Abstract
Temperature regulation is an indispensable physiological activity critical for animal survival. However, relatively little is known about the origin of thermoregulatory regimes in a phylogenetic context, or the genetic mechanisms driving the evolution of these regimes. Using bats as a study system, we examined the evolution of three thermoregulatory regimes (hibernation, daily heterothermy, and homeothermy) in relation to the evolution of leptin, a protein implicated in regulation of torpor bouts in mammals, including bats. A threshold model was used to test for a correlation between lineages with positively selected lep, the gene encoding leptin, and the thermoregulatory regimes of those lineages. Although evidence for episodic positive selection of lep was found, positive selection was not correlated with lineages of heterothermic bats, a finding that contradicts results from previous studies. Evidence from our ancestral state reconstructions suggests that the most recent common ancestor of bats used daily heterothermy and that the presence of hibernation is highly unlikely at this node. Hibernation likely evolved independently at least four times in bats—once in the common ancestor of Vespertilionidae and Molossidae, once in the clade containing Rhinolophidae and Rhinopomatidae, and again independently in the lineages leading to Taphozous melanopogon and Mystacina tuberculata. Our reconstructions revealed that thermoregulatory regimes never transitioned directly from hibernation to homeothermy, or the reverse, in the evolutionary history of bats. This, in addition to recent evidence that heterothermy is best described along a continuum, suggests that thermoregulatory regimes in mammals are best represented as an ordered continuous trait (homeothermy ← → daily torpor ← → hibernation) rather than as the three discrete regimes that evolve in an unordered fashion. These results have important implications for methodological approaches in future physiological and evolutionary research.
Keywords: ancestral state reconstruction, bats, hibernation, homeothermy, leptin, thermoregulation, torpor
1. INTRODUCTION
In mammals, the evolution of endothermy—the use of metabolically produced heat to maintain T B (body temperature)—has enabled survival of many different taxa across a variety of ecological niches and ecosystems (Fristoe et al., 2015; Hillenius & Ruben, 2004; Scholander, Hock, Walters, & Johnson, 1950). Homeothermy is defined by maintenance of a relatively constant T B over time (Ivanov, 2006), and endothermy and homeothermy are frequently linked in mammals. Endothermic heterothermy, characterized by a combination of metabolically produced heat and the use of torpor—a physiological state characterized by the reduction in metabolism, and subsequently T b, below basal levels—has enabled the survival of mammalian taxa globally, especially in extremely cold climates, highly variable climates, or regions with periodic constraints on food and water availability (Geiser & Stawski, 2011; Geiser & Turbill, 2009; Tattersall et al., 2012). The duration of discrete torpor bouts is often used to define the two traditionally recognized forms of heterothermy in mammals: hibernation and daily heterothermy, also known as daily torpor (Geiser, 2004; Ruf & Geiser, 2015). Hibernators are capable of multiday torpor bouts and drastic Tb reduction, while daily heterotherms exclusively use shorter (<24 hr), shallower torpor bouts (Geiser, 2004; Ruf & Geiser, 2015). However, this binary categorization of heterothermy has been debated, with some authors arguing that hard boundaries between hibernation and daily torpor do not exist (Boyles et al., 2013; Canale, Levesque, & Lovegrove, 2012; Geiser & Ruf, 1995; Ruf & Geiser, 2015; van Breukelen & Martin, 2015). As new methods are developed, support for a continuum of heterothermic abilities has been growing but a consensus has not been reached (Boyles, Bennett, Mohammed, & Alagaili, 2017; Levesque, Nowack, & Stawski, 2016).
Although the common ancestor of mammals was traditionally presumed to be homeothermic (Crompton, Taylor, Jagger, & a, 1978), some recent authors have argued against this interpretation on physiological and/or behavioral grounds (Grigg, Beard, & Augee, 2004; Lovegrove, 2012). Grigg et al (2004) suggested that endothermic heterothermy may be ancestral to homeothermy in mammals because endothermic heterothermy would provide a reasonable intermediate along the transition from ectothermy to full endothermic homeothermy, a shift that would have required a many‐fold increase in metabolism (Geiser & Stawski, 2011).
Transitions to new thermoregulatory regimes over evolutionary time require physiological changes. Leptin, a hormone encoded by the lep gene, influences thermoregulation and satiety by signaling to the brain the degree of adiposity available for energy intake (Dodd et al., 2014; Enriori, Sinnayah, & Simonds, 2011; Kaiyala, Ogimoto, & Nelson, 2015; Zhang et al., 2011). Due to the role it plays in regulating satiety and metabolic activity, leptin concentrations may also be impacted by diet (Clarke & Connor, 2014; Harvey et al., 2000; Weigle et al., 2005). Leptin function is correlated with post‐hibernation weight gain in arctic ground squirrels (Boyer et al., 1997), pre‐hibernation weight gain in male woodchucks (Concannon, Levac, & Rawson, 2001), and feeding activity of at least one homeothermic bat (Scotophilus heathii; Srivastava & Krishna, 2008). Exon 3 of lep may be particularly important because it contains 29 amino acid variants with functional significance (Yuan et al., 2011). Some hibernating bats show structural alterations in exon 3 of lep that may cause leptin to become more physiologically active (He et al., 2010). Outside of the relationship leptin has with food intake and temperature regulation, leptin has a mechanistically independent function in initiating torpor bouts in some species (Stehling, Doring, & Ertl, 1996; Swoap, 2008). As part of a complex matrix of gene–gene interactions, sympathetic activation of white adipose tissue during fasting triggers a dramatic decrease in circulating leptin which is thought to initiate a torpor bout (Swoap, 2008). Entrance into torpor for Siberian hamsters is triggered by leptin (Freeman, Lewis, & Kauffman, 2004), and regulation of T B is impacted by leptin in homeothermic rats (Stehling et al., 1996).
Bats (Chiroptera) represent a useful model for comparative evaluation of the evolutionary genomics of thermoregulatory regimes because they are a large monophyletic group with nearly 1,400 extant species, and show considerable variation in thermoregulatory regimes across the phylogeny including homeothermy, daily torpor, and hibernation (Lyman, 1970; Simmons, 2005; Stawski, Willis, & Geiser, 2014). Bats are also among the most diverse mammalian clades with respect to diet, with various lineages specialized as insectivores, carnivores, frugivores, nectarivores, omnivores, and even sanguinivores (Fenton & Simmons., 2015; Hill & Smith., 1984).
Consistent with studies on other mammals, leptin in bats influences torpor abilities, feeding activity, and energy balance (Banerjee, Udin, & Krishna, 2011; He et al., 2010; Kronfeld‐Schor, Richardson, & Silvia, 2000; Srivastava & Krishna, 2008; Yuan et al., 2011; Zhu et al., 2014). Ancestral state reconstruction based on the evolution of the lep gene (Yuan et al., 2011) and circumstantial evidence based on the abundance of tropical and subtropical heterothermic bats (Cory Toussaint, McKechnie, & Merwe, 2010; Geiser & Stawski, 2011; Liu & Karasov, 2011; Stawski, Turbill, & Geiser, 2009; Turbill, Law, & Geiser, 2003) have been used to suggest that the most recent common ancestor of bats was heterothermic. However, it is unclear if this ancestor used hibernation or daily torpor.
To better understand the unique evolutionary history of thermoregulation, we examined the relationship between thermoregulatory regime of extant taxa and selection on lep exon 3. Yuan et al. (2011) found that leptin has undergone positive selection in heterothermic bat lineages, and therefore associated it with the evolution of torpor. However, by using a lep exon 3 gene tree instead of a species tree, Yuan et al. (2011) effectively treated two nonindependent variables (branch length and lep selection) as independent. Coalescence theory suggests that the use of gene trees to calculate ω, a measure of positive selection, may bias results because gene trees do not necessarily mirror species trees, and may have alternative branching patterns and lengths compared to the species tree (Diekmann & Pereira‐Leal, 2016). To reexamine the findings of Yuan et al. (2011), we hypothesized that lep exon 3 has undergone episodic positive selection in heterothermic lineages. Episodic positive selection is defined as positive selection which occurs in a subset of lineages. We tested this hypothesis in the context of a species tree for relevant bat taxa. We also reconstructed the ancestral thermoregulatory regimes of bats with revised species‐level data compared to previous family‐level analyses (Yuan et al., 2011). By evaluating the number of transitions between regimes across the phylogeny through stochastic character mapping, we also evaluated the validity of categorizing thermoregulatory regimes as three discrete character states. Finally, to address other known effects of leptin, we tested for correlation between lineages with positively selected lep exon 3 and the diets of those lineages.
2. MATERIALS AND METHODS
2.1. Taxon sampling
To test for positive selection of lep exon 3, 31 species of bats with known thermoregulatory regimes were sampled for the lep exon 3 gene, including representatives from nine families (Table 1). Thirteen of these species hibernate, five use daily torpor, and 13 are homeothermic (Table 1). Lep exon 3 sequences for 27 of these species were downloaded from GenBank, and the other four, Pteropus hypomelanus, Nyctimene major, Macroglossus minimus, and Syconyteris australis, were sequenced in the Sackler Institute for Comparative Genomics. Lep exon 3 sequences for three outgroups were also included, Homo sapiens (human), Capra hircus (goat), and Galeopterus variegatus (Sunda colugo; Table 1). Species‐specific data on thermoregulatory regimes and dietary preferences were taken from the primary literature (Table 1, dietary preferences only listed for species in this dataset). The resulting dataset included 34 taxa, which we used for analyses of positive selection of lep exon 3 and models evaluating the relationship between positive selection of lep exon 3 and thermoregulatory strategies or diet.
Table 1.
Overview of the thermoregulatory regimes and diet of bat species included in this study
| Taxa | TR | TR reference | Diet | Diet reference | Accession # | Loan Institution: ID |
|---|---|---|---|---|---|---|
| Bats | ||||||
| Anoura geoffroyi a | Daily Torporb | Audet and Thomas (1997), Avila‐Flores and Medellín (2004), Stawski et al. (2014) | Insectivorous | Baker, Jones, and Carter (1976), Ortega and Alarcón‐D (2008) | GU230833 | |
| Carollia perspicillata | Daily Torpor | Audet and Thomas (1997) | ||||
| Dermanura gnoma a | Daily Torporb | Audet and Thomas (1997), Avila‐Flores and Medellín (2004), Stawski et al. (2014) | Frugivorous | Solari et al. (2009), Rojas, Vale, Ferrero, and Navarro, (2011) | GU230832 | |
| Erophylla bombifrons | Daily Torpor | Rodriguez‐Duran (1995) | ||||
| Eumops perotis | Daily Torpor | Leitner (1966) | ||||
| Glossophaga soricina | Daily Torpor | Rasweiler (1973) | ||||
| Lasiurus seminolus | Daily Torpor | Genoud (1993) | ||||
| Macroglossus minimus a | Daily Torpor | Bartels, Law, and Geiser (1998), Geiser (2006) | Nectarivorous | Nowak (1994), Hollar and Springer (1997) | AMCC: CEF801 | |
| Megaloglossus woermanni | Daily Torpor | Kulzer and Storf (1980) | ||||
| Monophyllus redmani | Daily Torpor | Rodriguez‐Duran (1995) | ||||
| Mops condylurus | Daily Torpor | Maloney, Bronner, and Buffenstein (1999) | ||||
| Myotis daubentonii | Daily Torpor | Dietz and Kalko (2006) | ||||
| Nycteris thebaica | Daily Torpor | Cory Toussaint and McKechnie (2012) | ||||
| Nyctimene albiventer | Daily Torpor | Bartholomew, Dawson, and Lasiewski (1970) | ||||
| Nyctimene robinsoni | Daily Torpor | Geiser (2006) | ||||
| Peropteryx macrotis | Daily Torpor | Genoud, Bonaccorso, and Anends (1990) | ||||
| Pternotus davyi | Daily Torpor | Czenze and Dunbar (2017) | ||||
| Rhinolophus megaphyllus | Daily Torpor | Young (2001) | ||||
| Scotophilus dinganii | Daily Torpor | Jacobs, Kelly, Mason, and Stoffberg (2007) | ||||
| Sturnira lilium | Daily Torpor | Audet and Thomas (1997) | ||||
| Syconycteris australis a | Daily Torpor | Geiser (2006) | Nectarivorous | Altringham (1996), Hollar and Springer (1997), Courts (1998) | Smithsonian: 585524 | |
| Tadarida teniotis a | Daily Torpor | Arlettaz et al. (2000) | Insectivorous | Freeman (1979), Rydell and Arlettaz (1994), Whitaker and Karataş (2009) | GU230839 | |
| Taphozous australis | Daily Torpor | Kulzer, Nelson, McKean, and Möhres (1970), Geiser (2006) | ||||
| Barbastella barbastellus | Hibernation | Pohl (1961), Russo et al. (2017) | ||||
| Chaerephon plicatus a | Hibernation | Vivier and Van Der Merwe (2007), Yuan et al. (2011) | Insectivorous | Freeman (1979), Bohmann et al. (2011), Kusuminda and Yapa (2017) | GU230836 | |
| Chalinolobus gouldii | Hibernation | Stawski and Currie (2016) | ||||
| Eptesicus fuscus a | Hibernation | Brack (2007) | Insectivorous | Agosta (2002) | NW_007370746 | |
| Hipposideros armiger a | Hibernation | Liu and Karasov (2011) | Insectivorous | Whitaker and Karataş (2009), Weterings, Wardenaar, Dunn, and Umponstira (2015) | NW_017731447 | |
| Lasionycteris noctivagans | Hibernation | Izor (1979) | ||||
| Lasiurus borealis | Hibernation | Dunbar and Tomasi (2006) | ||||
| Lasiurus cinereus | Hibernation | Cryan (2003), Willis, Brigham, and Geiser (2006) | ||||
| Miniopterus fuliginosus a | Hibernation | Kimura and Uchida (1983), Yuan et al. (2011) | Insectivorous | Hu, Wei, Zhu, Wang, and Zhang (2011) | GU230844 | |
| Miniopterus natalensis a | Hibernation | Van Der Merwe (1973) | Insectivorous | Naidoo, Mackey, and , Schoeman MC, (2011) | NW_015504548 | |
| Miniopterus schreibersii | Hibernation | Kulzer et al. (1970), Geiser (2006) | ||||
| Myotis adversus | Hibernation | Kulzer et al. (1970) | ||||
| Myotis brandtii a | Hibernation | Villanueva‐Cañas et al. (2014) | Insectivorous | Vaughan (1997), Whitaker and Karataş (2009) | NW_005360677 | |
| Myotis davidii a | Hibernation | Villanueva‐Cañas et al. (2014) | Insectivorous | Zhang et al. (2013) | NW_006296405 | |
| Myotis leibii | Hibernation | Best and Jennings (1997) | ||||
| Myotis lucifugus a | Hibernation | Brack (2007) | Insectivorous | Belwood and Fenton (1976) | NW_005871121 | |
| Myotis myotis | Hibernation | Pohl (1961), Harmata (1987), Koteja, Jurczyszyn, and Woloszyn (2001) | ||||
| Myotis nattereri | Hibernation | Hope and Jones (2012) | ||||
| Myotis ricketti a | Hibernation | Zhang et al. (2014) | Piscivorous | Ma et al. (2003) | GU230846 | |
| Myotis septentrionalis | Hibernation | Brack (2007) | ||||
| Myotis sodalis | Hibernation | Brack (2007) | ||||
| Myotis velifer | Hibernation | Tinkle and Patterson (1965), Riedesel and Williams (1976) | ||||
| Myotis vivesi | Hibernation | Salinas, Herrera, Flores‐Martínez, and Johnston (2014) | ||||
| Nyctalus noctula | Hibernation | Ransome (1990), Arlettaz et al. (2000) | ||||
| Mystacina tuberculata | Hibernation | Czenze, Brigham, Hickey, and Parsons (2017a) | ||||
| Nyctophilus geoffroyi | Hibernation | Turbill et al. (2003) | ||||
| Nyctophilus gouldi | Hibernation | Turbill et al. (2003) | ||||
| Pipistrellus pipistrellus | Hibernation | Kayser (1964), Kulzer (1965) | ||||
| Plecotus auritus | Hibernation | Eisentraut (1956) | ||||
| Rhinolophus ferrumequinum a | Hibernation | Park, Jones, and Ransome (1999); Chen, Yuan, and Sun (2008) | Insectivorous | Vaughan (1997), Whitaker and Karataş (2009) | GU230845 | |
| Rhinolophus hipposideros | Hibernation | Harmata (1987) | ||||
| Rhinopoma microphyllum a | Hibernation | Levin and Kronfeld‐schor (2012), Stawski et al. (2014) | Insectivorous | Sharifi and Hemmati (2002) | GU230830 | |
| Scotophilus heathii a | Hibernation | Rashid, Irfan, Nadeem, and Shabbir (2016) | Insectivorous | Jacobs, Eick, Schoeman, and Matthee (2006) | GU230843 | |
| Tadarida aegyptiaca | Hibernation | Geiser and Stawski (2011) | ||||
| Tadarida brasiliensis | Hibernation | Herreid (1963), Herreid and Schmidt‐Nielsen (1966) | ||||
| Taphozous melanopogon a | Hibernation | Kulzer (1965) | Insectivorous | Hu et al. (2011), Weterings et al. (2015) | GU230842 | |
| Carollia brevicauda a | Homeothermy | Avila‐Flores and Medellín (2004) | Frugivorous | Fleming (1991) | GU230829 | |
| Cynopterus sphinx a | Homeothermyb | Banerjee, Meenakumari, and Krishna (2007), Stawski et al. (2014) | Frugivorous | Ruby, Nathan, Balasingh, and Kunz (2000) | GU230842 | |
| Dobsonia viridis a | Homeothermyb | Stawski et al. (2014) | Frugivorous | Bonaccorso, Winkelmann, Dumont, and Bat (2002) | GU230840 | |
| Eidolon helvum a | Homeothermy | Zaidan (1980) | Frugivorous | Richter and Cumming (2006) | GU230838 | |
| Eonycteris spelaea a | Homeothermy | Krutzsch (1979) | Nectarivorous | Fenton (1990), Bumrungsri et al. (2013) | GU230848 | |
| Macroderma gigas | Homeothermy | Lyman (1970) | ||||
| Mormoops blainvilli | Homeothermy | Bonaccorso et al. (1992), Rodriguez‐Duran (1995) | ||||
| Nyctimene major a | Homeothermy | Bartholomew et al. (1970) | Frugivorous | Freeman (1995) | AMCC: PRS2767 | |
| Pteronotus parnelli a | Homeothermy | Bonaccorso et al. (1992); Stawski et al. (2014) | Insectivorous | Fenton (1990), Brigham (1991), Rojas et al. (2011) | GU230831 | |
| Pteronotus personatus | Homeothermy | Bonaccorso et al. (1992), Stawski et al. (2014) | ||||
| Pteronotus quadridens | Homeothermy | Rodriguez‐Duran (1995) | ||||
| Pteropus alecto a | Homeothermyb | McNab and Bonaccorso (2001) | Herbivorous | Zhang et al. (2013) | NW_006434839 | |
| Pteropus giganteus a | Homeothermy | Kulzer (1965) | Frugivorous | Nowak (1994), Vaughan (1997) | GU230837 | |
| Pteropus hypomelanus a | Homeothermy | Ochoa‐Acuña and Kunz (1999) | Frugivorous | Heard and Whittier (1997) | AMCC: PER 1 | |
| Pteropus vampyrus a | Homeothermy | McNab and Armstrong (2001) | Herbivorous | Stier and Mildenstein (2005) | NW_011888814 | |
| Rousettus aegyptiacus a | Homeothermy | Noll (1979) | Frugivorous | Korine, Izhaki, and Arad (1999) | NW_015494499 | |
| Rousettus leschenaultii a | Homeothermyb | Noll (1979), Stawski et al. (2014) | Frugivorous | Raghuram, Thangadurai, and Gopukumar (2009) | GU230847 | |
| Outgroups | ||||||
| Capra hircus a | Homeothermy | Kaciuba‐Usciz.xl;lexo, Jessen, Feistkorn, and Brzezinska (1987) | Folivorous | Genin and Pijoan (1993) | AM114397 | |
| Galeopterus variegatus a | Homeothermy | Herbivorous | Dzulhelmi and Abdullah (2009) | NW_007735418 | ||
| Homo sapiens a | Homeothermy | Mekjavic and Eiken (2006) | Omnivorous | D63519 | ||
TR: thermoregulatory regime.
Included in the dataset for the tests of positive selection of lep exon 3.
Species‐specific thermoregulatory regime is undocumented in the literature. The most likely thermoregulatory regime was inferred based on associated literature and family‐level summaries of thermal physiology (Stawski et al., 2014).
A larger dataset including 76 bat taxa and three outgroups was used for the ancestral state reconstruction of thermoregulatory regimes. Here, we were not limited by the requirement of having leptin sequences, which allowed for greater coverage of the phylogeny. Species were selected for the ancestral state reconstruction based on availability of data describing their thermoregulatory regime and presence in the published phylogeny that was used in the analyses (i.e., Amador, Arévalo, & Almeida, 2016). Data on the thermoregulatory regime of each species were taken from the primary literature by searching databases (Google Scholar and Web of Science) between the dates of September 2015 and August 2018, using keywords “bats,” “thermoregulation,” “thermoregulatory regimes,” “temperature regulation,” “hibernation,” “torpor,” “daily torpor,” “daily heterothermy,” “heterothermy,” “homeothermy,” “metabolism,” and “Chiroptera.” These data are summarized in Table 1.
DNA was extracted and lep exon 3 was amplified and sequenced for four bat species for the tests for positive selection—Pteronotus hypomelanus, Nyctimene major, Macroglossus minimus, and Syconycteris australis. All laboratory work was conducted in the Sackler Institute for Comparative Genomics. DNA was extracted from either wing punches or tissues of museum specimens (Table 1) using the Qiagen DNeasy Blood and Tissue Extraction Kit. In order to amplify lep exon 3 in these species, a primer pair (F1—AGAAGGGAGGGAGGACTCAAC, R1—GCTTCAGCACCCAGGGCTG) was developed on the flanking region of the consensus sequence from a multiple alignment of published lep sequences from Rousettus leschenaultii, Pteronotus giganteus, Eonycteris spelaea, Eidolon helvum, Dobsonia viridis, and Cynopterus sphinx which was made by eye in Geneious (Kearse et al., 2012).
The polymerase chain reaction (PCR) was carried out using illustraTM puReTaq Ready‐To‐Go PCR Beads Kit. Amplification was performed in a 25 µl reaction volume. This consisted of 20.7 µl nuclease‐free water, 0.3 µl bovine serum albumin, 1 µl 10× solution of forward primer, 1 µl 10× solution of reverse primer, 2 µl template, and one bead containing recombinant puReTaq DNA polymerase. PCR conditions were as follows: an initial denaturation phase at 95°C for 5 min, 25 cycles with a denaturation phase at 95°C for 30 s, an annealing phase at 57°C for 30 s, and an extension phase at 72°C for 45 s. The wells were then stored in a refrigerator at 4°C. PCR products were purified with AgenCourt AMPure XP. PCR products were sequenced using Sanger sequencing (Smith & Hood, 1987) following protocol from the BigDye® Terminator v3.1 Cycle Sequencing Kit.
Sequences were assembled and edited within Geneious (Kearse et al., 2012). Ends of forward and reverse sequences were trimmed with an error set to 0.01. Trimmed regions were ignored and forward and reverse sequences were assembled using de novo assembly, as outlined by the Geneious manual (Biomatters, 2017). The forward and reverse sequences of lep for Nyctimene major did not assemble through de novo assembly. Therefore, these were mapped to the reference sequence from which the primers were built. Reads were then manually edited to maximize the coverage and identity between the forward and reverse sequences. Lep exon 3 was trimmed to the open reading frame (ORF), and stop codons were removed from the tail. ORFs from all species in the dataset were then aligned by eye in Geneious (Kearse et al., 2012).
2.2. Branch–sites under positive selection
To identify lineages that have experienced episodic positive selection of lep exon 3, we ran a mixed‐effects model of evolution (MEME) to detect a subset of branch–sites under episodic positive selection (Murrell et al., 2012). MEME uses a fixed‐effect model to explain the distribution and variation of ω across sites, and a random‐effects model to explain variation in the distribution of ω across branches (Kosakovsky Pond et al., 2011; Murrell et al., 2012; Nielsen & Yang, 1998). MEME was used here because other tests, which usually average ω over branches and sites, often miss positive selection when it occurs in a subset of branch–sites (Yang & Nielsen, 2002). When evaluating if a branch–site is under positive selection, MEME considers the specific model of molecular evolution, which here was the TRN93 model (Tamura & Nei, 1993), differences in codon frequencies, and ω.
The test for branch–sites under episodic positive selection, MEME, was performed within the DataMonkey web server (Delport, Poon, Frost, & Kosakovsky Pond, 2010) using the aligned lep exon 3 sequences, the automatic substitution model selection tool, and a user‐specified tree from Amador et al. (2016). This 807 taxa phylogeny was calibrated using 44 key fossils, inferred using nine nuclear and mitochondrial genes, and shows support for the majority of currently recognized bat clades (Amador et al., 2016). The Amador et al. (2016) tree was chosen for this study because it represents the most genus‐ and species‐level diversity, 90% and 64%, respectively, compared to other phylogenies (Amador et al., 2016). The tree was pruned using the ape package in R (Paradis, Claude, & Strimmer, 2004) to only include the 31 bats and three outgroup taxa in our dataset. The model of molecular evolution that best fit these data was determined to be the TRN93 model (Tamura & Nei, 1993) using the automatic function available in DataMonkey (Delport et al., 2010).
The significance threshold was set to 0.1 and a log‐ratio test (LRT) was performed, comparing the alternative model to the null model. The alternative model allows for positive selection in a subset of branch–sites, while the null model does not allow for positive selection in a subset of branch–sites. For each branch, an empirical Bayes factor (EBF) for having ω > 1 was calculated with an associated posterior probability.
Mixed‐effects model of evolution only detects branch–sites under episodic positive selection, not lineages with gene‐wide positive selection. Therefore, to confirm that lep exon 3 is indeed under positive selection in the branches detected by MEME, we tested for gene‐wide episodic positive selection using BUSTED, a branch–site unrestricted statistical test for episodic positive selection (Murrell et al., 2015). BUSTED uses a LRT to detect evidence of episodic positive selection, when the rate of non‐synonymous to synonymous substitutions at branch–sites is transiently greater in the foreground branches compared to background (Murrell et al., 2015). Foreground branches are the lineages hypothesized to be under positive selection, and the background branches are all other branches in the phylogeny. This model assumes that the gene evolves under the general time reversal model (Tavaré, 1986).
We also used BUSTED to test for episodic positive selection by binning branch–sites into three ω categories representative of either purifying, neutral, or positive selection. Purifying selection is defined by having a ω < 1, indicating that there is strong selection to maintain the sequence over evolutionary time. Neutral selection is defined by having a ω approximately equal to 1, indicating that there is neither strong selection for the maintenance of a sequence over time nor selection for changes to that sequence. In the unconstrained model, both foreground and background branches can evolve under positive selection. In the null models, neither foreground nor background branches are allowed to evolve through positive selection. This analysis required, as input, the same alignment of lep exon 3 sequences used in the MEME analysis, and the pruned, user‐specified phylogeny from Amador et al. (2016).
Two analyses to test for gene‐wide positive selection were performed. In the first analysis, we tested for selection along branches where evidence for positive selection was previously detected (Figure 1a; Yuan et al., 2011). In the second analysis, we selected foreground branches based on lineages with evidence for positive selection from the MEME results (Figure 1b). Here, branches were considered foreground if they met the following criteria based on the MEME output: A branch had at least one codon site with an EBF > 3 for having ω > 1, and a posterior probability >0.25 for having ω > 1. These criteria conform with the guidelines for interpreting EBF values from Kass and Raftery (1995). In addition to the LRT calculation, Akaike information criteria (AIC) scores were calculated. AIC statistically quantifies the quality of each model by considering the optimum log likelihood (l) and the number of parameters (p) (AIC = −2l + 2p), enabling model comparison.
Figure 1.
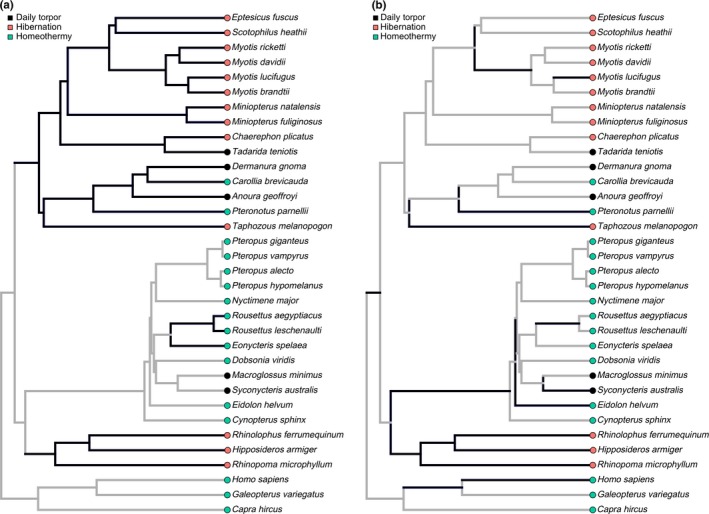
BUSTED hypotheses tests for positive selection of lep exon 3 across bats with different thermoregulatory regimes (black = daily torpor; pink = hibernation; green = homeothermy). Topology of tree generated from Amador et al. (2016). Bolded lineages represent the lineages tested for positive selection (foreground branches). Gray lineages represent the background. (a) Foreground branches correspond with those found to have evidence of positive selection by Yuan et al. (2011). (b) Foreground branches represent lineages detected by MEME to have evidence of positive selection
2.3. Correlation between positive selection and phenotypic traits
To determine whether evolution of lep exon 3 is correlated with evolution of thermoregulatory regimes, we ran a threshold model (Felsenstein, 2012). Here, a discrete trait is presumed to change state when an underlying variable, the liability, crosses a certain threshold (Felsenstein, 2005, 2012 ). This liability is assumed to have a multivariate normal distribution and to evolve under Brownian motion (Felsenstein, 2005, 2012 ). Contrasting the commonly used continuous‐time Markov (Mkn) model of discrete character evolution (Lewis, 2001; Pagel, 1994), character states under the threshold model are inherently ordered and the evolution of discrete character states is not memoryless (Felsenstein, 2005, 2012 ); the character state at one node is influenced by the character state at previous nodes. We categorized thermoregulatory regimes into three states: hibernation, daily torpor, and homeothermy. The presence (1) or absence (0) of positive selection in lep exon 3 served as the liability. Species were considered to have undergone positive selection if lep exon 3 was determined to be under positive selection per the results from MEME and BUSTED. Only data for the terminal branches were considered, as character states at internal nodes are necessarily unknown.
Parameters for the model were estimated with a Bayesian Markov chain Monte Carlo (MCMC) approach using the threshBayes function in R, with default priors and liabilities (Revell, 2012). We ran the chain for 3 million generations, thinned to 1,000 samples per chain, to account for autocorrelation, and discarded the first 500,000 as burn‐in. To quantify the relationship between lep evolution and TR, a correlation coefficient was calculated between the liability and the thermoregulatory regime. Finally, the highest posterior densities (HPDs) were estimated from the correlation coefficients to determine whether the correlation between traits statistically differed from 0, indicating a statistically significant relationship between the two variables. To test the alternative hypothesis that lep exon 3 evolution is correlated with diet, we used the same methods as above but used diet as the discrete trait evolving under the liability.
2.4. Ancestral state reconstruction of thermoregulatory regimes
To model the ancestral thermoregulatory regimes of bats, we first used the Mkn model of discrete character evolution (Lewis, 2001; Pagel, 1994) with the states hibernation, daily torpor, and homeothermy. It was important to test which transition rate matrix best described the data. Transition matrices describe the rate of transitioning from state i to state j. Here, we tested the data to fit one of three transition matrices: (a) equal transitions between all states (equal rates), (b) different transition rates between, but not among, pairs of states (symmetric), and (c) different transition rates between and among pairs of states (all rates different). We also tested each transition matrix under different transformations to determine whether transition rates varied overtime. We ran these tests for 100 iterations within the fitDiscrete function in the geiger package in R (Harmon, Weir, & Brock, 2008). The model with the lowest weighted AIC score was subsequently chosen to run the ancestral state reconstruction. The symmetric model under a kappa transformation had the best fit to these data. This suggests that, given our data, transition rates vary over time depending on the number of speciation events between two species and that the transition rate between one pair of character states is identical in the forward and reverse but pairs of states can have different transition rates. No transformations were indicated by the data, indicating that the transition rate does not vary over time.
Given these parameters, we ran an ancestral state reconstruction using the Ace function from the ape package in R (Figure 2) with maximum likelihood estimation to obtain probabilities of states at interior nodes (Paradis et al., 2004). Stochastic character mapping (Bollback, 2006; Huelsenbeck, Nielsen, & Bollback, 2003) was also used to estimate states at interior nodes and to determine how well the chosen parameters matched the real data. Stochastic character maps were built using the make.simmap function in the phytools package in R (Revell, 2012). Fifty thousand simulations were run using the parameters described previously. A Q–Q plot was generated to compare the Mkn model to the stochastic character map to evaluate the goodness of fit. The stochastic character map was then used to quantify the number of transitions between states across simulations.
Figure 2.
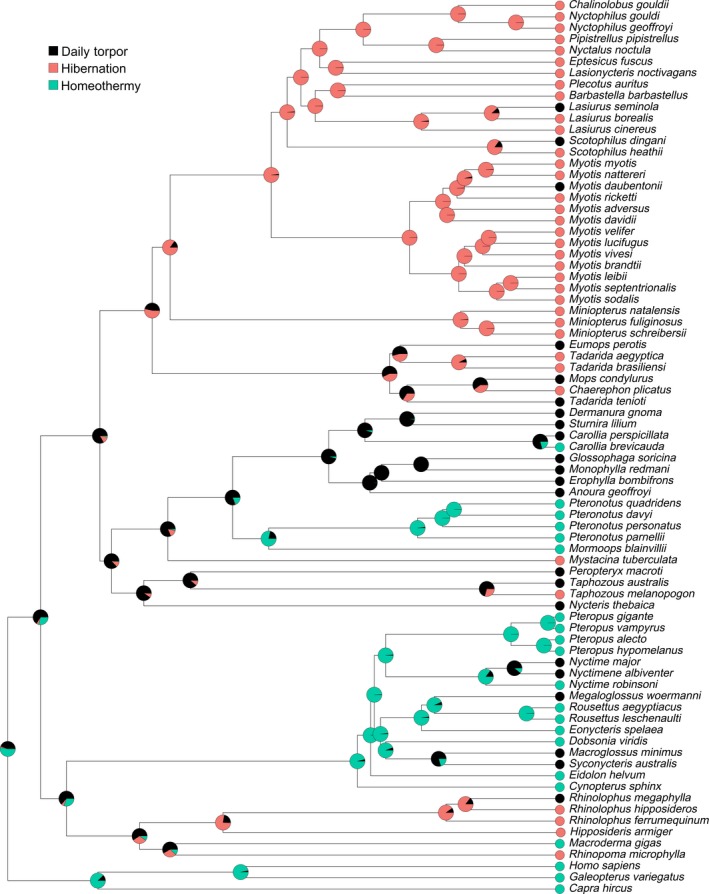
Ancestral state reconstruction under the Mkn model. Topology of tree generated from Amador et al. (2016). Pie charts represent the marginal likelihoods of each thermoregulatory regime at a given node
3. RESULTS
3.1. Data collection
We obtained new lep sequences for four bats, Pteropus hypomelanus (484 bp), Nyctimene major (487 bp), Macroglossus minimus (788 bp), and Syconyteris australis (489 bp). These were aligned to the other sequences in the dataset. Once aligned, sequences were trimmed to the coding region of lep exon 3 at 357 bp across 34 taxa.
3.2. Measuring positive selection
From the MEME results, evidence for positive selection was detected in 15 branches (Table 2) over a total of 10 codons (Table 3). When these lineages were selected as the foreground branches in the BUSTED analyses, evidence for episodic positive selection was detected (p = 0.009; Figure 1b). Positive selection was not detected when the foreground branches were selected according to those previously found by Yuan et al. (2011) to be under positive selection (p = 0.220).
Table 2.
Branch–Sites with evidence of positive selection in lep exon 3
| Branch | TR | Sites | EBF | Posterior probability |
|---|---|---|---|---|
| Node 3 | —a | 75 | 210.2 | 1 |
| Node 8 | —a | 7 | 15.3 | 0.56 |
| Node 10 | —a | 5 | >1,000 | 1 |
| 6 | >1,000 | 1 | ||
| 7 | 11.8 | 0.5 | ||
| 75 | 15 | 0.86 | ||
| 108 | 46.2 | 0.39 | ||
| Node 13 | —a | 75 | 209 | 1 |
| Node 26 | —a | 78 | 319.6 | 0.99 |
| 86 | 42.1 | 1 | ||
| Node 57 | —a | 86 | 436.3 | 1 |
| Eidolon helvum | Homeothermic | 86 | >1,000 | 1 |
| Hipposideros armiger | Hibernation | 7 | 23.8 | 0.67 |
| 75 | 48.4 | 0.95 | ||
| 78 | 3.6 | 0.61 | ||
| 108 | 529.1 | 0.88 | ||
| Homo sapiens | Homeothermic | 91 | >1,000 | 1 |
| Myotis lucifugus | Hibernation | 6 | >1,000 | 1 |
| Pteronotus parnellii | Homeothermic | 4 | >1,000 | 1 |
| 56 | >1,000 | 1 | ||
| 86 | 23.2 | 1 | ||
| Rhinolophus ferrumequinum | Hibernation | 7 | 17.3 | 0.59 |
| 78 | 3.6 | 0.61 | ||
| 108 | 206.5 | 0.74 | ||
| Rhinopoma microphyllum | Hibernation | 4 | >1,000 | 1 |
| 7 | 36.2 | 0.75 | ||
| 78 | 234 | 0.99 | ||
| Syconycteris australis | Daily Torpor | 86 | >1,000 | 1 |
| Taphozous melanopogon | Hibernation | 6 | >1,000 | 1 |
| 75 | >1,000 | 1 |
EBF: empirical Bayes factor for having ω > 1; Posterior: posterior probability; TR: thermoregulatory regime.
Not Applicable.
Table 3.
Lep exon 3 codons with evidence of positive selection in bats
| Codon | α | Unconstrained β+ | ω | p‐Value |
|---|---|---|---|---|
| 4 | 0 | 7.21 | ∞ | 0.03 |
| 5 | 2.04 | 48.00 | 23.53 | 0.04 |
| 6 | 0 | 5.64 | ∞ | 0.03 |
| 7 | 0.56 | 43.45 | 77.59 | 0.05 |
| 56 | 0 | 207.15 | ∞ | 0.01 |
| 75 | 0 | 5.50 | ∞ | 0.02 |
| 78 | 0 | 5.84 | ∞ | 0.07 |
| 86 | 0 | 1.37 | ∞ | 0.08 |
| 91 | 0.66 | 11.68 | 17.70 | 0.06 |
| 108 | 0 | 38.31 | ∞ | <0.001 |
α: maximum likelihood estimation (MLE) of synonymous rate; β+: unconstrained MLE of non–synonymous rate.
3.3. Correlation between positive selection and phenotypic traits
No relationship was found between thermoregulatory regimes and lineages with positive selection of lep exon 3 (95% HPD = −0.271 to 0.600), or between positively selected lineages and diet (95% HPD = −0.222 to 0.692).
3.4. Ancestral state reconstruction
The scaled likelihoods from the Mkn model (Supporting Information Table S1) and the posterior probabilities from stochastic character mapping were highly correlated (ρ = 0.985, p = 2.2 × 10−16; Figures 2 and 3). The state of the most recent common ancestor of bats could not be fully resolved in either model (Daily Torpor, logL = 0.660, posterior = 0.714; Hibernation, logL = 0.005, posterior = 0.154; Homeothermy, logL = 0.289 posterior = 0.131). In the Mkn model (Figure 2), the transition rate between daily torpor and hibernation was similar to that between daily torpor and homeothermy (MLE = 0.054 ± 0.012 and MLE = 0.052 ± 0.014, respectively), while no transitions were found between hibernation and homeothermy. Across the 50,000 stochastic simulations, an average of 33 state changes occurred per tree (summarized in Figure 3). Over each tree, an average of eight transitions occurred from daily torpor to hibernation, and 10 transitions occurred from hibernation to daily torpor. An average of eight transitions occurred from daily torpor to homeothermy, and seven transitions from homeothermy to daily torpor. Transitions between hibernation and homeothermy, in either direction, never occurred across all simulations. Across all regimes and simulations, the mean proportion of time spent in each state was 0.308, 0.436, and 0.256 for daily torpor, hibernation, and homeothermy, respectively. Here, time was measured by the proportion of branch lengths for which lineages are predicted to use a specific regime in the phylogeny averaged over all simulations.
Figure 3.
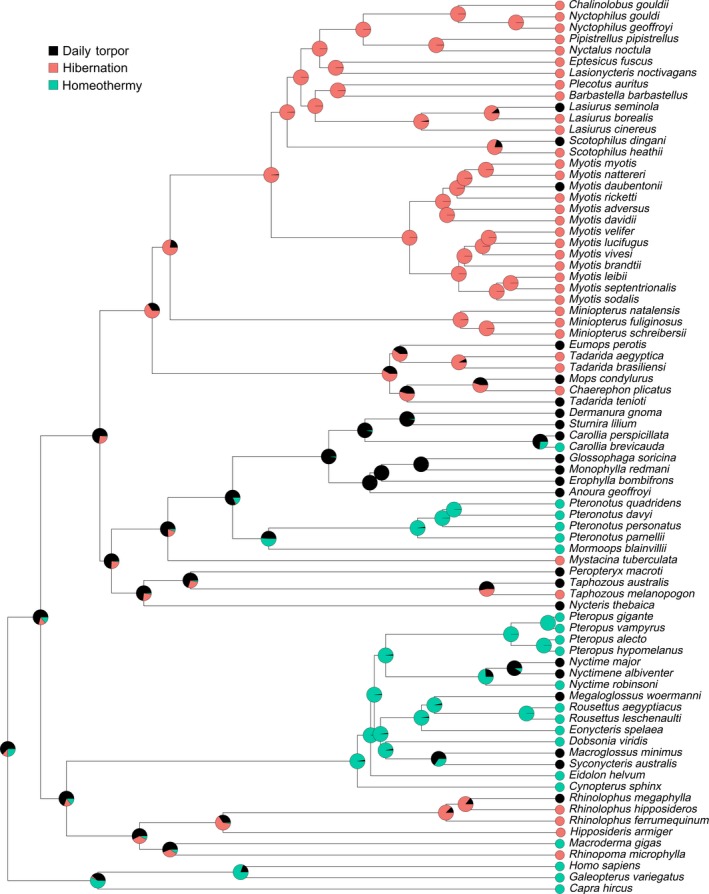
Ancestral state reconstruction from stochastic character mapping. Topology of tree generated from Amador et al. (2016). Pie charts represent the posterior probability of each thermoregulatory regime at a given node
Based on the ancestral state reconstruction, hibernation appears to have evolved four times in bats. Vespertilionidae and Molossidae both have taxa that use hibernation. Their most recent common ancestor may have used hibernation (logL = 0.533). The MRCA of Vespertilionids likely hibernated (logL = 0.850), and multiple internal nodes in Molossidae suggest a heterothermic ancestor. Our results suggest that hibernation evolved at least once in this group. Our data suggest that hibernation evolved independently at least three other times, in the species Taphozous melanopogon (Family Emballonuridae) and Mystacina tuberculata (Family Mystacinidae), and again in the clade comprised of Rhinopomatidae and Rhinolophidae. However, the node(s) at which hibernation arose in these groups remains ambiguous.
4. DISCUSSION
We found that the common ancestor of bats most likely used daily heterothermy and is very unlikely to have used hibernation. We also found that leptin evolution is not associated with the evolution of thermoregulatory regimes in bats. Twente and Twente (1964) hypothesized that the most recent common ancestor of bats was homeothermic and that heterothermy evolved secondarily as an adaptation to survive cold climates (Bieber & Ruf, 2009; Geiser & Turbill, 2009; Kortner & Geiser, 2000). However, daily heterothermy remains adaptive even at warmer temperatures because it increases energy savings and long‐term survival (Geiser & Stawski, 2011; Stawski & Geiser, 2012). Flexible use of torpor may have enhanced the ability of some bats to survive in warm and/or tropical climates, such as the environments likely encountered throughout the Eocene when bats likely originated (Amador et al., 2016; Czenze & Dunbar, 2017; Czenze, Brigham, Hickey, & Parsons, 2017b; Meredith et al., 2011; O'Leary et al., 2013; Simmons, 2005; Simmons & Geisler, 1998; Simmons, Seymour, Habersetzer, & Gunnell, 2008; Teeling, 2005).
Supporting our results, recent work estimating the ancestral thermoregulatory regimes of bats (Yuan et al., 2011) and evidence for the commonality of heterothermy in bats (Geiser & Stawski, 2011) suggests that heterothermy was the ancestral state for Chiroptera and that homeothermy was secondarily derived (Geiser & Stawski, 2011; Yuan et al., 2011). This scenario mirrors the hypothesis that the common ancestor of all mammals was heterothermic (Grigg et al., 2004; Lovegrove, 2012). However, until now, the question of which heterothermic regime was used by the ancestor of bats—hibernation or daily heterothermy—was unresolved. Here, we show evidence that the ancestor of bats was likely a daily heterotherm.
Consistent with the lack of evolutionary advantages that a hibernator would be expected to accrue during the early Eocene, a relatively warm time period characterized by widespread tropical and subtropical conditions, our results suggest that the common ancestor of bats did not hibernate (Humphries, Thomas, & Speakman, 2002). Although our results suggested a marginal likelihood that the ancestor of bats was a homeotherm, this seems unlikely based on previous studies (e.g., Geiser & Stawski, 2011; Yuan et al., 2011). Taken together with the high likelihood of daily heterothermy at this node, we argue that the most recent common ancestor of bats was a daily heterotherm. Our reconstruction therefore also indicates several reversals back to daily heterothermy. Pteronotus davyi, Nyctimene major, Macroglossus minimus, and Syconyteris australis all represent reversals back to daily heterothermy after their lineages evolved homeothermy. However, due to inconsistent methods for measuring T b and the setting of arbitrary thresholds to determine a torpid state (Levesque et al., 2016), some of the bats categorized as homeothermic in our study may in fact be heterothermic. This may alter the interpretation of reversals back to heterothermy. Increased data collection following consistent operationalized definitions of torpor should be performed for more species and recollected for species currently identified as homeothermic. Our suspicion is that many bats thought to be endothermic are actually facultative homeotherms under some conditions (e.g., see Czenze & Dunbar, 2017). Future work should also focus on other species across the mammal phylogeny in order to reconstruct the ancestral states at deeper nodes.
Our analyses suggest that hibernation evolved approximately four times in Chiroptera—at the base of Vespertilionidae and Molossidae, in the species Taphozous melanopogon and Mystacina tuberculata from the families Emballonuridae and Mystacinidae, and in the clade comprised of Rhinolophidae and Rhinopomatidae. Our analyses also suggest that the MRCA of Rhinolophidae used hibernation; however, it is unclear if this is derived or ancestral. Conservatively, we suggest that hibernation arose at least once in this group. Similarly, we suggest that hibernation arose at least once in the clade comprised of Vespertilionids and Molossids. We found no evidence for reversals in the hibernation phenotype—no lineages that lost and subsequently regained the ability to hibernate.
Significant evidence for positive selection of lep was detected in some lineages of Chiroptera, but this had no correlation with the thermoregulatory regimes of those lineages (Figure 1b). Compared to results from Yuan et al. (2011), our dataset included 11 additional bat taxa, and the thermoregulatory regimes of Carollia brevicauda and Pteronotus parnellii (Figure 4) were reclassified to be consistent with the literature (Avila‐Flores & Medellín, 2004; Bonaccorso, Arends, & Genoud, 1992). Leptin evolution is impacted by pleiotropic effects on other physiological and developmental processes beyond thermoregulation. Leptin can effect thyroid function (Ghamari‐Langroudi et al., 2010) and bone development (Crespi & Denver, 2016), induction of mitosis (Gat‐Yablonski & Phillip, 2008), and immune and stress responses (Ahima & Osei, 2004; Procaccini, Lourenco, Matarese, & La, 2009). The potential for positive selection of leptin for alternative traits (Carey, Andrews, & Martin, 2003; Jastroch et al., 2016; Yang et al., 2008) makes it difficult to find correlations between positive selection of lep and a singular function. Therefore, in retrospect it is perhaps not surprising that we found no evidence for a tight correlation between leptin and chiropteran thermoregulatory regimes, and also found no correlation between leptin selection and diet despite the known influence of high‐fat diets on leptin functioning (Frederich et al., 1995; Koch et al., 2014).
Figure 4.

Pteronotus parnellii photograph taken by Brock and Sherri Fenton in a cave in the western end of Cuba
Complex genomic mechanisms and associated physiological alterations to organ systems and physiological functions across species with different thermoregulatory regimes suggest that the evolution of thermoregulatory regimes may intrinsically have no correlation with positive selection of a single gene (Andrews, 2004; Grabek et al., 2011; Hindle, Grabek, & Epperson, 2014; Morin & Storey, 2009; Villanueva‐Cañas, Faherty, Yoder, & Albà, 2014). A non‐synonymous substitution at codon site 91 in exon 3, found here to be under positive selection, was previously inferred to cause a functional difference in hibernating bats compared to homeothermic bats (He et al., 2010). However, we found that this substitution in the sequence of the hibernating bat is shared with Homo sapiens, a homeothermic species. Therefore, a direct relationship to thermoregulatory regime and sequence variation cannot be made for this site. Recent evidence suggests that thermoregulatory regimes are mostly influenced by the regulation of gene expression, rather than the sequence specificity of protein‐coding genes (Geiser & Stawski, 2011; Grabek, Martin, & Hindle, 2015; Morin & Storey, 2009; Schwartz, Hampton, & Andrews, 2013; Yan, Barnes, Kohl, & Marr, 2008).
In our ancestral state reconstruction, zero direct transitions occurred between hibernation and homeothermy. In this model, we assumed a priori that character states are not ordered. Therefore, the lack of transitions between hibernation and homeothermy is not an artifact of the model. This suggests that thermoregulatory regimes may be better represented as an ordered trait (with daily torpor as a necessary intermediate between homeothermy and hibernation) rather than as an unordered trait. Recent evidence suggests that heterothermy exists along a continuum (Boyles et al., 2013; Dunbar & Brigham, 2010; Lovegrove, 2012; Wilz & Heldmaier, 2000). If true, this suggests that there is an inherent order to the evolution of thermoregulatory regimes, which our results indicate. Our results suggest that future research on mammalian thermoregulation should treat thermoregulatory regimes as an ordered and possibly continuous trait.
The genomics underlying thermoregulation in mammals remains largely unclear. Future research should aim to sequence whole genomes of mammals that vary in thermoregulatory regime. Comparing these data in a phylogenetic framework would enable a more complete understanding of the genomic components involved in the evolution of thermoregulatory regimes. Our results revealed that leptin does not appear to be directly involved in the evolution of thermoregulatory regimes but many candidate genes including LEPR (Rezai‐Zadeh et al., 2014), MEF2 (Tessier & Storey, 2010), and G0S2 (Jessen et al., 2016) have yet to be examined in a similar framework. Such studies will reveal the importance these candidate genes have in the evolution of thermoregulation across diverse taxa.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
M.E.L, F.T.B., and N.B.S. contributed to the concept and design of project. M.E.L. performed laboratory work, data collection, and analyses, and drafted the manuscript. All authors contributed to manuscript preparation and approval of final draft.
DATA ACCESSIBILITY
Sequence data are available on GENBANK (https://www.ncbi.nlm.nih.gov/genbank/) with accession numbers listed in Table 1. Log likelihoods for the thermoregulatory regime at each internal node in the phylogeny under the Mkn model are available in Supporting Information Table S1. All other data can be accessed through Dryad or by contacting Margaret Lazzeroni.
Supporting information
ACKNOWLEDGMENTS
This research was supported through the Theodore Roosevelt Memorial Grant, the Ecology Evolution and Environmental Biology Department's MA Student Travel Grant from Columbia University, and the Earth Institute Travel Grant. Thanks also Rebecca Hersch in the Sackler Institute for Comparative Genomics for helping with laboratory work, and Angelo Soto‐Centeno for the many discussions that inspired this project.
Lazzeroni ME, Burbrink FT, Simmons NB. Hibernation in bats (Mammalia: Chiroptera) did not evolve through positive selection of leptin. Ecol Evol. 2018;8:12576–12596. 10.1002/ece3.4674
REFERENCES
- Agosta, S. J. (2002). Habitat use, diet and roost selection by the big brown bat (Eptesicus fuscus) in North America: A case for conserving an abundant species. Mammal Review, 32, 179–198. 10.1046/j.1365-2907.2002.00103.x [DOI] [Google Scholar]
- Ahima, R. S. , & Osei, S. Y. (2004). Leptin signaling. Physiology & Behavior, 81, 223–241. 10.1016/j.physbeh.2004.02.014 [DOI] [PubMed] [Google Scholar]
- Altringham, J. (1996). Bats: Biology and behaviour. Oxford, UK: Oxford University Press. [Google Scholar]
- Amador, L. I. , Arévalo, R. L. M. , & Almeida, F. C. (2016). Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. Journal of Mammalian Evolution, 25(1), 37–70. 10.1007/s10914-016-9363-8 [DOI] [Google Scholar]
- Andrews, M. T. (2004). Genes controlling the metabolic switch in hibernating mammals. Biochemical Society Transactions, 32, 1021–1024. 10.1042/BST0321021 [DOI] [PubMed] [Google Scholar]
- Arlettaz, R. , Ruchet, C. , Aeschimann, J. , Brun, E. , Genoud, M. , & Vogel, P. (2000). Physiological traits affecting the distribution and wintering strategy of the bat Tadarida teniotis. Ecology, 81, 1004–1014. 10.2307/177174 [DOI] [Google Scholar]
- Audet, D. , & Thomas, D. W. (1997). Facultative hypothermia as a thermoregulatory strategy in the phyllostomid bats, Carollia perspicillata and Sturnira lilium . Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 167, 146–152. 10.1007/s003600050058 [DOI] [PubMed] [Google Scholar]
- Avila‐Flores, R. , & Medellín, R. A. (2004). Ecological, taxonomic, and physiological correlates of cave use by Mexican bats. Journal of Mammalogy, 85, 675–687. 10.1644/BOS-127 [DOI] [Google Scholar]
- Baker, R. J. , Jones, J. K. , & Carter, D. C. (1976). Biology of bats of the New World family Phyllostomatidae. Special publications the Museum Texas Tech University.
- Banerjee, A. , Meenakumari, K. J. , & Krishna, A. (2007). Relationship between delayed embryonic development and metabolic factors and fat deposition in fruit bat Cynopterus sphinx . Reproduction, Fertility, and Development, 19(5), 626–633. [DOI] [PubMed] [Google Scholar]
- Banerjee, A. , Udin, S. , & Krishna, A. (2011). Regulation of leptin synthesis in white adipose tissue of the female fruit bat, Cynopterus sphinx: Role of melatonin with or without insulin. Experimental Physiology, 96, 216–225. 10.1113/expphysiol.2010.055129 [DOI] [PubMed] [Google Scholar]
- Bartels, W. , Law, B. S. , & Geiser, F. (1998). Daily torpor and energetics in a tropical mammal, the northern blossom‐bat Macroglossus minimus (Megachiroptera). Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 168, 233–239. 10.1007/s003600050141 [DOI] [PubMed] [Google Scholar]
- Bartholomew, G. A. , Dawson, W. R. , & Lasiewski, R. C. (1970). Thermoregulation and heterothermy in some of the smaller flying foxes (Megachiroptera) of New Guinea. Zeitschrift Für Vergleichende Physiologie, 70, 196–209. 10.1007/BF00297716 [DOI] [Google Scholar]
- Belwood, J. J. , & Fenton, M. B. (1976). Variation in the diet of Myotis lucifugus (Chiroptera: Vespertilionidae). Canadian Journal of Zoology, 54, 1674–1678. 10.1139/z76-194 [DOI] [Google Scholar]
- Best, T. L. , & Jennings, J. B. (1997). Myotis leibii . American Society of Mammalogists, 547, 1–6. [Google Scholar]
- Bieber, C. , & Ruf, T. (2009). Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften, 96, 165–171. 10.1007/s00114-008-0471-z [DOI] [PubMed] [Google Scholar]
- Biomatters (2017). Geneious 10.1 Manual (pp. 1–264).
- Bohmann, K. , Monadjem, A. , Lehmkuhl Noer, C. , Rasmussen, M. , Zeale, M. R. K. , Clare, E. , … Gilbert, M. T. P. (2011). Molecular diet analysis of two African free‐tailed bats (molossidae) using high throughput sequencing. PLoS ONE, 6(6), e21441 10.1371/journal.pone.0021441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollback, J. P. (2006). SIMMAP: Stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics, 7, 88 10.1186/1471-2105-7-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso, F. J. , Arends, A. , Genoud, M. , Cantoni, D. , & Morton, T. (1992). Thermal ecology of moustached and ghost‐faced bats (Mormoopidae) in Venezuela. Journal of Mammalogy, 73, 365–378. 10.2307/1382071 [DOI] [Google Scholar]
- Bonaccorso, F. J. , Winkelmann, J. R. , Dumont, E. R. , & Bat, F. F. (2002). Home range of Dobsonia minor (Pteropodidae): A solitary, foliage‐roosting fruit bat in Papua New Guinea. Biotropica, 34, 127–135. 10.1111/j.1744-7429.2002.tb00248.x [DOI] [Google Scholar]
- Boyer, B. B. , Ormseth, O. A. , Buck, L. , Nicolson, M. , Pelleymounter, M. A. , & Barnes, B. M. (1997). Leptin prevents posthibernation weight gain but does not reduce energy expenditure in Arctic ground squirrels. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 118, 405–412. 10.1016/S0742-8413(97)00172-2 [DOI] [PubMed] [Google Scholar]
- Boyles, J. G. , Bennett, N. C. , Mohammed, O. B. , & Alagaili, A. N. (2017). Torpor patterns in Desert Hedgehogs (Paraechinus aethiopicus) represent another new point along a thermoregulatory continuum. Physiological and Biochemical Zoology, 90, 445–452. 10.1086/691542 [DOI] [PubMed] [Google Scholar]
- Boyles, J. G. , Thompson, A. B. , McKechnie, A. E. , Malan, E. , Humphries, M. M. , & Careau, V. (2013). A global heterothermic continuum in mammals. Global Ecology and Biogeography, 22, 1029–1039. 10.1111/geb.12077 [DOI] [Google Scholar]
- Brack, V. (2007). Temperatures and locations used by hibernating bats, including Myotis sodalis (Indiana bat), in a limestone mine: Implications for conservation and management. Environmental Management, 40, 739–746. 10.1007/s00267-006-0274-y [DOI] [PubMed] [Google Scholar]
- Brigham, R. M. (1991). Prey detection, dietary niche breadth, and body size in bats: Why are aerial insectivorous bats so small? American Naturalist, 137, 693–703. [Google Scholar]
- Bumrungsri, S. , Lang, D. , Harrower, C. , Sripaoraya, E. , Kitpipit, K. , & Racey, P. A. (2013). The dawn bat, Eonycteris spelaea Dobson (Chiroptera: Pteropodidae) feeds mainly on pollen of economically important food plants in Thailand. Acta Chiropterologica, 15, 95–104. 10.3161/150811013X667894 [DOI] [Google Scholar]
- Canale, C. I. , Levesque, D. L. , & Lovegrove, B. G. (2012). Tropical heterothermy: Does the exception prove the rule or force a re‐definition In T. Ruf. C. Bieber. W. Arnold. & E. Millesi. (Eds.), Living in a seasonal world (pp. 29–40). Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Carey, H. V. , Andrews, M. T. , & Martin, S. L. (2003). Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiological Reviews, 83, 1153–1181. 10.1152/physrev.00008.2003 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Yuan, L. , Sun, M. , Zhang, L. , & Zhang, S. (2008). Screening of hibernation‐related genes in the brain of Rhinolophus ferrumequinum during hibernation. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 149, 388–393. 10.1016/j.cbpb.2007.10.011 [DOI] [PubMed] [Google Scholar]
- Clarke, A. , & Connor, M. I. O. (2014). Diet and body temperature in mammals and birds. Global Ecology and Biogeography, 23, 1000–1008. 10.1111/geb.12185 [DOI] [Google Scholar]
- Concannon, P. , Levac, K. , Rawson, R. , Tennant, B. , & Bensadoun, A. (2001). Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 281, R951–R959. 10.1152/ajpregu.2001.281.3.R951 [DOI] [PubMed] [Google Scholar]
- Cory Toussaint, D. , & McKechnie, A. E. (2012). Interspecific variation in thermoregulation among three sympatric bats inhabiting a hot, semi‐arid environment. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 182, 1129–1140. 10.1007/s00360-012-0683-6 [DOI] [PubMed] [Google Scholar]
- Cory Toussaint, D. , McKechnie, A. E. , & van der Merwe, M. (2010). Heterothermy in free‐ranging male Egyptian Free‐tailed bats (Tadarida aegyptiaca) in a subtropical climate. Mammalian Biology, 75, 466–470. 10.1016/j.mambio.2009.06.001 [DOI] [Google Scholar]
- Courts, S. E. (1998). Dietary strategies of Old World Fruit Bats (Megachiroptera, Pteropodidae): How do they obtain sufficient protein? Mammal Review, 28, 185–194. 10.1046/j.1365-2907.1998.00033.x [DOI] [Google Scholar]
- Crespi, E. J. , & Denver, R. J. (2016). Leptin (ob gene) of the South African clawed frog Xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America, 103, 10092–10097. 10.1073/pnas.0507519103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton, A. W. , Taylor, C. R. , & Jagger, J. A. (1978). Evolution of homeothermy in mammals. Nature, 272, 333–336. 10.1038/272333a0 [DOI] [PubMed] [Google Scholar]
- Cryan, P. M. (2003). Sex differences in the thermoregulation and evaporative water loss of a heterothermic bat, Lasiurus cinereus, during its spring migration. Journal of Experimental Biology, 206, 3381–3390. 10.1242/jeb.00574 [DOI] [PubMed] [Google Scholar]
- Czenze, Z. J. , Brigham, R. M. , Hickey, A. J. R. , & Parsons, S. (2017a). Winter climate affects torpor patterns and roost choice in New Zealand lesser short‐tailed bats. Journal of Zoology, 303, 236–243. 10.1111/jzo.12486 [DOI] [Google Scholar]
- Czenze, Z. J. , Brigham, R. M. , Hickey, A. J. R. , & Parsons, S. (2017b). Stressful summers? Torpor expression differs between high‐ and low‐latitude populations of bats. Journal of Mammalogy, 98, 1249–1255. 10.1093/jmammal/gyx071 [DOI] [Google Scholar]
- Czenze, Z. J. , & Dunbar, M. B. (2017). Hot bats go cold: Heterothermy in neotropical bats. Canadian Journal of Zoology, 95, 909–912. [Google Scholar]
- Delport, W. , Poon, A. F. Y. , Frost, S. D. W. , & Kosakovsky Pond, S. L. (2010). Datamonkey 2010: A suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics, 26, 2455–2457. 10.1093/bioinformatics/btq429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann, Y. , & Pereira‐Leal, J. B. (2016). Gene tree affects inference of sites under selection by the branch‐site test of positive selection. Evolutionary Bioinformatics, 11, 11–17. 10.4137/EBO.S30902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz, M. , & Kalko, E. K. V. (2006). Seasonal changes in daily torpor patterns of free‐ranging female and male Daubenton’s bats (Myotis daubentonii). Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 176, 223–231. 10.1007/s00360-005-0043-x [DOI] [PubMed] [Google Scholar]
- Dodd, G. T. , Worth, A. A. , Nunn, N. , Korpal, A. K. , Bechtold, D. A. , Allison, M. B. , … Luckman, S. M. (2014). The thermogenic effect of leptin is dependent on a distinct population of prolactin‐releasing peptide neurons in the dorsomedial hypothalamus. Cell Metabolism, 20, 639–649. 10.1016/j.cmet.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, M. B. , & Brigham, R. M. (2010). Thermoregulatory variation among populations of bats along a latitudinal gradient. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 180, 885–893. 10.1007/s00360-010-0457-y [DOI] [PubMed] [Google Scholar]
- Dunbar, M. B. , & Tomasi, T. E. (2006). Arousal patterns, metabolic rate, and an energy budget of eastern red bats (Lasiurus borealis) in winter. Journal of Mammalogy, 87, 1096–1102. 10.1644/05-MAMM-A-254R3.1 [DOI] [Google Scholar]
- Dzulhelmi, M. N. , & Abdullah, M. T. (2009). Foraging ecology of the sunda colugo (Galeopterus variegatus) in bako national park, Sarawak, Malaysia. Malayan Nature Journal, 61, 285–294. [Google Scholar]
- Eisentraut, M. (1956). Der Winterschlaf mit seinen ökologischen und physiologischen Begleiterscheinungen.
- Enriori, P. J. , Sinnayah, P. , Simonds, S. E. , Garcia Rudaz, C. , & Cowley, M. A. (2011). Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. Journal of Neuroscience, 31, 12189–12197. 10.1523/JNEUROSCI.2336-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. (2005). Using the quantitative genetic threshold model for inferences between and within species. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 360, 1427–1434. 10.1098/rstb.2005.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. (2012). A comparative method for both discrete and continuous characters using the threshold model. American Naturalist, 179, 145–156. 10.1086/663681 [DOI] [PubMed] [Google Scholar]
- Fenton, M. B. (1990). The foraging behaviour and ecology of animal‐eating bats. Canadian Journal of Zoology, 68, 411–422. 10.1139/z90-061 [DOI] [Google Scholar]
- Fenton, B. M. , & Simmons, N. B. (2015). Bats: A world of science and mystery. Chicago, IL: University of Chicago Press. [Google Scholar]
- Fleming, T. H. (1991). The relationship between body size, diet, and habitat use in frugivorous bats, genus Carollia (Phyllostomidae). Journal of Mammalogy, 72, 493–501. 10.2307/1382132 [DOI] [Google Scholar]
- Frederich, R. C. , Hamann, A. , Anderson, S. , Löllmann, B. , Lowell, B. B. , & Flier, J. S. (1995). Leptin levels reflect body lipid‐content in mice – Evidence for diet‐induced resistance to leptin action. Nature Medicine, 1, 1311–1314. 10.1038/nm1295-1311 [DOI] [PubMed] [Google Scholar]
- Freeman, P. W. (1979). Beetle‐eating and moth‐eating molossid bats. Journal of Mammalogy, 60, 467–479. [Google Scholar]
- Freeman, P. W. (1995) Nectarivorous feeding mechanisms in bats. Biological Journal of the Linnean Society, 56(3), 439–463. 10.1111/j.1095-8312.1995.tb01104.x [DOI] [Google Scholar]
- Freeman, D. A. , Lewis, D. A. , Kauffman, A. S. , Blum, R. M. , & Dark, J. (2004). Reduced leptin concentrations are permissive for display of torpor in Siberian hamsters. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 1650, 97–103. 10.1152/ajpregu.00716.2003 [DOI] [PubMed] [Google Scholar]
- Fristoe, T. S. , Burger, J. R. , Balk, M. A. , Khaliq, I. , Hof, C. , & Brown, J. H. (2015). Metabolic heat production and thermal conductance are mass‐independent adaptations to thermal environment in birds and mammals. Proceedings of the National Academy of Sciences of the United States of America, 112, 15934–15939. 10.1073/pnas.1521662112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat‐Yablonski, G. , & Phillip, M. (2008). Leptin and regulation of linear growth. Current Opinion in Clinical Nutrition and Metabolic Care, 11, 303–308. 10.1097/MCO.0b013e3282f795cf [DOI] [PubMed] [Google Scholar]
- Geiser, F. (2004). Metabolic rate and body temperature reduction during hibernation and daily torpor. Annual Review of Physiology, 66, 239–274. 10.1146/annurev.physiol.66.032102.115105 [DOI] [PubMed] [Google Scholar]
- Geiser, F. (2006). Energetic, thermal biology and torpor in Australian bats In Zubaid A., McCracken G. F., & Kunz T. (Eds.), Function and evolutionary ecology of bats (pp. 1–22). Oxford, UK: Oxford University Press. [Google Scholar]
- Geiser, F. , & Ruf, T. (1995). Hibernation versus daily torpor in mammals and birds: Physiological variables and classification of torpor patterns. Physiological Zoology, 68, 935–966. 10.1086/physzool.68.6.30163788 [DOI] [Google Scholar]
- Geiser, F. , & Stawski, C. (2011). Hibernation and torpor in tropical and subtropical bats in relations to energetics, extinctions and the evolution of endothermy. PLoS ONE, 51, 337–348. 10.1093/icb/icr042 [DOI] [PubMed] [Google Scholar]
- Geiser, F. , & Turbill, C. (2009). Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften, 96, 1235–1240. 10.1007/s00114-009-0583-0 [DOI] [PubMed] [Google Scholar]
- Genin, D. , & Pijoan, A. P. (1993). Seasonality of goat diet and plant acceptabilities in the coastal scrub of Baja California, Mexico. Small Ruminant Research, 10, 1–11. 10.1016/0921-4488(93)90102-N [DOI] [Google Scholar]
- Genoud, M. (1993). Temperature regulation in subtropical tree bats. Comparative Biochemistry and Physiology Part A: Physiology, 104, 321–331. 10.1016/0300-9629(93)90324-W [DOI] [PubMed] [Google Scholar]
- Genoud, M. , Bonaccorso, F. J. , & Anends, A. (1990). Rate of metabolism and temperature regulation in two small tropical insectivorous bats (Peropteryx macrotis and Natalus tumidirostris). Comparative Biochemistry and Physiology Part A: Physiology, 97, 229–234. 10.1016/0300-9629(90)90177-t [DOI] [Google Scholar]
- Ghamari‐Langroudi, M. , Vella, K. R. , Srisai, D. , Sugrue, M. L. , Hollenberg, A. N. , & Cone, R. D. (2010). Regulation of thyrotropin‐releasing hormone‐expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Molecular Endocrinology, 24, 2366–2381. 10.1210/me.2010-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabek, K. R. , Karimpour‐Fard, A. , Epperson, L. E. , Hindle, A. , Hunter, L. E. , & Martin, S. L. (2011). Multistate proteomics analysis reveals novel strategies used by a hibernator to precondition the heart and conserve ATP for winter heterothermy. Physiological Genomics, 43, 1263–1275. 10.1152/physiolgenomics.00125.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabek, K. R. , Martin, S. L. , & Hindle, A. G. (2015). Proteomics approaches shed new light on hibernation physiology. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 185, 607–627. 10.1007/s00360-015-0905-9 [DOI] [PubMed] [Google Scholar]
- Grigg, G. C. , Beard, L. A. , & Augee, M. L. (2004). The evolution of endothermy and its diversity in Mammals and Birds. Physiological and Biochemical Zoology, 77, 982–997. 10.1086/425188 [DOI] [PubMed] [Google Scholar]
- Harmata, W. (1987). The frequency of winter sleep interruptions in two species of bats hibernating in limestone tunnels. Acta Theriologica (Warszawa), 32, 21–31. 10.4098/AT.arch.87-23 [DOI] [Google Scholar]
- Harmon, L. J. , Weir, J. T. , Brock, C. D. , Glor, R. E. , & Challenger, W. (2008). GEIGER: Investigating evolutionary radiations. Bioinformatics, 24, 129–131. 10.1093/bioinformatics/btm538 [DOI] [PubMed] [Google Scholar]
- Harvey, J. , McKay, N. G. , Walker, K. S. , Van derKaay, J. , Downes, C. P. , & Ashford, M. L. J. (2000). Essential role of phosphoinositide 3‐kinase in leptin‐induced K ATP channel activation in the rat CRI‐G1 insulinoma cell line. Journal of Biological Chemistry, 275, 4660–4669. 10.1074/jbc.275.7.4660 [DOI] [PubMed] [Google Scholar]
- He, L. , Pan, Y. , He, G. , Lin, B. , Liao, C.‐C. , Zuo, X. , & Yuan, L. (2010). Structural and functional studies of leptins from hibernating and non‐hibernating bats. General and Comparative Endocrinology, 168, 29–35. 10.1016/j.ygcen.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Heard, D. J. , & Whittier, D. A. (1997). Hematologic and plasma biochemical reference values for three flying fox species (Pteropus sp.). American Association of Zoo Veterinarians, 28, 464–470. [PubMed] [Google Scholar]
- Herreid, C. F. I. (1963). Temperature regulation of Mexican free‐tailed bats in cave habitats. Journal of Mammalogy, 44, 560–573. 10.2307/1377140 [DOI] [Google Scholar]
- Herreid, C. F. II , & Schmidt‐Nielsen, K. (1966). Oxygen consumption temperature and water loss in bats from different environments. American Journal of Physiology, 211, 1108–1112. 10.1152/ajplegacy.1966.211.5.1108 [DOI] [PubMed] [Google Scholar]
- Hill, J. E. , & Smith, J. D. (1984). Bats: A natural history. Austin, TX: University of Texas Press. [Google Scholar]
- Hillenius, W. J. , & Ruben, J. A. (2004). The evolution of endothermy in terrestrial vertebrates: Who? When? Why? Physiological and Biochemical Zoology, 77, 1019–1042. 10.1086/425185 [DOI] [PubMed] [Google Scholar]
- Hindle, A. G. , Grabek, K. R. , Epperson, L. E. , Karimpour‐Fard, A. , & Martin, S. L. (2014). Metabolic changes associated with the long winter fast dominate the liver proteome in 13‐lined ground squirrels. Physiological Genomics, 46, 348–361. 10.1152/physiolgenomics.00190.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollar, L. J. , & Springer, M. S. (1997). Old World fruitbat phylogeny: Evidence for convergent evolution and an endemic African clade. Proceedings of the National Academy of Sciences of the United States of America, 94, 5716–5721. 10.1073/pnas.94.11.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope, P. R. , & Jones, G. (2012). Warming up for dinner: Torpor and arousal in hibernating Natterer’s bats (Myotis nattereri) studied by radio telemetry. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 182, 569–578. 10.1007/s00360-011-0631-x [DOI] [PubMed] [Google Scholar]
- Hu, K. L. , Wei, L. , Zhu, T. T. , Wang, X. Z. , & Zhang, L. B. (2011). Dietary composition, echolocation pulses, and morphological measurements of the long‐fingered bat Miniopterus fuliginosus. Zoological Research, 32, 163–167. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck, J. P. , Nielsen, R. , & Bollback, J. P. (2003). Stochastic mapping of morphological characters. Systematic Biology, 52, 131–158. 10.1080/10635150390192780 [DOI] [PubMed] [Google Scholar]
- Humphries, M. M. , Thomas, D. W. , & Speakman, J. R. (2002). Climate‐mediated energetic constraints on the distribution of hibernating mammals. Nature, 418, 313–316. 10.1038/nature00903.1 [DOI] [PubMed] [Google Scholar]
- Ivanov, K. P. (2006). The development of the concepts of homeothermy and thermoregulation. Journal of Thermal Biology, 31, 24–29. 10.1016/j.jtherbio.2005.12.005 [DOI] [Google Scholar]
- Izor, R. J. (1979). Winter range of the silver‐haired bat. Journal of Mammalogy, 60, 641–643. 10.2307/1380114 [DOI] [Google Scholar]
- Jacobs, D. S. , Eick, G. N. , Schoeman, C. , & Matthee, C. A. (2006). Cryptic species in an insectivorous bat, Scotophilus dinganii . Journal of Mammalogy, 87, 161–170. 10.1644/04-MAMM-A-132R2.1 [DOI] [Google Scholar]
- Jacobs, D. S. , Kelly, E. J. , Mason, M. , & Stoffberg, S. (2007). Thermoregulation in two free‐ranging subtropical insectivorous bat species: Scotophilus species (Vespertilionidae). Canadian Journal of Zoology, 85, 883–890. 10.1139/Z07-067 [DOI] [Google Scholar]
- Jastroch, M. , Giroud, S. , Barrett, P. , Geiser, F. , Heldmaier, G. , & Herwig, A. (2016). Seasonal control of mammalian energy balance: Recent advances in the understanding of daily torpor and hibernation. Journal of Neuroendocrinology, 28, 1–6. 10.1111/jne.12437 [DOI] [PubMed] [Google Scholar]
- Jessen, N. , Nielsen, T. S. , Vendelbo, M. H. , Viggers, R. , Støen, O.‐G. , Evans, A. , & Frøbert, O. (2016). Pronounced expression of the lipolytic inhibitor G0/G1 Switch Gene 2 (G0S2) in adipose tissue from brown bears (Ursus arctos) prior to hibernation. Physiological Reports, 4, e12781 10.14814/phy2.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaciuba‐Usciz.xl;lexo, H. , Jessen, C. , Feistkorn, G. , & Brzezinska, Z. (1987). Work performance, thermoregulation and muscle metabolism in thyroidectomized goats (Capra hircus). Comparative Biochemistry and Physiology Part A: Physiology, 87, 915–921. 10.1016/0300-9629(87)90015-6 [DOI] [PubMed] [Google Scholar]
- Kaiyala, K. J. , Ogimoto, K. , Nelson, J. T. , Schwartz, M. W. , & Morton, G. J. (2015). Leptin signaling is required for adaptive changes in food intake, but not energy expenditure, in response to different thermal conditions. PLoS ONE, 10, 1–19. 10.1371/journal.pone.0119391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass, R. E. , & Raftery, A. E. (1995). Bayes factors. Journal of American Statistical Association, 90, 773–795. 10.1080/01621459.1995.10476572 [DOI] [Google Scholar]
- Kayser, C. H. (1964). La dépense d’énergie des mammiferes en hibernation. Archives Des Sciences Physiologiques, 18, 137–150. [PubMed] [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , … Drummond, A. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K. , & Uchida, T. A. (1983). Ultrastructural observations of delayed implantation in the Japanese long‐Fingered Bat, Miniopterus‐Schreibersii‐Fuliginosus. Journal of Reproduction and Fertility, 69, 187–193. 10.1530/jrf.0.0690187 [DOI] [PubMed] [Google Scholar]
- Koch, C. E. , Lowe, C. , Pretz, D. , Steger, J. , Williams, L. M. , & Tups, A. (2014). High‐fat diet induces leptin resistance in leptin‐deficient mice. Journal of Neuroendocrinology, 26, 58–67. 10.1111/jne.12131 [DOI] [PubMed] [Google Scholar]
- Korine, C. , Izhaki, I. , & Arad, Z. (1999). Is the Egyptian fruit‐bat Rousettus aegyptiacus a pest in Israel? An analysis of the bat’s diet and implications for its conservation. Biological Conservation, 88, 301–306. 10.1016/S0006-3207(98)00126-8 [DOI] [Google Scholar]
- Kortner, G. , & Geiser, F. (2000). Torpor and activity patterns in free‐ranging sugar gliders Petaurus breviceps (Marsupialia). Oecologia, 123, 350–357. 10.1007/s004420051021 [DOI] [PubMed] [Google Scholar]
- Kosakovsky Pond, S. L. , Murrell, B. , Fourment, M. , Frost, S. D. W. , Delport, W. , & Scheffler, K. (2011). A random effects branch‐site model for detecting episodic diversifying selection. Molecular Biology and Evolution, 28, 3033–3043. 10.1093/molbev/msr125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteja, P. , Jurczyszyn, M. , & Woloszyn, B. W. (2001). Energy balance of hibernating mouse‐eared bat Myotis myotis: A study with a TOBEC instrument. Acta Theriologica (Warszawa), 46, 1–12. 10.1007/BF03192411 [DOI] [Google Scholar]
- Kronfeld‐Schor, N. , Richardson, C. , Silvia, B. A. , Kunz, T. H. , & Widmaier, E. P. (2000). Dissociation of leptin secretion and adiposity during prehibernatory fattening in little brown bats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 279, R1277–R1281. 10.1159/000088358 [DOI] [PubMed] [Google Scholar]
- Krutzsch, P. H. (1979). Male reproductive patterns in nonhibernating bats. Journal of Reproduction and Fertility, 56, 333–344. 10.1530/jrf.0.0560369 [DOI] [PubMed] [Google Scholar]
- Kulzer, E. , Nelson, J. E. , McKean, J. L. , & Möhres, F. P. (1970). Untersuchungen Uber die Temperaturregulation australischer Fledermause (Microchiroptera). Zeitschrift Für Vergleichende Physiologie, 69, 426–451. 10.1007/BF00333769 [DOI] [Google Scholar]
- Kulzer, E. , & Storf, R. (1980). Schlaf‐Lethargie bei dem afrikanischen Langzungenflughund Megaloglossus woermanni Pagenstecher. Zeitschrift Für Säugetierkunde, 45, 23–29. [Google Scholar]
- Kulzer, E. (1965). Temperaturregulation bei fledermausen (Chiroptera) aus verschiedenen klimazonen.
- Kusuminda, T. , & Yapa, W. B. (2017). First record of wrinkle‐lipped free‐tailed bat Chaerephon plicatus Buchannan, 1800 (Mammalia: Chiroptera: Molossidae) colony in Sri Lanka, with notes on echolocation calls and taxonomy. Journal of Threatened Taxa, 9, 10115–10120. [Google Scholar]
- Leitner, P. (1966). Body temperature, oxygen consumption, heart rate and shivering in the California mastiff bat, Eumops perotis. Comparative Biochemistry and Physiology, 19, 431–443. 10.1016/0010-406X(66)90152-6 [DOI] [PubMed] [Google Scholar]
- Levesque, D. L. , Nowack, J. , & Stawski, C. (2016). Modelling mammalian energetics: The heterothermy problem. Climate Change Responses, 3, 7 10.1186/s40665-016-0022-3 [DOI] [Google Scholar]
- Levin, E. , & Kronfeld‐schor, N. (2012). Summer torpor and sexual segregation in the subtropical bat Rhinopom In Giroud S., Turbill C., & Ruf T. (Eds.), Living in a seasonal world (pp. 481–491). Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Lewis, P. O. (2001). A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology, 50, 913–925. 10.1080/106351501753462876 [DOI] [PubMed] [Google Scholar]
- Liu, J. N. , & Karasov, W. H. (2011). Hibernation in warm hibernacula by free‐ranging Formosan leaf‐nosed bats, Hipposideros terasensis, in subtropical Taiwan. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 181, 125–135. 10.1007/s00360-010-0509-3 [DOI] [PubMed] [Google Scholar]
- Lovegrove, B. G. (2012). The evolution of endothermy in Cenozoic mammals: A plesiomorphic‐apomorphic continuum. Biological Reviews, 87(1), 128–162. 10.1111/j.1469-185X.2011.00188.x [DOI] [PubMed] [Google Scholar]
- Lyman, C. P. (1970). Thermoregulation and metabolism in bats In Wimsatt W.A. (Ed.), Biology of bats (pp. 301–330). Cambridge, MA: Academic Press. [Google Scholar]
- Ma, J. , Jones, G. , Zhang, S. , Shen, J. , Metzner, W. , Zhang, L. , & Liang, B. (2003). Dietary analysis confirms that Rickett’s big‐footed bat (Myotis ricketti) is a piscivore. Journal of Zoology, 261, 245–248. 10.1017/S095283690300414x [DOI] [Google Scholar]
- Maloney, S. K. , Bronner, G. N. , & Buffenstein, R. (1999). Thermoregulation in the Angolan Free‐Tailed Bat Mops condylurus: A Small Mammal That Uses Hot Roosts. Physiological and Biochemical Zoology, 72, 385–396. 10.1086/316677 [DOI] [PubMed] [Google Scholar]
- McNab, B. K. , & Armstrong, M. I. (2001). Sexual dimorphism and scaling of energetics in flying foxes of the genus Pteropus . Journal of Mammalogy, 82, 709 [DOI] [Google Scholar]
- McNab, B. K. , & Bonaccorso, F. J. (2001). The metabolism of New Guinean pteropodid bats. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 171, 201–214. 10.1007/s003600000163 [DOI] [PubMed] [Google Scholar]
- Mekjavic, I. B. , & Eiken, O. (2006) Contribution of thermal and nonthermal factors to the regulation of body temperature in humans. Journal of Applied Physiology, 100, 2065–2072. 10.1152/japplphysiol.01118.2005 [DOI] [PubMed] [Google Scholar]
- Meredith, R. W. , Janecka, J. E. , Gatesy, J. , Ryder, O. A. , Fisher, C. A. , Teeling, E. C. , … Murphy, W. J. (2011). Impacts of the cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science, 334, 521–524. 10.1126/science.1211028 [DOI] [PubMed] [Google Scholar]
- Morin, P. , & Storey, K. B. (2009). Mammalian hibernation: Differential gene expression and novel application of epigenetic controls. International Journal of Developmental Biology, 53, 433–442. 10.1387/ijdb.082643pm [DOI] [PubMed] [Google Scholar]
- Murrell, B. , Weaver, S. , Smith, M. D. , Wertheim, J. O. , Murrell, S. , Aylward, A. , … Kosakovsky Pond, S. L. (2015). Gene‐wide identification of episodic selection. Molecular Biology and Evolution, 32, 1365–1371. 10.1093/molbev/msv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, B. , Wertheim, J. O. , Moola, S. , Weighill, T. , Scheffler, K. , & Kosakovsky Pond, S. L. (2012). Detecting individual sites subject to episodic diversifying selection. PLoS Genetics, 8(7), e1002764 10.1371/journal.pgen.1002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo, S. , Mackey, R. L. , & Schoeman, M. C. (2011). Foraging ecology of insectivorous bats (Chiroptera) at polluted and an unpolluted river in an urban landscape. Durban Natural Science Museum Novitates, 34, 21–28. 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- Nielsen, R. , & Yang, Z. (1998). Likelihood models for detecting positively selected amino acid sites and applications to the HIV‐1 envelope gene. Genetics, 148, 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll, U. G. (1979). Body temperature, oxygen consumption, noradrenaline response and cardiovascular adaptations in the flying fox, Rousettus aegyptiacus . Comparative Biochemistry and Physiology Part A: Physiology, 63, 79–88. 10.1016/0300-9629(79)90631-5 [DOI] [Google Scholar]
- Nowak, R. M. (1994). Walker’s bats of the world. Baltimore, MD: JHU Press. [Google Scholar]
- Ochoa‐Acuña, H. , & Kunz, T. H. (1999). Thermoregulatory behavior in the small island flying fox, Pteropus hypomelanus (Chiroptera: Pteropodidae). Journal of Thermal Biology, 24, 15–20. 10.1016/S0306-4565(98)00033-3 [DOI] [Google Scholar]
- O'Leary, M. A. , Bloch, J. I. , Flynn, J. J. , Gaudin, T. J. , Giallombardo, A. , Giannini, N. P. , … Cirranello, A. l. (2013). The placental mammal ancestor and the post‐K‐Pg radiation of placentals. Science, 339, 662–667. 10.1126/science.1229237 [DOI] [PubMed] [Google Scholar]
- Ortega, J. , & Alarcón‐D, I. (2008). Anoura geoffroyi (Chiroptera: Phyllostomidae). Mammalian Species, 818, 1–7. 10.1644/818.1.Key [DOI] [Google Scholar]
- Pagel, M. (1994). Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proceedings of the Royal Society of London, 255, 37–45. 10.1098/rsta.1892.0001 [DOI] [Google Scholar]
- Paradis, E. , Claude, J. , & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290. 10.1093/bioinformatics/btg412 [DOI] [PubMed] [Google Scholar]
- Park, K. J. , Jones, G. , & Ransome, R. D. (1999). Winter activity of a population of greater horseshoe bats (Rhinolophus ferrumequinum). Journal of Zoology, 248, 419–427. 10.1111/j.1469-7998.1999.tb01041.x [DOI] [Google Scholar]
- Pohl, H. (1961). Temperaturregulation und Tagesperiodik des Stoffwechsels bei Winterschläfern.
- Procaccini, C. , Lourenco, E. V. , Matarese, G. , & La, C. A. (2009). Leptin signaling: A key pathway in immune responses. NIH Public Access, 4, 22–30. 10.2174/157436209787048711.Leptin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram, H. , Thangadurai, C. , Gopukumar, N. , Nathar, K. , & Sripathi, K. (2009). The role of olfaction and vision in the foraging behaviour of an echolocating megachiropteran fruit bat, Rousettus leschenaulti (Pteropodidae). Mammalian Biology, 74, 9–14. 10.1016/j.mambio.2008.02.008 [DOI] [Google Scholar]
- Ransome, R. (1990). Natural History of Hibernating Bats.
- Rashid, N. , Irfan, M. , Nadeem, M. S. , & Shabbir, A. (2016). Comparative seasonal haematology of two bat species, Scotophilus heathii and Pipistrellus pipistrellus, in a Subtropical Area of Pakistan. Pakistan Journal of Zoology, 48, 1503–1510. [Google Scholar]
- Rasweiler, J. J. I. (1973). Care and management of the long‐tongued bat, Glossophaga soricina (Chiroptera: Phyllostomatidae), in the laboratory, with observations on estivation induced by food deprivation. Journal of Mammalogy, 54, 391–404. [PubMed] [Google Scholar]
- Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. 10.1111/j.2041-210X.2011.00169.x [DOI] [Google Scholar]
- Rezai‐Zadeh, K. , Yu, S. , Jiang, Y. , Laque, A. , Schwartzenburg, C. , Morrison, C. D. , … Münzberg, H. (2014). Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Molecular Metabolism, 3, 681–693. 10.1016/j.molmet.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, H. V. , & Cumming, G. S. (2006). Food availability and annual migration of the straw‐colored fruit bat (Eidolon helvum). Journal of Zoology, 268, 35–44. 10.1111/j.1469-7998.2005.00020.x [DOI] [Google Scholar]
- Riedesel, M. L. , & Williams, B. A. (1976). Continuous 24‐hour oxygen consumption studies of myotis velifer. Biochem Physiol, 54A, 95–99. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Duran, A. (1995). Metabolic rates and thermal conductance in four species of neotropical bats roosting in hot caves. Comparative Biochemistry and Physiology, 110, 347–355. 10.1016/0300-9629(94)00174-R [DOI] [PubMed] [Google Scholar]
- Rojas, D. , Vale, Á. , Ferrero, V. , & Navarro, L. (2011). When did plants become important to leaf‐nosed bats? Diversification of feeding habits in the family Phyllostomidae. Molecular Ecology, 20, 2217–2228. 10.1111/j.1365-294X.2011.05082.x [DOI] [PubMed] [Google Scholar]
- Ruby, J. , Nathan, P. T. , Balasingh, J. , & Kunz, T. H. (2000) Chemical composition of fruits and leaves eaten by short‐nosed fruit bat, Cynopterus sphinx.
- Ruf, T. , & Geiser, F. (2015). Daily torpor and hibernation in birds and mammals. Biological Reviews, 90, 1–70. 10.1111/brv.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, D. , Cistrone, L. , Budinski, I. , Console, G. , Della Corte, M. , Milighetti, C. , … Ancillotto, L. (2017). Sociality influences thermoregulation and roost switching in a forest bat using ephemeral roosts. Ecology and Evolution, 7, 5310–5321. 10.1002/ece3.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydell, J. , & Arlettaz, R. (1994). Low‐frequency echolocation enables the bat Tadarida teniotis to feed on tympanate insects. Proceedings of the Royal Society of London. Series B: Biological Sciences, 257, 175–178. [DOI] [PubMed] [Google Scholar]
- Salinas, R. V. B. , Herrera, M. L. G. , Flores‐Martínez, J. J. , & Johnston, D. S. (2014). Winter and summer torpor in a free‐ranging subtropical desert bat: The fishing myotis (Myotis vivesi). Acta Chiropterologica, 16, 327–336. 10.3161/150811014X687288 [DOI] [Google Scholar]
- Scholander, P. F. , Hock, R. , Walters, V. , & Johnson, F. (1950). Heat regulation in some arctic and tropical mammals and birds. Biological Bulletin, 99, 237–258. 10.2307/1538741 [DOI] [PubMed] [Google Scholar]
- Schwartz, C. , Hampton, M. , & Andrews, M. T. (2013). Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS ONE, 8(3), e58427 10.1371/journal.pone.0058427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi, M. , & Hemmati, Z. (2002). Variation in the diet of the Greater Mouse‐tailed Bat, Rhinopoma microphyllum (Chiroptera: Rhinopomatidae) in south‐western Iran In dry habitats, large and seasonally rich insect patches may support large colonies of bats, 7140.
- Simmons, N. B. (2005). An eocene big bang for bats. Evolution (New York), 307, 527–529. [DOI] [PubMed] [Google Scholar]
- Simmons, N. B. , & Geisler, J. H. (1998). Phylogenetic relationships of Icaronycteris, Archaeonycteris, Hassianycteris, and Palaeochiropteryx to extant bat lineages, with comments on the evolution of echolocation and foraging strategies in Microchiroptera. Bulletin of the American Museum of Natural History, 55, 4–182. [Google Scholar]
- Simmons, N. B. , Seymour, K. L. , Habersetzer, J. , & Gunnell, G. F. (2008). Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature, 451, 818–821. 10.1038/nature06549 [DOI] [PubMed] [Google Scholar]
- Smith, L. , & Hood, L. (1987). Mapping and sequencing the human genome: How to proceed. Nature Biotechnology, 5, 933–939. 10.1038/nbt0987-933 [DOI] [Google Scholar]
- Solari, S. , Hoofer, S. R. , Larsen, P. A. , Brown, A. D. , Bull, R. J. , Guerrero, J. A. , … Baker, R. J. (2009). Operational criteria for genetically defined species: analysis of the diversification of the small fruit‐eating bats, Dermanura (Phyllostomidae: Stenodermatinae). Acta Chiropterologica, 11, 279–288. 10.3161/150811009X485521 [DOI] [Google Scholar]
- Srivastava, R. K. , & Krishna, A. (2008). Seasonal adiposity, correlative changes in metabolic factors and unique reproductive activity in a vespertilionid bat, Scotophilus heathi . Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 309, 94–110. 10.1002/jez.440 [DOI] [PubMed] [Google Scholar]
- Stawski, C. , & Currie, S. E. (2016). Effect of roost choice on winter torpor patterns of a free‐ranging insectivorous bat. Australian Journal of Zoology, 64, 132–137. 10.1071/ZO16030 [DOI] [Google Scholar]
- Stawski, C. , & Geiser, F. (2012). Will temperature effects or phenotypic plasticity determine the thermal response of a heterothermic tropical bat to climate change? PLoS ONE, 7, e40278 10.1371/journal.pone.0040278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski, C. , Turbill, C. , & Geiser, F. (2009). Hibernation by a free‐ranging subtropical bat (Nyctophilus bifax). Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 179, 433–441. 10.1007/s00360-008-0328-y [DOI] [PubMed] [Google Scholar]
- Stawski, C. , Willis, C. K. R. , & Geiser, F. (2014). The importance of temporal heterothermy in bats. Journal of Zoology, 292, 86–100. 10.1111/jzo.12105 [DOI] [Google Scholar]
- Stehling, O. , Doring, H. , Ertl, J. , Preibisch, G. , & Schmidt, I. (1996). Leptin reduces juvenile fat stores by altering the circadian cycle of energy expenditure. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 40, R1770–R1774. 10.1152/ajpregu.1996.271.6.R1770 [DOI] [PubMed] [Google Scholar]
- Stier, S. C. , & Mildenstein, T. L. (2005). Dietary habits of the world’ s largest bats: The Philippine Flying Foxes, Acerodon jubatus and Pteropus vampyrus lanensis. Journal of Mammalogy, 86, 719–728. [Google Scholar]
- Swoap, S. J. (2008). The pharmacology and molecular mechanisms underlying temperature regulation and torpor. Biochemical Pharmacology, 76, 817–824. 10.1016/j.bcp.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , & Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10, 512–526. 10.1093/molbev/msl149 [DOI] [PubMed] [Google Scholar]
- Tattersall, G. J. , Sinclair, B. J. , Withers, P. C. , Fields, P. A. , Seebacher, F. , Cooper, C. E. , & Maloney, S. K. (2012). Coping with thermal challenges: Physiological adaptations to environmental temperatures. Comprehensive Physiology, 2, 2151–2202. 10.1002/cphy.c110055 [DOI] [PubMed] [Google Scholar]
- Tavaré, S. (1986). Some probabilistic and statistical problems in the analysis of DNA sequences. American Mathematical Society Lectures on Mathematics in the Life Sciences, 17, 57–86. [Google Scholar]
- Teeling, E. C. (2005) A molecular phylogeny of bats illuminates biogeography and the fossil record. Science, 307, 580–584. [DOI] [PubMed] [Google Scholar]
- Tessier, S. N. , & Storey, K. B. (2010). Expression of myocyte enhancer factor‐2 and downstream genes in ground squirrel skeletal muscle during hibernation. Molecular and Cellular Biochemistry, 344, 151–162. 10.1007/s11010-010-0538-y [DOI] [PubMed] [Google Scholar]
- Tinkle, D. W. , & Patterson, I. G. (1965). A study of hibernating populations of myotis velifer in Northwestern Texas. Journal of Mammalogy, 46, 612–633. 10.2307/1377932 [DOI] [PubMed] [Google Scholar]
- Turbill, C. , Law, B. S. , & Geiser, F. (2003). Summer torpor in a free‐ranging bat from subtropical Australia. Journal of Thermal Biology, 28, 223–226. 10.1016/S0306-4565(02)00067-0 [DOI] [Google Scholar]
- Twente, J. W. , & Twente, J. A. (1964). An hypothesis concerning the evolution of heterothermy in bats.
- van Breukelen, F. , & Martin, S. L. (2015). The hibernation continuum: Physiological and molecular aspects of metabolic plasticity in mammals. Physiology (Bethesda), 30, 273–281. 10.1152/physiol.00010.2015 [DOI] [PubMed] [Google Scholar]
- Van Der Merwe, M. (1973). Aspects of temperature and humidity in preferred hibernation sites of the natal clinging bat Miniopterus Schreibersi Natalensis (A. Smith, 1834). Zoologica Africana, 8, 121–134. 10.1080/00445096.1973.11447471 [DOI] [Google Scholar]
- Vaughan, N. (1997). The diets of British bats (Chiroptera). Mammal Review, 27, 77–94. 10.1111/j.1365-2907.1997.tb00373.x [DOI] [Google Scholar]
- Villanueva‐Cañas, J. L. , Faherty, S. L. , Yoder, A. D. , & Albà, M. M. (2014). Comparative genomics of mammalian hibernators using gene networks. Integrative and Comparative Biology, 54, 452–462. 10.1093/icb/icu048 [DOI] [PubMed] [Google Scholar]
- Vivier, L. , & Van Der Merwe, M. (2007). The incidence of torpor in winter and summer in the Angolan free‐tailed bat, Mops condylurus (Microchiroptera: Molossidae), in a subtropical environment, Mpumulanga, South Africa. African Zoology, 42, 50–58. 10.3377/1562-7020(2007)42[50:TIOTIW]2.0.CO;2. [DOI] [Google Scholar]
- Weigle, D. S. , Breen, P. A. , Matthys, C. C. , Callahan, H. S. , Meeuws, K. E. , Burden, V. R. , & Purnell, J. Q. (2005). A high‐protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. American Journal of Clinical Nutrition, 82, 41–48. 10.1093/ajcn/82.1.41 [DOI] [PubMed] [Google Scholar]
- Weterings, R. , Wardenaar, J. , Dunn, S. , & Umponstira, C. (2015). Dietary analysis of five insectivorous bat species from Kamphaeng Phet, Thailand. Raffles Bulletin of Zoology, 63, 91–96. 10.3161/150811010X537927 [DOI] [Google Scholar]
- Whitaker, J. O. , & Karataş, A. (2009). Food and feeding habits of some bats from Turkey. Acta Chiropterologica, 11, 393–403. 10.3161/150811009X485611 [DOI] [Google Scholar]
- Willis, C. K. R. , Brigham, R. M. , & Geiser, F. (2006) Deep, prolonged torpor by pregnant, free‐ranging bats. Naturwissenschaften, 93(2), 80–83. 10.1007/s00114-005-0063-0 [DOI] [PubMed] [Google Scholar]
- Wilz, M. , & Heldmaier, G. (2000). Comparison of hibernation, estivation and daily torpor in the edible dormouse, Glis glis. Journal of Comparative Physiology B, 170, 511–521. 10.1007/s003600000129 [DOI] [PubMed] [Google Scholar]
- Yan, J. , Barnes, B. M. , Kohl, F. , & Marr, T. G. (2008). Modulation of gene expression in hibernating arctic ground squirrels. Physiological Genomics, 32, 170–181. 10.1152/physiolgenomics.00075.2007 [DOI] [PubMed] [Google Scholar]
- Yang, Z. , & Nielsen, R. (2002). Codon‐substitution models for detecting molecular adaptation at individual sites along specific lineages. Molecular Biology and Evolution, 19, 908–917. 10.1093/oxfordjournals.molbev.a004148 [DOI] [PubMed] [Google Scholar]
- Yang, J. , Wang, Z. L. , Zhao, X. Q. , Wang, D. P. , Qi, D. L. , Xu, B. H. , … Tian, H. F. (2008). Natural selection and adaptive evolution of leptin in the Ochotona family driven by the cold environmental stress. PLoS ONE, 3(1), e1472 10.1371/journal.pone.0001472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, R. A. (2001). The eastern horseshoe bat, Rhinolophus megaphyllus, in south‐east Queensland, Australia: Colony demography and dynamics, activity levels, seasonal weight changes, and capture‐recapture analyses. Wildlife Research, 28(4), 425–434. [Google Scholar]
- Yuan, L. , Zhao, X. , Lin, B. , Rossiter, S. J. , He, L. , Zuo, X. , … Zhang, S. (2011). Adaptive evolution of leptin in heterothermic bats. PLoS ONE, 6(11), e27189 10.1371/journal.pone.0027189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidan, N. K. K. S. (1980). Aging phenomena: Relationships among different levels of organization. New York, NY: Plenum Press. [Google Scholar]
- Zhang, G. , Cowled, C. , Shi, Z. , Huang, Z. , Bishop‐Lilly, K. A. , Fang, X. , … Wang, J. (2013) Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science, 339(6118), 456–460. 10.1126/science.1230835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Kerman, I. A. , Laque, A. , Nguyen, P. , Faouzi, M. , Louis, G. W. , … Munzberg, H. (2011). Leptin‐receptor‐expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. Journal of Neuroscience, 31, 1873–1884. 10.1523/JNEUROSCI.3223-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Pan, Y.‐H. , Yin, Q. , Yang, T. , Dong, D. , Liao, C.‐C. , & Zhang, S. (2014). Critical roles of mitochondria in brain activities of torpid Myotis ricketti bats revealed by a proteomic approach. Journal of Proteomics, 105, 266–284. 10.1016/j.jprot.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Zhu, T. , Yuan, L. , Jones, G. , Hua, P. , He, G. , Chen, J. , & Zhang, S. (2014). OB‐RL silencing inhibits the thermoregulatory ability of Great Roundleaf Bats (Hipposideros armiger). General and Comparative Endocrinology, 204, 80–87. 10.1016/j.ygcen.2014.04.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data are available on GENBANK (https://www.ncbi.nlm.nih.gov/genbank/) with accession numbers listed in Table 1. Log likelihoods for the thermoregulatory regime at each internal node in the phylogeny under the Mkn model are available in Supporting Information Table S1. All other data can be accessed through Dryad or by contacting Margaret Lazzeroni.


