Brussard, & Barrett, 2003; Smith et al., 2018). We may therefore predict that the specific person administering the foraging assay paired with unpredictable human behavior should induce plasticity in risk‐taking. Future work should compare risk‐taking both in wild and captive coyote systems, across a gradient of threat, to help elucidate how variation in anthropogenic disturbance regimes contribute to variance in habituation rates over time.
To conclude, the relationship among parent and offspring can dictate how future generations will navigate predicted ecological conditions (Badyaev & Uller, 2009; Duckworth et al., 2015; Wolf et al., 1998). The predictability of environmental cues and the transmission of those cues to offspring are particularly intriguing in an anthropogenic context, in which parents modify their phenotype according to human influence. As a result, we may expect that parents with accumulating anthropogenic experiences over multiple reproductive bouts may produce offspring that have an optimal phenotype suited to environments with increased human densities. Our results provide evidence to suggest that parental effects reduce fear in anthropogenic settings within as little as two generations. This is likely due to strong parental influence, in which parental habituation level is an ever‐present cue that offspring use to modify their fear responses toward humans. It remains unclear, however, whether reductions in fear of humans have fitness consequences for developing coyotes in the wild, as is the case for other taxa (De Meester et al., 2018; Hall et al., 2015). In addition, it is still uncertain whether such traits observed during development are consistent across multiple life stages. Future work addressing the stability of pup traits across life stages is critical to determining the importance of early developmental experiences and parental effects on individual fitness.
CONFLICT OF INTEREST
The authors do not have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
C.J.S. conceived the research, conducted field work, analyzed data, created figures, and wrote the manuscript. J.K.Y. conceived the research, conducted field work, provided comments on the manuscript, and provided funding. E.V.L. conceived the research and provided comments on the manuscript. R.M.S. conceived the research, assisted in endocrine lab work, provided comments on the manuscript and provided funding. J.M.M. conceived the research and provided comments on the manuscript.
DATA ACCESSIBILITY
The datasets used for these analyses, as well as the R script used to perform the analyses, are deposited in the Dryad Digital Repository as https://doi.org/10.5061/dryad.pr00820.
Supporting information
ACKNOWLEDGEMENTS
We thank Bruce Patterson, Trevor Price, Stacey Brummer, Jeff Schultz, Mike Davis, Erika Stevenson, and members of the Mateo and Santymire laboratories for their input in project development and analyses, as well as on grounds assistance with study animals. Special thanks to Danielle Schell, Brandon Flack, and other volunteers for providing data collection assistance, behavioral observations, and laboratory analyses. This work was supported by two University of Chicago Hinds Fund fellowships (awarded to C.J.S.), a GAANN Fellowship from the University of Chicago (awarded to C.J.S.), the United Negro College Fund (UNCF)‐Merck Fellowship (awarded to C.J.S.), and the NSF Graduate Research Fellowship (awarded to C.J.S.). This research was also supported in part by the intramural research program of the U.S. Department of Agriculture, National Wildlife Research Center. Institutional Animal Care and Use Committees (IACUC) at the University of Chicago (protocol no. 72184), the NWRC (protocol no. QA‐1818), and the Lincoln Park Zoo Research Committee approved this study.
Schell CJ, Young JK, Lonsdorf EV, Santymire RM, Mateo JM. Parental habituation to human disturbance over time reduces fear of humans in coyote offspring. Ecol Evol. 2018;8:12965–12980. 10.1002/ece3.4741
REFERENCES
- Acker, M. , Mastromonaco, G. , & Schulte‐Hostedde, A. I. (2018). The effects of body region, season and external arsenic application on hair cortisol concentration. Conservation Physiology, 6(1), 1–9. 10.1093/conphys/coy037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya‐Ajoy, Y. G. , & Dingemanse, N. J. (2017). Repeatability, heritability, and age‐dependence of seasonal plasticity in aggressiveness in a wild passerine bird. Journal of Animal Ecology, 86(2), 227–238. 10.1111/1365-2656.12621. [DOI] [PubMed] [Google Scholar]
- Badyaev, A. V. , & Uller, T. (2009). Parental effects in ecology and evolution: Mechanisms, processes and implications. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1520), 1169–1177. 10.1098/rstb.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, L. P. , Stanton, L. , & Benson‐Amram, S. (2018). The cognition of ‘nuisance’ species. Animal Behaviour, 10.1016/j.anbehav.2018.05.005. [DOI] [Google Scholar]
- Bartoń, K. (2018). MuMIn: Multi‐model inference. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://cran.r-project.org/web/packages/MuMIn/index.html [Google Scholar]
- Bateman, P. W. , & Fleming, P. A. (2014). Does human pedestrian behaviour influence risk assessment in a successful mammal urban adapter? Journal of Zoology, 294(2), 93–98. 10.1111/jzo.12156. [DOI] [Google Scholar]
- Bekoff, M. , & Wells, M. C. (1982). Behavioral ecology of coyotes: Social organization, rearing patterns, space use, and resource defense. Zeitschrift Für Tierpsychologie, 60(4), 281–305. 10.1111/j.1439-0310.1982.tb01087.x. [DOI] [Google Scholar]
- Bekoff, M. , & Wells, M. C. (1986). Social ecology and behavior of coyotes. Advances in the Study of Behavior, 16(C), 251–338. 10.1016/S0065-3454(08)60193-X. [DOI] [Google Scholar]
- Bell, A. M. (2007). Future directions in behavioural syndromes research. Proceedings of the Royal Society B: Biological Sciences, 274(1611), 755–761. 10.1098/rspb.2006.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard, M. F. , & McCauley, S. J. (2008). Integrating across life‐history stages: consequences of natal habitat effects on dispersal. The American Naturalist, 171(5), 553–567. 10.1086/587072. [DOI] [PubMed] [Google Scholar]
- Benson, J. F. , Mills, K. J. , Loveless, K. M. , & Patterson, B. R. (2013). Genetic and environmental influences on pup mortality risk for wolves and coyotes within a Canis hybrid zone. Biological Conservation, 166, 133–141. 10.1016/j.biocon.2013.06.018. [DOI] [Google Scholar]
- Blackwell, B. F. , DeVault, T. L. , Fernández‐Juricic, E. , Gese, E. M. , Gilbert‐Norton, L. , & Breck, S. W. (2016). No single solution: Application of behavioural principles in mitigating human–wildlife conflict. Animal Behaviour, 120, 245–254. 10.1016/j.anbehav.2016.07.013. [DOI] [Google Scholar]
- Blumstein, D. T. (2006). Developing an evolutionary ecology of fear: How life history and natural history traits affect disturbance tolerance in birds. Animal Behaviour, 71(2), 389–399. 10.1016/j.anbehav.2005.05.010. [DOI] [Google Scholar]
- Blumstein, D. T. , Fuong, H. , & Palmer, E. (2017). Social security: Social relationship strength and connectedness influence how marmots respond to alarm calls. Behavioral Ecology and Sociobiology, 71(10), 145 10.1007/s00265-017-2374-5. [DOI] [Google Scholar]
- Bókony, V. , Kulcsár, A. , Tóth, Z. , & Liker, A. (2012). Personality traits and behavioral syndromes in differently urbanized populations of house sparrows (Passer domesticus). PLoS ONE, 7(5), 10.1371/journal.pone.0036639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonduriansky, R. , & Day, T. (2009). Nongenetic inheritance and its evolutionary implications. Annual Review of Ecology, Evolution, and Systematics, 40(1), 103–125. 10.1146/annurev.ecolsys.39.110707.173441. [DOI] [Google Scholar]
- Bonier, F. (2012). Hormones in the city: Endocrine ecology of urban birds. Hormones and Behavior, 61(5), 763–772. 10.1016/j.yhbeh.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Boulton, K. , Couto, E. , Grimmer, A. J. , Earley, R. L. , Canario, A. V. M. , Wilson, A. J. , & Walling, C. A. (2015). How integrated are behavioral and endocrine stress response traits? A repeated measures approach to testing the stress‐coping style model. Ecology and Evolution, 5(3), 618–633. 10.1002/ece3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton, K. , Grimmer, A. J. , Rosenthal, G. G. , Walling, C. A. , & Wilson, A. J. (2014). How stable are personalities? A multivariate view of behavioural variation over long and short timescales in the sheepshead swordtail. Xiphophorus Birchmanni. Behavioral Ecology and Sociobiology, 68(5), 791–803. 10.1007/s00265-014-1692-0. [DOI] [Google Scholar]
- Brommer, J. E. , Karell, P. , Ahola, K. , & Karstinen, T. (2014). Residual correlations, and not individual properties, determine a nest defense boldness syndrome. Behavioral Ecology, 25(4), 802–812. 10.1093/beheco/aru057. [DOI] [Google Scholar]
- Burgess, S. C. , & Marshall, D. J. (2014). Adaptive parental effects: The importance of estimating environmental predictability and offspring fitness appropriately. Oikos, 123(7), 769–776. 10.1111/oik.01235. [DOI] [Google Scholar]
- Careau, V. , Réale, D. , Humphries, M. M. , & Thomas, D. W. (2010). The pace of life under artificial selection: personality, energy expenditure, and longevity are correlated in domestic dogs. The American Naturalist, 175(6), 753–758. 10.1086/652435. [DOI] [PubMed] [Google Scholar]
- Carere, C. , Caramaschi, D. , & Fawcett, T. W. (2010). Covariation between personalities and individual differences in coping with stress: Converging evidence and hypotheses cal and behavioural responses to stress: A review of the evidence across vertebrates. Current Zoology, 56(6), 728–741. [Google Scholar]
- Carere, C. , Drent, P. J. , Koolhaas, J. M. , & Groothuis, T. G. G. (2005). Epigenetic effects on personality traits: Early food provisioning and sibling competition. Behaviour, 142(9), 1329–1355. 10.1163/156853905774539328. [DOI] [Google Scholar]
- Carlitz, E. H. D. , Kirschbaum, C. , Stalder, T. , & van Schaik, C. P. (2014). Hair as a long‐term retrospective cortisol calendar in orang‐utans (Pongo spp.): New perspectives for stress monitoring in captive management and conservation. General and Comparative Endocrinology, 195, 151–156. 10.1016/j.ygcen.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Carrete, M. , Martínez‐Padilla, J. , Rodríguez‐Martínez, S. , Rebolo‐Ifrán, N. , Palma, A. , & Tella, J. L. (2016). Heritability of fear of humans in urban and rural populations of a bird species. Scientific Reports, 6(1), 31060 10.1038/srep31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrete, M. , & Tella, J. L. (2011). Inter‐individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS ONE, 6(4), e18859 10.1371/journal.pone.0018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrete, M. , & Tella, J. L. (2013). High individual consistency in fear of humans throughout the adult lifespan of rural and urban burrowing owls. Scientific Reports, 3(1), 3524 10.1038/srep03524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrete, M. , & Tella, J. L. (2017). Behavioral correlations associated with fear of humans differ between rural and urban burrowing owls. Frontiers in Ecology and Evolution, 5(May), 1–9. 10.3389/fevo.2017.00054. [DOI] [Google Scholar]
- Carter, A. W. , Paitz, R. T. , McGhee, K. E. , & Bowden, R. M. (2016). Turtle hatchlings show behavioral types that are robust to developmental manipulations. Physiology and Behavior, 155, 46–55. 10.1016/j.physbeh.2015.11.034. [DOI] [PubMed] [Google Scholar]
- Champagne, F. A. (2008). Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in Neuroendocrinology, 29(3), 386–397. 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne, F. A. , & Curley, J. P. (2009). The Trans‐Generational Influence of Maternal Care on Offspring Gene Expression and Behavior in Rodents In Maestripieri D., & Mateo J. (Eds.), Maternal effects in mammals, 1st ed. (pp. 182–202). Chicago, IL: University of Chicago Press. [Google Scholar]
- Charmantier, A. , Demeyrier, V. , Lambrechts, M. , Perret, S. , & Grégoire, A. (2017). Urbanization is associated with divergence in pace‐of‐life in great tits. Frontiers in Ecology and Evolution, 5, 10.3389/fevo.2017.00053. [DOI] [Google Scholar]
- Clinchy, M. , Zanette, L. Y. , Roberts, D. , Suraci, J. P. , Buesching, C. D. , Newman, C. , & Macdonald, D. W. (2016). Fear of the human “super predator” far exceeds the fear of large carnivores in a model mesocarnivore. Behavioral Ecology, 284(1857), arw117 10.1093/beheco/arw117. [DOI] [Google Scholar]
- Cole, E. F. , & Quinn, J. L. (2014). Shy birds play it safe: Personality in captivity predicts risk responsiveness during reproduction in the wild. Biology Letters, 10(5), 8–11. 10.1098/rsbl.2014.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, M. O. , Weaver, M. J. , Hutton, P. , & McGraw, K. J. (2017). The effects of urbanization and human disturbance on problem solving in juvenile house finches (Haemorhous mexicanus). Behavioral Ecology and Sociobiology, 71(5), 85 10.1007/s00265-017-2304-6. [DOI] [Google Scholar]
- Crean, A. J. , Dwyer, J. M. , & Marshall, D. J. (2013). Adaptive paternal effects? Experimental evidence that the paternal environment affects offspring performance. Ecology, 94(11), 2575–2582. 10.1890/13-0184.1. [DOI] [PubMed] [Google Scholar]
- Crino, O. L. , Prather, C. T. , Driscoll, S. C. , Good, J. M. , & Breuner, C. W. (2014). Developmental stress increases reproductive success in male zebra finches. Proceedings of the Royal Society B: Biological Sciences, 281(1795), 20141266–20141266. 10.1098/rspb.2014.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammhahn, M. , & Almeling, L. (2012). Is risk taking during foraging a personality trait? A field test for cross‐context consistency in boldness. Animal Behaviour, 84(5), 131–1139. 10.1016/j.anbehav.2012.08.014. [DOI] [Google Scholar]
- Dantzer, B. , Newman, A. E. M. , Boonstra, R. , Palme, R. , Boutin, S. , Humphries, M. M. , & McAdam, A. G. (2013). Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science, 340(6137), 1215–1217. 10.1126/science.1235765. [DOI] [PubMed] [Google Scholar]
- Darrow, P. A. , & Shivik, J. A. (2009). Bold, shy, and persistent: Variable coyote response to light and sound stimuli. Applied Animal Behaviour Science, 116(1), 82–87. 10.1016/j.applanim.2008.06.013 [DOI] [Google Scholar]
- Dawson, S. , & Jaeger, M. M. (2009). Cognitive inference and behavioral syndromes in the coyote (Canis latrans). Journal of Veterinary Behavior: Clinical Applications and Research, 4(2), 67–68. 10.1016/j.jveb.2008.09.005. [DOI] [Google Scholar]
- De Meester, G. , Lambreghts, Y. , Briesen, B. , Smeuninx, T. , Tadić, Z. , & Van Damme, R. (2018). Hunt or hide: How insularity and urbanization affect foraging decisions in lizards. Ethology, 124(4), 227–235. 10.1111/eth.12722. [DOI] [Google Scholar]
- de Villemereuil, P. (2012). Estimation of a biological trait heritability using the animal model. How to Use the MCMCglmm R Package, 1–36. Retrieved from http://devillemereuil.legtux.org/wp-content/uploads/2012/12/tuto_en.pdf
- Debeffe, L. , Lemaître, J. F. , Bergvall, U. A. , Hewison, A. J. M. , Gaillard, J. M. , Morellet, N. , … Kjellander, P. (2015). Short‐ and long‐term repeatability of docility in the roe deer: Sex and age matter. Animal Behaviour, 109, 53–63. 10.1016/j.anbehav.2015.08.003 [DOI] [Google Scholar]
- Dettmer, A. M. , Rosenberg, K. L. , Suomi, S. J. , Meyer, J. S. , & Novak, M. A. (2015). Associations between parity, hair hormone profiles during pregnancy and lactation, and infant development in rhesus monkeys (Macaca mulatta). PLoS ONE, 10(7), e0131692 10.1371/journal.pone.0131692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse, N. J. , & Araya‐Ajoy, Y. G. (2015). Interacting personalities: Behavioural ecology meets quantitative genetics. Trends in Ecology and Evolution, 30(2), 88–97. 10.1016/j.tree.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Dingemanse, N. J. , & Réale, D. (2005). Natural selection and animal personality. Behaviour, 142(9), 1159–1184. 10.1163/156853905774539445 [DOI] [Google Scholar]
- Ditchkoff, S. S. , Saalfeld, S. T. , & Gibson, C. J. (2006). Animal behavior in urban ecosystems: Modifications due to human‐induced stress. Urban Ecosystems, 9(1), 5–12. 10.1007/s11252-006-3262-3 [DOI] [Google Scholar]
- Dochtermann, N. A. , & Roff, D. A. (2010). Applying a quantitative genetics framework to behavioural syndrome research. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1560), 4013–4020. 10.1098/rstb.2010.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosmann, A. J. , Brooks, K. C. , & Mateo, J. M. (2015). Within‐individual correlations reveal link between a behavioral syndrome, condition, and cortisol in free‐ranging belding’s ground squirrels. Ethology, 121(2), 125–134. 10.1111/eth.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez, S. , Audet, J. N. , Rodriguez, J. R. , Kayello, L. , & Lefebvre, L. (2017). Innovativeness and the effects of urbanization on risk‐taking behaviors in wild Barbados birds. Animal Cognition, 20(1), 33–42. 10.1007/s10071-016-1007-0. [DOI] [PubMed] [Google Scholar]
- Duckworth, R. A. , Belloni, V. , & Anderson, S. R. (2015). Cycles of species replacement emerge from locally induced maternal effects on offspring behavior in a passerine bird. Science, 347(6224), 875–877. 10.1126/science.1260154. [DOI] [PubMed] [Google Scholar]
- English, S. , Bateman, A. W. , Mares, R. , Ozgul, A. , & Clutton‐Brock, T. H. (2014). Maternal, social and abiotic environmental effects on growth vary across life stages in a cooperative mammal. Journal of Animal Ecology, 83(2), 332–342. 10.1111/1365-2656.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks, L. A. , Jorgensen, M. J. , Bailey, J. N. , Breidenthal, S. E. , Grzywa, R. , & Laudenslager, M. L. (2011). Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology, 36(8), 1201–1208. 10.1016/j.psyneuen.2011.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress, J. C. , Ryon, J. , & McLeod, P. J. (1987). Coyote adult–pup interactions in the first 3 months. Canadian Journal of Zoology, 65(3), 760–763. 10.1139/z87-118 [DOI] [Google Scholar]
- Fisher, D. N. , James, A. , Rodríguez‐Muñoz, R. , & Tregenza, T. (2015). Behaviour in captivity predicts some aspects of natural behaviour, but not others, in a wild cricket population. Proceedings of the Royal Society B: Biological Sciences, 282(1809), 10.1098/rspb.2015.0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest, A. R. , Dender, M. G. E. , Pitcher, T. E. , & Semeniuk, C. A. D. (2017). The effects of paternal reproductive tactic and rearing environment on juvenile variation in growth as mediated through aggression and foraging behaviours of Chinook salmon (Oncorhynchus tshawytscha). Ethology, 123(5), 329–341. 10.1111/eth.12601 [DOI] [Google Scholar]
- Fox, J. , & Bouchet‐Valat, M. (2018). Rcmdr: R Commander. Retrieved from http://socserv.socsci.mcmaster.ca/jfox/Misc/Rcmdr/
- Galhardo, L. , Vitorino, A. , & Oliveira, R. F. (2012). Social familiarity modulates personality trait in a cichlid fish. Biology Letters, 8(6), 936–938. 10.1098/rsbl.2012.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gese, E. M. , Roberts, B. M. , & Knowlton, F. F. (2016). Nutritional effects on reproductive performance of captive adult female coyotes (Canis latrans). Animal Reproduction Science, 165, 69–75. 10.1016/j.anireprosci.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Gilbert‐Norton, L. B. , Leaver, L. A. , & Shivik, J. A. (2009). The effect of randomly altering the time and location of feeding on the behaviour of captive coyotes (Canis latrans). Applied Animal Behaviour Science, 120(3–4), 179–185. 10.1016/j.applanim.2009.06.007 [DOI] [Google Scholar]
- Golla, W. , Hofer, H. , & East Marion, L. (1999). Within‐litter sibling aggression in spotted hyaenas: Effect of maternal nursing, sex and age. Animal Behaviour, 58(4), 715–726. 10.1006/anbe.1999.1189 [DOI] [PubMed] [Google Scholar]
- Greenberg, J. R. , & Holekamp, K. E. (2017). Human disturbance affects personality development in a wild carnivore. Animal Behaviour, 132, 303–312. 10.1016/j.anbehav.2017.08.023 [DOI] [Google Scholar]
- Greggor, A. L. , Clayton, N. S. , Fulford, A. J. C. , & Thornton, A. (2016). Street smart: Faster approach towards litter in urban areas by highly neophobic corvids and less fearful birds. Animal Behaviour, 117, 123–133. 10.1016/j.anbehav.2016.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield, J. D. (2010). MCMC methods for multi‐response generalised linear mixed models. Journal of Statistical Software, 33(2), 1–22. Retrieved from http://ftp5.gwdg.de/pub/misc/cran/web/packages/MCMCglmm/vignettes/Overview.pdf 20808728 [Google Scholar]
- Hadfield, J. D. , & Nakagawa, S. (2010). General quantitative genetic methods for comparative biology: Phylogenies, taxonomies and multi‐trait models for continuous and categorical characters. Journal of Evolutionary Biology, 23(3), 494–508. 10.1111/j.1420-9101.2009.01915.x [DOI] [PubMed] [Google Scholar]
- Hafer, N. , Ebil, S. , Uller, T. , & Pike, N. (2011). Transgenerational effects of food availability on age at maturity and reproductive output in an asexual collembolan species. Biology Letters, 7(5), 755–758. 10.1098/rsbl.2011.0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, M. L. , van Asten, T. , Katsis, A. C. , Dingemanse, N. J. , Magrath, M. J. L. , & Mulder, R. A. (2015). Animal personality and pace‐of‐life syndromes: Do fast‐exploring fairy‐wrens die young? Frontiers in Ecology and Evolution, 3(March), 1–14. 10.3389/fevo.2015.00028 [DOI] [Google Scholar]
- Hennessy, C. A. , Dubach, J. , Gehrt, S. D. , Resources, N. , & Resources, N. (2012). Long‐term pair bonding and genetic evidence for monogamy among urban coyotes (Canis latrans). Journal of Mammalogy, 93(3), 732–742. 10.1644/11-MAMM-A-184.1 [DOI] [Google Scholar]
- Herborn, K. A. , Heidinger, B. J. , Alexander, L. , & Arnold, K. E. (2014). Personality predicts behavioral flexibility in a fluctuating, natural environment. Behavioral Ecology, 25(6), 1374–1379. 10.1093/beheco/aru131 [DOI] [Google Scholar]
- Herborn, K. A. , Macleod, R. , Miles, W. T. S. , Schofield, A. N. B. , Alexander, L. , & Arnold, K. E. (2010). Personality in captivity reflects personality in the wild. Animal Behaviour, 79(4), 835–843. 10.1016/j.anbehav.2009.12.026 [DOI] [Google Scholar]
- Hinde, K. , Skibiel, A. L. , Foster, A. B. , Del Rosso, L. , Mendoza, S. P. & Capitanio, J. P. (2014). Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology, 26, 269–281. 10.1093/beheco/aru186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. , & Trillmich, F. (2007). Sibling competition and cooperation in mammals: Challenges, developments and prospects. Behavioral Ecology and Sociobiology, 62(3), 299–307. 10.1007/s00265-007-0417-z [DOI] [Google Scholar]
- Kanda, L. L. , Louon, L. , & Straley, K. (2012). Stability in activity and boldness across time and context in captive siberian dwarf hamsters. Ethology, 118(6), 518–533. 10.1111/j.1439-0310.2012.02038.x [DOI] [Google Scholar]
- Kapoor, A. , Lubach, G. R. , Ziegler, T. E. , & Coe, C. L. (2016). Hormone levels in neonatal hair reflect prior maternal stress exposure during pregnancy. Psychoneuroendocrinology, 66, 111–117. 10.1016/j.psyneuen.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemme, K. , Kaiser, S. , & Sachser, N. (2007). Prenatal maternal programming determines testosterone response during social challenge. Hormones and Behavior, 51(3), 387–394. 10.1016/j.yhbeh.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Kimball, B. A. , Johnston, J. J. , Mason, J. R. , Zemlicka, D. E. , & Blom, F. S. (2000). Development of chemical coyote attractants for wildlife management applications In Salmon T. P., & Crabb A. C. (Eds.), 19th Vertebrate Pest Conference (pp. 304–309). San Diego, CA: University of California, Davis. [Google Scholar]
- Kimball, B. A. , Mason, J. R. , Blom, F. S. , Johnston, J. J. , & Zemlicka, D. E. (2000). Development and testing of seven new synthetic coyote attractants. Journal of Agricultural and Food Chemistry, 48(5), 1892–1897. 10.1021/jf990648z. [DOI] [PubMed] [Google Scholar]
- Kitchen, A. M. , & Knowlton, F. F. (2006). Cross‐fostering in coyotes: Evaluation of a potential conservation and research tool for canids. Biological Conservation, 129(2), 221–225. 10.1016/j.biocon.2005.10.036. [DOI] [Google Scholar]
- Laskowski, K. L. , & Bell, A. M. (2014). Strong personalities, not social niches, drive individual differences in social behaviours in sticklebacks. Animal Behaviour, 90, 287–295. 10.1016/j.anbehav.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager, M. L. , Jorgensen, M. J. , & Fairbanks, L. A. (2012). Developmental patterns of hair cortisol in male and female nonhuman primates: Lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology, 37(10), 1736–1739. 10.1016/j.psyneuen.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey, D. J. , Londono, G. A. , Ungvari‐Martin, J. , Hiersoux, M. R. , Jankowski, J. E. , Poulsen, J. R. , … Robinson, S. K. (2009). Urban mockingbirds quickly learn to identify individual humans. Proceedings of the National Academy of Sciences, 106(22), 8959–8962. 10.1073/pnas.0811422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, O. P. , Mcgowan, P. O. , & Sheriff, M. J. (2013). Maternal adversity and ecological stressors in natural populations: The role of stress axis programming in individuals, with implications for populations and communities. Functional Ecology, 27(1), 81–92. 10.1111/j.1365-2435.2012.02040.x [DOI] [Google Scholar]
- Lowry, H. , Lill, A. , & Wong, B. B. M. (2013). Behavioural responses of wildlife to urban environments. Biological Reviews, 88(3), 537–549. 10.1111/brv.12012 [DOI] [PubMed] [Google Scholar]
- Maestripieri, D. , & Mateo, J. (2009). The role of maternal effects in mammalian evolution and adaptation In Maestripieri D., & Mateo J. (Eds.), Maternal effects in mammals, 1st ed. (pp. 1–10). Chicago, IL: The University of Chicago Press. [Google Scholar]
- Margulis, S. W. , Nabong, M. , Alaks, G. , Walsh, A. , & Lacy, R. C. (2005). Effects of early experience on subsequent parental behaviour and reproductive success in oldfield mice, Peromyscus polionotus . Animal Behaviour, 69(1996), 627–634. 10.1016/j.anbehav.2004.04.021 [DOI] [Google Scholar]
- Marshall, D. J. (2008). Transgenerational plasticity in the sea: Context‐dependent maternal effects across the life history. Ecology, 89(2), 418–427. 10.1890/07-0449.1 [DOI] [PubMed] [Google Scholar]
- Marshall, D. J. , & Keough, M. J. (2004). When the going gets rough: Effect of maternal size manipulation on larval quality. Marine Ecology Progress Series, 272, 301–305. 10.3354/meps272301 [DOI] [Google Scholar]
- Marshall, D. J. , & Uller, T. (2007). When is a maternal effect adaptive? Oikos, 116(12), 1957–1963. 10.1111/j.2007.0030-1299.16203.x [DOI] [Google Scholar]
- Martin, J. G. A. , & Réale, D. (2008). Temperament, risk assessment and habituation to novelty in eastern chipmunks. Tamias Striatus. Animal Behaviour, 75(1), 309–318. 10.1016/j.anbehav.2007.05.026. [DOI] [Google Scholar]
- Mason, G. , Burn, C. C. , Dallaire, J. A. , Kroshko, J. , McDonald Kinkaid, H. , & Jeschke, J. M. (2013). Plastic animals in cages: Behavioural flexibility and responses to captivity. Animal Behaviour, 85(5), 1113–1126. 10.1016/j.anbehav.2013.02.002. [DOI] [Google Scholar]
- Mazué, G. P. F. , Dechaume‐Moncharmont, F. X. , & Godin, J. G. J. (2015). Boldness‐exploration behavioral syndrome: Interfamily variability and repeatability of personality traits in the young of the convict cichlid (Amatitlania siquia). Behavioral Ecology, 26(3), 900–908. 10.1093/beheco/arv030. [DOI] [Google Scholar]
- McCowan, L. S. C. , Mainwaring, M. C. , Prior, N. H. , & Griffith, S. C. (2015). Personality in the wild zebra finch: Exploration, sociality, and reproduction. Behavioral Ecology, 26(3), 735–746. 10.1093/beheco/aru239 [DOI] [Google Scholar]
- Messier, F. , & Barrette, C. (1982). The social system of the coyote (Canis latrans) in a forested habitat. Canadian Journal of Zoology, 60(7), 1743–1753. 10.1139/z82-227. [DOI] [Google Scholar]
- Meyer, J. S. , & Novak, M. A. (2012). Minireview: Hair cortisol: A novel biomarker of hypothalamic‐pituitary‐ adrenocortical activity. Endocrinology, 153(9), 4120–4127. 10.1210/en.2012-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan, S. , Miles, D. B. , & Clobert, J. (2012). Hormonally mediated maternal effects, individual strategy and global change. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1596), 1647–1664. 10.1098/rstb.2012.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles, D. B. , Sinervo, B. , Hazard, L. C. , Svensson, E. I. , & Costa, D. (2007). Relating endocrinology, physiology and behaviour using species with alternative mating strategies. Functional Ecology, 21, 653–665. 10.1111/j.1365-2435.2007.01304.x [DOI] [Google Scholar]
- Miranda, A. C. (2017). Mechanisms of behavioural change in urban animals: The role of microevolution and phenotypic plasticity In Murgui E., & Hedblom M. (Eds.), Ecology and conservation of birds in urban environments (pp. 113–132). Cham, Switzerland: Springer International Publishing; 10.1007/978-3-319-43314-1_7 [DOI] [Google Scholar]
- Miranda, A. C. , Schielzeth, H. , Sonntag, T. , & Partecke, J. (2013). Urbanization and its effects on personality traits: A result of microevolution or phenotypic plasticity? Global Change Biology, 19(9), 2634–2644. 10.1111/gcb.12258. [DOI] [PubMed] [Google Scholar]
- Moll, R. J. , Cepek, J. D. , Lorch, P. D. , Dennis, P. M. , Robison, T. , Millspaugh, J. J. , & Montgomery, R. A. (2018). Humans and urban development mediate the sympatry of competing carnivores. Urban Ecosystems, 1–14, 10.1007/s11252-018-0758-6. [DOI] [Google Scholar]
- Montiglio, P.‐O. , Ferrari, C. , & Reale, D. (2013). Social niche specialization under constraints: Personality, social interactions and environmental heterogeneity. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1618), 20120343–20120343. 10.1098/rstb.2012.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau, T. A. (1998). The adaptive significance of maternal effects. Trends in Ecology & Evolution, 13(10), 403–407. 10.1016/S0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Mousseau, T. A. , Uller, T. , Wapstra, E. , & Badyaev, A. V. (2009). Evolution of maternal effects: Past and present. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1520), 1035–1038. 10.1098/rstb.2008.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, M. H. , Edwards, M. A. , Abercrombie, B. , & St. Clair, C. C. (2015). Poor health is associated with use of anthropogenic resources in an urban carnivore. Proceedings of the Royal Society B: Biological Sciences, 282(1806), 20150009 10.1098/rspb.2015.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. , & Schielzeth, H. (2010). Repeatability for Gaussian and non‐Gaussian data: A practical guide for biologists. Biological Reviews, 85(4), 935–956. 10.1111/j.1469-185X.2010.00141.x [DOI] [PubMed] [Google Scholar]
- Niemelä, P. T. , & Dingemanse, N. J. (2014). Artificial environments and the study of “adaptive” personalities. Trends in Ecology and Evolution, 29(5), 245–247. 10.1016/j.tree.2014.02.007 [DOI] [PubMed] [Google Scholar]
- O’Connor, C. M. , Norris, D. R. , Crossin, G. T. , & Cooke, S. J. (2014). Biological carryover effects: Linking common concepts and mechanisms in ecology and evolution. Ecosphere, 5(3), art28 10.1890/ES13-00388.1 [DOI] [Google Scholar]
- Patrick, S. C. , Charmantier, A. , & Weimerskirch, H. (2013). Differences in boldness are repeatable and heritable in a long‐lived marine predator. Ecology and Evolution, 3(13), 4291–4299. 10.1002/ece3.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick, S. C. , & Weimerskirch, H. (2015). Senescence rates and late adulthood reproductive success are strongly influenced by personality in a long‐lived seabird. Proceedings of the Royal Society B: Biological Sciences, 282(1799), 20141649–20141649. 10.1098/rspb.2014.1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perals, D. , Griffin, A. S. , Bartomeus, I. , & Sol, D. (2017). Revisiting the open‐field test: What does it really tell us about animal personality? Animal Behaviour, 123, 69–79. 10.1016/j.anbehav.2016.10.006 [DOI] [Google Scholar]
- Petelle, M. B. , Martin, J. G. A. , & Blumstein, D. T. (2015). Heritability and genetic correlations of personality traits in a wild population of yellow‐bellied marmots (Marmota flaviventris). Journal of Evolutionary Biology, 28(10), 1840–1848. 10.1111/jeb.12700 [DOI] [PubMed] [Google Scholar]
- Petelle, M. B. , McCoy, D. E. , Alejandro, V. , Martin, J. G. A. , & Blumstein, D. T. (2013). Development of boldness and docility in yellow‐bellied marmots. Animal Behaviour, 86(6), 1147–1154. 10.1016/j.anbehav.2013.09.016 [DOI] [Google Scholar]
- Plaistow, S. J. , & Benton, T. (2009). The influence of context‐dependent maternal effects on population dynamics: An experimental test. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1520), 1049–1058. 10.1098/rstb.2008.0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaistow, S. J. , St. Clair, J. J. H. , Grant, J. , & Benton, T. G. (2007). How to put all your eggs in one basket: empirical patterns of offspring provisioning throughout a mother’s lifetime. The American Naturalist, 170(4), 520–529. 10.1086/521238 [DOI] [PubMed] [Google Scholar]
- Poessel, S. A. , Gese, E. M. , & Young, J. K. (2017). Environmental factors influencing the occurrence of coyotes and conflicts in urban areas. Landscape and Urban Planning, 157, 259–269. 10.1016/j.landurbplan.2016.05.022 [DOI] [Google Scholar]
- Pooley, C. M. , & Marion, G. (2018). Bayesian model evidence as a practical alternative to deviance information criterion. Royal Society Open Science, 5(3), 171519 10.1098/rsos.171519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.r-project.org/ [Google Scholar]
- Rebolo‐Ifran, N. , Carrete, M. , Sanz‐Aguilar, A. , Rodriguez‐Martinez, S. , Cabezas, S. , Marchant, T. A. , … Tella, J. L. (2015). Links between fear of humans, stress and survival support a non‐random distribution of birds among urban and rural habitats. Scientific Reports, 5(1), 13723 10.1038/srep13723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks, B. N. , & Neale, J. C. C. (2001). Does paternal care of pups benefit breeding female coyotes? Southwestern Association of Naturalists, 46(1), 121–126. 10.2307/3672388 [DOI] [Google Scholar]
- Salaberger, T. , Millard, M. , Makarem, S. E. , Möstl, E. , Grünberger, V. , Krametter‐Frötscher, R. , … Palme, R. (2016). Influence of external factors on hair cortisol concentrations. General and Comparative Endocrinology, 233, 73–78. 10.1016/j.ygcen.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Samia, D. S. M. , Nakagawa, S. , Nomura, F. , Rangel, T. F. , & Blumstein, D. T. (2015). Increased tolerance to humans among disturbed wildlife. Nature Communications, 6(1), 8877 10.1038/ncomms9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, J. L. , Stott, I. , Young, A. J. , Vitikainen, E. I. K. , Hodge, S. J. , & Cant, M. A. (2015). The origins of consistent individual differences in cooperation in wild banded mongooses, Mungos mungo. Animal Behaviour, 107, 193–200. 10.1016/j.anbehav.2015.06.022 [DOI] [Google Scholar]
- Schell, C. J. , Young, J. K. , Lonsdorf, E. V. , Mateo, J. M. , & Santymire, R. M. (2016). Olfactory attractants and parity affect prenatal androgens and territoriality of coyote breeding pairs. Physiology & Behavior, 165, 43–54. 10.1016/j.physbeh.2016.06.038 [DOI] [PubMed] [Google Scholar]
- Schell, C. J. , Young, J. K. , Lonsdorf, E. V. , Mateo, J. M. , & Santymire, R. M. (2017). Investigation of techniques to measure cortisol and testosterone concentrations in coyote hair. Zoo Biology, 36(February), 220–225. 10.1002/zoo.21359 [DOI] [PubMed] [Google Scholar]
- Schell, C. J. , Young, J. K. , Lonsdorf, E. V. , Mateo, J. M. , & Santymire, R. M. (2018). It takes two: Evidence for reduced sexual conflict over parental care in a biparental canid. Journal of Mammalogy, 99(1), 75–88. 10.1093/jmammal/gyx150 [DOI] [Google Scholar]
- Schmidt, R. H. , & Timm, R. M. (2007). Bad dogs: Why do coyotes and other canids become unruly? (pp. 287–302). Proceedings of the 12th Wildlife Damage Management Conference [Google Scholar]
- Schultz, J. T. , & Young, J. K. (2018). Behavioral and spatial responses of captive coyotes to human activity. Applied Animal Behaviour Science, 205, 83–88. 10.1016/j.applanim.2018.05.021 [DOI] [Google Scholar]
- Schweitzer, C. , Schwabl, H. , Baran, N. M. , & Adkins‐Regan, E. (2014). Pair disruption in female zebra finches: Consequences for offspring phenotype and sensitivity to a social stressor. Animal Behaviour, 90, 195–204. 10.1016/j.anbehav.2014.01.022. [DOI] [Google Scholar]
- Séquin, E. S. , Jaeger, M. M. , Brussard, P. F. , & Barrett, R. H. (2003). Wariness of coyotes to camera traps relative to social status and territory boundaries. Canadian Journal of Zoology, 81(12), 2015–2025. 10.1139/z03-204 [DOI] [Google Scholar]
- Sheriff, M. J. , Krebs, C. J. , & Boonstra, R. (2010). The ghosts of predators past: Population cycles and the role of maternal programming under fluctuating predation risk. Ecology, 91(10), 2983–2994. 10.1890/09-1108.1 [DOI] [PubMed] [Google Scholar]
- Sheriff, M. J. , Bell, A. , Boonstra, R. , Dantzer, B. , Lavergne, S. G. , McGhee, K. E. , … Love, O. P. (2017). Integrating ecological and evolutionary context in the study of maternal stress. Integrative and Comparative Biology, 7, 1–13. 10.1093/icb/icx105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivik, J. A. , Wilson, R. R. , & Gilbert‐Norton, L. (2011). Will an artificial scent boundary prevent coyote intrusion? Wildlife Society Bulletin, 35(4), 494–497. 10.1002/wsb.68 [DOI] [Google Scholar]
- Smith, J. A. , Suraci, J. P. , Clinchy, M. , Crawford, A. , Roberts, D. , Zanette, L. Y. , & Wilmers, C. C. (2017). Fear of the human ‘super predator’ reduces feeding time in large carnivores. Proceedings of the Royal Society B: Biological Sciences, 284(1857), 20170433 10.1098/rspb.2017.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. A. , Thomas, A. C. , Levi, T. , Wang, Y. , & Wilmers, C. C. (2018). Human activity reduces niche partitioning among three widespread mesocarnivores. Oikos, 10.1111/oik.04592 [DOI] [Google Scholar]
- Sol, D. , Lapiedra, O. , & González‐Lagos, C. (2013). Behavioural adjustments for a life in the city. Animal Behaviour, 85(5), 1101–1112. 10.1016/j.anbehav.2013.01.023 [DOI] [Google Scholar]
- Sol, D. , Maspons, J. , Gonzalez‐Voyer, A. , Morales‐Castilla, I. , Garamszegi, L. Z. , & Møller, A. P. (2018). Risk‐taking behavior, urbanization and the pace of life in birds. Behavioral Ecology and Sociobiology, 72(3) 10.1007/s00265-018-2463-0 [DOI] [Google Scholar]
- Soulsbury, C. D. , & White, P. C. L. (2015). Human‐wildlife interactions in urban areas: A review of conflicts, benefits and opportunities. Wildlife Research, 42(7), 541–553. 10.1071/WR14229 [DOI] [Google Scholar]
- Spiegelhalter, D. J. , Best, N. G. , Carlin, B. P. , & Van Der Linde, A. (2002). Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society. Series B: Statistical Methodology, 64(4), 583–616. 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- Stalder, T. , & Kirschbaum, C. (2012). Analysis of cortisol in hair ‐ State of the art and future directions. Brain, Behavior, and Immunity, 26(7), 1019–1029. 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Stein, L. R. , & Bell, A. M. (2014). Paternal programming in sticklebacks. Animal Behaviour, 95, 165–171. 10.1016/j.anbehav.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, L. R. , & Bell, A. M. (2015). Consistent individual differences in paternal behavior: A field study of three‐spined stickleback. Behavioral Ecology and Sociobiology, 69(2), 227–236. 10.1007/s00265-014-1835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff, C. C. , & Vitousek, M. N. (2016). Endocrine flexibility: Optimizing phenotypes in a dynamic world? Trends in Ecology and Evolution, 31(6), 476–488. 10.1016/j.tree.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Taylor, R. W. , Boon, A. K. , Dantzer, B. , Réale, D. , Humphries, M. M. , Boutin, S. , … McAdam, A. G. (2012). Low heritabilities, but genetic and maternal correlations between red squirrel behaviours. Journal of Evolutionary Biology, 25(4), 614–624. 10.1111/j.1420-9101.2012.02456.x [DOI] [PubMed] [Google Scholar]
- Uchida, K. , Suzuki, K. , Shimamoto, T. , Yanagawa, H. , & Koizumi, I. (2016). Seasonal variation of flight initiation distance in Eurasian red squirrels in urban versus rural habitat. Journal of Zoology, 298(3), 225–231. 10.1111/jzo.12306 [DOI] [Google Scholar]
- Uller, T. (2008). Developmental plasticity and the evolution of parental effects. Trends in Ecology & Evolution, 23(8), 432–438. 10.1016/j.tree.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Uller, T. , Nakagawa, S. , & English, S. (2013). Weak evidence for anticipatory parental effects in plants and animals. Journal of Evolutionary Biology, 26(10), 2161–2170. 10.1111/jeb.12212 [DOI] [PubMed] [Google Scholar]
- Vincze, E. , Papp, S. , Preiszner, B. , Seress, G. , Bókony, V. , & Liker, A. (2016). Habituation to human disturbance is faster in urban than rural house sparrows. Behavioral Ecology, 27(5), 1304–1313. 10.1093/beheco/arw047 [DOI] [Google Scholar]
- Wahaj, S. A. , & Holekamp, K. E. (2006). Functions of sibling aggression in the spotted hyaena. Crocuta Crocuta. Animal Behaviour, 71(6), 1401–1409. 10.1016/j.anbehav.2005.11.011 [DOI] [Google Scholar]
- Wang, Y. , Smith, J. A. , & Wilmers, C. C. (2017). Residential development alters behavior, movement, and energetics in an apex predator, the puma. PLoS ONE, 12(10), 1–17. 10.1371/journal.pone.0184687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way, J. G. , Auger, P. J. , Ortega, I. M. , & Strauss, E. G. (2001). Eastern coyote denning behavior in an anthropogenic environment. Northeast Wildlife, 56, 18–30 [Google Scholar]
- Weaver, I. C. G. , Cervoni, N. , Champagne, F. A. , D’Alessio, A. C. , Sharma, S. , Seckl, J. R. , … Meaney, M. J. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854. 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Wester, V. L. , van der Wulp, N. R. P. , Koper, J. W. , de Rijke, Y. B. , & van Rossum, E. F. C. (2016). Hair cortisol and cortisone are decreased by natural sunlight. Psychoneuroendocrinology, 72, 94–96. 10.1016/j.psyneuen.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Wickman, H. (2009). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer‐Verlag New York; Retrieved from http://ggplot2.org [Google Scholar]
- Wilson, A. J. , Réale, D. , Clements, M. N. , Morrissey, M. M. , Postma, E. , Walling, C. A. , … Nussey, D. H. (2010). An ecologist’s guide to the animal model. Journal of Animal Ecology, 79(1), 13–26. 10.1111/j.1365-2656.2009.01639.x [DOI] [PubMed] [Google Scholar]
- Wolf, J. B. , Brodie, E. D. , Cheverud, J. M. , Moore, A. J. , & Wade, M. J. (1998). Evolutionary consequences of indirect genetic effects. Trends in Ecology and Evolution, 10.1016/S0169-5347(97)01233-0 [DOI] [PubMed] [Google Scholar]
- Young, J. K. , Mahe, M. , & Breck, S. (2015). Evaluating behavioral syndromes in coyotes (Canis latrans). Journal of Ethology, 33(2), 137–144. 10.1007/s10164-015-0422-z [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used for these analyses, as well as the R script used to perform the analyses, are deposited in the Dryad Digital Repository as https://doi.org/10.5061/dryad.pr00820.


