Abstract
Objectives
The deficit of Glyoxalase I (Glo1) and the subsequent increase in methylglyoxal (MG) has been reported to be one the five mechanisms by which hyperglycemia causes diabetic late complications. Aldo-keto reductases (AKR) have been shown to metabolize MG; however, the relative contribution of this superfamily to the detoxification of MG in vivo, particularly within the diabetic state, remains unknown.
Methods
CRISPR/Cas9-mediated genome editing was used to generate a Glo1 knock-out (Glo1−/−) mouse line. Streptozotocin was then applied to investigate metabolic changes under hyperglycemic conditions.
Results
Glo1−/− mice were viable and showed no elevated MG or MG-H1 levels under hyperglycemic conditions. It was subsequently found that the enzymatic efficiency of various oxidoreductases in the liver and kidney towards MG were increased in the Glo1−/− mice. The functional relevance of this was supported by the altered distribution of alternative detoxification products. Furthermore, it was shown that MG-dependent AKR activity is a potentially clinical relevant pathway in human patients suffering from diabetes.
Conclusions
These data suggest that in the absence of GLO1, AKR can effectively compensate to prevent the accumulation of MG. The combination of metabolic, enzymatic, and genetic factors, therefore, may provide a better means of identifying patients who are at risk for the development of late complications caused by elevated levels of MG.
Keywords: Advanced glycation end products, Glyoxalase 1, Reactive metabolites, Methylglyoxal, Diabetic complications, Aldo-keto reductases
Abbreviations: AGE, Advanced glycation end product; ALDH, Aldehyde dehydrogenase; AKR, Aldo-keto reductase; Glo1, Glyoxalase 1; Glo2, Glyoxalase 2; GSH, Glutathione; GSSG, Glutathione disulfide; HTA, Hemithioacetal; MDRD, Modification of Diet in Renal Disease; MG, Methylglyoxal; MG-H1, arginine-derived hydroimidazalone Nδ-(5-hydro-5-methyl- 4-imidazolon-2-yl)-ornithine; STZ, Streptozotocin; UACR, Urine Albumine-Creatinine Ratio
1. Introduction
Blood glucose and/or glycated hemoglobin (HbA1c) are poor predictors for the development of diabetic complications. The DCCT-trial showed that only 11% of the diabetes-related complications in type 1 diabetic patients can be explained by the duration of diabetes and the HbA1c levels [1]. Similar findings were published for cardiovascular death in patients with type 1 diabetes [2], as well as in patients with type 2 diabetes [3], [4] suggesting that there are other forces driving the development of long term diabetic complications.
Advanced glycation end products (AGEs) are a heterogeneous group of stable post-translational modifications formed by the non-enzymatic reaction of glucose, dicarbonyls and other saccharide derivatives with amino acids. Methylglyoxal (MG) is a highly potent glycating agent and the arginine-derived hydroimidazalone Nδ-(5-hydro-5-methyl- 4-imidazolon-2-yl)-ornithine (MG-H1), is the most physiologically relevant MG-derived AGE, typically accounting for >90% adducts formed in vitro and in in vivo [5]. Protein-bound and free AGEs have been shown to be significantly elevated in experimental models of diabetes and in diabetic patients [6], [7], [8], [9], in parallel with their precursors [10], [11]. Studies have also shown that there is an association between AGEs and diabetic micro- and macrovascular complications (Reviewed in [12]), an association which is independent of HbA1c. The reasons for this discrepancy may be due to the loss of detoxification capacity.
The Glyoxalase system is considered to be the major pathway by which MG is detoxified [5]. It consists of two enzymes, Glyoxalase 1 (Glo1) and Glyoxalase 2 (Glo2), and a catalytic amount of glutathione (GSH), which catalyzes the conversion of MG to d-lactate [5]. The importance of Glo1 in detoxifying MG and therefore the extent of glycation has been shown frequently [13], [14], [15], [16], [17]. The overexpression of Glo1 has been shown to prevent various types of cellular dysfunction in the progression of neuropathy, nephropathy and responsiveness to hypoxia, inflammation and angiogenesis in diabetes [18], [19], [20], [21], [22], [23]. The consequences resulting from the loss of Glo1, particularly in vivo, have been lacking as the genetic deletion of Glo1 has been reported to be embryonically lethal in humans and mice [24]. As such, a total loss of Glo1 in mammalian model organisms has been elusive until recently [25].
Other enzymes such as aldehyde dehydrogenases (ALDH) and aldo-keto reductases (AKR) have both been shown to metabolize MG to pyruvate and hydroxyacetone, respectively [26], [27]. In vivo, it has been shown that AKR-deficient mice have increased AGE content within cardiac tissue and increased formation of atherosclerotic lesions [28], while genetic deletion of AKR1b3 leads to the development of nephrogenic diabetes insipidus [29]. It has been shown in vitro that in the absence of Glo1, AKR can compensate with respect to MG detoxification [30]. However, it remains unknown whether this compensatory pathway is active in vivo, particularly within the diabetic state.
In this study, CRISPR/Cas9-mediated genome editing was used to generate a viable Glo1 knock-out (Glo1−/−) mouse line to investigate the phenotype of this mouse under basal conditions and under conditions in which glucose load is elevated, such as in STZ-diabetes. Two organs in which Glo1 and MG have been shown to be of importance, the liver and kidney, were studied, with the question of whether there is an organ specific effect resulting from the loss of Glo1. The clinical relevance of alternative MG detoxification pathways was subsequently studied in the erythrocytes of type 2 diabetic patients, with and without diabetic nephropathy.
2. Methods
2.1. Patients
All patients had been recruited in the study department for diabetes research at the University Hospital in Heidelberg and gave written informed consent. Recruitment and tests were part of the Heidelberg Study on Diabetes and Complications (HEIST-DiC), which had been approved by the local ethics committee (ethics number S-383/2016, ClinicalTrials.gov Identifier NCT03022721). Details can be found in the supplementary methods.
2.2. Mice
Male, wild-type C57BL/6N mice were purchased from Charles River Laboratories (Wilmington, MA, USA). The mice were maintained under standard laboratory conditions of 12 h light/12 h dark cycle and always had access to food and water. All procedures were approved by the Animal Care and Use Committee at the Regierungspräsidium Karlsruhe, Germany (G 319/14 and G295/15).
2.3. Generation of Glo1 knock-out (Glo1−/−) mouse
CRISPR-Cas9-mediated genome editing was used to generate Glo1−/− mice. A detailed description of CRISPR-Cas9-mediated genome editing can be found in the supplementary information. Briefly, a very efficient sgRNA (sgRNA 171, Sigma) was identified in vitro, injected into C57BL/6N zygotes and two independent Glo1-deficient alleles identified in the offspring and bred to homozygosity (Glo1Δ8 and Glo1Δ7, Figure 1A). The Glo1Δ8/Δ8 mice, subsequently referred to as Glo1−/−, were used for further analysis.
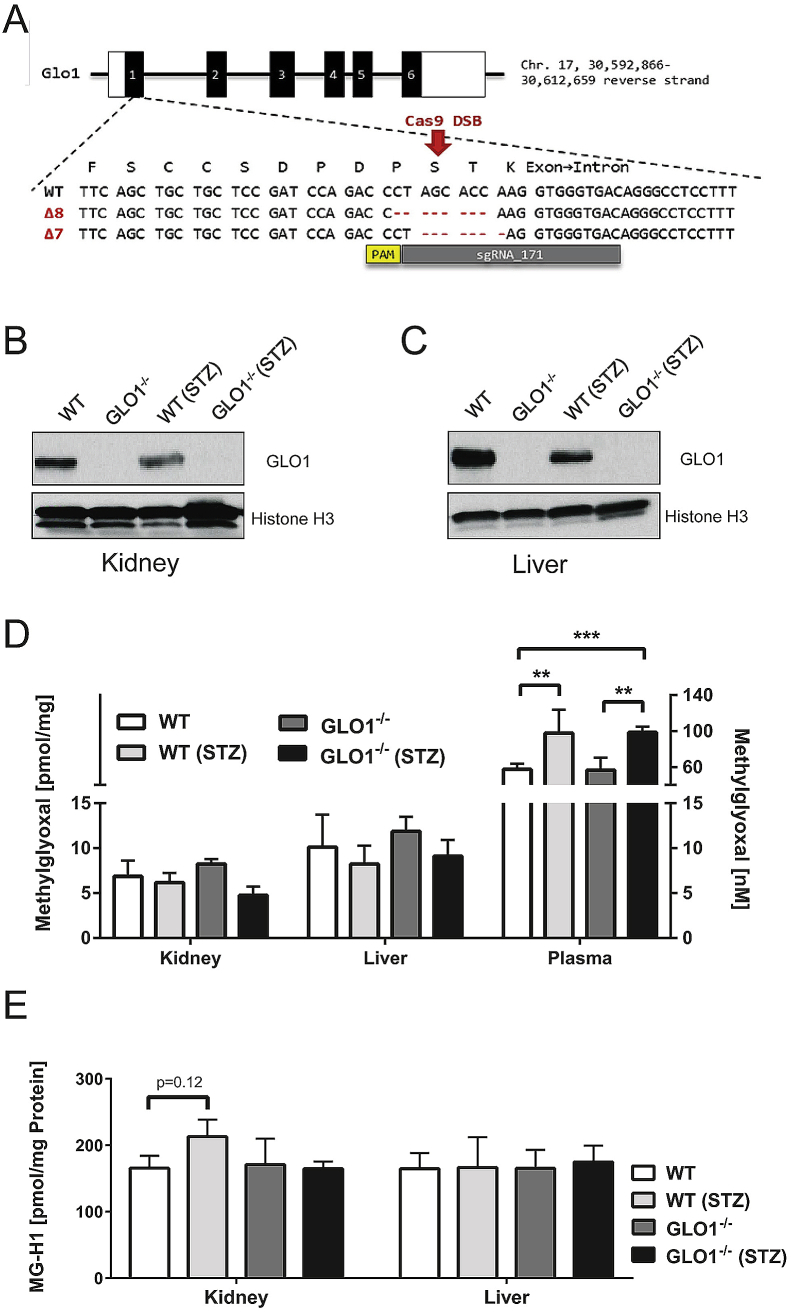
Figure 1.
Schematic overview of CRISPR/Cas9 genome editing in Glo1 locus and T7 assay of found tail DNA & quantitation of GLO1, MG and MG-H1 levels. (A)Glo1 sgRNA target locus in exon 1. The sgRNA binding sequence (sgRNA_171) is depicted in grey and is located on the reverse strand, the corresponding PAM sequence is marked in yellow. Below the WT sequence, the CRISPR/Cas9-mediated deletion of 8 and 7 base pairs leading to a frame shift in the respective Δ8 and Δ7 Glo1−/− mice mouse lines is are shown. B-C: Representative western blotting analysis of total cell extracts from kidney (B) and liver (C) of the appropriate mouse group probed with anti-GLO1 antibody and anti-Histone H3 antibody as a loading control. D: Intracellular MG-levels in whole organs of the appropriate group. E: Intracellular levels of MG-modified arginine (MG-H1) residues after exhaustive enzymatic digestion and determination via LC-MS/MS. Data represent mean ± S.E; *p < 0.05, **p < 0.01, ***p < 0.001 (n = 4).
2.4. Induction of streptozotocin (STZ)-induced diabetes
Details can be found in the supplementary methods. Briefly, diabetes was induced in 14–16 weeks old male WT and Glo1−/− mice by intraperitoneal injection of STZ (60 mg/kg) for five consecutive days, while age-matched controls received sodium citrate. Blood glucose levels were monitored and maintained in a range of 300–500 mg/dl. After 16 weeks, mice were sacrificed and glycated hemoglobin (HbA1c) was determined at 16 weeks by cation-exchange chromatography on a PolyCAT A column.
2.5. Assessment of kidney function
Urine was collected for 24 h using metabolic cages, and the total volume was determined. The albumin content of urine was measured using ICL Mouse Albumin ELISA, according to the manufacturer's instructions. Urine creatinine was determined by colorimetric assay (BioVision, Milpitas, CA) and ACR was subsequently calculated [31]. GFR was determined by transcutaneous measurements following intravenous injection of fluorescein-isothiocyanate-labelled sinistrin, as previously described [32].
2.6. Histological analysis
Sections of paraffin-embedded kidneys were stained with PAS. The area covered by mesangial cells in the PAS staining in glomeruli was determined relative to the total glomerular area as an average value of 20–30 glomeruli per animal using the WEKA segmentation tool (Image J).
2.7. Measurement of metabolites and post-translational modifications
The tissue content of MG and MG-H1 was determined by stable isotopic dilution, LC-MS/MS, as described previously [9], [33]. Hydroxyacetone, lactaldehyde [30], [34] and pyruvate [35] were determined as described previously with minor modifications that can be found in the supplementary methods. d-lactate was determined as described previously [36].
2.8. Enzymatic activity assays
Activity of GLO1, AKR and ALDH was determined in cytosolic fractions. Details can be found in the supplementary methods.
2.9. Western blotting
Proteins were isolated from pulverized tissue using RIPA buffer and stained with antibodies against GLO1 (ab81461, Abcam) and Histone H3 (4499, Cell Signaling Technology) after blotting. Details can be found in the supplementary methods.
2.10. Quantitative PCR (qPCR)
Total RNA from pulverized tissues was extracted and qPCR performed using a SYBR Green Master Mix (Thermo Fisher Scientific) on a LightCycler 480 Instrument II (Roche Diagnostics, Basel, Switzerland) was performed using the primer sequences given in Supplementary Table 1.
2.11. Statistical analysis
GraphPad Prism version 6.05 and Microsoft Excel were used for analysis. Data represents mean ± SD unless otherwise stated. Statistical significance was tested using ANOVA: ***p < 0.0001, **p < 0.001, *p < 0.05.
3. Results
3.1. Glo1−/− mice are viable and show no elevated MG or MG-H1 levels under hyperglycemic conditions
The genome editing in the Glo1 gene led to the complete loss of the GLO1 protein and activity in the kidney and liver of homozygous Glo1−/− mice lacking 8 nucleotides (Glo1Δ8/Δ8) in exon 1 (Table 1, Figure 1B–C) and in brain and heart (Supplementary Table 3, Supplementary Figure 2). Glo1−/− mice were viable and showed no obvious abnormalities and the Glo1Δ8 allele was segregated with the expected Mendelian frequency (Supplementary Table 3). Similar results were obtained in Glo1Δ7/Δ7 mice (Supplementary Table 4). Tissues from the nondiabetic Glo1−/− mice showed no increase in either MG or MG-H1, as compared to the nondiabetic WT (Figure 1D–E). Furthermore, in the diabetic mice there were also no increases except for MG-H1 in WT kidney, despite significant reductions in Glo1 activity (Table 1).
Table 1.
GLO1 activity in the kidney and liver of nondiabetic and diabetic, wild-type and Glo1−/−mice. GLO1 activity is described in units, where 1 unit is the amount of GLO1 that catalyzes the formation of 1 μmol of S-d-lactoylglutathione per min. Date represents mean ± S.E.; **p < 0.01,***p < 0.001 vs. nondiabetic (n = 4).
| Organ | GLO1 activity [U/mg] |
|||
|---|---|---|---|---|
| Wild-type |
Glo1−/− |
|||
| Nondiabetic | Diabetic | Nondiabetic | Diabetic | |
| Kidney | 4.02 ± 1.38 | 2.37 ± 0.56** | 0.04 ± 0.061 | 0.06 ± 0.043 |
| Liver | 10.61 ± 2.05 | 6.12 ± 0.83*** | 0.02 ± 0.006 | 0.01 ± 0.003 |
3.2. Manifestation of hyperglycemia-induced nephropathy is not changed in Glo1−/− mice
The diabetic mice showed elevated blood glucose level, HbA1c, as well as altered GTT and ITT, consistent with robust diabetic hyperglycemia (Supplementary Table 5). No differences were observed in the measured parameters between the diabetic WT and Glo1−/− mice. In the WT diabetic mice, Glo1 activity was significantly decreased by ∼41% as compared to nondiabetic WT mice (Table 1) and no Glo1 activity was observed in the kidneys and livers of the nondiabetic and diabetic Glo1−/− mice.
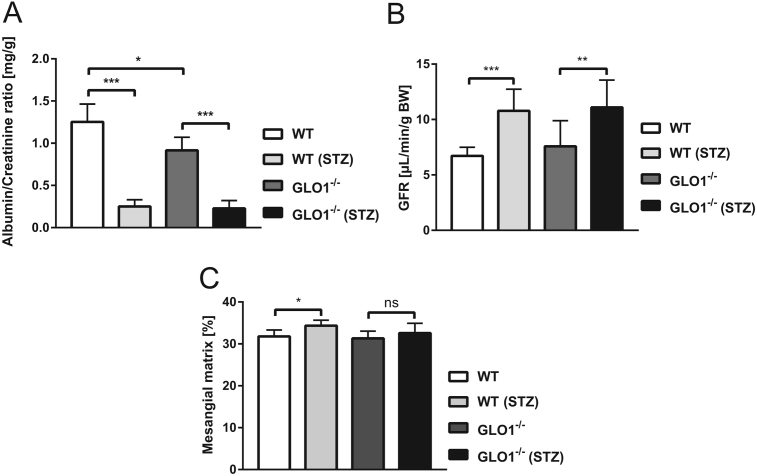
The albumin-creatinine ratio (ACR) was determined in the urine of nondiabetic and diabetic WT and Glo1−/− mice as to assess kidney function. In the diabetic WT and Glo1−/− mice, ACR excretion was significantly decreased by 75–80% as compared to the nondiabetic mice, suggesting that hyperfiltration, an indication of early nephropathy, was occurring. There was also a significant decrease 27% in ACR between the nondiabetic WT and Glo1−/− mice (Figure 2A).
Figure 2.
Characterization of a hyperglycemia-induced nephropathic phenotype. A: Albumin-Creatinine ratio (n = 4 animals ± S.E.). B: Glomerular filtration rate (GFR) normalized by body weight (n = 8–9 animals ± S.E.). C: Percentage of mesangial matrix area (n = 7–11 animals ± S.E.).
Glomerular filtration rate (GFR) was found to be significantly elevated in the WT and Glo1−/− diabetic mice as compared to the respective nondiabetic mice (Figure 2B). No differences were observed between the diabetic WT and Glo1−/− mice with respect to GFR.
Glomerular structural abnormalities, which define diabetic nephropathy, were assessed. In the WT diabetic mice, there was a significant increase in mesangial expansion (Figure 2C). In the diabetic Glo1−/− mice, mesangial expansion was also increased as compared to the nondiabetic Glo1−/− mice; however, the difference was non-significant. The degree of mesangial expansion in the diabetic Glo1−/− mice was lower as compared to the diabetic WT mice, but non-significant (Figure 2C).
3.3. Glo1−/− mice revealed increased enzymatic efficiency of various oxidoreductases in liver and kidney towards MG
To determine whether a compensation for Glo1 was occurring in the Glo1−/− mice, the mRNA profiles for AKR and ALDH subtypes were measured in the kidney and liver of the nondiabetic and diabetic WT and Glo1−/− mice.
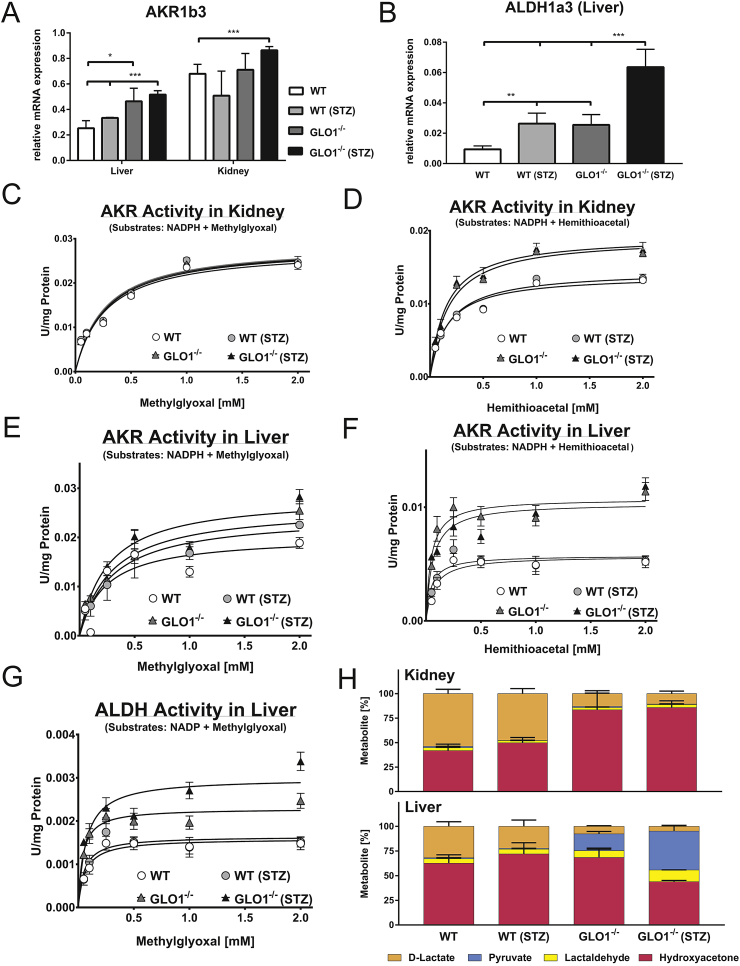
The mRNA expression of AKR1b3, a subtype which had previously been shown to be compensating Glo1 in Glo1−/− Schwann cells [30], was significantly increased by ∼2-fold in the nondiabetic Glo1−/− liver as compared to the WT (Figure 3A). In the diabetic livers, the expression of AKR1b3 was unchanged as compared to nondiabetic livers. In the kidneys, there were no significant differences in the expression of AKR1b3 between WT and Glo1−/− or nondiabetic and diabetic mice (Figure 3A). The screening of the liver showed that the expression of various ALDHs, such as ALDH1a3 (Figure 3B, Supplementary Figure 3), were increased in the Glo1−/− tissue as compared to the WT, and were increased further in the diabetic state. Comparable changes in the expression of AKR and ALDH were observed in the heart and brain of the Glo1−/− mice (Supplementary Figure 3).
Figure 3.
Analysis of alternative detoxification pathways for dicarbonyls. A: mRNA expression of AKR1b3 in liver and kidney of appropriate mouse subgroup. B: mRNA expression of ALDH1a3 in the liver of appropriate mouse subgroup. mRNA data are normalized to β-actin. C–F: Kinetic profiles of AKR-catalyzed reduction of HTA or MG in the kidney (C-D) or liver (E - F) of the appropriate mouse group. Kinetic profiles of ALDH-catalyzed reduction of MG in the liver (G) of the appropriate mouse group. Kinetic parameters are summarized in Supplementary Table 1. H: Metabolite distribution of MG detoxification in the liver and kidney of appropriate mouse group; absolute amounts of pyruvate, lactaldehyde, hydroxyacetone, as well as d-lactate are summarized in Supplementary Figure 4. All data represent mean ± S.E; *p < 0.05, **p < 0.01, ***p < 0.001 (n = 4).
It has previously been reported that the activation of an AKR-dependent compensatory pathway is not necessarily dependent upon changes in either mRNA or protein expression of AKR but on a change in the enzyme substrate specificity [30]. To establish whether this was the case, kinetic profiling of AKR activity was performed in whole tissue extracts from the kidney and liver. In the kidney, when MG was used as the substrate, no differences were observed between the WT and Glo1−/− or nondiabetic and diabetic mice (Figure 3C; Supplementary Table 6a). However, when HTA, the non-enzymatic product of MG, and GSH was used as the substrate, the maximal activity of AKR was significantly increased by ∼34% in the Glo1−/− kidneys as compared to the WT (Figure 3D; Supplementary Table 6a). In the diabetic kidneys, the kinetic parameters of HTA-dependent AKR activity were unchanged as compared to nondiabetic kidneys. For both MG- and HTA-dependent AKR activity in the kidney, there were no significant changes in substrate affinity (Supplementary Table 6a).
In the Glo1−/− liver, both maximal activity and substrate affinity of MG-dependent AKR activity were significantly increased by ∼55% and 60%, respectively as compared to the WT (Figure 3E, Supplementary Table 6b). In the WT diabetic liver, the maximal activity and substrate affinity were significantly increased to a similar extent as the nondiabetic Glo1−/−, whereas the kinetic parameters of diabetic Glo1−/− were unchanged. For HTA-dependent AKR activity, the maximal activity was significantly increased by ∼89% in the Glo1−/− liver, whilst substrate affinity was non-significantly decreased (Figure 3F, Supplementary Table 6b).
In addition to AKR activity, ALDH activity was also measured using MG as the substrate. In the kidneys of the WT and Glo1−/−, no activity at all was detectable (data not shown). In the liver, the ALDH activity was present, but overall, was lower than either MG or HTA-dependent AKR activity, consistent with the mRNA expression (Figure 3G). In the nondiabetic Glo1−/− liver, maximal activity was significantly increased by ∼44% as compared to nondiabetic WT liver (Figure 3G, Supplementary Table 6b).
3.4. Products of alternative detoxification of MG are elevated in kidney and liver of Glo1−/− mice
These findings would suggest that, in the absence of Glo1, the major pathway for the detoxification of MG is mediated by AKR. To prove this, the products of the alternative detoxification were screened in the tissues. In the kidney of the WT nondiabetic mice, the distribution of hydroxyacetone to d-lactate was approximately equal (Figure 3H). In the Glo1−/− nondiabetic mice, the relative proportion of d-lactate was reduced, while hydroxyacetone was the major product measured, contributing ∼83% of the total metabolites (Figure 3H). This would suggest that MG-dependent AKR detoxification is the major route for MG removal in the absence of Glo1. In diabetic mice, there were no significant changes in the distribution of the metabolites in the WT and Glo1−/− mice as compared to the respective nondiabetic mice. In the kidney, the relative proportions of pyruvate and lactaldehyde were minor, only accounting for <10% of the total metabolites measured (Figure 3H) and no differences between genotypes.
In the liver of the nondiabetic WT mice, hydroxyacetone accounting for ∼60% of the total metabolites measured, whereas d-lactate only accounted for ∼30% (Figure 3H). Minor products were lactaldehyde and pyruvate. In the liver of the Glo1−/− nondiabetic mice, hydroxyacetone content was increased, whilst the d-lactate was reduced to ∼7%. Pyruvate and lactaldehyde content were also increased, but their relative proportion to the total metabolites measured was only 20% (Figure 3H). In diabetic WT mice, there was a non-significant trend towards increased hydroxyacetone content in both the kidney and liver, as compared to the nondiabetic WT mice.
3.5. Evidence for AKR-dependent MG detoxification in the erythrocytes of type 2 diabetic patients without complications
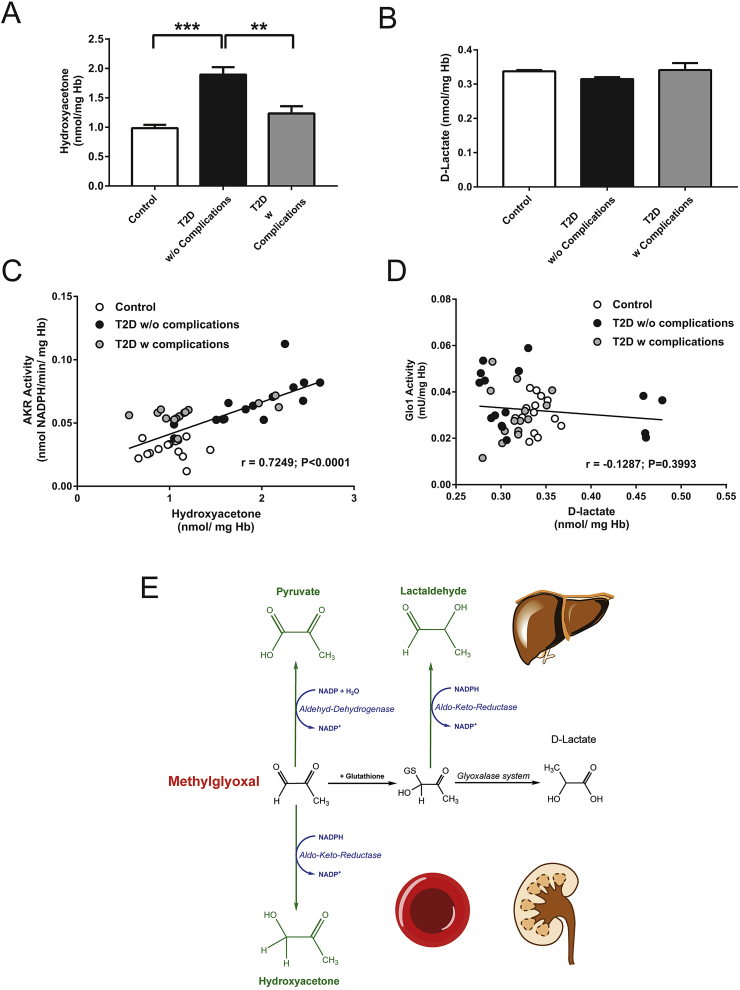
To determine whether MG-dependent AKR activity is a clinical relevant pathway in diabetes, red blood cells were isolated from healthy control and type 2 diabetic patients with and without nephropathy (Table 2). The activities of AKR and Glo1 were measured, as well as their respective detoxification products, hydroxyacetone and d-lactate. It was found that in general, the levels of hydroxyacetone were significantly higher (∼4-fold) than the levels of d-lactate (49.37 ± 20.24 vs. 11.92 ± 1.732 nmol/mg Hb; P < 0.0001). In the diabetic patients without complications, the levels of hydroxyacetone were significantly increased ∼2-fold, as compared to the healthy control patients (Figure 4A). There was a significant decrease of ∼1.5-fold between the diabetic patient with and without complications. With respect to d-lactate, no significant differences were observed between the patient groups (Figure 4B). Linear regression analysis showed that the hydroxyacetone content correlated positively with the MG-dependent AKR activity (r = 0.7249; P < 0.0001). Diabetic patients without complications therefore had the highest AKR activity, as well as the highest content of hydroxyacetone (Figure 4C). Glo1 activity did not significantly correlate with d-lactate concentration (r = −0.1287; P = 0.3993) and could not be used to differentiate between diabetic patients with or without complications (Figure 4D).
Table 2.
Mean baseline characteristics of the control and patient cohorts (T2D without complications; T2D with complications). All parameters were determined prior to collection. Data represents mean ± S.E.; *p < 0.05,***p < 0.001, vs. controls. Unless stated, all other characteristics were not significant (p > 0.05).
| Sex [%male] | Age [years] | BMI | blood glucose [mg/dL] | HbA1c [%] | GFR (MDRD) [mL/min] | uACR [mg/g] | |
|---|---|---|---|---|---|---|---|
| Control (15) | 40 | 59.5 ± 7.4 | 27.9 ± 5.2 | 97.1 ± 8.1 | 5.4 ± 0.4 | 94.1 ± 11.9 | 0.6 ± 1.9 |
| T2D without complications (15) | 40 | 64.2 ± 7.3 | 31.2 ± 7.3* | 141.4 ± 37.4* | 7.2 ± 1.3*** | 98.4 ± 20.8 | 0.4 ± 0.9 |
| T2D with complications (15) | 53.3 | 66.4 ± 5.6 | 32.7 ± 5.6* | 161.9 ± 33.3*** | 7.4 ± 0.7*** | 83.0 ± 32.9 | 15.3 ± 22.4* |
Figure 4.
AKR-dependent MG detoxification in human erythrocytes. Concentration of Hydroxyacetone (A) and d-lactate (B) in the RBCs from healthy controls and type 2 diabetic patients (T2D) with or without late diabetic complications. Data represent mean ± SEM (N = 15 per group); ***p < 0.001. Correlation of hydroxyacetone and AKR activity (C) or d-lactate and GLO1 activity (D) in the RBCs from healthy controls and type 2 diabetic patients (T2D) with or without late diabetic complications. Data represents mean ± SEM (N = 15 per group). E: Proposed detoxification mechanisms taking place in liver and kidney of Glo-deficient mice and men.
4. Discussion
Elevated MG has been shown to be a clinical feature in patients with diabetes [18], [38], [39]. However, within the clinical context, the extent to which Glo1 activity is reduced remains unclear with the majority of studies either reporting no differences or an increase in activity [38], [40], [41]. The levels of d-lactate in the urine and plasma of diabetic patients has been shown to be elevated [42], [43]. Therefore, it is possible that the increased d-lactate concentrations reflect the flux of MG formation. Interestingly, the measurement of Glo1-dependent detoxification of MG is insufficient to predict the complications of diabetes. This is supported by the clinical data in this study, which showed that AKR activity and hydroxyacetone could be used to differentiate between diabetic patients with and without complications, whereas Glo1 and d-lactate could not. Surprisingly, it has been shown that Glo1 activity in RBCs is higher in diabetic patients with complications such as retinopathy, nephropathy and neuropathy [38]. The size of the current study cohort precludes the overinterpretation of these findings; however, it could be suggested that diabetic patients without complications are protected due to AKR detoxification of MG, whereas patients with complications have lost this capacity. Interestingly, a similar up-regulation of both AKR and Glo1 has also recently been reported in the glomeruli of diabetic patients who are protected against diabetic nephropathy [44].
In vitro, the inhibition or silencing of Glo1 [13], [14], [15], [16], [17] has led to the development of a cause-and-effect model between its activity and levels of MG. In vivo, it has been shown that a Glo1 knock-down mouse had elevated MG-H1 content within the kidney and a nephropathic phenotype which is characteristic of diabetes [37]. However, in the current study, it was shown that the Glo1−/− mouse shared none of these pathologies. In the diabetic state, there were also no differences. Furthermore, while ACR, glomerular filtration and mesangial matrix expansion were increased in the diabetic mice, suggestive of early diabetic nephropathy, no sign of aggravation or any major differences were observed between the WT and Glo1−/− mice, despite the loss of Glo1 activity. The discrepancies observed between the knock-down and knock-out Glo1 mice could be due to the level of Glo1 enzyme present in the system. In the study of Giacco et al., there was residual Glo1 activity [37], whereas in Glo1−/− mice analyzed in the current study, Glo1 enzyme activity was entirely abrogated in all tissues from embryonic stage on which may induce different levels and quality of compensatory mechanisms to reduce dicarbonyl stress. On the other hand, a reduction of 50% in Glo1 activity has previously been shown to be sufficient to induce a pathological condition. It would therefore be expected that 100% reduction would also be able to induce a similar effect, unless AKR compensatory pathway is having a beneficial effect which is greater than just detoxification of MG alone. Indeed, it was shown in vitro that the loss of Glo1 leads to changes in the GSH/GSSG ratio [30] which would suggest that the cells had a greater capacity to handle cellular stress which is independent of MG. Furthermore, it would suggest that the cause-and-effect model that has developed for Glo1 and MG in the last decade cannot be recapitulated in the Glo1-deficient mouse model under STZ-evoked chronic hyperglycemia. However, the conversion of MG to hydroxyacetone is not mandatory a beneficial effect regarding MG detoxification. Acetone/Acetol monooxygenase is able (re-)catalyze hydroxyacetone back to MG, which can be potentially interpreted as a vicious circle due to the loss of important cellular co-factors such as NADPH via those pathways [26].
The major substrate for Glo1 is MG and as such it is considered to be the major route by which it is detoxified [5]. In the current study, the detoxification products in the tissues of the WT mice were distributed equally between hydroxyacetone and d-lactate, particularly within the liver, suggesting that the dependency upon Glo1 for MG is not necessarily correct in all cell types. In an experimental kinetic model system Baba et al. suggested that at low concentrations of MG, AKR accounts for 40% and Glo1 accounts for 60% of MG detoxification [28]. However, due to a methodological error, the authors may have underestimated the Glo1 dependent metabolism. Despite this, one should consider that AKR superfamily consists of approximately 60 members that have potentially the capacity to detoxify MG and/or hemithioacetal in vivo [28]. In the absence of Glo1, the dependency for AKR is increased, particularly in the kidney, whereas in the liver, the increased pyruvate content would suggest a contribution from ALDH towards MG detoxification. The differences observed between the tissues could be due to the heterogeneous expression profile of the two enzymes (Figure 4E), which is consistent with the fact that AKR is highest expressed in the renal medulla. In STZ-evoked diabetes, the distribution of the detoxification products was unchanged as compared to the nondiabetic mice, although a non-significant trend towards increased hydroxyacetone production in both the kidney and liver was observed. The lack of a significant change in the WT diabetic tissue would not be unexpected given that the activity of Glo1 is only reduced by 40% in both tissues, meaning that the remaining activity is sufficient to deal with any elevation in MG, whilst in the diabetic Glo1−/− mice, particularly with respect to the kidney, AKR continues to provide adequate protection in the absence of Glo1. This would therefore explain the lack of an increase in either MG or MG-H1 in the diabetic mice. However, the possibility of other adaptive mechanisms besides alternative detoxification is possible. For instance the upregulation of triosephosphate isomerase and shift of this pathway towards glyceraldehyde 3-phosphate could be beneficial to prevent enhanced MG production. Surprisingly triosephosphate isomerase activity was unchanged in the liver, whereas the mRNA content was increased (Supplementary Figure 7).
The failure to acknowledge the detoxification capacity of AKRs towards MG, particularly within the context of diabetes, has likely resulted from the fact that AKRs have generally been studied within the context of the polyol pathway, where it produces sorbitol during hyperglycemic episodes in various tissues [45]. Clinical trials to prevent the development of diabetic complications with AKR inhibitors, such as Sorbinil or Epalrestat, have proven to be unsuccessful [46], [47]. This could be because inhibition is leading to the loss of detoxification capacity in the MG pathway. Based upon the kinetic properties of AKR it could be argued that this detoxification capacity is not as efficient as Glo1. However, unlike Glo1, AKR is not dependent upon GSH as a catalytic cofactor. This dependency makes Glo1 activity highly susceptible to changes in GSH [5]. In contrast, AKR requires NADPH as a cofactor [48]. Unlike the NAD+/NADH ratio, which is regulated by rates of glycolysis and respiration, the ratio of NADPH-to-NADP+ is reflective of the synthetic capacity; as such, AKR activity would be unaffected by fluctuations in the cofactor ratio due to changes in metabolic rate and capacity. This would mean that regardless of changes in energy homeostasis the activity of AKR would be maintained, thereby providing the cell with a constant means for the detoxification of MG. The relevance of AKR-dependent detoxification of MG in diabetes has previously been shown by Baba et al. [28]. The loss of AKR1b3 in apoE-knock-out mice not only increased AGE accumulation but also the formation of atherosclerotic lesions. However, Glo1 knock-down mice on the apoE background did not show any differences in either MG-derived AGEs or enhanced formation of atherosclerosis [49]. These studies would suggest that the loss of Glo1 alone is insufficient to trigger the development of complications in diabetes. The decrease in Glo1 activity may provide an early warning sign for a detrimental biochemical change, while the subsequent loss of AKR may provide the additional stress to push the system into cellular dysfunction. The combined loss of these two defence pathways may therefore provide a suitable model for studying the contribution of MG accumulation in diabetes.
The findings from this study provide evidence for the in vivo role of AKR in the detoxification of MG. The use of this compensatory pathway, in the absence of Glo1, may provide a partial explanation for the heterogeneity in the development of late complications within the general diabetic population. The screening of alternative detoxification products, as well as the measurement of enzymatic activities would allow for the grouping of patients in regard to their detoxification capacity. Furthermore, the measurement of polymorphism may also contribute an additional means for making this assessment [50]. The combination of metabolic, enzymatic and genetic factors may therefore provide a better approach to identify those patients which are at risk for the development of late complications and in doing so, provide a more robust means of treatment.
Author's contribution
D.S. designed and validated all aspects of the genome editing strategy for generation of Glo1−/− mice, supervised and took part in the maintenance of the diabetic mice, urine and organ collection, performed all analyses for assessments of nephropathy and was involved in designing the study.
J.M. performed the biochemical and molecular analysis.
Y.O. contributed to molecular tools for generating and genotyping of Glo1−/− mice
N.V. was responsible for the IHC analysis of the tissues from the Glo1−/− mice
S.K. & J.B.G. were responsible for the recruitment and assessment of patients and the collection of blood samples.
P.P.N, T.F., and M.F. conceived, designed and supervised the study.
All authors approved the final version of the manuscript to be published.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG; SFB1118, FOR 2289). We thank Xenia Tolksdorf and Christin Matka for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.09.005.
Conflict of interest
The authors have no conflicts of interest with the contents of this manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lind M., Odén A., Fahlén M., Eliasson B. The shape of the metabolic memory of HbA1c: re-analysing the DCCT with respect to time-dependent effects. Diabetologia. 2010;53:1093–1098. doi: 10.1007/s00125-010-1706-z. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39:686–693. [Google Scholar]
- 3.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England) 1998;352:837–853. [PubMed] [Google Scholar]
- 4.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein H.C., Miller M.E., Byington R.P., Goff D.C., Jr, Bigger J.T., Buse J.B. Effects of intensive glucose lowering in type 2 diabetes. New England Journal of Medicine. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbani N., Thornalley P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids. 2012;42:1133–1142. doi: 10.1007/s00726-010-0783-0. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N., Babaei-Jadidi R., Howell S.K., Beisswenger P.J., Thornalley P.J. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia. 2005;48:1590–1603. doi: 10.1007/s00125-005-1810-7. [DOI] [PubMed] [Google Scholar]
- 7.Karachalias N., Babaei-Jadidi R., Rabbani N., Thornalley P.J. Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia. 2010;53:1506–1516. doi: 10.1007/s00125-010-1722-z. [DOI] [PubMed] [Google Scholar]
- 8.Agalou S., Ahmed N., Babaei-Jadidi R., Dawnay A., Thornalley P.J. Profound mishandling of protein glycation degradation products in uremia and dialysis. Journal of the American Society of Nephrology. 2005;16:1471–1485. doi: 10.1681/ASN.2004080635. [DOI] [PubMed] [Google Scholar]
- 9.Thornalley P.J., Battah S., Ahmed N., Karachalias N., Agalou S., Babaei-Jadidi R. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochemial Journal. 2003;592:581–592. doi: 10.1042/BJ20030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Eupen M.G.A., Schram M.T., Colhoun H.M., Hanssen N.M., Niessen H.W., Tarnow L. The methylglyoxal-derived AGE tetrahydropyrimidine is increased in plasma of individuals with type 1 diabetes mellitus and in atherosclerotic lesions and is associated with sVCAM-1. Diabetologia. 2013;56:1845–1855. doi: 10.1007/s00125-013-2919-8. [DOI] [PubMed] [Google Scholar]
- 11.van Eupen M.G.A., Schram M.T., Colhoun H.M., Scheijen J.L., Stehouwer C.D., Schalkwijk C.G. Plasma levels of advanced glycation endproducts are associated with type 1 diabetes and coronary artery calcification. Cardiovascular Diabetology. 2013;12:149. doi: 10.1186/1475-2840-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groener J.B., Oikonomou D., Cheko R., Kender Z., Zemva J., Kihm L. Methylglyoxal and advanced glycation end products in patients with diabetes – what we know so far and the missing links. Experimental and Clinical Endocrinology & Diabetes. 2017 doi: 10.1055/s-0043-106443. [DOI] [PubMed] [Google Scholar]
- 13.Dobler D., Ahmed N., Song L., Eboigbodin K.E., Thornalley P.J. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes. 2006;55:1961–1969. doi: 10.2337/db05-1634. [DOI] [PubMed] [Google Scholar]
- 14.Stratmann B., Engelbrecht B., Espelage B.C., Klusmeier N., Tiemann J., Gawlowski T. Glyoxalase 1-knockdown in human aortic endothelial cells – effect on the proteome and endothelial function estimates. Scientific Reports. 2016;6:37737. doi: 10.1038/srep37737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tikellis C., Pickering R.J., Tsorotes D., Huet O., Cooper M.E., Jandeleit-Dahm K. Dicarbonyl stress in the absence of hyperglycemia increases endothelial inflammation and atherogenesis similar to that observed in diabetes. Diabetes. 2014;63:3915–3925. doi: 10.2337/db13-0932. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara M., Thornalley P.J., Giardino I., Beisswenger P., Thorpe S.R., Onorato J. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. Journal of Clinical Investigation. 1998;101:1142–1147. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morcos M., Du X., Pfisterer F., Hutter H., Sayed A.A., Thornalley P. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis Elegans. Aging Cell. 2008;1:260–269. doi: 10.1111/j.1474-9726.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 18.Bierhaus A., Fleming T., Stoyanov S., Leffler A., Babes A., Neacsu C. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nature Medicine. 2012;18:926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 19.Yao D., Taguchi T., Matsumura T., Pestell R., Edelstein D., Giardino I. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. Journal of Biological Chemistry. 2007;282:31038–31045. doi: 10.1074/jbc.M704703200. [DOI] [PubMed] [Google Scholar]
- 20.Ceradini D.J., Yao D., Grogan R.H., Callaghan M.J., Edelstein D., Brownlee M. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. Journal of Biological Chemistry. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao D., Brownlee M. 2009. Hyperglycemia-induced reactive oxygen species increase expression of RAGE and RAGE ligands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed U., Dobler D., Larkin S.J., Rabbani N., Thornalley P.J. Reversal of hyperglycemia-induced angiogenesis deficit of human endothelial cells by overexpression of glyoxalase 1 in vitro. Annals of the New York Academy of Sciences. 2008;1126:262–264. doi: 10.1196/annals.1433.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwers O., Niessen P.M., Haenen G., Miyata T., Brownlee M., Stehouwer C.D. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shafie A., Xue M., Barker G., Zehnder D., Thornalley P.J., Rabbani N. Reappraisal of putative glyoxalase 1-deficient mouse and dicarbonyl stress on embryonic stem cells in vitro. Biochemical Journal. 2016;473:4255–4270. doi: 10.1042/BCJ20160691. [DOI] [PubMed] [Google Scholar]
- 25.Jang S., Kwon D.M., Kwon K., Park C. Generation and characterization of mouse knockout for glyoxalase 1. Biochemical and Biophysical Research Communications. 2017;490:460–465. doi: 10.1016/j.bbrc.2017.06.063. [DOI] [PubMed] [Google Scholar]
- 26.Vander Jagt D.L., Robinson B., Taylor K.K., Hunsaker L.A. Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications. Journal of Biological Chemistry. 1992;267:4364–4369. [PubMed] [Google Scholar]
- 27.Vander Jagt D.L., Hunsaker L.A. Methylglyoxal metabolism and diabetic complications: roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chemico-Biological Interactions. 2003;143–144:341–351. doi: 10.1016/s0009-2797(02)00212-0. [DOI] [PubMed] [Google Scholar]
- 28.Baba S.P., Barski O.A., Ahmed Y., O`Toole T.E., Conklin D.J., Bhatnagar A. Reductive metabolism of AGE precursors: a metabolic route for preventing AGE accumulation in cardiovascular tissue. Diabetes. 2009;58:2486–2497. doi: 10.2337/db09-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho H.T., Chung S.K., Law J.W., Ko B.C., Tam S.C., Brooks H.L. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Molecular and Cellular Biology. 2000;20:5840–5846. doi: 10.1128/mcb.20.16.5840-5846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgenstern J., Fleming T., Schumacher D., Eckstein V., Freichel M., Herzig S. Loss of Glyoxalase 1 induces compensatory mechanism to achieve dicarbonyl detoxification in mammalian Schwann cells. Journal of Biological Chemistry. 2017;292:3224–3238. doi: 10.1074/jbc.M116.760132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi Z., Fujita H., Jin J., Davis L.S., Wang Y., Fogo A.B. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54:2628–2637. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber A., Shulhevich Y., Geraci S., Hesser J., Stsepankou D., Neudecker S. Transcutaneous measurement of renal function in conscious mice. American Journal of Pysiology, Renal Physiology. 2012;303:F783–F788. doi: 10.1152/ajprenal.00279.2012. [DOI] [PubMed] [Google Scholar]
- 33.Rabbani N., Thornalley P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nature Protocols. 2014;9:1969–1979. doi: 10.1038/nprot.2014.129. [DOI] [PubMed] [Google Scholar]
- 34.Beilin E., Baker L.J., Culbert P., Toltl N.P. Quantitation of acetol in common pharmaceutical excipients using LC-MS. Journal of Pharmaceutical and Biomedical Analysis. 2008;46:316–321. doi: 10.1016/j.jpba.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Chuang C.-K., Wang T.-J., Yeung C.-Y., Lin D.S., Lin H.Y., Liu H.L. A method for lactate and pyruvate determination in filter-paper dried blood spots. Journal of Chromatography A. 2009;1216:8947–8952. doi: 10.1016/j.chroma.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 36.McLellan A.C., Phillips S.A., Thornalley P.J. Fluorimetric assay of D-lactate. Analytical Biochemistry. 1992;16:12–16. doi: 10.1016/s0003-2697(05)80004-1. [DOI] [PubMed] [Google Scholar]
- 37.Giacco F., Du X., D'Agati V.D., Milne R., Sui G., Geoffrion M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes. 2014;63:291–299. doi: 10.2337/db13-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLellan A.C., Thornalley P.J., Benn J., Sonksen P.H. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clinical Science (London) 1994;87:21–29. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- 39.Fleming T., Cuny J., Nawroth G., Djuric Z., Humpert P.M., Zeier M. Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia. 2012;55:1151–1155. doi: 10.1007/s00125-012-2452-1. [DOI] [PubMed] [Google Scholar]
- 40.Thornalley P.J., Hooper N.I., Jennings P.E., Florkowski C.M., Jones A.F., Lunec J. The human red blood cell glyoxalase system in diabetes mellitus. Diabetes Research and Clinical Practice. 1989;7:115–120. doi: 10.1016/0168-8227(89)90101-0. [DOI] [PubMed] [Google Scholar]
- 41.Skapare E., Konrade I., Liepinsh E., Strele I., Makrecka M., Bierhaus A. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. Journal of Diabetic Complications. 2013;27:262–267. doi: 10.1016/j.jdiacomp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Chou C.-K., Lee Y.-T., Chen S.-M., Hsieh C.W., Huang T.C., Li Y.C. Elevated urinary D-lactate levels in patients with diabetes and microalbuminuria. Journal of Pharmaceutical and Biomedical Analysis. 2015;116:65–70. doi: 10.1016/j.jpba.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Scheijen J.L.J.M., Hanssen N.M.J., van de Waarenburg M.P.H., Jonkers D.M., Stehouwer C.D., Schalkwijk C.G. L(+) and D(-) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of L(+) and D(-) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Experimental Diabetes Research. 2012;2012:234812. doi: 10.1155/2012/234812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi W., Keenan H.A., Li Q., Ishikado A., Kannt A., Sadowski T. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nature Medicine. 2017;23:753–762. doi: 10.1038/nm.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabbay K.H. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Annual Review of Medicine. 1975;26:521–536. doi: 10.1146/annurev.me.26.020175.002513. [DOI] [PubMed] [Google Scholar]
- 46.Schemmel K.E., Padiyara R.S., D'Souza J.J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: a review. Journal of Diabetic Complications. 2010;24:354–360. doi: 10.1016/j.jdiacomp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Hu X., Li S., Yang G., Liu H., Boden G., Li L. Efficacy and safety of aldose reductase inhibitor for the treatment of diabetic cardiovascular autonomic neuropathy: systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barski O.A., Tipparaju S.M., Bhatnagar A. The Aldo-Keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metabolism Reviews. 2008;40:553–624. doi: 10.1080/03602530802431439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wortmann M., Hakimi M., Fleming T., Peters A.S., Sijmonsma T.P., Herzig S. A Glyoxalase-1 knockdown does not have major short term effects on energy expenditure and atherosclerosis in mice. Journal of Diabetes Research. 2016;2016:1–8. doi: 10.1155/2016/2981639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oikonomou D., Groener J.B., Cheko R., Kender Z., Kihm L., Fleming T. Genetic polymorphisms of antioxidant and antiglycation enzymes and diabetic complications. How much can we learn from the genes? Experimental and Clinical Endocrinology & Diabetes. 2017 doi: 10.1055/s-0043-106442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.