Abstract
Introduction
A cornerstone of kidney disease management is participation in guideline-recommended health behaviors. However, the relationship of these health behaviors with outcomes, and the identification of barriers to health behavior engagement, have not been described among younger and older adults with chronic kidney disease.
Methods
Data from a cohort study of 5499 individuals with chronic kidney disease was used to identify health behavior patterns with latent class analysis stratified by age <65 and ≥65 years. Cox models, stratified by diabetes, assessed the association of health behavior patterns with chronic kidney disease (CKD) progression, atherosclerotic events, and death. Logistic regression was used to assess for barriers to health behavior engagement.
Results
Three health behavior patterns were identified: 1 “healthy” pattern, and 2 “less healthy” patterns comprising 1 pattern with more obesity and sedentary activity and 1 with more smoking and less obesity. Less healthy patterns were associated with an increased hazard of poor outcomes. Among participants <65 years of age, the less healthy patterns (vs. healthy pattern) was associated with an increased hazard of death in diabetic individuals (hazard ratio [HR] = 2.17, 95% confidence interval [CI] = 1.09–4.29; and HR = 2.50, 95% CI = 1.39–4.50) and cardiovascular events among nondiabetic individuals (HR = 1.49, 95% CI = 1.04–2.43; and HR = 2.97, 95% CI = 1.49–5.90). Individuals with the more obese/sedentary pattern had an increased risk of CKD progression in those who were diabetic (HR = 1.34, 95% CI = 1.13–1.59). Among older adults, the less healthy patterns were associated with increased risk of death (HR = 2.97, 95% CI = 1.43–6.19; and HR = 3.47, 95% CI = 1.48–8.11) in those who were nondiabetic. Potential barriers to recommended health behaviors include lower health literacy and self-efficacy.
Conclusion
Identifying health behavior patterns and barriers may help target high-risk groups for strategies to increase participation in health behaviors.
Keywords: all-cause death cardiovascular outcomes, chronic renal failure, chronic renal insufficiency, CKD progression, health behavior, self-management
See Commentary on Page 11
The number of adults 65 years and older in the United States is projected to be more than 20% of the population by the year 2030.1 With each advancing decade of life, CKD incidence and prevalence increases.2 CKD increases the risk of developing cardiovascular disease (CVD), progressing to end-stage renal disease (ESRD), and premature death.2, 3 Age is an important effect modifier of these risk associations, with older adults less frequently progressing to ESRD but experiencing more cardiovascular mortality than younger adults.4, 5
Current CKD guidelines are not age specific and broadly recommend patients to actively participate in health behaviors to manage their disease and mitigate its sequelae; these behaviors include monitoring blood pressure, abstaining from smoking, maintaining a healthy weight, consuming a healthy diet, and participating in physical activity.6, 7, 8 The effectiveness of these health behavior recommendations in CKD is relatively unknown, as they were informed by research in the general population.9 In addition, to date, there are limited data examining the health behavior practices of adults with CKD.10 In particular, even though older adults likely face unique challenges to health behavior engagement, such as higher rates of comorbid conditions, frailty, and decreased functional status, little consideration has been given to whether adoption of health behaviors differs for older (vs. younger) adults. Such information is important to understand gaps in care and to develop age-appropriate recommendations and interventions to help all patients with CKD. The primary goals of this study were to examine the level of engagement in recommended health behaviors, to identify potential barriers to this engagement, and to elucidate the association of clustering of health behaviors with important outcomes separately among younger and older adults with CKD.
Methods
This study utilized data from the Chronic Renal Insufficiency Cohort (CRIC) Study, a multicenter, prospective, observational cohort of 5,499 men and women with mild-to-moderate CKD in the United States.11, 12 Participants were recruited from 7 clinical centers during 2 phases of the study: (i) phase I, from 2003 to 2008 (3939 participants); and (ii) phase III, from 2013 to 2015 (1560 participants). The study protocol was approved by the institutional review boards of all participating centers and is in accordance with the Declaration of Helsinki. All participants provided informed consent. The full inclusion and exclusion criteria have been previously reported.11, 12 In brief, major eligibility criteria for phase I included adults aged 21 to 74 years with age-based estimated glomerular filtration rate (eGFR) of 20 to 70 ml/min per 1.73 m2; entry into phase III required eGFR 45 to 70 ml/min per 1.73 m2 and age 45 to 79 years. Exclusion criteria included inability to provide consent and the presence of certain severe conditions.
The selection of recommended health behavior measures was guided by previous literature and guidelines (Table 1).8, 13, 14 Briefly, body mass index (BMI) served as a proxy of weight maintenance, was dichotomized into “recommended” for a BMI of 20 to <25 kg/m2 and “not recommended” if otherwise.8, 9 A “healthy” diet score (range 0–4) was constructed from a 12-month diet history15 based on fruits, vegetables, fish, grains, and sweets/sweetened beverages.9 A diet score >2 was “recommended” and ≤2 “not recommended.” Reported weekly physical activity was categorized as “recommended” for moderate activity ≥150 minutes or vigorous ≥75 minutes, or moderate plus vigorous ≥150 minutes9 and “not recommended” if otherwise. Past use or never use of tobacco was “recommended” and current use “not recommended.” A mean blood pressure was a proxy for adherence to antihypertensive treatment, with ≤140 mm Hg systolic blood pressure and ≤90 mm Hg diastolic blood pressure defined as “recommended” and >140/90 mm Hg as “not recommended.”16 A hemoglobin A1c (HbA1c) value among individuals with diabetes was a proxy for blood glucose management, with ≤7.0% “recommended” and >7.0% “not recommended.”17
Table 1.
Measures of Health Behaviors in CKD
| Health Behavior Measures | Recommended | Not recommended |
|---|---|---|
| Cigarette smoking | Never/former | Current |
| Body mass index (kg/m2) | 20 to <25 | <20 or ≥25 |
| Physical activity (min/wk) | ≥150 moderate, ≥75 vigorous, or moderate + vigorous ≥150 | Less than recommended |
| Healthy diet scorea | 3 or 4 | ≤ 2 |
| Blood pressure (mm Hg) | ≤140/90 | >140/90 |
| Blood glucose control | HbA1c ≤7% | HbA1c >7% |
Healthy diet score: based on 12-month diet history of fruits, vegetables, fish, grains, and sweets/sweetened beverages. A diet score of 3 or 4 was “recommended,” and ≤2 was “not recommended.”
Other Study Variables
Age, gender, race/ethnicity, education, income, insurance, duration of CKD awareness, and medical history were collected through self-report. Comorbidities reported were used to construct an index using the Charlson Comorbidity Index scoring system.18 Assessments of health literacy, physical health, general health status, depressive symptoms, and cognitive function were collected through validated questionnaires. Health literacy was determined by a score on the short version of the Test of Functional Health Literacy Assessment.19 Reported health status (including mental and physical health) and health-related quality of life were collected using the Kidney Disease Quality of Life Short Form 36.20 The Beck Depression Inventory, which is widely used in CKD, assessed symptoms of depression.21, 22, 23 Global cognitive function was assessed with the Modified Mini-Mental State Examination.24 Several measures were available only in the phase III Cohort, including social support, which was collected using the Lubben Social Network Scale,25 general disease management self-efficacy, and self-efficacy in the patient−provider interaction, which were both assessed using 5-item validated instruments.26, 27 Blood samples were used to measure HbA1c, and to estimate eGFR with a CRIC-specific equation using serum creatinine and cystatin C.28 Cystatin C may be less related to muscle mass than creatinine, and therefore may have a particular advantage in older adults.29 A 24-hour urine collection was used to assess proteinuria.
The following outcomes were evaluated: (i) CKD progression, defined as a 50% decrease in eGFR from baseline or occurrence of ESRD (incident dialysis or kidney transplantation); (ii) atherosclerotic cardiovascular events (myocardial infarction, stroke, or peripheral arterial disease); and (iii) death from any cause. Progression of CKD was defined by achieving the composite endpoint of development of ESRD or halving of eGFR from baseline. Ascertainment of time to eGFR halving was imputed assuming a linear decline in kidney function between annual visits.30 End-stage renal disease was defined as the initiation of maintenance dialysis or kidney transplantation, and was ascertained through self-report or report from family members of deceased participants. Information collected on ESRD was supplemented by data from the United States Renal Data System. Cardiovascular events were adjudicated by 2 physician reviewers using hospital records.11 Deaths were ascertained from reports by next of kin, death certificates, obituaries, reviews of hospital records, and the Social Security Death Master File. Participants were followed until the occurrence of death, withdrawal from the study, or the end of the follow-up period (mid-2014).
Statistical Analysis
We described the study population using mean, SD, or median and interquartile range for continuous variables, and frequency and proportion for categorical variables. The Pearson χ2 or Fisher exact test and analysis of variance or Kruskal−Wallis test were used to compare categorical and continuous variables, respectively. We examined for patterns of health behavior engagement, as many CKD patients engage only in certain behaviors. To account for clustering of behaviors, we used latent class analysis (LCA), a well-validated statistical technique that uses mixture modeling to identify unobserved clusters based on variables of interest (i.e., the measures of health behaviors) without mandating consideration of the outcome.31 To determine the optimal number of clusters (or patterns), latent class models with successive numbers of patterns were compared, and the best-fitting model was chosen. Our previous publication describes this process in more detail.32 A priori, we chose to stratify the LCA by age greater or less than 65 years, as many studies have demonstrated effect modification at 65 years of age in relation to the clinical outcomes of interest. Further information on the age-stratified LCA is provided in the Supplementary Data. A sensitivity LCA stratified by age group was performed with the recommended blood pressure defined as ≤130 mm Hg systolic pressure and ≤80 mm Hg diastolic pressure.
To evaluate for potential barriers to health behavior engagement, we analyzed the association of psychosocial and clinical factors in multinomial logistic regression models with reported prevalence ratios to approximate relative risk. The covariates included in the regression models were thought to influence participation in health behaviors and/or have been associated with self-management behaviors in prior studies. Potential barriers previously identified in other chronic diseases include inadequate social support, low self-efficacy, physical limitations or low levels of physical functioning, presence of depression, low level of health literacy, lack of knowledge about one’s medical conditions, and presence of comorbid diseases.33, 34, 35, 36, 37, 38, 39, 40 Subjective well-being has also been linked to self-management in individuals with ESRD.41 In addition, social and systems-based characteristics, such as uninsured/underinsured, may play major roles in increasing complexity of patients’ ability to adopt health behaviors.42, 43 Education, income, and insurance were included as key measures of social and systems-based characteristics. Cognition was included, as it may influence the ability or motivation of individuals with CKD to participate in health behaviors.
Cox proportional hazards models were used to generate crude and adjusted HRs using the phase I cohort only, stratified by diabetes, given the observed differences of CKD progression within the CRIC population. Insufficient follow-up time did not allow for analyses using the phase III cohort. Sample sizes included in each analytical step are provided in Supplementary Figure S1. Secondary analyses were performed using Cox proportional hazards models to estimate the risk of outcomes with individual measures of health behaviors. For each outcome, hierarchical modeling was performed with sequential adjustment for socio-demographic covariates including gender, race/ethnicity, clinical center, education, eGFR, proteinuria, diabetes, and history of CVD. In the secondary analyses, a final adjustment was made for the individual measures of health behaviors. For analyses of cardiovascular events, death was a competing event. The assumptions of proportionality were tested in the fully adjusted model; if violated, the covariates were modified by an interaction with time, and for the health behavior patterns, HRs were reported in intervals of follow-up time. Statistical analyses were conducted using STATA version 14.2, using the LCA Stata Plugin, Version 1.2 and the LCA Bootstrap, Version 1.0 (Penn State Methodology Center, University Park, PA).44, 45, 46
Results
Characteristics of the study participants are listed in Tables 2 and 3. Participants <65 years of age had a higher level of education, income, cognition, and fewer comorbidities, compared to those ≥65 years. The younger age group reported lower disease self-efficacy and more depressive symptoms. Three similar patterns of health behavior engagement were identified among participants <65 and ≥65 years of age based on model fit indices (Supplementary Table S1). In both age groups, the 3 health behavior patterns were characterized as follows: 1 “healthy” pattern, and 2 “less healthy” patterns comprising 1 pattern with more obesity and sedentary activity (more obese/sedentary) and the other with more smoking and less obesity (less obese/smoker) (Supplementary Figure S2). In both age groups, individuals with the healthy pattern had high levels of engagement in recommended health behaviors; those with the more obese/sedentary pattern had low levels of recommended BMI and physical activity; and those with the less obese/smoker pattern had high levels of smoking and recommended BMI. Blood glucose control was similar across the patterns. Among individuals <65 years of age, the healthy pattern was the most prevalent (55.7%), the more obese/sedentary pattern the next most prevalent (40.1%), and the less obese/smoker pattern the least prevalent (4.2%). Among the older age group, the most prevalent was the more obese/sedentary pattern (47.9%), the healthy pattern was less prevalent (42.3%), and, the less obese/smoker pattern was the least prevalent (9.8%).
Table 2.
Demographic characteristics of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohorts overall, and by age group (<65 years and ≥65 years)
| Characteristic | Age <65 years |
Age ≥65 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 3552) | Healthy pattern n = 1979 (55.7%) |
More obese/sedentary pattern n = 1423 (40.1%) |
Less obese/smoker pattern n = 150 (4.2%) |
P valuea | Overall (N = 1947) | Healthy pattern n = 823 (42.3%) |
More obese/sedentary pattern n = 933 (47.9%) |
Less obese/smoker pattern N = 191 (9.8%) |
P value | |
| Age, mean (SD) | 54 (9) | 54 (9) | 53 (9) | 53 (8) | <0.001 | 70 (3) | 70 (4) | 70 (3) | 69 (3) | 0.01 |
| Gender, male, % | 55 | 56 | 54 | 59 | 0.19 | 42 | 61 | 56 | 57 | 0.10 |
| Race, % | <0.001 | 0.001 | ||||||||
| Non-Hispanic white | 39 | 41 | 37 | 30 | 45.9 | 50.3 | 43.6 | 37.0 | ||
| Non-Hispanic black | 45 | 42 | 47 | 65 | 42 | 38 | 44 | 52 | ||
| Hispanic | 12 | 13 | 12 | 3 | 9 | 8 | 10 | 7 | ||
| Other | 4 | 4 | 4 | 2 | 3 | 4 | 3 | 3 | ||
| Education, % | <0.001 | <0.001 | ||||||||
| High school or less | 36 | 33 | 39 | 50 | 41 | 33 | 46 | 49 | ||
| College or more | 64 | 67 | 61 | 50 | 59 | 67 | 54 | 51 | ||
| Insurance, % | <0.001 | 0.01 | ||||||||
| Medicaid | 24 | 20 | 29 | 42 | 13 | 10 | 15 | 15 | ||
| Medicare/VA | 42 | 42 | 41 | 40 | 80 | 82 | 79 | 80 | ||
| Private/commercial | 34 | 38 | 30 | 19 | 7 | 9 | 6 | 5 | ||
| Income, % | <0.001 | <0.001 | ||||||||
| ≤$20,000 | 37 | 33 | 40 | 52 | 33 | 26 | 36 | 50 | ||
| $20–50,000 | 26 | 26 | 27 | 23 | 35 | 35 | 36 | 27 | ||
| >$50,000 | 37 | 41 | 22 | 25 | 32 | 39 | 28 | 23 | ||
Values for continuous data given as mean (SD); values for categorical data given as percentage.
P value for test of the difference across behavior engagement patterns.
Table 3.
Clinical characteristics of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohorts overall, and by age group (<65 years and ≥65 years)
| Characteristic | Age <65 years |
Age ≥65 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 3552) | Healthy pattern n = 1979 (55.7%) |
More obese/sedentary pattern n = 1423 (40.1%) |
Less obese/smoker pattern n = 150 (4.2%) |
P valuea | Overall (n = 1947) | Healthy pattern n = 823 (42.3%) |
More obese/sedentary pattern n = 933 (47.9%) |
Less obese/smoker pattern n = 191 (9.8%) |
P value | |
| Adequate Health Literacyb, % | 87 | 87 | 88 | 84 | 0.29 | 83 | 83 | 81 | 75 | 0.08 |
| Duration of CKD awareness, % | 0.15 | 0.45 | ||||||||
| Unknown/≤1 yr | 45 | 46 | 43 | 42 | 48 | 48 | 49 | 53 | ||
| >1 yr | 56 | 54 | 57 | 58 | 52 | 52 | 51 | 47 | ||
| Disease self-efficacy score, mean (SD) | 40 (9) | 41 (9) | 39 (10) | 39 (10) | 0.03 | 42 (8) | 43 (7) | 40 (9) | 42 (7) | 0.001 |
| Patient provider efficacy score, mean (SD) | 21 (4) | 21 (4) | 21 (4) | 21 (4) | 0.73 | 21 (4) | 22 (4) | 21 (4) | 21 (5) | 0.09 |
| Social Support scorec, mean (SD) | N/A | N/A | N/A | N/A | 16 (6.) | 17 (6) | 16 (6) | 15 (6) | 0.02 | |
| Reported general health excellent/good, % | 57 | 62 | 51 | 57 | <0.001 | 64 | 73 | 58 | 59 | <0.001 |
| Cognition score, mean (SD) | 92 (8) | 92 (8) | 92 (8) | 91 (8) | 0.08 | 89 (9) | 91 (8) | 89 (10) | 88 (10) | <0.001 |
| BDI depressive symptom score, mean (SD) | 9 (9) | 8 (8) | 10 (9) | 11 (9) | <0.001 | 7 (7) | 6 (6) | 8 (7) | 8 (6) | <0.001 |
| BMI, kg/m2, mean (SD) | 33 (8) | 33 (8) | 33 (9) | 29 (7) | <0.001 | 32 (7) | 31 (6) | 34 (7) | 27 (6) | <0.001 |
| Any CVD, % | 29 | 28 | 31 | 35 | <0.001 | 42 | 38 | 44 | 48 | 0.01 |
| eGFR, ml/min per 1.73 m2, mean (SD) | 50 (18) | 51 (17) | 48 (18) | 46 (17) | <0.001 | 47 (15) | 49 (15) | 46 (15) | 46 (15) | <0.001 |
| Proteinuria, g/24 h, mean (SD) | 1.2 (2.5) | 1.1 (2.3) | 1.3 (2.6) | 1.3 (2.2) | <0.001 | 0.7 (1.5) | 0.6 (1.1) | 0.7 (1.6) | 0.9 (1.1) | 0.22 |
| Charlson Comorbidity Index score, mean (SD) | 4.9 (2.1) | 4.9 (2.1) | 4.9 (2.1) | 5.1 (2.0) | 0.52 | 6.8 (1.7) | 6.6 (1.7) | 7.0 (1.7) | 6.6 (1.7) | <0.001 |
| KDQOL Mental Composite Score, mean (SD) | 49.1 (10.9) | 49.9 (10.4) | 48.2 (11.3) | 48.0 (11.2) | <0.001 | 52.4 (9.5) | 53.4 (8.6) | 51.4 (10.1) | 52.8 (9.5) | <0.001 |
| KDQOL Physical Composite Score, mean (SD) | 41.1 (11.7) | 42.3 (11.3) | 39.5 (12.1) | 40.9 (11.3) | <0.001 | 40.5 (11.1) | 43.1 (10.4) | 38.3 (11.1) | 39.7 (11.4) | <0.001 |
| Total number of medications, mean (SD) | 8.8 (4.8) | 8.9 (4.8) | 8.8 (4.8) | 7.8 (4.4) | 0.02 | 10.3 (4.5) | 10.1 (4.4) | 10.6 (4.5) | 9.8 (5.1) | 0.003 |
| Health behavior measures, % | ||||||||||
| Recommended body mass index | 14 | 12 | 14 | 33 | <0.001 | 11 | 14 | 0 | 55 | <0.001 |
| Nonsmoking | 85 | 97 | 77 | 0 | <0.001 | 92 | 94 | 100 | 42 | <0.001 |
| Controlled blood pressure | 72 | 77 | 65 | 61 | <0.001 | 72 | 73 | 70 | 73 | 0.29 |
| Recommended diet | 33 | 58 | 5 | 6 | <0.001 | 31 | 33 | 32 | 20 | 0.004 |
| Recommended physical activity | 41 | 66 | 0 | 100 | <0.001 | 42 | 100 | 0 | 0 | <0.001 |
| HbA1c <7.0% (if diabetic) | 36 | 37 | 37 | 30 | 0.52 | 51 | 53 | 49 | 57 | 0.29 |
Values for continuous data given as mean (SD); values for categorical data given as percentage. BDI, Beck Depression Inventory; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; KDQOL, Kidney Disease Quality of Life; NA, not available.
P value for test of the difference across behavior engagement patterns.
Adequate health literacy was defined by score >23 on the Short Test of Functional Health Literacy.19
Assessed only in older adults.
In both age groups, individuals with the healthy pattern had the highest levels of education, income, self-efficacy, renal function, cognition, and physical functioning, and the lowest levels of depressive symptoms and CVD history (Tables 2 and 3). Comorbidity scores were similar across the health behavior patterns. In older adults, the less healthy patterns, compared to the healthy pattern, were associated with inadequate health literacy, less social support, as well as lower mental and physical functioning (Supplementary Table S2).
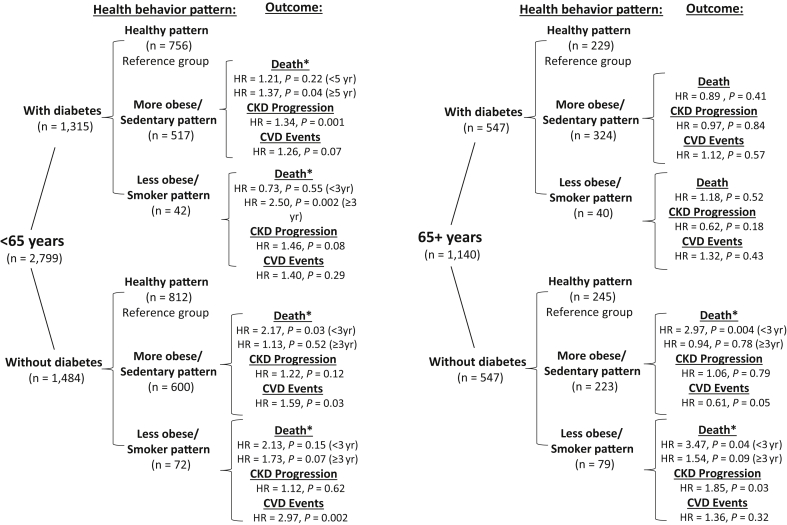
Overall, event rates were increased in individuals with the less healthy patterns, and among those with diabetes (Supplementary Table S3). The proportional hazards assumption was violated for death among those <65 years of age; therefore the HR and 95% CI are reported in intervals of follow-up time in which the hazard appeared to change (before and after 3 years for those without diabetes and 5 years for those with diabetes). Results are reported for the final multivariable-adjusted model (Figures 1 and 2; Supplementary Table S4). Among individuals without diabetes, increased risk of death was seen in the more obese/sedentary pattern (HR = 2.17, 95% CI = 1.09–4.29, <3 years of follow-up), and of cardiovascular events in both the more obese/sedentary and less obese/smoker patterns (HR = 1.59, 95% CI = 1.04–2.43, and HR = 2.97, 95% CI = 1.49–5.90, respectively). Among individuals with diabetes, increased risk for death was observed among the more obese/sedentary and less obese/smoker patterns (HR = 1.37, 95% CI = 1.01–1.86 and HR = 2.50, 95% CI = 1.39–4.50, respectively for >5 years of follow-up) and CKD progression in the more obese/sedentary pattern (HR = 1.34, 95% CI = 1.13–1.59). The proportional hazards assumption was also violated for the outcome of death among older adults without diabetes, and the HR (95% CI) reported before and after 3 years of follow-up time. Increased rates of death were seen in the more obese/sedentary and less obese/smoker patterns (HR = 2.97, 95% CI = 1.43–6.19 and HR = 3.47, 95% CI = 1.48–8.11, respectively for <3 years), and CKD progression for the less obese/smoker pattern (HR = 1.85, 95% CI = 1.07–3.22).
Figure 1.
Tree display of sample size by age group, diabetes status, and health behavior patterns with reported hazard ratios (HR) and P values for each clinical outcome. Reference group is the healthy pattern group. ∗HR is reported from baseline to 3 years of follow-up and thereafter for participants without diabetes and from baseline to 5 years of follow-up for those with diabetes, as the proportionality of hazard assumption was violated, with the hazard of death appearing to change at selected intervals.
Figure 2.
(a,b) Forest plot of hazard ratios (HRs) (95% confidence intervals [CIs]) of clinical outcomes by health behavior pattern for participants without diabetes (ND) (white rows) and those with diabetes (DM) (gray rows) for participants (a) <65 years of age and (b) ≥65 years of age. Reference group is the healthy pattern group for both DM and ND. HR is reported from baseline to 3 years of follow-up and after for ND and from baseline to 5 years of follow-up and thereafter for DM, as the proportionality of hazards assumption was violated, with the hazard of death appearing to change at selected intervals.
Individual Health Behaviors
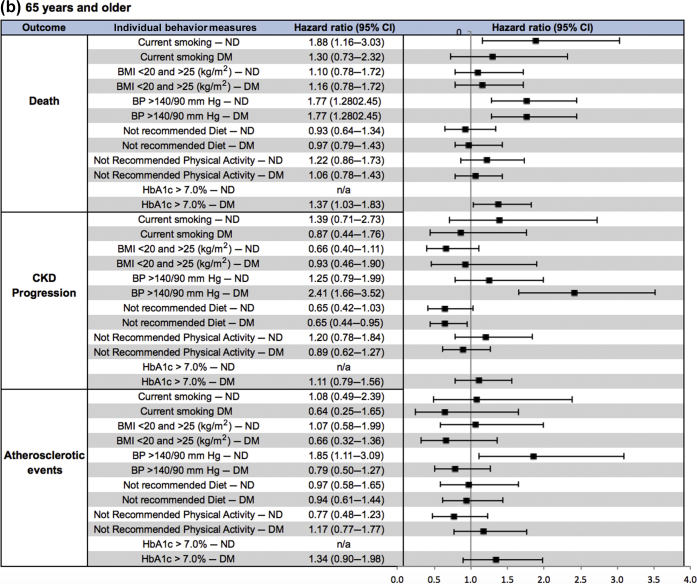
In individuals <65 years of age without diabetes, current smoking and uncontrolled blood pressure were associated with increased risk of death (HR = 2.62, 95% CI = 1.81–3.79, and HR = 1.53, 95% CI = 1.06–2.19, respectively) as well as increased risk of atherosclerotic events (HR = 2.60, 95% CI = 1.57–4.29, and HR = 2.11, 95% CI = 1.33–3.35, respectively) (Figure 3, Supplementary Table S5). Among individuals <65 years of age with diabetes, current smoking and less physical activity were associated with increased risk of death (HR = 1.47, 95% CI = 1.10–1.97, and HR = 1.40, 95% CI = 1.09–1.80, respectively); uncontrolled blood pressure and less healthy diet were associated with increased risk of CKD progression (HR = 1.65, 95% CI = 1.34–2.04, and HR = 1.32, 95% CI = 1.08–1.62, respectively); and less physical activity and increased HbA1c were associated with increased risk of atherosclerotic events (HR = 1.41, 95% CI = 1.06–1.87, and HR = 1.65, 95% CI = 1.21–2.23, respectively). Of note, a BMI <20 kg/m2 or ≥25 kg/m2 was associated with decreased risk of CKD progression in those with and without diabetes (HR = 0.66, 95% CI = 0.49–0.89, and HR = 0.49, 95% CI = 0.36–0.67).
Figure 3.
(a,) Forest plot of hazard ratios (HRs) (95% confidence intervals [CIs]) of clinical outcomes by individual measures of health behaviors for participants without diabetes (ND) (white rows) and those with diabetes (DM) (gray rows) among participants (a) <65 years of age and (b) ≥65 years of age.
In individuals ≥65 years of age without diabetes, current smoking was associated with increased risk of death (HR = 1.88, 95% CI = 1.16–3.03), and uncontrolled blood pressure was associated with increased atherosclerotic events (HR = 1.85, 95% CI = 1.11–3.09) (Figure 3, Supplementary Table S5). Among individuals ≥65 years of age with diabetes, uncontrolled blood glucose and blood pressure were associated with increased risk of death (HR = 1.37, 95% CI = 1.03–1.83, and HR = 1.77, 95% CI = 1.28–2.45, respectively), and uncontrolled blood pressure was associated with increased CKD progression (HR = 2.41, 95% CI = 1.66–3.52). Notably, a BMI <20 kg/m2 or ≥25 kg/m2 was associated with decreased risk of death (HR = 0.55, 95% CI = 0.33–0.92), and a less healthy diet was associated with decreased risk of CKD progression (HR = 0.65, 95% CI = 0.44–0.95).
In the sensitivity analyses in which recommended blood pressure was defined as ≤130 mm Hg systolic and ≤80 mm Hg diastolic, slightly different behavior patterns were identified from the primary analysis (Supplementary Tables S6–S11). Behavior patterns in individuals with a lower prevalence of recommended health behaviors were associated with poorer clinical characteristics, and the health behavior patterns with less obesity and more smoking similarly trended toward an increased hazard of poor clinical outcomes, with a significant finding for CKD progression for those <65 years of age.
Conclusion
In a multicenter, diverse cohort of individuals with CKD, we identified 3 similar patterns of health behavior engagement among younger and older adults. The health behavior engagement patterns consisted of 1 “healthy” pattern, and 2 “less healthy” patterns comprising 1 pattern with more obesity and sedentary activity and another with more smoking and less obesity. Overall, the less healthy behavior patterns were associated with increased hazard of poor clinical outcomes. Potential barriers to recommended health behavior engagement included social determinants of health, as well as depressive symptoms, inadequate health literacy, poor social support, and a higher burden of comorbidities.
Previous studies regarding other chronic diseases have shown a link between health behaviors and poor outcomes.47, 48 This is the first study to examine the health behavior pattern–outcome link separately among older and younger adults with CKD. We explored for patterns of health behavior engagement, as we thought it imperative to recognize that participation in health behaviors cluster at the individual level. We found that clustering of certain health behaviors in less healthy behavior patterns was associated with increased risk of poor outcomes, but differentially, depending on diabetes status. Among younger adults, the less healthy behavior patterns were associated with increased risk of CKD progression in only those with diabetes and increased atherosclerotic events in those without diabetes. Among the older adults, less healthy behavior patterns were associated with increased risk of death and CKD progression in only those without diabetes. There were no statistically significant associations for older adults with concomitant diabetes. This finding suggests that the presence of diabetes may largely explain the association in this age group. Alternatively, the lack of a significant finding could also be related to reduced power among this smaller sample of older adults.
In addition to exploring the health behavior pattern–outcome link, we investigated the association of individual health behaviors to further explore which health behaviors could potentially be driving the risk of poor outcomes. Previously, within the CKD population, several individual health behaviors, such as smoking, blood pressure, and physical activity, have been shown to be associated with outcomes, but these behaviors have not been specifically explored in different age groups.49, 50 In this study, the strongest associations between individual health behaviors and outcomes existed for current smoking and higher blood pressure. There were paradoxical findings with the recommended BMI of <20 kg/m2 or ≥25 kg/m2, in that it was associated with a decreased risk of death among older adults with diabetes, which has not yet been reported,51 and a decreased risk of CKD progression in younger adults; however, the published reports of BMI contributing to CKD progression have been conflicting.52, 53 Furthermore, a diet that did not fall in the recommended range was differentially associated with CKD progression between age groups with diabetes, as well as an increased risk among younger adults and a decreased risk in older adults. The available studies on the influence of diet have yet to demonstrate a consistent association with CKD-related outcomes.54
Based on this study’s results, and if future studies support the findings, health care providers caring for CKD patients could tailor their management recommendations. For example, ensuring that we prioritize blood pressure control regardless of age, as it was a central component of the healthy behavior pattern in both age groups, and as an individual measure, it appears to be protective for death, CKD progression, and atherosclerotic events. In addition, a focus on increasing physical activity as an intervention target would be beneficial, since the health behavior patterns in both age groups with sedentary activity were associated with poorer outcomes across diabetes strata. In addition, even though the prevalence of smoking is quite low, smoking cessation is likely a prime intervention target for individuals <65 years of age in the health behavior pattern with less obesity and more smoking, as this group already participates in increased physical activity. At this time, weight management should likely not be a focus of CKD management recommendations, as it remains unclear which BMI is most appropriate in predialysis CKD.
Potential barriers identified in this study, such as less education, lower self-efficacy, and more depressive symptoms, have also been identified in other chronic disease populations, but had yet to be explored for differences between younger and older adults.34, 38 Age-specific barriers are important to consider, because certain clinical and psychosocial factors may affect older adults’ ability to adopt health behaviors. In particular, older adults with CKD have higher rates of comorbid conditions, frailty, and decreased functional status, which could further challenge their ability to engage in the recommended health behaviors.29, 55, 56 In this study, we demonstrate that a higher burden of comorbidities and medications, lower social support, inadequate health literacy, lower physical functioning, and lower efficacy in the patient−provider interaction are associated with less healthy behavior engagement patterns.
A logical first step to enhance the ability of individuals with CKD to participate in recommended health behaviors is to address the barriers to behavior engagement. The more readily modifiable barriers include physical functioning, self-efficacy, social support, health literacy, and depressive symptoms, which could serve as potential targets for intervention. Specifically addressing health literacy could be particularly beneficial as it has been associated with better outcomes in other chronic diseases,57 can lead to greater self-efficacy, which in turn leads to improved self-management, which includes participation in recommended health behaviors.58 In addition, future directions in CKD management could further explore the role of self-efficacy in the patient−provider relationship, as well as conducting randomized clinical trials of health behavior promotion programs, exploring the role of supporting health behaviors to address poor outcomes.
Unique to this study is the assessment of engagement in health behaviors, identification of potential barriers to health behavior engagement, and description of the association of health behaviors to important clinical outcomes separately for younger and older adults with CKD. Strengths of this study includes the wealth of data obtained from the CRIC Study, which represents the leading causes of CKD, a broad spectrum of ages, and diverse racial/ethnic groups. The data also allowed for the assessment of psychosocial, clinical and sociodemographic factors that have been minimally explored in relation to health behaviors and, more broadly, self-management in CKD.
Despite these strengths, there are limitations to discuss. This study used baseline measures and therefore could not assess whether the severity of kidney disease or other important covariates such as cognitive decline and depression mediate the association with clinical outcomes. It will be important to investigate how psychosocial characteristics and change in renal function influence health behavior engagement in a longitudinal manner in order to potentially identify the reasons for the excess mortality and the cognitive and functional decline unique to older adults with CKD. Furthermore, the generalization of our study results could be limited, as the CRIC Study population likely differs from the overall CKD population. The prevalence of adequate health literacy among older participants within the CRIC Study was somewhat higher (75.2%–83.2%) than national levels (71%).59 However, the engagement in most of the health behaviors was similar to that in a national sample of older adults.60
In conclusion, we identified 3 similar patterns of engagement in health behaviors among younger and older adults with CKD that varied in association with clinical outcomes. In both age groups, individuals with less healthy behavior patterns had an increased hazard of poor clinical outcomes, but differed depending on diabetes status. Identification of the health behavior patterns and barriers to health behavior engagement may help target high-risk groups for strategies to increase participation in health behaviors.
Disclosure
AHA declares lecture fees from Kyowa Hakko Kirin. MW declares consulting fees from Akebia, Amag, Amgen, Ardelyx, Diasorin, Keryx, and grant support from Shire. All the other authors declared no competing interests.
Acknowledgments
SJS was supported by a training grant from the National Institutes of Health (NIH): F32-DK113681-01A1. ACR was supported by NIH/NIDDK K23DK094829. JWN was supported by NIH/NIDDK K23 DK097183, R01 DK115844. AHA was supported by NIH/NIDDK R01DK107566 and R01 DK104730.
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the following: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The funding for the CRIC Study supported the collection of the data that was analyzed for this study. The funders had no role in the study design, analysis and interpretation of the data, writing the manuscript, or the decision to submit for publication.
Footnotes
Supplementary Data. Latent class analysis.
Figure S1. Analytical steps and associated sample sizes for each step.
Figure S2. Response probabilities (x-axis) of recommended health behaviors (y-axis), given behavior engagement pattern, (A) <65 years and (B) 65+ years. Pattern 1 (healthy pattern), pattern 2 (more obese/sedentary pattern), pattern 3 (less obese/smoker pattern).
Table S1. Model fit indices of successive latent-class models by age group.
Table S2. Multinomial logistic regression model results of the association of the 3 behavior engagement patterns with selected demographic, psychosocial, and clinical factors among age groups in the 5499 CRIC Study participants.
Table S3. Events, event rate (per 100 person-years) reported for the phase I CRIC population (N = 3939), stratified by diabetes and age (<65 years and ≥65 years of age).
Table S4. Associations of health behavior engagement patterns with clinical outcomes by <65 years and ≥65 years of age in the phase I CRIC population (N = 3939), stratified by diabetes.
Table S5. Associations of individual health behavior measures among adults with CKD <65 years and ≥65 years, stratified by diabetes.
Table S6. Model fit indices of successive latent-class models by age group, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Table S7. Behaviors of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohort participants <65 years, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Table S8. Health behaviors of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohort participants ≥65 years, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Table S9. Clinical characteristics of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohorts by age group (<65 years and ≥65 years), in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Table S10. Associations of health behavior engagement patterns from the sensitivity LCA and clinical outcomes in the phase I CRIC population (N = 3939) <65 years, stratified by diabetes.
Table S11. Associations of health behavior engagement patterns from the sensitivity LCA and clinical outcomes in the phase I CRIC population (N = 3939) ≥65 years, stratified by diabetes.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Contributor Information
Sarah J. Schrauben, Email: Sarah.Schrauben@uphs.upenn.edu.
CRIC Study Investigators:
Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jian He, John W. Kusek, James P. Lash, Panduranga S. Rao, Mahboob Rahman, and Raymond R. Townsend
Supplementary Material
Latent class analysis.
Analytical steps and associated sample sizes for each step.
Response probabilities (x-axis) of recommended health behaviors (y-axis), given behavior engagement pattern, (A) <65 years and (B) 65+ years. Pattern 1 (healthy pattern), pattern 2 (more obese/sedentary pattern), pattern 3 (less obese/smoker pattern).
Model fit indices of successive latent-class models by age group.
Multinomial logistic regression model results of the association of the 3 behavior engagement patterns with selected demographic, psychosocial, and clinical factors among age groups in the 5499 CRIC Study participants.
Events, event rate (per 100 person-years) reported for the phase I CRIC population (N = 3939), stratified by diabetes and age (<65 years and ≥65 years of age).
Associations of health behavior engagement patterns with clinical outcomes by <65 years and ≥65 years of age in the phase I CRIC population (N = 3939), stratified by diabetes.
Associations of individual health behavior measures among adults with CKD <65 years and ≥65 years, stratified by diabetes.
Model fit indices of successive latent-class models by age group, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Behaviors of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohort participants <65 years, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Health behaviors of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohort participants ≥65 years, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Clinical characteristics of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohorts by age group (<65 years and ≥65 years), in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Associations of health behavior engagement patterns from the sensitivity LCA and clinical outcomes in the phase I CRIC population (N = 3939) <65 years, stratified by diabetes.
Associations of health behavior engagement patterns from the sensitivity LCA and clinical outcomes in the phase I CRIC population (N = 3939) ≥65 years, stratified by diabetes.
References
- 1.Ortman J.M., Velkoff V.A., Hogan H. U.S Department of Commerce. U.S Census Bureau; Suitland, MD: 2014. An aging nation: the older population in the United States. [Google Scholar]
- 2.Stevens L., Li S., Wang C. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2010;55(suppl 2):S23–S33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go A., Chertow G., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Hemmelgarn B., Zhang J., Manns B. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69:2155–2161. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 5.O'Hare A., Choi A.I., D B. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 6.National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation Kidney Disease Outcomes Quality Initiative: KDOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kid Dis. 2004;43(5 suppl 1):S1–S290. [PubMed] [Google Scholar]
- 8.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chornic Kidney Disease Guideline Development Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clincal practice guidelines. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Jones D., Hong Y., Labarthe D. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 10.Bowling C.B., Vandenberg A.E., Phillips L.S. Older patients' perspectives on managing complexity in CKD self-management. Clin J Am Soc Nephrol. 2017;12:635–643. doi: 10.2215/CJN.06850616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman H.I., Appel L.J., Chertow G.M. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 12.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Chort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Kidney Foundation KDOQI Clinical Practice Guidelines for Diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation K/DOQI Clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1–S290. [PubMed] [Google Scholar]
- 15.National Cancer Institute . National Cancer Institute; 2002. Diet History Questionnaire. Bethesda, MD: National Institutes of Health, Applied Research Program. [Google Scholar]
- 16.KDIGO Clinical Pratice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:341–342. [Google Scholar]
- 17.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3 doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 18.Charlson M., Pompei P., Ales K., MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Baker D.W., Williams M.V., Parker R.M. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38:33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 20.Ricardo A.C., Hacker E., Lora C.M. Validation of the Kidney Disease Quality of Life Short Form 36 (KDQOL-36) US Spanish and English versions in a cohort of Hispanics with chronic kidney disease. Ethn Dis. 2013;23:202–209. [PMC free article] [PubMed] [Google Scholar]
- 21.Craven J.L., Rodin G.M., Littlefield C. The Beck Depression Inventory as a screening device for major depression in renal dialysis patients. Int J Psychiatry Med. 1988;18:365–374. doi: 10.2190/m1tx-v1ej-e43l-rklf. [DOI] [PubMed] [Google Scholar]
- 22.Watnick S W.P., Damadura T., Ganzini L. Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis. 2005;46:919–924. doi: 10.1053/j.ajkd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Hedayati SS B.H., Kuchibhathla M., Kimmel P.L., Dzczech L.A. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69:1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 24.Teng E.L., Chui H.C. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 25.Lubben J., Blozik E., Gillmann G. Performance of an abbreviated version of the Lubben Social Network Scale among three European Community-dwelling older adult populations. Gerontologist. 2006;46:503–513. doi: 10.1093/geront/46.4.503. [DOI] [PubMed] [Google Scholar]
- 26.Maly R., Frank J., Marshall G. Perceived Efficacy in Patient-Physician Interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc. 1998;46:889–894. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 27.Lorig K., Stewart A., Ritter P. Sage Publications; Thousand Oaks, CA: 1996. Outcome Measures for Health Education and Other Health Care Interventions. [Google Scholar]
- 28.Anderson A., Yang W., Hsu C. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens L., Levey A. Chronic kidney disease in the elderly—how to assess risk. N Engl J Med. 2005;352:2122–2124. doi: 10.1056/NEJMe058035. [DOI] [PubMed] [Google Scholar]
- 30.Yang W., Xie D., Anderson A.H. Association of kidney diseaes outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2014;63:236–243. doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanza S.T., Bray B.C., LM C. An introduction to latent class and latent transition analysis. In: Weiner I.B., editor. Vol 2. John Wiley and Sons; Hoboken, NJ: 2013. pp. 691–716. (Handbook of Psychology, Second Edition). [Google Scholar]
- 32.Schrauben S., Hsu J., Rosas S. CKD self-management: phenotypes and associations with clinical outcomes. Am J Kidney Dis. 2018;72:360–370. doi: 10.1053/j.ajkd.2018.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albright T.L., Parchman M., Burge S.K., RRNeST Investigators Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354–360. [PubMed] [Google Scholar]
- 34.Glasgow R., Toobert D., Gillette C. Psychosocial barriers to diabetes self-managment and quality of life. Diabetes Spectrum. 2001;14:33–41. [Google Scholar]
- 35.Riegel B., Carlson B. Facilitators and barriers to heart failure self-care. Patient Educ Couns. 2002;45:287–295. doi: 10.1016/s0738-3991(01)00165-3. [DOI] [PubMed] [Google Scholar]
- 36.Lorig K.R., Soebl D.S., Ritteer P.L. Effect of a self-management program on patients with chronic disease. Effect Clin Pract. 2001;4:256–262. [PubMed] [Google Scholar]
- 37.Wolf M.S., Gazmararian J.A., Baker D.W. Health literacy and functional health status among older adults. Arch Intern Med. 2005;165:1946–1952. doi: 10.1001/archinte.165.17.1946. [DOI] [PubMed] [Google Scholar]
- 38.Bayliss E.A., Ellis J.L., Steiner J.F. Barriers to self-management and quality-of-life outcomes in seniors with multimorbidities. Ann Fam Med. 2007;5:395–402. doi: 10.1370/afm.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Vargas P., Tong A., Phoon R. Knowledge deficit of patients with stage 1-4 CKD: a focus group study. Nephrology. 2014;19:234–243. doi: 10.1111/nep.12206. [DOI] [PubMed] [Google Scholar]
- 40.Palmer S.C., Hanson C.S., Craig J.C. Dietary and fluid restrictions in CKD: a thematic syntehsis of patient views from qualitative studies. Am J Kidney Dis. 2015;65:559–573. doi: 10.1053/j.ajkd.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Curtin R.B., Mapes D., Schatell D., Burrows-Hudson S. Self-management in patients with end stage renal disease: exploring domains and dimensions. Nephrol Nurs J. 2005;32:389–398. [PubMed] [Google Scholar]
- 42.Loeb D.F., Binswanger I.A., Candrian C., Bayliss E.A. Primary care physician insights into a typology of the complex patient in primary care. Ann Fam Med. 2015;13:451–455. doi: 10.1370/afm.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant R.W., Ashburner J.M., Hong C.S. Defining patient complexity from the primary care physician's perspective: a cohort study. Ann Intern Med. 2011;155:797–804. doi: 10.7326/0003-4819-155-12-201112200-00001. [DOI] [PubMed] [Google Scholar]
- 44.StataCorp . StataCorp LP; College Station, TX: 2015. Stata Statistical Software: Release 14. [Google Scholar]
- 45.Lanza S, Dziak JJ, Huang L, et al. LCA Stata plugin users' guide (Version 1.2). University Park, PA: The Methodology Center, Penn State. Available at: methodology.psu.edu; 2015.
- 46.Huang L, Dziak JJ, Wagner AT, Lanza ST. LCA Bootstrap Stata function users' guide (Version 1.0). University Park, PA: The Methodology Center, Penn State. Available at: http://methodology.psu.edu. Accessed August 13, 2018.
- 47.Marti C., Gerogiopoulou V., Giamouzis G. Patient-reported selective adherence to heart failure self-care recommendations: a prospective cohort study: the Atlanta Cardiomyopathy Consortium. Congest Heart Fail. 2013;19:16–24. doi: 10.1111/j.1751-7133.2012.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fries J. Measuring and monitoring success in compressing morbidity. Ann intern Med. 2003;139:455–459. doi: 10.7326/0003-4819-139-5_part_2-200309021-00015. [DOI] [PubMed] [Google Scholar]
- 49.Ricardo A., Anderson C., Yang W. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65:412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He J., Whelton P. Elevated systolic blood pressure and risk of cardiovasuclar and renal disease. Overview of evidence form observational epidemiological studies and randomized controlled trials. Am Heart J. 1999;138:S211–S219. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 51.Rahimlu M., Shab-Bidar S., Djafarian K. Body mass index and all-cause mortality in chronic kidney disease: a dose-response meta-analysis of observational studies. J Ren Nutr. 2017;27:225–232. doi: 10.1053/j.jrn.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Othman M., Kawar B., El Nahas A. Influence of obesity on progression of non-diabetic chronic kidney disease: a retrospective cohort study. Nephron Clin Pract. 2009;113:c16–c23. doi: 10.1159/000228071. [DOI] [PubMed] [Google Scholar]
- 53.Khedr A., Khedr E., House A. Body mass index and the risk of progression of chronic kidney disease. J Ren Nutr. 2011;21:455–461. doi: 10.1053/j.jrn.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Jain N., Reilly R. Effects of dietary interventions on incidence and progression of CKD. Nat Rev Nephrol. 2014;10:712–724. doi: 10.1038/nrneph.2014.192. [DOI] [PubMed] [Google Scholar]
- 55.Fried L.F., Lee J.S., Shlipak M. Chronic kidney disease and functional limitation in older people: health, aging, and body composition study. J Am Geriatr Soc. 2006;54:750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 56.Bowling C., Sawyer P., Campbell R. Impact of chronic kidney disease on activities of daily living in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:689–694. doi: 10.1093/gerona/glr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schillinger D., Grumbach K., Piette J. Association of health literacy with diabetes outcomes. JAMA. 2002;288:475–482. doi: 10.1001/jama.288.4.475. [DOI] [PubMed] [Google Scholar]
- 58.Buchbinder R., Batterham R., Cicireillo S. Health literacy: what is it and why is it important to measure? J Rheumatol. 2011;38:1791–1797. doi: 10.3899/jrheum.110406. [DOI] [PubMed] [Google Scholar]
- 59.Kutner M., Greenberg E., Jin Y., Paulsen C. Vol NCES 2006-483. US Department of Education. National Center for Education Statistics; Washington, DC: 2006. (The Health Literacy of America's Adults: Results from the 2003 National Assessment of Adult Literacy). [Google Scholar]
- 60.Centers for Disease Control and Prevention . U.S. Department of Helath and Human Services; Atlanta, GA: 2013. The state of aging and health in America 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Latent class analysis.
Analytical steps and associated sample sizes for each step.
Response probabilities (x-axis) of recommended health behaviors (y-axis), given behavior engagement pattern, (A) <65 years and (B) 65+ years. Pattern 1 (healthy pattern), pattern 2 (more obese/sedentary pattern), pattern 3 (less obese/smoker pattern).
Model fit indices of successive latent-class models by age group.
Multinomial logistic regression model results of the association of the 3 behavior engagement patterns with selected demographic, psychosocial, and clinical factors among age groups in the 5499 CRIC Study participants.
Events, event rate (per 100 person-years) reported for the phase I CRIC population (N = 3939), stratified by diabetes and age (<65 years and ≥65 years of age).
Associations of health behavior engagement patterns with clinical outcomes by <65 years and ≥65 years of age in the phase I CRIC population (N = 3939), stratified by diabetes.
Associations of individual health behavior measures among adults with CKD <65 years and ≥65 years, stratified by diabetes.
Model fit indices of successive latent-class models by age group, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Behaviors of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohort participants <65 years, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Health behaviors of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohort participants ≥65 years, in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Clinical characteristics of latent class-defined health behavior engagement patterns in the CRIC phase I and III cohorts by age group (<65 years and ≥65 years), in the sensitivity LCA with controlled BP ≤130/80 mm Hg.
Associations of health behavior engagement patterns from the sensitivity LCA and clinical outcomes in the phase I CRIC population (N = 3939) <65 years, stratified by diabetes.
Associations of health behavior engagement patterns from the sensitivity LCA and clinical outcomes in the phase I CRIC population (N = 3939) ≥65 years, stratified by diabetes.