Abstract
Introduction
High-dose corticosteroids remain the first-line therapy for focal and segmental glomerulosclerosis (FSGS), whereas calcineurin inhibitors (CNIs) are reserved for those patients resistant to corticosteroid therapy.
Methods
This is a retrospective cohort analysis in patients with primary FSGS diagnosed between 2007 and 2014. According to the administered treatment, patients were segregated into 3 groups: high-dose prednisone, first-line CNIs plus low-dose prednisone, and rescue CNIs. Cumulative corticosteroid doses were compared as well as response to therapy and long-term renal survival by Cox regression analysis.
Results
A total of 66 patients were included (39 treated with high-dose prednisone, 11 treated with first-line CNI, 16 treated with high-dose prednisone followed by rescue CNI). Cumulative doses of prednisone in the high-dose group were 9.3 g (interquartile range [IQR] = 7.5−12.5 g), compared to 2.5 g (IQR = 1.82−3.12 g) in the first-line CNI plus low-dose corticosteroid group and 13.8 g (IQR = 9.2−15.8 g) rescue CNI groups, respectively (P < 0.001). Time under corticosteroid management was also higher in the high-dose prednisone group compared to the first-line CNI group. There was a response to treatment in 76.9%, 72.7%, and 87.5% of high-dose prednisone, first-line CNI and rescue CNI groups, with complete remission in 48.7%, 36.4%, and 31.3% respectively. There was no difference in relapse incidence after treatment (48.4%, 44.4%, and 46.7%) or in 5-year renal survival (87.2%, 81.8%, and 87.5%). Baseline proteinuria, biopsy chronicity score, and response to therapy were independent predictors of renal survival.
Conclusion
An initial CNI plus low-dose corticosteroid approach in primary FSGS reduces corticosteroid exposure with a response-to-therapy rate similar to that of the currently recommended high-dose corticosteroid regimen. These findings justify a randomized trial to formally test this hypothesis.
Keywords: calcineurin inhibitor, corticosteroids, focal segmental glomerulosclerosis, FSGS, nephrotic syndrome
See Commentary on Page 8
FSGS constitutes the main cause of nephrotic syndrome in some regions of the world, including the United States and Canada.1, 2, 3 It is also the most common primary glomerular disease leading to end-stage renal disease in the United States.4, 5
The goal of therapy in FSGS is to induce a complete or partial remission of proteinuria with long-term preservation of renal function. Both complete and partial remission have been associated with improved long-term survival.6, 7, 8, 9, 10, 11, 12, 13
High-dose corticosteroids remain the first-line therapy for primary FSGS when associated with the nephrotic syndrome,14 with response rates varying from 42% to 62%.9, 10, 11, 12, 13 Based on the observation that prolonged corticosteroid courses (>16 weeks) are associated with a better response,10 current guidelines recommend treatment with high-dose corticosteroids (prednisone 1 mg/kg per day and up to 80 mg/d) for 4 to 16 weeks with subsequent slow tapering over a few months. Steroid resistance is defined as little or no reduction in proteinuria after 12 to 16 weeks of treatment.14
CNIs as a first-line therapy are reserved for patients at high risk for steroid-induced complications, such as those with diabetes, uncontrolled psychiatric disorders, or severe osteoporosis.14 Recently, FSGS CNI first-line therapy was reported to be numerically superior to glucocorticoids in preventing ESRD in an observational cohort15; however, the authors provided no data on response rates, number of relapses, and glucocorticoid exposure. Two additional small reports have reported a response in 6 of 6 and in 6 of 7 patients treated with first-line tacrolimus16 or cyclosporine,17 respectively.
Reduction of corticosteroid exposure is currently a relevant focus in the management of glomerular diseases, given that high-dose corticosteroids are known to be associated with a multiplicity of adverse effects, especially when prescribed in prolonged courses and high doses, as is the case for FSGS.18, 19
In the last few years, our center started managing patients with primary FSGS with CNI associated with low-dose corticosteroids as a first-line therapy, with the goal of reducing corticosteroid exposure. The aim of this study was to compare response to therapy and cumulative corticosteroid exposure between patients with first-line CNI, those with high-dose corticosteroids, and those with CNI added for steroid-resistant disease.
Methods
Subjects
In this retrospective cohort study, we reviewed the charts of all patients with biopsy-proven primary FSGS who were seen at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City from 2007 to 2014 and who completed at least 24 months of follow-up.
Primary FSGS was defined by clinical and histopathological criteria. Clinical criteria included presentation with nephrotic syndrome and absence of a recognized secondary etiology (e.g., infectious agents, drugs, morbid obesity, etc.). Histopathological criteria included diffuse foot process effacement >80% by electron microscopy (available in 29 patients) and absence of secondary FSGS features by light microscopy examination.
Treatment Groups
Patients were divided into 3 treatment groups: high-dose prednisone, first-line CNI plus low-dose prednisone (first-line CNI), and first-line high-dose prednisone plus added CNI rescue for steroid-resistant disease (rescue CNI). Patients treated with high-dose prednisone received 0.8 to 1.0 mg/kg prednisone for at least 12 weeks with subsequent tapering. The first-line CNI group received treatment with either cyclosporine or tacrolimus, aiming for serum levels between 100 and 180 ng/ml and between 5 and 7 ng/ml, respectively, with 0.10 to 0.15 mg/kg per day of prednisone. The rescue CNI group received 0.8 to 1.0 mg/kg prednisone for 12 weeks, the time at which proteinuria was evaluated and, if there was a reduction of <25%, a CNI was added to the therapy.
Outcomes
The 3 groups were evaluated for cumulative prednisone dose, complete remission, partial remission, time to relapse, progression to end-stage renal disease (ESRD), and adverse events as registered in the patient records.
Complete remission (CR) was defined as stable renal function (no less than 15% of baseline estimated glomerular filtration rate) plus 24-hour urine protein-to-creatinine ratio <0.3 g/g. Partial remission (PR) was defined as stable renal function plus 50% reduction of proteinuria to subnephrotic range. Relapse was defined as doubling of the previous urine protein-to-creatinine ratio to a proteinuria >3.5 g/g observed on 2 consecutive visits, with or without features of the nephrotic syndrome. Doubling of serum creatinine was defined as doubling of the lowest serum creatinine value achieved during the first 6 months of therapy. End-stage renal disease was defined as estimated glomerular filtration rate of <10 ml/min or requirement of renal replacement therapy.
Predictors
We obtained data on patients’ clinical courses. Baseline evaluation was considered at the time of the diagnostic kidney biopsy. Data collected included age, gender, body mass index, mean arterial pressure, serum creatinine and proteinuria (quantified by 24-hour collections) at baseline and every 6 months during follow-up, hemoglobin, platelets, hematuria (defined as >5 erythrocytes per high-power field), serum albumin, triglycerides, cholesterol, and serum complement C3 and C4.
Histopathological parameters included percentage of global/segmental sclerosis, mesangial expansion, interstitial fibrosis, tubular atrophy, and vascular and immunofluorescence findings. A chronicity score was calculated based on a recently proposed scale20 that considers total glomerulosclerosis (global plus segmental), interstitial fibrosis, tubular atrophy, and intimal thickening. Treatment data were collected throughout the duration of each patient’s clinical course.
Statistical Analysis
Data distribution was evaluated by the Shapiro−Wilk test. Baseline data are described as number and relative frequencies or as median and interquartile range, as appropriate. For baseline comparisons, the χ2, Fisher exact, or Kruskal−Wallis test was used as appropriate. For response to therapy outcomes (complete/partial remission), patients were censored at 24 months of follow-up and compared by log-rank test for time to outcome. For end-stage renal disease, we used Cox proportional hazards models over the entire duration of follow-up. To obtain independent predictors for outcome, we first used a univariate model, and all variables with P < 0.100 were included in the final multivariate analysis, which included age, gender, baseline creatinine and proteinuria, response to therapy, and chronicity score.
All analyses were performed with JMP Pro 12.0 Statistical Software (SAS Institute Inc., Cary, NC) and GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Statistical significance was considered with a P value <0.05.
Results
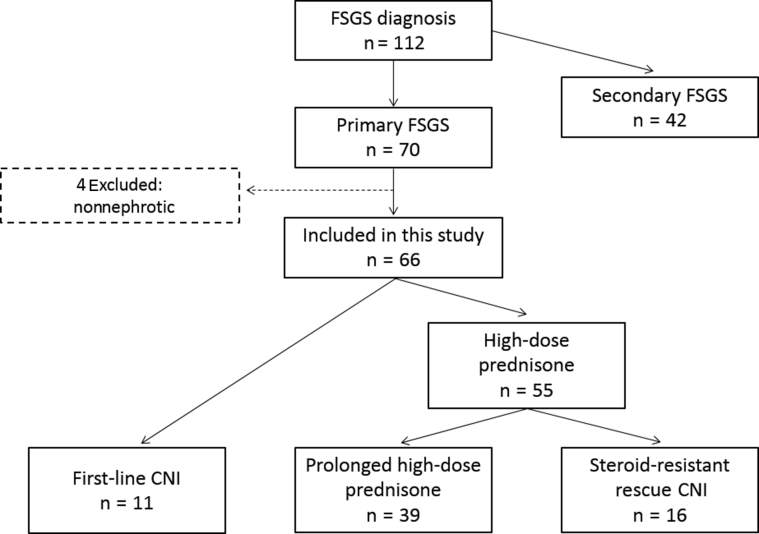
From 2007 to 2014, a total of 112 patients were diagnosed with FSGS. Of these patients, 70 (63%) were classified as having primary FSGS. Among them, 4 patients had nonnephrotic proteinuria and were excluded. A total of 66 patients were selected for the study and divided into 3 treatment groups (Figure 1): high-dose prednisone (n = 39), first-line CNI (n = 11), and rescue CNI (n = 16). The median follow-up for this cohort was 51 months (IQR = 30−77).
Figure 1.
Patient flowchart.
Baseline Characteristics
Baseline characteristics are shown in Table 1. Patients in the high-dose prednisone group were older (P = 0.041), but there were no significant differences in any other variables such as proteinuria, serum creatinine, or coexistence of acute kidney injury at presentation. Histopathological characteristics are shown in Table 2. There were no differences in the FSGS subtypes according to the Columbia classification, the percentage of sclerosis, interstitial fibrosis, or tubular atrophy.
Table 1.
Characteristics of the studied cohort
| Variable | High-dose prednisone n = 39 |
First-line CNI n = 11 |
Rescue CNI n = 16 |
P value |
|---|---|---|---|---|
| Age, yr | 37 (24–46) | 23 (18–31) | 29 (24–41) | 0.041 |
| Male, n (%) | 21 (53.8) | 3 (36.4) | 8 (50.0) | 0.592 |
| Body mass index, kg/m2 | 25.6 (23.0–28.7) | 28.3 (24.7–33.5) | 26.9 (24.4–31.4) | 0.257 |
| Mean arterial pressure, mm Hg | 97 (87–107) | 92 (83–103) | 98 (91–103) | 0.669 |
| Hemoglobin, g/dl | 14.9 (13.0–16.9) | 13.7 (12.4–16.9) | 15.4 (14.4–16.5) | 0.508 |
| Platelets, per mm3 | 287 (214–376) | 304 (216–370) | 310 (268–381) | 0.790 |
| Serum creatinine, mg/dl | 1.3 (0.7–1.8) | 1.3 (0.6–1.7) | 1.0 (0.6–1.4) | 0.639 |
| Estimated GFR, ml/min per 1.73 m2 | 60 (41–115) | 58 (48–122) | 79 (66–112) | 0.495 |
| Proteinuria, g/24 h | 4.9 (3.5–8.0) | 7.4 (3.6–11.9) | 6.7 (3.8–11.0) | 0.449 |
| Protein to creatinine ratio, g/g | 3.7 (3.2–7.3) | 4.9 (3.7–7.6) | 5.8 (3.6–8.7) | 0.359 |
| Protein to creatinine ratio at CNI start, g/ga | — | 4.9 (3.7–7.6) | 5.7 (4.1–9.3) | 0.544 |
| Serum albumin, g/dl | 2.6 (1.6–2.9) | 2.9 (2.3–3.3) | 2.4 (1.0–2.8) | 0.072 |
| Triglycerides, mg/dl | 261 (211–329) | 314 (201–341) | 270 (219–316) | 0.927 |
| Cholesterol, mg/dl | 262 (198–338) | 282 (212–341) | 325 (253–483) | 0.188 |
| Acute kidney injury, n (%) | 3 (7.7) | 1 (9.1) | 1 (6.3) | 0.962 |
CNI, calcineurin inhibitor; GFR, glomerular filtration rate estimated from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Protein-to-creatinine ratio at CNI start in the rescue CNI group represents the proteinuria after steroid treatment when the definition for resistance disease was met.
Table 2.
Histological parameters of the cohort
| Variable | High-dose prednisone n = 39 |
First-line CNI n = 11 |
Rescue CNI n = 16 |
P value |
|---|---|---|---|---|
| Columbia classification | 0.233 | |||
| NOS | 18 (45.2) | 4 (36.4) | 4 (25.0) | 0.360 |
| Tip | 6 (15.4) | 2 (18.2) | 5 (31.3) | 0.422 |
| Perihiliar | 11 (28.2) | 4 (36.4) | 3 (18.8) | 0.635 |
| Cellular | 4 (25.6) | 1 (9.1) | 4 (25.0) | 0.367 |
| Global sclerosis (%) | 10 (0–22) | 14 (0–33) | 3 (0–16) | 0.358 |
| Segmental sclerosis (%) | 17 (4–30) | 14 (7–27) | 13 (7–28) | 0.898 |
| Interstitial fibrosis (%) | 20 (10–40) | 10 (10–50) | 13 (10–38) | 0.741 |
| Tubular atrophy (%) | 20 (10–40) | 10 (5–50) | 13 (6–38) | 0.749 |
| Vascular subintimal fibrosis | 17 (43.6) | 3 (27.3) | 6 (37.5) | 0.610 |
| Vascular medial hyperplasia | 12 (30.8) | 2 (18.2) | 5 (31.3) | 0.696 |
CNI, calcineurin inhibitor; NOS, not otherwise specified variant.
Treatment and Corticosteroid Exposure
There were no differences in adjunctive therapies (angiotensin-converting enzyme inhibitors, statins) among the 3 groups (Table 3). Median average cyclosporine or tacrolimus serum levels were 134 ng/ml (IQR = 92−186 ng/ml) and 5.2 ng/ml (IQR = 4.0−7.9 ng/ml), respectively, in the first-line CNI group, and 128 ng/ml (IQR = 92−172 ng/ml) and 5.8 ng/ml (IQR = 4.4−7.3 ng/ml), respectively, in the rescue-CNI group (P = 0.698 and P = 0.771). The total duration under CNI treatment was 12 months (6−16 months) and 16 months (9−25 months) in the first-line CNI and rescue CNI group, respectively.
Table 3.
Immunosuppression and adjunctive treatment
| Variable | High-dose prednisone n = 39 |
First-line CNI n = 11 |
Rescue CNI n = 16 |
P value |
|---|---|---|---|---|
| Prednisone | ||||
| Months on prednisone | 13 (7–22) | 8 (6–12) | 24 (12–28) | <0.001 |
| Cumulative prednisone, g | 9.3 (7.5–12.5) | 2.5 (1.8–3.1) | 13.8 (9.2–15.8) | <0.001 |
| Cumulative prednisone, mg/kg | 134 (112–180) | 36 (26–39) | 177 (145–209) | <0.001 |
| Months to taper <10 mg | 7 (6–12) | 6 (4–7) | 11 (6–18) | 0.003 |
| Calcineurin inhibitor | ||||
| Tacrolimus | — | 5 (45.5) | 8 (50.0) | 0.816 |
| Avg plasma level, ng/dl | — | 5.2 (4.0–7.9) | 5.8 (4.4–7.3) | 0.698 |
| Cyclosporine | — | 6 (54.5) | 8 (50.0) | 0.816 |
| Avg plasma level, ng/dl | — | 134 (92–186) | 128 (92–172) | 0.771 |
| Months on CNI | — | 12 (6–16) | 16 (9–25) | 0.174 |
| Adjunctive treatment | ||||
| RAAS blockade | 33 (84.6) | 9 (81.8) | 15 (93.8) | 0.596 |
| Statins | 21 (53.8) | 5 (45.5) | 10 (62.5) | 0.676 |
Avg, average; CNI, calcineurin inhibitor; RAAS, renin−angiotensin−aldosterone system.
Cumulative doses of prednisone in the high-dose corticosteroid group were 9.3g (IQR = 7.5−12.5g) compared to 2.5 g (IQR = 1.82−3.12g) in the first-line CNI with low-dose corticosteroid group and 1.8 g (IQR = 9.2−15.8g) in the rescue CNI group, respectively (P < 0.001). This was also reflected in the tapering months to a prednisone dose <10 mg: 7 months (IQR = 6−12 months) in the high-dose prednisone group, 6 months (IQR = 4−7 months) in the first-line CNI group, and 11 months (IQR = 6−18 months) in the rescue CNI group (P < 0.001). The total duration of prednisone exposure was 13 months (min 7, max 22 months), 8 months (min 6, max 12 months), and 24 months (min 12, max 28 months) for the high-dose prednisone, first-line CNI, and rescue CNI groups, respectively.
Treatment Outcomes
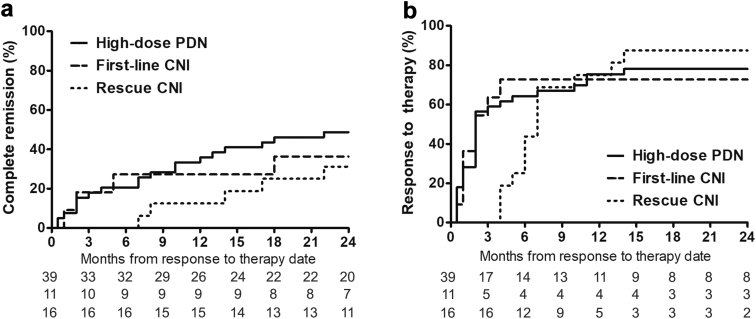
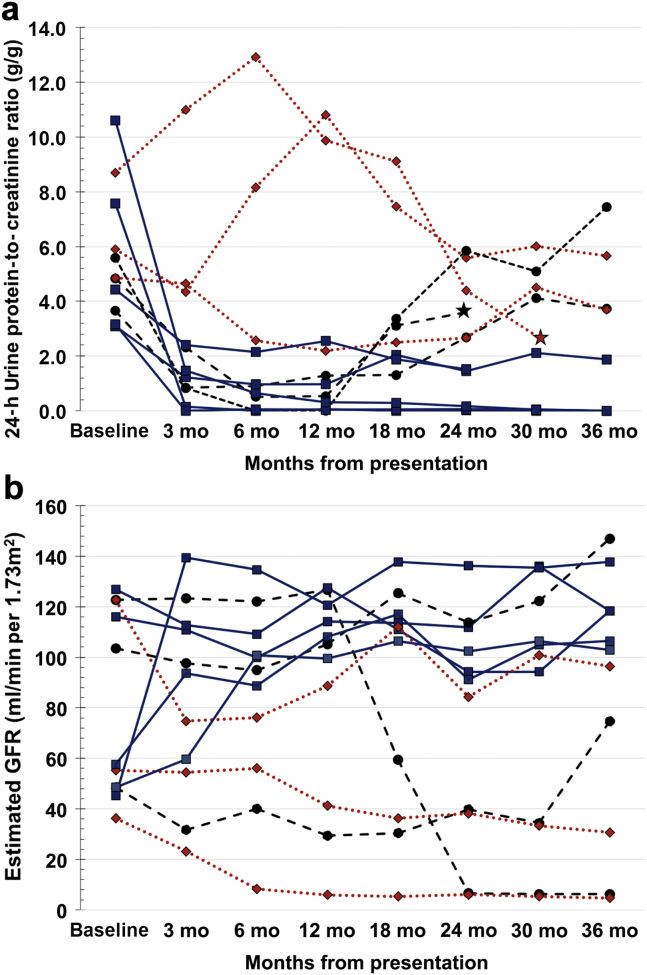
Response to treatment (either complete or partial response) was censored at 24 months from the start of therapy. At this point in time, a response to treatment was observed in 30 (76.9%), 8 (72.7%), and 14 (87.5%) patients in the high-dose prednisone, first-line CNI, and rescue CNI groups, respectively (Figure 2). Complete remission was observed in 19 (48.7%), 4 (36.4%), and 5 (31.3%) patients in the high-dose prednisone, first-line CNI, and rescue CNI groups (Figure 2), whereas partial remission occurred in 11 (28.2%), 4 (36.4%), and 9 (56.3%) patients, respectively. The individual course of proteinuria and estimated glomerular filtration rate from patients in the first-line CNI group is shown in Figure 3.
Figure 2.
(a) Complete remission and (b) response to therapy.
Figure 3.
(a) Individual 24-hour urine protein-to-creatinine ratio and (b) estimated glomerular filtration rate in the first-line calcineurin inhibitor (CNI) group. Continuous lines represent patients who responded to therapy; dotted lines, patients with resistant disease; dashed lines, patients with initial response followed by relapse; stars, patients who progressed to end-stage renal disease.
The median time to first response to therapy was 2 (IQR = 1−10 months), 2 (IQR = 1−4 months), and 4 (IQR = 3−7 months) months after the start of therapy in the high-dose prednisone, first-line CNI, and rescue CNI groups, respectively (log-rank test P = 0.901).
Among the first-line CNI group, there were no differences in response to therapy between cyclosporine-treated patients (2 complete and 2 partial responses) and tacrolimus-treated patients (2 complete and 2 partial responses).
Relapses
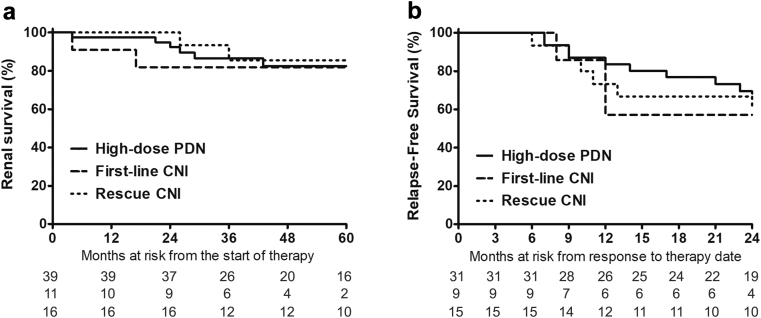
A total of 26 (47.3%) relapses occurred in 55 patients who responded to therapy during a median of 25 months (12−46 months) time-at−risk of relapse. The incidence of relapse was 48.4%, 44.4%, and 46.7% in the high-dose prednisone, first-line CNI, and rescue CNI groups, respectively (Figure 4). There were no differences in time to relapse among the groups (P = 0.548).
Figure 4.
Renal (a) survival and (b) disease relapses. CNI, calcineurin inhibitor; PDN, prednisone.
Renal Function
There were no differences in the estimated glomerular filtration rate slope in the first 2 years among the groups: high-dose prednisone, −1.5 (−5.1 to +8.9) ml/min per year; first-line CNI, −4.1 (−17.1 to +16.5) ml/min per year; and rescue CNI, −3.0 (−14.0 to +23.9) ml/min per year (P = 0.496). The 5-year renal survival in the whole cohort was 82.4%. A total of 5 (12.8%), 2 (18.2%), and 2 (12.5%) patients doubled their serum creatinine, whereas 3 (7.7%), 2 (18.2%), and 1 (6.3%) progressed to ESRD (Figure 4). The independent renal survival predictors in the Cox multivariate model (Table 4) were the presentation proteinuria (hazard ratio [HR] = 1.26, 95% confidence interval [CI] = 1.05−1.51), chronicity score of the diagnostic biopsy (HR = 1.56, 95% CI = 1.05−2.31), and a complete response to therapy (HR = 0.02, 95% CI = 0.01−0.21).
Table 4.
Cox regression analysis for factors associated with progression to end-stage renal disease
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, per yr | 0.979 | 0.934–1.026 | 0.373 | — | — | — |
| Male gender, versus female | 0.588 | 0.176–1.967 | 0.389 | — | — | — |
| Creatinine, per mg/dl | 1.622 | 1.064–2.474 | 0.025 | — | — | — |
| Proteinuria, per g/g | 1.111 | 0.992–1.244 | 0.070 | 1.259 | 1.052–1.507 | 0.012 |
| Chronicity score, per point | 1.510 | 1.122–2.033 | 0.007 | 1.558 | 1.052–2.305 | 0.027 |
| Response to treatment | ||||||
| No response | 1.000 | Ref | Ref | 1.000 | Ref | Ref |
| Partial response | 0.214 | 0.060–0.768 | 0.018 | 0.356 | 0.077–1.652 | 0.187 |
| Complete response | 0.026 | 0.003–0.220 | 0.001 | 0.023 | 0.001–0.212 | 0.002 |
CI, confidence interval; HR, hazard ratio; Ref, reference.
Adverse Events
All the adverse events reported during the first 36 months of follow-up are shown in Table 5. There were no differences in serious adverse events leading to hospitalization. The number of total infectious events not requiring hospitalization was higher but not statistically significant in the high-dose prednisone group. Renal events as a group, including acute kidney injury and uncontrolled blood pressure, were more frequent in the CNI-first (P = 0.0928) and rescue CNI (P = 0.0266) groups. Ophthalmic events were observed exclusively in the high-prednisone group.
Table 5.
Adverse events reported during treatment and follow-up
| Variable | Steroids n = 39 |
CNI first n = 11 |
Rescue CNI n = 16 |
|---|---|---|---|
| Events requiring hospitalization | 8 (20.5) | 2 (18.2) | 5 (31.3) |
| Pneumonia | 4 (10.3) | 1 (9.0) | 1 (6.3) |
| Bacteremia | 1 (2.6) | 1 (9.0) | 2 (12.5) |
| Cytomegalovirus colitis | 1 (2.6) | — | — |
| Acute cholecystitis | 1 (2.6) | — | 1 (6.3) |
| Acute cerebrovascular event | — | — | 1 (6.3) |
| Upper GI hemorrhage | 1 (2.6) | — | — |
| Infectious events (not hospitalized) | 18 (46.2) | 3 (27.3) | 4 (25.0) |
| Upper respiratory tract infection | 10 (25.6) | 2 (18.2) | 2 (12.5) |
| Acute diarrhea | 4 (10.3) | 1 (9.0) | 1 (6.3) |
| Cellulitis | 3 (7.7) | — | 1 (6.3) |
| Oral candidiasis | 1 (2.6) | — | — |
| Renal events | |||
| Acute kidney injury | 4 (10.3) | 1 (9.0) | 4 (25.0) |
| Uncontrolled blood pressure | 1 (2.6) | 3 (27.3) | 3 (18.8) |
| Metabolic events | |||
| Weight gain >10% baseline | 3 (7.7) | 1 (9.0) | 1 (6.3) |
| Exogenous Cushing features | 5 (12.8) | 1 (9.0) | 3 (18.8) |
| Secondary diabetes | 1 (2.6) | — | 1 (6.3) |
| Ophthalmic events | |||
| Cataract | 1 (2.6) | — | — |
| Glaucoma | 1 (2.6) | — | — |
| Others | |||
| Acidopeptic symptoms | 4 (10.3) | 1 (9.0) | 3 (18.8) |
| Psychiatric symptomsa | 5 (12.8) | — | 2 (12.5) |
| Inferior vena cava thrombosis | 1 (2.6) | — | — |
GI, gastrointestinal.
Psychiatric symptoms included depression or anxiety requiring pharmacological intervention.
Discussion
Calcineurin inhibitor use in FSGS treatment has been reserved mainly for steroid-resistant patients, whereas first-line therapy has scarcely been studied. Here we report a similar efficacy of a first-line CNI approach in terms of response to therapy compared to a high-dose prednisone regimen. Furthermore, the first-line CNI therapy was associated with 4 times lower exposure to corticosteroids and a relapse rate similar to that associated with high-dose corticosteroid therapy.
Focal and segmental glomerulosclerosis remains the most common nephrotic syndrome etiology in many countries, comprising close to 40% of cases in adults.1, 21 However, evidence for treatment proceeds from small randomized controlled trials8, 22, 23, 24 and observational studies.6, 10, 12, 13, 15, 25, 26, 27 Prolonged courses of treatment, based mainly on high-dose corticosteroids (1 mg/kg for 8−16 weeks) have resulted in response rates that range from 40% to 70% of patients; however, steroid exposure from currently recommended regimens14 remains among the highest for primary glomerulonephritis with the consequent risk for development of steroid adverse events.18, 19
A recent focus in the management of glomerular diseases is the decrease in steroid exposure. Here we showed that a CNI-first approach decreases prednisone exposure up to 4 times with similar rates of response to treatment. A previous report by Laurin et al.15 suggested that a CNI-first approach is effective in preventing progression to ESRD. Small reports have shown a partial response in 6 of 6 patients treated with tacrolimus alone16 and in 6 of 7 patients treated with cyclosporine combined with steroids.17 We observed a response rate of 73% with this approach and complete remission rates of 36%. These numbers are relatively high, which may relate to the young age of our cohort.
The prevalence of steroid resistance has been estimated at 30% to 40%. In this cohort, 36% of our patients were classified as steroid resistant. We observed a response to CNI in 88% of these patients. This group of patients received a rescue CNI approach combined with low-dose corticosteroids, yet the cumulative prednisone dose was the highest among those in the 3 treatment groups. Treatment response was higher than those reported for corticosteroid-resistant patients, which have been estimated between 48% and 83%.23, 24, 26, 28, 29 The differences may be due to different definitions of steroid-resistant disease, as we defined resistance after 12 weeks of high-dose corticosteroid treatment, whereas others suggest waiting up to 16 weeks. It has not been studied whether a CNI-first approach may benefit patients deemed to be steroid resistant; however, predictors of steroid resistance have not been completely defined, and it is therefore difficult to predict treatment resistance in advance.
Relapse after CNI suspension has been a major hurdle preventing first-line CNI use. The incidence of relapse has been reported to be between 53% and 78% of patients once CNI inhibitors are suspended in steroid-resistant patients.8, 22, 23, 26, 28 We observed a similar relapse rate between patients treated with a prolonged course of high-dose corticosteroids and those with a CNI-first approach.
Five-year progression to ESRD has been reported in 20% to 30% of patients.6, 8, 11, 12, 13, 22, 25, 27, 30 Those who respond to treatment are less likely to progress compared to those without response or without treatment.6, 8, 9, 10, 11, 12, 13, 15, 27 In this study, none of the patients who responded to treatment progressed to ESRD, and this constituted an independent renal survival predictor. Although there were no differences in progression to ESRD between the high-dose prednisone and the first-line CNI groups, a numerically higher percentage of patients in the CNI-first group progressed to ESRD. A possible impact of a first-line CNI approach in preventing ESRD progression has been reported by Laurin et al.15 in 90 patients treated with a CNI-first approach (HR = 0.42, 95% CI = 0.15−1.18). However, a larger, long-term study is required to fully address the effect of CNI-first treatment on hard renal outcomes.
As it is not our local practice, a CNI approach without steroids was not evaluated in this study. As we reported, even the CNI-first combined with low-dose prednisone group had a median 2.5-g cumulative prednisone dose, and adverse events attributable to corticosteroid use were still observed. An exploratory study is currently underway to address this approach (AURONA trial, NCT03598036).
Finally, as previously reported, the best independent predictors for long-term prognosis were the grade of proteinuria at presentation, the chronicity score observed in the diagnostic biopsy (which includes the percentage of glomerular sclerosis, tubular atrophy, and interstitial fibrosis), and the patient’s response to therapy.
This study has limitations. Due to its retrospective nature and even when baseline characteristics were similar, a time bias cannot be excluded, as patients with the CNI-approach were treated in the most recent years. The effect of supportive therapies such as arterial pressure control, renin−angiotensin−aldosterone system blockade, and lipid control were not completely assessed and controlled for. As previously mentioned, the number of patients was small for determining hard renal endpoints. The follow-up was close to 5 years, which may limit its power to determine long-term prognosis, especially for the CNI groups in whom prolonged CNI toxicity may be of concern.
In conclusion, a first-line CNI plus low-dose prednisone approach in FSGS reduces corticosteroid exposure with therapy response rates similar to those associated with the currently recommended high-dose corticosteroid regimen. However, long-term preservation of renal function with this approach still remains to be addressed. These findings justify a randomized clinical trial to formally test this hypothesis.
Disclosure
All the authors declared no competing interests.
Acknowledgements
We thank Instituto Mexicano de Investigaciones Nefrológicas for their support for the publication of the present work.
References
- 1.Haas M., Meehan S.M., Karrison T.G., Spargo B.H. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis. 1997;30:621–631. doi: 10.1016/s0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 2.Korbet S.M., Genchi R.M., Borok R.Z., Schwartz M.M. The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis. 1996;27:647–651. doi: 10.1016/s0272-6386(96)90098-0. [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy M.M., Hogan S.L., Thompson B.D. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol Dial Transplant. 2018;33:661–669. doi: 10.1093/ndt/gfx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.USRDS: United States Renal Data System 2017 Annual Data Report. https://www.usrds.org/ Available at: Accessed October 1, 2018.
- 5.O’Shaughnessy M.M., Montez-Rath M.E., Lafayette R.A., Winkelmayer W.C. Patient characteristics and outcomes by GN subtype in ESRD. Clin J Am Soc Nephrol. 2015;10:1170–1178. doi: 10.2215/CJN.11261114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rydel J.J., Korbet S.M., Borok R.Z., Schwartz M.M. Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis. 1995;25:534–542. doi: 10.1016/0272-6386(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 7.Korbet S.M. Primary focal segmental glomerulosclerosis. J Am Soc Nephrol. 1998;9:1333–1340. doi: 10.1681/ASN.V971333. [DOI] [PubMed] [Google Scholar]
- 8.Cattran D.C., Appel G.B., Hebert L.A. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 9.Pei Y., Cattran D., Delmore T. Evidence suggesting under-treatment in adults with idiopathic focal segmental glomerulosclerosis. Regional Glomerulonephritis Registry Study. Am J Med. 1987;82:938–944. doi: 10.1016/0002-9343(87)90155-0. [DOI] [PubMed] [Google Scholar]
- 10.Ponticelli C., Villa M., Banfi G. Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? Am J Kidney Dis. 1999;34:618–625. doi: 10.1016/S0272-6386(99)70384-7. [DOI] [PubMed] [Google Scholar]
- 11.Troyanov S. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 12.Chun M.J. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15:2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 13.Deegens J.K.J., Steenbergen E.J., Borm G.F., Wetzels J.F.M. Pathological variants of focal segmental glomerulosclerosis in an adult Dutch population epidemiology and outcome. Nephrol Dial Transplant. 2007;23:186–192. doi: 10.1093/ndt/gfm523. [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 15.Laurin L.P., Gasim A.M., Poulton C.J. Treatment with glucocorticoids or calcineurin inhibitors in primary FSGS. Clin J Am Soc Nephrol. 2016;11:386–394. doi: 10.2215/CJN.07110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan N., Dhaygude A., Owen J. Treatment of focal and segmental glomerulosclerosis in adults with tacrolimus monotherapy. Nephrol Dial Transplant. 2004;19:3062–3067. doi: 10.1093/ndt/gfh536. [DOI] [PubMed] [Google Scholar]
- 17.Goumenos D.S., Tsagalis G., El Nahas A.M. Immunosuppressive treatment of idiopathic focal segmental glomerulosclerosis: a five-year follow-up study. Nephron Clin Pract. 2006;104:c75–c82. doi: 10.1159/000093993. [DOI] [PubMed] [Google Scholar]
- 18.Hoes J.N., Jacobs J.W.G., Verstappen S.M.M. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann Rheum Dis. 2009;68:1833–1838. doi: 10.1136/ard.2008.100008. [DOI] [PubMed] [Google Scholar]
- 19.Fardet L., Fève B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74:1731–1745. doi: 10.1007/s40265-014-0282-9. [DOI] [PubMed] [Google Scholar]
- 20.Sethi S., D’Agati V.D., Nast C.C. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91:787–789. doi: 10.1016/j.kint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 21.O’Shaughnessy M.M., Hogan S.L., Poulton C.J. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986–2015. Clin J Am Soc Nephrol. 2017;12:614–623. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponticelli C., Rizzoni G., Edefonti A. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int. 1993;43:1377–1384. doi: 10.1038/ki.1993.194. [DOI] [PubMed] [Google Scholar]
- 23.Heering P., Braun N., Müllejans R. Cyclosporine A and chlorambucil in the treatment of idiopathic focal segmental glomerulosclerosis. Am J Kidney Dis. 2004;43:10–18. doi: 10.1053/j.ajkd.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran R., Kumar V., Rathi M. Tacrolimus therapy in adult-onset steroid-resistant nephrotic syndrome due to a focal segmental glomerulosclerosis single-center experience. Nephrol Dial Transplant. 2014;29:1918–1924. doi: 10.1093/ndt/gfu097. [DOI] [PubMed] [Google Scholar]
- 25.Chitalia V.C., Wells J.E., Robson R.A. Predicting renal survival in primary focal glomerulosclerosis from the time of presentation. Kidney Int. 1999;56:2236–2242. doi: 10.1038/sj.ki.4491164. [DOI] [PubMed] [Google Scholar]
- 26.Meyrier A., Noël L.H., Auriche P. Long-term renal tolerance of cyclosporin A treatment in adult idiopathic nephrotic syndrome. Collaborative Group of the Société de Néphrologie. Kidney Int. 1994;45:1446–1456. doi: 10.1038/ki.1994.189. [DOI] [PubMed] [Google Scholar]
- 27.Stirling C.M., Mathieson P., Boulton-Jones J.M. Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. Q J Med. 2005;98:443–449. doi: 10.1093/qjmed/hci072. [DOI] [PubMed] [Google Scholar]
- 28.Segarra A., Vila J., Pou L. Combined therapy of tacrolimus and corticosteroids in cyclosporin-resistant or -dependent idiopathic focal glomerulosclerosis: a preliminary uncontrolled study with prospective follow-up. Nephrol Dial Transplant. 2002;17:655–662. doi: 10.1093/ndt/17.4.655. [DOI] [PubMed] [Google Scholar]
- 29.Gipson D.S., Trachtman H., Kaskel F.J. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korbet S.M., Schwartz M.M., Lewis E.J. Primary focal segmental glomerulosclerosis: clinical course and response to therapy. Am J Kidney Dis. 1994;23:773–783. doi: 10.1016/s0272-6386(12)80128-4. [DOI] [PubMed] [Google Scholar]