See Clinical Research on Page 1424 in Volume 3, Issue 6
Acute kidney injury (AKI) is defined by Kidney Disease: Improving Global Outcome (KDIGO) consensus criteria based on increase in serum creatinine (SCr) and decrease in urine output, which aim to capture an abrupt drop in glomerular filtration rate (GFR).1 Although most instances of injury to kidney tubules are accompanied by a drop in GFR and a subsequent increase in SCr, there are settings in which there is a discordance between the two. For example, during intensive blood pressure control or therapy with inhibitors of the renin−angiotensin−aldosterone system, AKI defined by SCr increase is not accompanied by kidney injury and causes false alarm.2, 3 Perhaps a bigger problem in sepsis-related AKI is that SCr might not increase despite injury to the kidneys. This could be due to a decrease in SCr production or its dilution from i.v. fluid administration. It could also be due to unfavorable kinetics of SCr, which does not increase for 24 to 48 hours after kidney injury has occurred. This is problematic, as patients could be exposed to nephrotoxins that exacerbate tubular injury while SCr has not yet detected AKI.
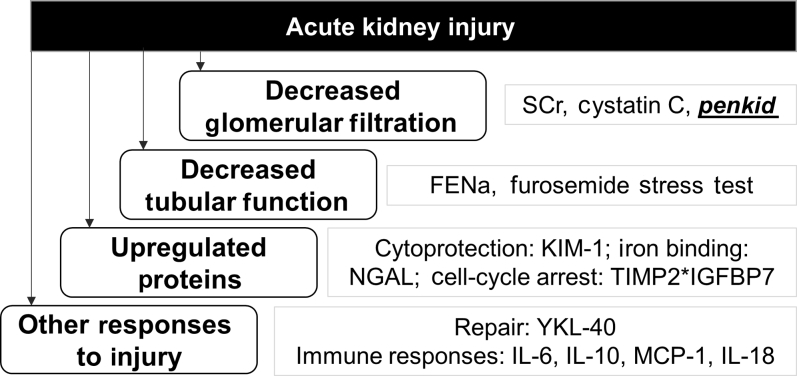
Many biomarkers that directly measure injury to kidney tubules are being tested to overcome these limitations of SCr. More than 1000 articles evaluating biomarkers in AKI were published in the past decade.4 Prominent among these are neutrophil gelatinase−associated lipocalin (NGAL), kidney injury molecule−1, interleukin-18, and, most recently, the product of tissue inhibitor of metalloproteinase−2 and insulin-like growth factor-binding protein−7 (TIMP2*IGFBP7). These biomarkers aim to overcome the limitations of SCr by directly measuring tubular injury or function rather than GFR (Figure 1). Among these, only TIMP-2*IGFBP-7 is approved for diagnostic use in the United States, whereas NGAL is available in the European Union, Canada, and various Asian and South American countries.
Figure 1.
Selected biomarkers of acute kidney injury. FENa, fractional excretion of sodium; IGFBP-7, insulin-like growth factor binding protein–7; IL, interleukin; KIM-1, kidney injury molecule–1; MCP-1, monocyte chemotactic protein 1; NGAL, neutrophil gelatinase-associated lipocalin; SCr, serum creatinine; TIMP2, tissue inhibitor of metalloproteinases–2.
In the study published in the November 2018 issue of Kidney International Reports, Hollinger et al.5 present results of a novel biomarker proenkephalin A 119-159 (penkid) in patients admitted to the intensive care unit (ICU) with sepsis and septic shock. Penkid is a 5-kDa, stable breakdown product of enkephalins, accumulates in the blood in settings of reduced GFR, and is associated with AKI and mortality in patients with sepsis and heart failure.6, 7 In this study, the authors evaluated penkid in two distinct ICU cohorts: the Kid-SSS study (n = 583) and the FROG-ICU study (n = 536). In the Kid-SSS cohort, 60% of participants were on mechanical ventilation, 58% were on vasopressors, and 28-day mortality among study participants was 22%. Penkid was measured in blood samples collected within 24 hours of ICU admission. The primary outcome was occurrence of major adverse kidney events (MAKE) at 7 days, which was a composite of death, dialysis, or persistent renal dysfunction. Penkid was 3-fold higher in those who experienced MAKE, with a standardized odds ratio of 3.3 (1.8–6) after controlling for key confounders such as age, sex, and estimated GFR (eGFR), but not for changes in SCr or urine output. Penkid showed an area under the receiver operating characteristic curve (AUC) for MAKE of 0.84 (95% confidence interval, 0.80–0.87), which was similar to the AUC of SCr (0.83 [0.80–0.87]). In 212 (37%) patients with a renal component of a Sequential Organ Failure Assessment (SOFA) score of 0 (SCr ≤ 1.2 mg/dl), the authors demonstrated that penkid had a better AUC than SCr (0.78 vs. 0.64, P < 0.001). Because patient harm could occur from failure to identify AKI using SCr-based definition in sepsis, penkid could help risk stratify this subgroup missed by SCr. For example, in those with admission SCr ≤ 1.2 mg/dl, <5% of those with penkid values <84.2 experienced MAKE, whereas about 20% of those with penkid value >84.2 experienced this outcome.
This study has several strengths. First, the authors show consistent association of penkid with MAKE in 2 separate cohorts. However, it should be noted that none of the predictions (such as AUC) of derivation cohort were tested in the validation cohort. Second, the authors present detailed results comparing the discriminative ability of penkid to existing biomarkers. The most important limitation is that penkid is a marker of GFR, not tubular injury. Thus, it is expected to have many of the same limitations of other markers of GFR such as SCr and cystatin C. Not surprisingly, the overall performance of SCr and penkid for AKI was similar in this and prior studies.7, 8 The authors identified a subset of patients with low SCr in whom penkid outperformed SCr, but its performance in this subgroup was much lower than in the overall cohort (AUC, 0.78). In this subgroup, only 1 in 5 participants with a penkid level above the cut-off experienced MAKE outcomes. Thus, 4 in 5 patients will incorrectly be classified as at risk for MAKE with this biomarker. Second, the authors did not compare penkid to any of the other biomarkers of AKI such as neutrophil gelatinase-associated lipocalin (NGAL) or TIMP2*IGFBP7. However, in a recent study, penkid showed a higher AUC as compared with TIMP2*IGFBP7 for AKI (AUC, 0.91 vs. 0.67) and for renal replacement therapy (0.78 vs. 0.68).9 Another study showed that, in septic patients, penkid had a higher AUC than NGAL for AKI (0.73 vs. 0.68) and for renal replacement therapy (0.87 vs. 0.74).7 In patients with sepsis in the emergency department, penkid had a similar AUC to that of NGAL (see Supplementary Reference).
No AKI biomarker has emerged as a reliable diagnostic test for clinical use in human AKI. Despite excellent performance in animal studies and small human studies, these biomarkers showed underwhelming performance in large human AKI cohorts. This could be due to the heterogeneity of human AKI, in which no single biomarker could capture all the different subtypes of AKI, or due to comparison of novel biomarkers to serum creatinine, which itself is an imperfect marker of AKI, as noted above. Moreover, in the absence of drug therapies for AKI and only minor improvements in prediction above SCr, the cost of these novel biomarkers is perhaps too burdensome for widespread clinical use. Implementation studies of AKI biomarkers, that is, demonstration that integration of these biomarkers in clinical care improves care or reduces cost, are also lacking. It is unclear whether penkid has overcome any of these limitations of AKI biomarkers. Penkid is also a marker of GFR similar to SCr, and its clinical utility over this existing and widely available biomarker remains to be demonstrated.
Disclosure
The author declared no competing interests.
Acknowledgments
DGM is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K23DK117065. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary Reference.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 2.Moledina D.G., Parikh C.R. Phenotyping of acute kidney injury: beyond serum creatinine. Semin Nephrol. 2018;38:3–11. doi: 10.1016/j.semnephrol.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W.R., Craven T.E., Malhotra R. Kidney damage biomarkers and incident chronic kidney disease during blood pressure reduction: a case-control study. Ann Intern Med. 2018;169:610–618. doi: 10.7326/M18-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh C.R., Mansour S.G. Perspective on clinical application of biomarkers in AKI. J Am Soc Nephrol. 2017;28:1677–1685. doi: 10.1681/ASN.2016101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollinger A., Wittebole X., François B. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int Rep. 2018;3:1424–1433. doi: 10.1016/j.ekir.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng L.L., Squire I.B., Jones D.J.L. Proenkephalin, renal dysfunction, and prognosis in patients with acute heart failure: a GREAT Network study. J Am Coll Cardiol. 2017;69:56–69. doi: 10.1016/j.jacc.2016.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Kim H., Hur M., Lee S. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Ann Lab Med. 2017;37:388–397. doi: 10.3343/alm.2017.37.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah K.S., Taub P., Patel M. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin Nephrol. 2015;83:29–35. doi: 10.5414/cn108387. [DOI] [PubMed] [Google Scholar]
- 9.Gayat E., Touchard C., Hollinger A., Vieillard-Baron A., Mebazaa A., Legrand M. Back-to-back comparison of PenKid with NephroCheck(R) to predict acute kidney injury at admission in intensive care unit: a brief report. Crit Care. 2018;22:24. doi: 10.1186/s13054-018-1945-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.